Abstract
Little is known about the influence of maternal inflammation on neonatal outcome. Production of IL-1β in the lungs of newborn infants is associated with bronchopulmonary dysplasia. Using bitransgenic (bi-TG) mice in which human (h) IL-1β is expressed with a doxycycline-inducible system controlled by the Clara cell secretory protein promoter, we have shown that hIL-1β expression causes a bronchopulmonary dysplasia–like illness in infant mice. To study the hypothesis that maternal hIL-1β production modifies the response of the newborn to hIL-1β, doxycycline was administered to bi-TG and control dams from Embryonic Day 0, inducing production of hIL-1β by the bi-TG dams before hIL-1β production started in their bi-TG fetuses, or from Embryonic Day 15, inducing simultaneous production of hIL-1β by both the bi-TG dams and their bi-TG fetuses. In addition to the lungs, hIL-1β was expressed at low levels in the uteri of bi-TG dams. Maternal inflammation preceding fetal inflammation increased the survival and growth of hIL-1β–expressing pups, enhanced alveolarization, and protected the airways against remodeling and goblet cell hyperplasia. Maternal hIL-1β production preceding fetal hIL-1β production caused silencing of several inflammatory genes, including CXC and CC chemokines, murine IL-1β, serum amyloid A3, and Toll-like receptors 2 and 4, and suppressed the expression of chitinase-like lectins Ym1 and Ym2 in the lungs of infant mice. Maternal inflammation protects the newborn against subsequent hIL-1β–induced lung inflammation and injury. In contrast, induction of hIL-1β production simultaneously in bi-TG dams and their fetuses offered no protection against inflammatory lung disease in the neonate.
Keywords: lung morphogenesis, cytokine, chemokine, premature birth
CLINICAL RELEVANCE.
Bronchopulmonary dysplasia (BPD) is the major inflammatory lung disease in premature infants. Although maternal inflammation is a common antecedent of premature birth, little is known about the influence of maternal inflammation on the development of BPD. The present results show that maternal inflammation preceding inflammation in the fetal lung protects the newborn against inflammatory lung disease. These unexpected findings help us to understand the complexity of the responses of newborn infants to perinatal inflammation and infection.
Preterm infants born at less than 30 weeks of gestational age are at a high risk of severe respiratory morbidity. Due to pulmonary immaturity and lack of surfactant, they often suffer from respiratory distress syndrome (RDS) after birth. Approximately 30% of these very premature infants develop bronchopulmonary dysplasia (BPD), a chronic lung disease defined as need of supplemental oxygen beyond 36 weeks postmenstrual age (1). This illness is characterized histologically by inflammation and abnormal alveolar and vascular development of the lung (2). In spite of advances in neonatal care, BPD remains an important cause of chronic pulmonary morbidity, poor growth, and mortality in infants.
Because early preterm birth is often preceded by intrauterine infection, the majority of infants born at less than 30 weeks of gestation have been exposed to antenatal inflammation. The frequency of positive cultures of chorioamnionic tissue among women with spontaneous labor at less than 30 weeks of gestation exceeds 70% (3). Women with positive fetal membrane cultures have histological findings of leukocytosis in the fetal membranes, and high levels of inflammatory cytokines, such as IL-1β, TNF, and IL-6, in amniotic fluid (3, 4). Intrauterine infection sometimes begins early in pregnancy, and remains undetected for months before preterm delivery. For example, amniotic fluid concentration of IL-6 is elevated already at 15 to 20 weeks of gestation in some pregnancies ending in preterm delivery at 23 to 30 weeks (5, 6). In addition to intrauterine infections, extrauterine infections, such as periodontitis (7) and pneumonia (8), are also associated with preterm delivery.
Antenatal exposure to inflammation may modulate the respiratory outcome of premature infants. For example, histologic chorioamnionitis is associated with a lower risk of RDS in neonates born at a gestational age between 23 and 32 weeks (9, 10). Experimental studies indicate that fetal exposure to inflammation accelerates the maturation of the fetal lung, and thereby protects the newborn against RDS. Intra-amniotic administration of IL-1 or endotoxin induces surfactant production and improves gas exchange and lung mechanics in preterm animals (11, 12). Exposure to inflammation may thus be advantageous for the pulmonary adaptation in the early postnatal period. The impact of antenatal inflammation on the development of the chronic lung disease, BPD, is less clear. Some clinical studies suggest that chorioamnionitis (9) or increased concentrations of inflammatory cytokines, such as IL-1β, IL-6, TNF, or IL-8 in amniotic fluid (13) or in cord blood (14), are linked with the development of BPD. However, other studies have not found a relationship between antenatal inflammation and BPD (10). Moreover, Van Marter and colleagues (15) found that chorioamnionitis decreased the risk of BPD. The reasons for these discrepanicies are unclear, but may be related to differences in the patient populations (e.g., varying gestational age, time and invasiveness of antenatal inflammation, reception of postnatal therapies injurious to the lung, such as oxygen administration, ventilator treatment, or possible postnatal infection).
IL-1β is a central inflammatory cytokine that is present in the amniotic fluid of women with chorioamnionitis and preterm labor (16). Increased levels of IL-1β are also found in amniotic fluid and tracheal aspirates of infants developing BPD (13, 17). To study the role of inflammation in perinatal lung injury independent of confounding factors, such as preterm birth, oxygen therapy, or ventilator-associated injury, we developed a bitransgenic (bi-TG) Clara cell secretory protein (CCSP)/reverse tetracycline transactivator (rtTA)–(tet operator [tetO])7–IL-1β mouse in which mature human (h) IL-1β is expressed in the lung epithelium with a doxycycline-inducible system controlled by the rat CCSP promoter. We have shown that, whereas chronic hIL-1β expression in the lungs of adult mice causes inflammation, emphysema, and airway remodeling (18), perinatal production of hIL-1β is sufficient to cause a pulmonary disease in the newborn mouse that clinically and histologically closely resembles BPD (19), suggesting that inflammation in the lung per se is an important pathogenetic factor in BPD.
Little is known about the influence of maternal inflammation on the development of inflammatory lung diseases, such as BPD, in the newborn. We hypothesized that maternal inflammation during pregnancy modifies the responses of the offspring to inflammatory insult. To study this hypothesis, we compared hIL-1β–induced immune responses and lung injury in the progeny of dams producing hIL-1β before hIL-1β production in their bi-TG fetuses, in the progeny of dams producing hIL-1β at the same time as their bi-TG fetuses, and in the progeny of control dams. Doxycycline administration induced hIL-β production not only in the lungs, but at low levels also in the uteri of pregnant bi-TG dams. The present study shows, for the first time, that maternal hIL-1β production preceding fetal hIL-1β production causes silencing of inflammatory genes in the lungs of the offspring, protecting them against hIL-1β–induced lung disease and poor growth, as well as reducing mortality. Simultaneous hIL-1β production in the dams and fetuses, on the other hand, had no effect on lung inflammation or injury in the infant mice.
MATERIALS AND METHODS
Conditional Expression of IL-1β in the Mouse Lung
Bi-TG CCSP-rtTA/(tetO)7CMV–IL-1β mice expressing mature hIL-1β in the lung epithelium under conditional control were generated by mating CCSP-rtTA+/+ activator transgenic control dams (20) expressing the rtTA under the control of the rat CCSP promoter with (tetO)7CMV–IL-1β+/− males in which the tetracycline-responsive promoter (tetO)7CMV drives the expression of active, mature hIL-1β (18), or by mating bi-TG CCSP-rtTA/(tetO)7CMV–IL-1β dams with wild-type males. Littermate single transgenic CCSP-rtTA+/− mice were used as controls. We chose to use these mice as controls to be able to study specifically the effects of hIL-1β expression on the lung phenotype (21). All mice were genotyped by PCR analysis of genomic tail DNA using primers specific for transgene constructs, as previously described (18). The mice were in FVB/N background.
Doxycycline (Sigma, St. Louis, MO) administration to pregnant mice in drinking water at a concentration of 0.5 mg/ml was started on plug date, counted as Embryonic Day (E) 0, or at E15, and was continued until either E14, day of birth (counted as Postnatal Day [PN] 0), or PN7, as indicated. The light-sensitive doxycycline solution was protected from light by covering cage bottles with aluminum foil, and the solution was changed three times per week. The CCSP promoter is inactive in the fetus until E14, and is thereafter active throughout fetal and postnatal life (22). This means that hIL-1β production can be induced by doxycycline in the bi-TG dams at any time, whereas hIL-1β production in bi-TG fetuses is only inducible after E14, as previously shown (19). This feature of the transgenic system makes it possible to study how maternal hIL-1β production, either before or simultaneous with fetal hIL-1β production, modifies the responses of the newborn to IL-1β.
Animal Care
The mice were housed in pathogen-free conditions, and all experiments were conducted in accordance with Animal Research Ethics Committee guidelines at the University of Gothenburg. All animals had access to water and laboratory chow ad libitum. Blood samples from pregnant dams at E14 were collected using EDTA as anticoagulant by heart punction after anesthesia with intraperitoneal injection of a mixture of ketamine, xylazine, and acepromazine. The lungs, the uteri, the placentas, and the fetal membranes were also collected. Blood smears were prepared, and the remaining blood was centrifuged at 1,000 × g for 10 minutes to separate the plasma and the blood cell fraction. For lung sample collection from infant mice, pups were anesthetized by intraperitoneal injection with a mixture of ketamine, xylazine, and acepromazine on PN0 or PN7, the abdomen was opened, and the animal was exsanguinated by transection of abdominal vessels.
RNA Isolation and Quantitative RT-PCR
Total RNA from fetal lung tissue, fetal membranes, maternal blood cells, maternal liver, maternal uterus, and placentas was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, and treated with RNase-free DNase (DNA-free; Ambion, Austin, TX). Total RNA (1 μg) was reverse transcribed (Omniscript; Qiagen, Hilden, Germany), and cDNA was analyzed by real-time PCR using gene-specific, intron-spanning primers. For quantification of lipocalin2 (24p3; the mouse homolog of human neutrophil gelatinase–associated lipocalin), monocyte chemoattractant protein-1 (CCL2), monocyte chemoattractant protein-3 (CCL7), keratinocyte chemokine (the mouse homolog of human CXC chemokine growth-related oncogene-α, CXCL1), LPS-induced CXC chemokine (the mouse homolog of human epithelial–derived neutrophil-activating peptide-78, CXCL5), CXC chemokine receptor (CXCR)-2, matrix metalloproteinase (MMP)-9, MMP-12, murine (m) IL-1β, IL-1 receptor type (R) I, IL-1RII, IL-1 receptor antagonist (IL-1Ra), S100 calcium-binding protein A (S100A) 8 (myeloid-related protein-8), S100A9 (myeloid-related protein-14), serum amyloid A3 (SAA3), Toll-like receptor (TLR) 2, TLR4, Ym1 (chitinase 3–like 3) and Ym2 (chitinase 3–like 4) mRNA levels, PCR was performed on 20 ng of cDNA, using Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) and an Mx3000P real-time PCR instrument (Stratagene). Specificity of PCR was confirmed by gel analysis during reaction optimization and melting curve analysis of each amplification product. Relative quantification of starting amounts of mRNA was performed with Mx3000P instrument software from amplification curves using standard curves obtained from dilution series of cDNA. The results were normalized to β-actin mRNA levels. Primer sequences used (forward and reverse, respectively, 5′ to 3′) were as follows:
24p3: ACA ACT GAA TGG GTG GTG AGT GTG
AGA AGA GGC TCC AGA TGC TCC TTG
β-actin: TCC GTA AAG ACC TCT ATG CCA ACA
CTC AGG AGG AGC AAT GAT CTT GAT
CCL2: GCT CTC TCT TCC TCC ACC ACC AT
GCT CTC CAG CCT ACT CAT TGG GAT
CCL7: TCT GCC ACG CTT CTG TGC CT
GCT CTT GAG ATT CCT CTT GGG GAT
CXCL1: AAA CCG AAG TCA TAG CCA CAC TCA
CTT GGG GAC ACC TTT TAG CAT CTT
CXCL5: GCT GGC ATT TCT GTT GCT GTT CA
ATG ACT TCC ACC GTA GGG CAC TGT
CXCR2: CCT CAG ACT TTT GGC TTC CTC GT
CGC AGT GTG AAC CCG TAG CAG A
MMP-9: TTC GCA GAC CAA GAG GGT TTT C
AAG ATG TCG TGT GAG TTC CAG GGC
MMP-12: CTG TCT TTG ACC CAC TTC GCC A
TCC TGC CTC ACA TCA TAC CTC CAG T
mIL-1β: AGC CCA TCC TCT GTG ACT CA
TGT CGT TGC TTG GTT CTC CT
IL-1RI: TGG AGG GAC AGT TTG GAT ACA AG
ATC AGC CTC CTG CTT TTC TTT AC
IL-1RII: GAT AAC CTG CTG GTG TGT GA
TCT GTC CAT TGA GGT GGA GA
IL-1Ra: ATA GTG TGT TCT TGG GCA TCC A
TGT CTT CTT CTT TGT TCT TGC TCA G
S100A8: GAG CAA CCT CAT TGA TGT CTA
TGC ATT GTC ACT ATT GAT GTC CA
S100A9: GCC AAC AAA GCA CCT TCT CAG AT
GCC ATC AGC ATC ATA CAC TCC TCA A
SAA3: TGC TCG GGG GAA CTA TGA TGC T
CCA CTC GTT GGC AAA CTG GTC A
TLR2: CCG AAA CCT CAG ACA AAG CGT CA
TCA CAC ACC CCA GAA GCA TCA CAT
TLR4: GCA AAG TCC CTG ATG ACA TTC CTT
CCA CAG CCA CCA GAT TCT CTA AA
Ym1: GCT CAT TGT GGG ATT TCC AGC A
CCT CAG TGG CTC CTT CAT TCA GAA
Ym2: TTG GAG GAT GGA AGT TTG GAC CT
TGA CGG TTC TGA GGA GTA GAG ACC A
hIL-1β and Total Protein Measurement
The tissue (fetal lung and maternal uterus) was homogenized in PBS containing protease inhibitor (Complete Protease Inhibitor; Roche Diagnostics, Basel, Switzerland) and centrifuged at 10,000 × g for 10 minutes to remove cell debris. Supernatant was used for analysis. Total protein concentration was measured using the bicinchoninic acid method according to the manufacturer's instructions (Sigma). Concentration of hIL-1β was measured using DuoSet hIL-1β ELISA development kit (R&D Systems, Abingdon, UK), specific for hIL-1β, with no cross-reactivity with mIL-1β. Assay standard concentration range was 3.9–250.0 pg/ml. Concentration of hIL-1β in maternal plasma and maternal uterus, and placenta homogenates, was measured using Quantikine HS hIL-1β ELISA development kit (R&D Systems), specific for hIL-1β, with no cross-reactivity with mIL-1β. Assay standard concentration range was 0.125–8.000 pg/ml.
Lung Histology and Immunohistochemistry
Lungs were inflation-fixed as previously described (18). The chest cavity was opened and the trachea exposed. A blunt cannula was inserted and tied to the trachea, and the lungs were inflated by instillation of PBS-buffered 4% paraformaldehyde fixative at a pressure of 25 cm H2O. After overnight fixation at +4°C, the tissue was rinsed in PBS and graded ethanol, dehydrated, and processed through conventional paraffin embedding. Tissue sections (5 μm) were stained with hematoxylin and eosin, or Alcian blue/periodic acid-Schiff (PAS) (pH 2.5) (23) and counterstained with Mayer's hematoxylin.
Immunohistochemistry for neutrophils and macrophages was performed using monoclonal rat anti-mouse neutrophils, clone 7/4 (Serotec, Oxford, UK) and monoclonal rat anti-mouse Mac3, clone M3/84 (BD Biosciences Pharmingen, San Diego, CA) antibodies, respectively. Biotinylated secondary antibodies, rabbit anti-rat, and avidin–biotin peroxidase (Vectastain Elite ABC) with NovaRed were used according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA). Sections were slightly counterstained with Mayer's hematoxylin.
Cell Counts
Maternal and fetal lung sections were immunostained using primary antibodies specific for neutrophils and macrophages. The numbers of stained cells were counted in 10 random high-power fields (×40 lens) in each section (four animals or more per group), and average numbers of positive cells per square millimeter were calculated. The number of white cells in maternal blood was counted using a hemacytometer. Differential leukocyte counts were performed from blood smears stained with Wright's Giemsa stain.
Morphometric Analysis of Airspace Size and Septal Wall Thickness
Quantification of distal airspace size at PN7 was performed from hematoxylin and eosin–stained lung tissue sections, using the mean chord length as a measure of alveolar size, as previously described (19). A minimum of eight representative, nonoverlapping fields from lungs of four to nine mice of each genotype and treatment group were acquired in 8-bit greyscale, using a 20× lens, at a final magnification of 1.89 pixels/μm using a Nikon Eclipse E800 microscope and DXM1200 digital camera (Nikon, Tokyo, Japan). Areas of bronchiolar airways and blood vessels were excluded from the analysis. Chord length analysis was performed using the public domain program NIH Image with a chord length macro (available from the U.S. National Institutes of Health at http://rsb.info.nih.gov/nih-image). To obtain consistent results, images were thresholded using equal threshold levels for all treatment groups. Binarized, inverted lung micrographs were subjected to a logical “AND” operation with horizontal and vertical grids of straight lines. The lengths of lines overlying alveolar space were then averaged as the mean chord length. Chord lines touching the edges of the image fields were excluded from analysis, hence the calculated mean chord length is representative of line segments touching air space walls at both ends. The same binarized images were used to assess the thickness of saccular/alveolar septa, as previously described (24). Ten nonoverlapping image fields per animal, with a minimum of five animals in each treatment and genotype group, were analyzed. Straight lines (∼60–80 per field) were drawn at 90° angles across the narrowest segments (to minimize the number of tangential sections) of septa of distal airspaces. The mean length of lines crossing the septa was determined using NIH Image software.
Goblet Cell Quantification
The percentage of goblet cells in the epithelium of 12–20 airways in at least four mice from each group was assessed at PN7 on Alcian blue/PAS-stained lung tissue sections. The distribution of airways having less than 10%, 11–40%, 41–80%, or more than 81% PAS-positive cells in the epithelium was then calculated. The trachea and the primary bronchi were not included in this analysis.
Statistical Analysis
Measurement values are expressed as mean (±SEM). Groups were compared with Student's two-tailed unpaired t test or with the Mann-Whitney U-test, as appropriate. Neonatal mortality data were analyzed using Kaplan-Meier survival analysis and logrank (Mantel-Cox) test. P values less than 0.05 were considered statistically significant.
RESULTS
Pulmonary Phenotype and Systemic Inflammation in hIL-1β–Expressing Dams
All dams appeared healthy during the experiments, and maintained a normal weight and activity level. In the present model, hIL-1β production in the lungs increased rapidly during the first 2 days after initiation of doxycycline administration, and reached a plateau of 79 (±12) ng hIL-1β/mg protein after 5 days of doxycycline treatment (18). Because prolonged pulmonary production of hIL-1β in adult mice causes emphysema (18), we studied whether hIL-1β expression in the lungs of pregnant mice causes alveolar destruction. Pregnant control and bi-TG dams were given doxycycline from the beginning of gestation until E14. The alveolar chord length was similar in both groups of dams (control dam, 37.4 ± 2.3 μm, versus bi-TG dam, 39.4 ± 2.5 μm; not significant [NS]; n = 4). Thus, pulmonary hIL-1β production in the maternal lung during the first 14 days of pregnancy did not cause airspace enlargement in the dams.
Immunostaining of lung sections with specific antibodies against neutrophils and macrophages revealed that hIL-1β–producing dams had a pulmonary inflammation characterized by an increased number of neutrophils (control dam, 9.1 ± 1.9/mm2, versus bi-TG dam, 57.4 ± 10.1/mm2; P ≤ 0.01; n = 4) and macrophages (control dam, 22.6 ± 2.7/mm2, versus bi-TG dam, 65.2 ± 4.9/mm2; P ≤ 0.01; n = 4).
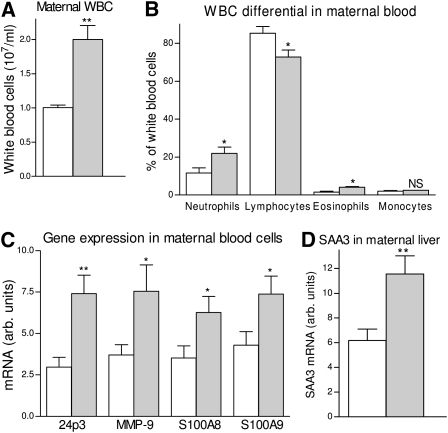
The levels of hIL-1β in the plasma of bi-TG dams were below the detection limit of the assay, implying that very little, if any, hIL-1β produced in the lung epithelium reached into the circulation. The bi-TG dams had a mild systemic inflammation. They had a higher white blood cell count than the control dams (P ≤ 0.01; Figure 1A), as well as an altered differential count that revealed increased percentages of neutrophils and eosinophils, and a decreased percentage of lymphocytes (P ≤ 0.05; Figure 1B). Accordingly, the mRNA expression of several genes associated with inflammation, including 24p3, MMP-9, S100A8, and S100A9, was higher in the blood cells of bi-TG dams than in control dams (Figure 1C). The mRNA expression of the acute-phase protein, SAA3, in maternal liver was higher in bi-TG dams than in control dams (P ≤ 0.01; Figure 1D), further confirming the presence of a systemic inflammatory response in hIL-1β–expressing dams.
Figure 1.
Maternal inflammation. Pregnant bitransgenic (bi-TG) and control dams received doxycycline in drinking water from Embryonic Day (E) 0 until the dams were killed at E14. (A) White blood cell (WBC) counts in dams. Bi-TG dams had a significantly higher number of circulating white blood cells than control animals. (B) Differential leukocyte counts in the blood of dams. Human (h) IL-1β–producing dams had higher percentages of neutrophils and eosinophils, but a lower percentage of lymphocytes than control animals. (C) mRNA expression of inflammatory genes in the blood cells of dams. Bi-TG dams had higher expression of lipocalin2 (24p3), MMP-9, S100 calcium-binding protein A (S100A) 8, and S100A9 than control dams. (D) mRNA expression of SAA3 in maternal liver. Bi-TG dams had higher expression of SAA3 than controls. Open bars, control dams; gray bars, bi-TG dams; n ≥ 4 in each group; *P ≤ 0.05, **P ≤ 0.01 control dams versus bi-TG dams.
hIL-1β Expression and Production in the Uteri of Bi-TG Dams
The expression of hIL-1β in the present transgenic system is driven by the rat CCSP promoter. CCSP or uteroglobin, expressed at high levels in the Clara cells in the airways, is also expressed in the pregnant or pseudopregnant uterus of mammals (25–27). We therefore studied whether hIL-1β was expressed in the uteri of bi-TG dams that had received doxycycline from E0 until being killed at E14. hIL-1β mRNA expression was present in the uteri of these mice. The level of hIL-1β in the uterine homogenates of these mice was 21.0 (±2.7) pg/ml, or 0.87 (±0.25) pg/mg protein (n = 10). No mRNA expression of hIL-1β was detected in the placentas or fetal membranes.
Inflammation in Uterus, Placenta, and Fetal Membrane
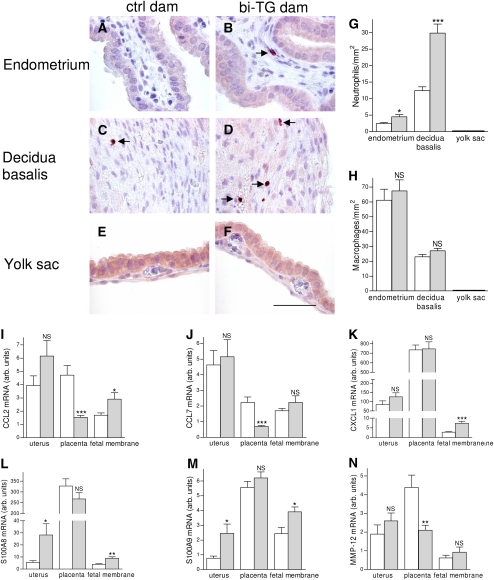
To assess whether hIL-1β production in the uterus caused inflammation in uterine or gestational tissues of bi-TG dams, we counted the numbers of neutrophils and macrophages in uteri, placentas, and fetal membranes from both control and bi-TG dams given doxycycline from E0 until being killed at E14. Neutrophils in the uterus and placenta were seen mostly in the endometrium and decidua basalis, respectively (Figures 2A–2D). The numbers of neutrophils were higher in the endometrium and decidua basalis of bi-TG dams than in those of control dams (Figures 2A–2D and 2G). The numbers of macrophages in the uterus or placenta were not significantly affected by hIL-1β expression in the uterus (Figure 2H). No neutrophils or macrophages were detected in the fetal membranes (yolk sac and amnion) in either maternal group (Figures 2E–2G). The expression of monocyte-attractant CC chemokines, CCL2 and CCL7, and of MMP-12, was lower in placentas from bi-TG dams than in those from control dams, but no such difference was detected in the uteri (Figures 2I–2J and 2N). In both maternal genotypes, the expression of the neutrophil-attractant CXC chemokine, CXCL1, in the placentas was roughly eightfold higher than in the uterus, and roughly 80-fold higher than in the fetal membranes (Figure 2K). The mRNA expression of CXCL1 in the uteri and placentas was not influenced by the genotype of the dam, whereas the expression of CXCL1 was higher in the fetal membranes (yolk sac and amnion) of bi-TG dams than in those of control dams (Figure 2K). The expression of S100A8 and S100A9 was higher in uteri and fetal membranes of bi-TG dams than in control animals, but no difference was seen in the placentas (Figures 2L–2M).
Figure 2.
Maternal inflammation. Pregnant bi-TG and control dams received doxycycline in drinking water from E0 until dams were killed at E14. (A–F) Immunohistochemical staining for neutrophils in the endometrium, decidua basalis, and yolk sac. (G) Neutrophil counts in uterine and gestational tissues. Bi-TG dams had significantly higher numbers of neutrophils in the endometrium and decidua basalis, but no neutrophils were seen in the fetal membranes (yolk sac and amnion). (H) Macrophage counts in uterine and gestational tissues. The number of macrophages in endometrium, decidua basalis, or fetal membranes (yolk sac and amnion) was not affected by maternal genotype. No macrophages were detected in the fetal membranes. (I–N) mRNA expression of inflammatory genes in uteri, placentas, and fetal membranes (yolk sac and amnion). Expression of S100A8 and S100A9 was increased in the uteri of bi-TG dams, whereas the expression of CCL2, CCL7, and MMP-12 was decreased in placentas from bi-TG dams. Open bars, control dams; gray bars, bi-TG dams; NS, not significant; n ≥ 5; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 control dams versus bi-TG dams. Scale bar, 50 μm.
hIL-1β Production in the Lungs of Bi-TG Pups of Control and Bi-TG Dams
When doxycycline is administered to the dams from the beginning of pregnancy, hIL-1β mRNA expression and protein production in the lungs of bi-TG fetuses start at E14, rapidly increase thereafter, and reach a plateau at E16.5 (19). To confirm that maternal transgenic status did not influence the expression of hIL-1β in response to doxycycline in bi-TG offspring, the levels of mRNA expression and protein production were measured in bi-TG pups of control and bi-TG dams. All dams received doxycycline from the beginning of pregnancy until death of the pups at PN7. Both hIL-1β mRNA expression (bi-TG pups of control dam, 5.6 ± 0.9 [n = 6], versus bi-TG pups of bi-TG dam, 5.3 ± 0.8 [n = 6]; NS) and hIL-1β protein level (bi-TG pups of control dam, 146.9 ± 7.3 ng hIL-1β/mg protein [n = 8], versus bi-TG pups of bi-TG dam, 163.6 ± 10.2 ng hIL-1β/mg protein [n = 5]; NS) were similar in the two groups of hIL-1β–producing pups. As expected, hIL-1β mRNA expression and hIL-1β protein production were not detected in the lungs of any of the control pups in either maternal group.
Maternal hIL-1β Expression Affected the Growth and Survival of hIL-1β–Expressing Infant Mice
In all groups of dams, pups expressing hIL-1β were asymptomatic during the first days of life, but developed respiratory symptoms (chest retractions) after PN3–PN4, at which time their weight gain also started to decrease compared with that of control littermates. The respiratory symptoms were more obvious in the bi-TG pups of both groups of control dams and those of bi-TG dams given doxycycline from E15 than in bi-TG pups of bi-TG dams given doxycycline from E0.
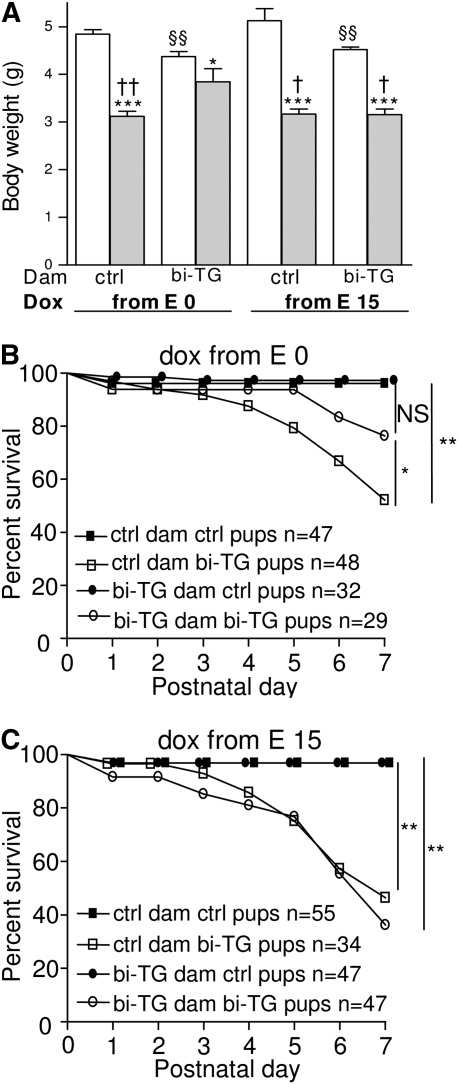
The birth weights of hIL-1β–expressing pups and their control littermates did not differ (data not shown). In all maternal groups, pups expressing hIL-1β had lower body weight at PN7 than their control littermates (Figure 3A). Interestingly, maternal hIL-1β production affected the weight of the control pups, as control animals born to bi-TG dams were smaller than those born to control dams (P ≤ 0.01; Figure 3A). In spite of this, bi-TG pups of bi-TG dams given doxycycline from E0 weighed more than all other groups of bi-TG pups (Figure 3A).
Figure 3.
Influence of maternal hIL-1β production on postnatal growth and mortality of the offspring. Pregnant bi-TG and control dams were given doxycycline in drinking water from E0 or E15 until the pups were killed at Postnatal Day (PN) 7. (A) Weight of pups at PN7. In all maternal groups, bi-TG pups were smaller than control animals. Bi-TG pups of hIL-1β–expressing dams given doxycycline from E0 weighed more than those of control dams and those of hIL-1β–expressing dams given doxycycline from E15 (open bars, control pups; gray bars, bi-TG pups; n ≥ 15 in each group). (B) Survival of the offspring of dams given doxycycline from E0 until pups were killed on PN7. The survival of bi-TG pups of bi-TG dams was significantly better than that of pups of control dams (P = 0.02). (C) Survival of offspring of dams given doxycycline from E15 until the pups were killed on PN7. No difference in survival was seen between the two groups of bi-TG pups. *P ≤ 0.05, ***P ≤ 0.001, bi-TG pups versus control pups within each group of pups; §§P ≤ 0.01, control pups of bi-TG dam versus control pups of control dam; †P ≤ 0.05, ††P ≤ 0.01, compared with bi-TG pups of bi-TG dam given doxycycline from E0. ctrl, control; dox, doxycycline.
In all groups of mice, one to three pups died during the immediate postnatal period (PN0–2). When doxycycline was administered from E0, the mortality of bi-TG pups of control dams increased after PN3, whereas very few bi-TG pups of hIL-1β–expressing dams died before PN5 (Figure 3B). With this doxycycline treatment, the survival of bi-TG pups of hIL-1β–expressing dams was better than that of bi-TG pups of control dams (bi-TG pups of bi-TG dams, 76%, versus bi-TG pups of control dams, 52%; P = 0.02; Figure 3B). In contrast, when doxycycline was administered from E15, the survival of the pups did not differ significantly between the two groups of dams (bi-TG pups of bi-TG dams, 36%, versus bi-TG pups of control dams, 48%; NS; Figure 3C). The survival of hIL-1β–expressing pups of bi-TG dams given doxycycline from E0 was better than that of those of bi-TG dams given doxycycline from E15 (bi-TG pups of bi-TG dams given doxycycline from E0, 76% versus bi-TG pups of bi-TG dams given doxycycline from E15, 36%; P = 0.001; Figures 3B and 3C).
Influence of Maternal hIL-1β Expression on Postnatal Lung Morphogenesis in hIL-1β–Expressing Newborn Mice
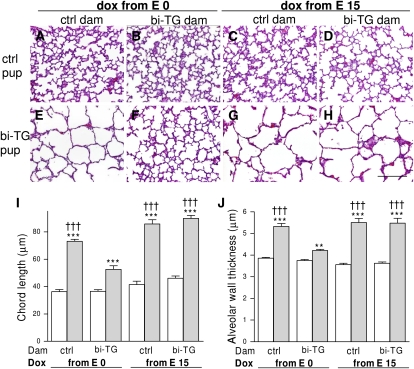
Alveolar septation was underway in control pups at PN7, independent of maternal genotype and time of doxycycline administration (Figures 4A–4D). Distal airspaces were large, and septation was lacking in the lungs of bi-TG pups of both groups of control dams and of bi-TG dam given doxycycline from E15 (Figures 4E, 4G, and 4H). In contrast, airspaces appeared smaller and more septa were present in the lungs of bi-TG pups of hIL-1β–expressing dams given doxycycline from E0 (Figure 4F). These results were confirmed by measurement of alveolar chord length. Although the chord lengths of all bi-TG pups in all four groups of dams were significantly greater than those of their control littermates (P ≤ 0.001), the chord length of bi-TG pups of hIL-1β–expressing dams given doxycycline from E0 was less (P ≤ 0.001) than that of bi-TG pups in the other groups (Figure 4I), indicating better alveolarization in pups of dams in which hIL-1β expression preceded fetal hIL-1β expression. There were no differences in chord length between control pups of control and bi-TG dams in either doxycycline treatment group.
Figure 4.
Alveolar septation and wall thickness in infant mice. Doxycycline was administered to pregnant bi-TG and control dams in drinking water from E0 or E15 until the pups were killed at PN7. (A–H) Pulmonary histology at PN7 (hematoxylin and eosin). Alveolar septation was underway in control mice (A–D). Septation was lacking in bi-TG pups of control dams given doxycycline from E0 (E), as well as in bi-TG pups of both control and bi-TG dams given doxycycline from E15 (G and H), whereas bi-TG pups of hIL-1β–expressing dams given doxycycline from E0 had smaller airspaces and more septa (F). (I) Chord length of the lungs of infant mice. The chord lengths were greater in hIL-1β–producing pups in all maternal groups than in control littermates. Maternal hIL-1β production preceding fetal hIL-1β production improved alveolar septation in bi-TG pups compared with the other groups of bi-TG pups. (J) Alveolar wall thickness in infant mice. Alveolar walls were thicker in bi-TG pups in all maternal groups than in control littermates. Maternal hIL-1β production preceding fetal hIL-1β ameliorated alveolar wall thinning in bi-TG offspring compared with the other groups of bi-TG pups. Open bars, control pups; gray bars, bi-TG pups; n ≥ 5 in each group; **P ≤ 0.01, ***P ≤ 0.001, bi-TG pups versus control pups; †††P ≤ 0.001, compared with bi-TG pups of bi-TG dams given doxycycline from E0. Scale bar, 200 μm.
Alveolar wall thickness was similar in all groups of control pups (Figure 4J). hIL-1β–expressing newborn mice born to both groups of control dams and those born to bi-TG dams given doxycycline from E15 had between 38 and 54% thicker alveolar walls than corresponding control animals (P ≤ 0.001; Figure 4J), whereas the walls in hIL-1β–expressing pups of dams with maternal hIL-1β expression preceding the fetal expression were only 12% thicker than those of control animals (P ≤ 0.01; Figure 4J). Thus, alveolar thinning was better in hIL-1β–expressing pups of hIL-1β–expressing dams given doxycycline from E0 than in the other groups of bi-TG pups (P ≤ 0.001; Figure 4J).
Maternal hIL-1β Expression Modulated Inflammation Induced by hIL-1β in the Alveolar Regions of the Lungs of Infant Mice
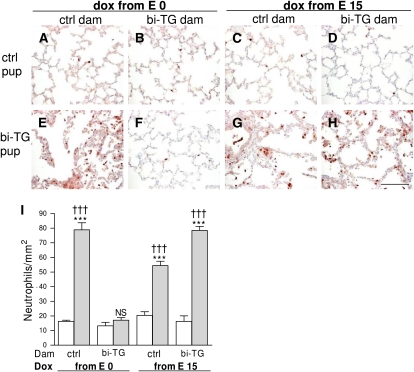
To assess inflammation in the alveolar regions in infant mice at PN7, immunostaining of lung sections was performed with specific antibodies against neutrophils and macrophages and the numbers of stained cells were counted in the alveolar lumen and alveolar walls. Areas of airways and blood vessels were excluded from this analysis. hIL-1β–expressing pups of bi-TG dams given doxycycline from E0 (Figures 5F and 5I) had fewer neutrophils in their alveolar regions than all other groups of hIL-1β–expressing pups (P ≤ 0.001; Figures 5E and 5G–5I). In fact, hIL-1β production in the lungs of pups of hIL-1β–expressing dams given doxycycline from E0 did not induce neutrophil infiltration into the alveoli or alveolar walls, as the numbers of neutrophils in the lungs of these mice (NS; Figures 5F and 5I) were similar to those in control mice (Figures 5A–5D and 5I).
Figure 5.
Neutrophils in the alveolar regions of infant mice. Pregnant bi-TG and control dams received doxycycline in drinking water from E0 or E15 until the pups were killed at PN7. (A–H) Immunohistochemical staining for neutrophils. More neutrophils were seen in the alveoli and alveolar walls of bi-TG pups (E, G, H) than in controls (A–D), except in the case of bi-TG pups of bi-TG dams given doxycycline from E0 (F). (I) Neutrophil counts in the lungs of the offspring. Neutrophil infiltration in response to pulmonary hIL-1β production in the infant mice was abrogated in pups of bi-TG given doxycycline from E0. Open bars, control pups; gray bars, bi-TG pups; n ≥ 4 in each group; ***P ≤ 0.001, bi-TG pups versus control pups; †††P ≤ 0.001, compared with bi-TG pups of bi-TG dam given doxycycline from E0. Scale bar, 100 μm. NS, not significant.
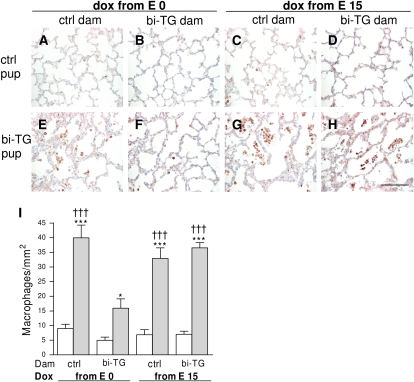
Fetal hIL-1β production resulted in infiltration of the lungs with macrophages in all groups of bi-TG offspring (Figure 6). Maternal hIL-1β production preceding fetal hIL-1β production suppressed the numbers of macrophages in the alveolar areas of the bi-TG offspring (P ≤ 0.001; Figures 6F and 6I) compared with the bi-TG offspring in the other groups (Figures 6E and 6G–6I).
Figure 6.
Macrophages in the alveolar regions of infant mice. Pregnant bi-TG and control dams received doxycycline in drinking water from E0 or E15 until the pups were killed at PN7. (A–H) Immunohistochemical staining for macrophages. More macrophages were seen in both groups of bi-TG offspring of control dams (E and G) and in bi-TG offspring of bi-TG dam given doxycycline from E15 (H) than in bi-TG pups of bi-TG dams given doxycycline from E0 (F) and in all groups of control pups (A–D). (I) Macrophage counts in the lungs of offspring. Maternal hIL-1β production preceding fetal hIL-1β production inhibited infiltration of the alveoli and alveolar walls of bi-TG pups with macrophages. Open bars, control pups; gray bars, bi-TG pups; n ≥ 4 in each group; *P ≤ 0.05, ***P ≤ 0.001, bi-TG pups versus control pups; †††P ≤ 0.001, compared with bi-TG pups of bi-TG dam given doxycycline from E0. Scale bar, 100 μm.
Maternal hIL-1β Production Influenced Airway Inflammation and Remodeling and the Expression of Ym1 and Ym2 in the Lungs of Infant Mice
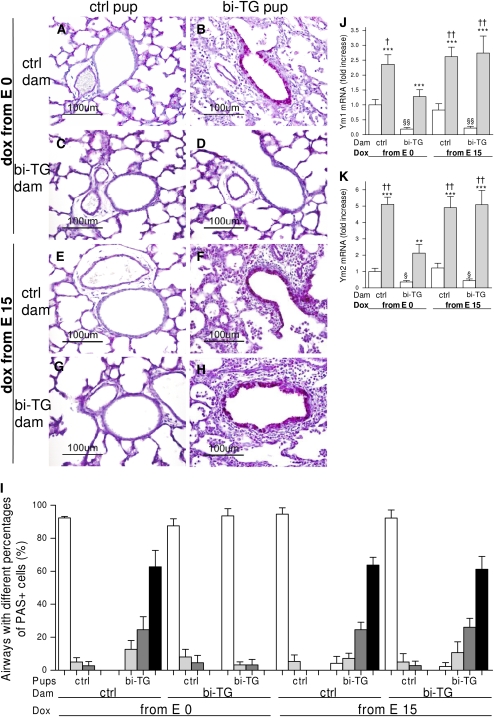
Airway structure was similar in all groups of control pups (Figures 7A–7D). Inflammatory cells accumulated in and around the airways of hIL-1β–expressing offspring of single transgenic dams (Figures 7E and 7G) and of bi-TG dams given doxycycline from E15 (Figure 7H), whereas few inflammatory cells were seen in the airways of hIL-1β–expressing offspring of bi-TG dams given doxycycline from E0 (Figure 7F). In addition, the airways of bi-TG offspring of control dams and of bi-TG dams given doxycycline from E15 were thicker and had more goblet cell hyperplasia (Figures 7E and 7G) than those of bi-TG offspring of bi-TG dams given doxycycline from E0 (Figure 7F). The suppressive effect of maternal IL-1β production on subsequent hIL-1β–induced goblet cell hyperplasia in the pups was confirmed when the proportion of PAS-positive cells in the epithelium of the airways was evaluated in the different groups (Figure 7I). The distribution of airways having less than 10%, 11–40%, 41–80%, or more than 81% PAS-positive cells in the epithelium was similar in bi-TG pups born to bi-TG dams given doxycycline from E0 and in the control pups of all groups (Figure 7I). Between 61 and 64% of the airways in bi-TG pups born to control dams and those born to bi-TG dams given doxycycline from E15 had more than 81% PAS-positive cells, whereas no airways in bi-TG pups born to bi-TG dams given doxycycline from E0 had more than 81% PAS-positive cells (Figure 7I). Thus, maternal hIL-1β production preceding fetal hIL-1β production protected the airways against inflammation, remodeling, and goblet cell hyperplasia.
Figure 7.
Airway remodeling, goblet cell hyperplasia, and expression of chitinase-like lectins, Ym1 and Ym2, in the lungs of infant mice. Pregnant bi-TG and control dams received doxycycline in drinking water from E0 or E15 until the pups were killed at PN7. (A–H) Airway inflammation and remodeling. The airways of all control pups appeared normal (A, C, E, and G). Bi-TG pups of bi-TG dams given doxycycline from E0 had less inflammation in and around the airways, thinner airway walls, and less goblet cell hyperplasia (D) than bi-TG pups of the other three groups of dams (B, F, H). (I) Distribution (%) of airways with different proportions of goblet cells in the epithelium in each genotype and treatment group at PN7 (open bars, airways having ≤10% goblet cells; gray bars, airways having 11–40% goblet cells; dark gray bars, airways having 41–80% goblet cells; black bars, airways having ≥81% goblet cells). In bi-TG pups of bi-TG dams given doxycycline from E0, few airways had goblet cells, whereas most cells were goblet cells in the epithelium of the majority of the airways in the other three groups of bi-TG pups. The expression of Ym1 and Ym2 was increased in all groups of bi-TG infant mice compared with control animals, but the increase was blunted in bi-TG pups of bi-TG dams given doxycycline from E0. Control pups of both groups of bi-TG dams had lower expression of Ym1 and Ym2 than those of control dams. The mRNA expression levels are shown relative to the mRNA expression level of control pups of control dams given doxycycline from E0, which was set to 1. Open bars, control pups; gray bars, bi-TG pups; n ≥ 4 in each group; **P ≤ 0.01, ***P ≤ 0.001, bi-TG pups versus control pups; §P ≤ 0.05, §§P ≤ 0.01, control pups of bi-TG dam versus control pups of control dam; †P ≤ 0.05, ††P ≤ 0.01, compared with bi-TG pups of bi-TG dam given doxycycline from E0. PAS, periodic acid-Schiff.
The expression of chitinase-like lectins, Ym1 and Ym2, was up-regulated in all groups of hIL-β–expressing pups compared with all control groups, but the increase was blunted in hIL-1β–expressing pups previously exposed to maternal hIL-1β production (Figures 7J and 7K). Interestingly, maternal hIL-1β production down-regulated the expression of Ym1 and Ym2 in both groups of control pups (Figures 7J and 7K).
Maternal hIL-1β Expression Modified the Expression of Inflammatory Genes in the Lungs of Infant Mice
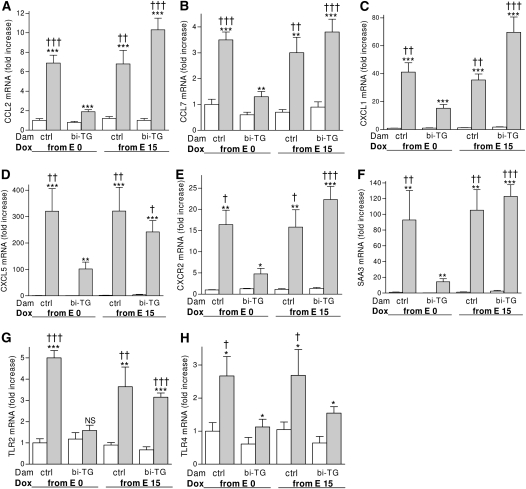
The differences in neutrophil and macrophage counts in the lungs of newborn mice in the four maternal groups prompted us to study the expression of CC and CXC chemokines that regulate the influx of these inflammatory cells. As seen in Figure 8, hIL-1β production in the lungs of newborn mice increased the mRNA expression of the CC chemokines, CCL2 and CCL7, and the CXC chemokines, CXCL1 and CXCL5, as well as of the chemokine receptor, CXCR2, in their lungs compared with control littermates in all maternal groups. Importantly, the expression of these chemokines was strongly inhibited in hIL-1β–expressing pups of hIL-1β–expressing dams given doxycycline from E0 compared with hIL-1β–expressing pups in the other three groups (Figures 8A–8E).
Figure 8.
Expression of inflammatory genes in the lungs of infant mice. Pregnant bi-TG and control dams were given doxycycline in drinking water from E0 or E15 until the pups were killed at PN7. Maternal hIL-1β production preceding the fetuses' own hIL-1β production inhibited the expression of inflammatory genes in response to hIL-1β in the lungs of the infant mice. The mRNA expression levels are shown relative to the mRNA expression level of control pups of control dams given doxycycline from E0, which was set to 1. Open bars, control pups; gray bars, bi-TG pups; n ≥ 4 in each group; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, bi-TG pups versus control pups; †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001, compared with bi-TG pups of bi-TG dam given doxycycline from E0. TLR, Toll-like receptor.
To further assess the impact of maternal hIL-1β production on the expression of inflammatory genes in hIL-1β–producing offspring, the expression of the acute-phase protein, SAA3, and of TLR2 and TLR4, was studied. Although hIL-1β production in the lungs of newborn mice induced the expression of SAA3 in the lungs of the offspring of control and bi-TG dams, maternal hIL-1β production preceding fetal hIL-1β production reduced the expression of SAA3 in hIL-1β–expressing infant mice compared with hL-1β–expressing pups in the other groups (Figure 8F). The expression of TLR2 was induced in bi-TG offspring of control dams or of bi-TG dams given doxycycline from E15, but not in bi-TG offspring of hIL-1β–producing dams given doxycycline from E0 (Figure 8G). Thus, maternal hIL-1β production preceding fetal hIL-1β production inhibited the induction of these inflammatory genes by hIL-1β in the offspring. The mRNA expression of TLR4 was higher in hIL-1β–producing mice born to control dams than in hIL-1β–producing offspring of bi-TG dams (Figure 8H). There was no difference in the mRNA expression of any of the analyzed genes between the four groups of control mice.
Expression of mIL-1β, IL-1RI, and IL-1RII, and IL-1Ra
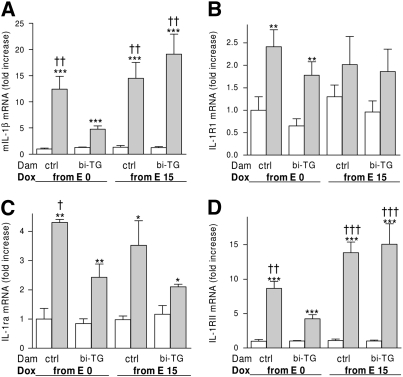
The expression of mIL-1β was increased by hIL-1β expression in all groups of bi-TG pups (P ≤ 0.001). However, this increase was blunted in bi-TG mice born to bi-TG dams receiving doxycycline from E0 compared with all other groups of bi-TG pups (P ≤ 0.01; Figure 9A). The expression of IL-1RI and IL-1RII, and of IL-1Ra, was measured to test whether the tolerance to hIL-1β of bi-TG pups of hIL-1β–producing dams given doxycycline from E0 was due to a down-regulation in IL-1RI expression or to increased expression of IL-1Ra or of IL-1RII. Instead, we found that maternal production of hIL-1β preceding fetal hIL-1β production inhibited the expression of IL-1RII in response to hIL-1β in the offspring (Figure 9D). IL-1Ra expression was increased by hIL-1β in all groups of bi-TG pups (Figure 9C), whereas IL-1RI expression was up-regulated only in the bi-TG offspring of control and bi-TG dams given doxycycline from E0 (Figure 9B). Thus the dampened inflammatory response in the bi-TG offspring of bi-TG dams given doxycycline from E0 was not due to regulation of IL-1RI, IL-1RII, or IL-1Ra.
Figure 9.
Expression of murine (m) IL-1β, IL-1 receptor type (R) RI, IL-1RII, and IL-1 receptor antagonist (IL-1Ra) in the lungs of infant mice. Pregnant bi-TG and control dams were given doxycycline in drinking water from E0 or E15 until the pups were killed at PN7. (A, B, and D) Maternal hIL-1β production preceding fetal hIL-1β production inhibited the mRNA expression of mIL-1β and IL-1RII, but not of IL-1RI, in response to hIL-1β in the offspring. (C) IL-1Ra mRNA expression was up-regulated in all groups of bi-TG mice compared with control animals. Bi-TG pups of bi-TG dams given doxycycline from E0 had less IL-1Ra than bi-TG pups of control dams given doxycycline from E0. The mRNA expression levels are shown relative to the mRNA expression level of control pups of control dams given doxycycline from E0, which was set to 1. Open bars, control pups; gray bars, bi-TG pups; n ≥ 4 in each group; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, bi-TG pups versus control pups; †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001, compared with bi-TG pups of bi-TG dam given doxycycline from E0.
DISCUSSION
The present study shows, for the first time, that maternal inflammation preceding fetal inflammation protects the offspring against inflammatory lung disease. We have previously shown that perinatal hIL-1β production in the fetal and newborn lung causes a BPD-like illness in infant mice, characterized by pulmonary inflammation, disrupted alveolar development, airway remodeling, poor growth, and increased postnatal mortality (19). We now show that hIL-1β–expressing pups exposed to prior maternal hIL-1 production have less infiltration of the lungs with neutrophils and macrophages, better alveolar septation and alveolar wall thinning, less airway thickening and goblet cell hyperplasia, better growth, and higher survival rates than hIL-1β–expressing pups born to control dams. Interestingly, the resistance of the infant mice to hIL-1β–induced inflammation and injury was lost if hIL-1β production was initiated at the same time in both the dam and the fetuses. In this case, the mortality, poor growth, pulmonary inflammation, abnormal lung morphogenesis, and airway remodeling of hIL-1β–expressing mice born to hIL-1β–expressing dams were similar to those of hIL-1β–expressing mice born to control dams.
hIL-1β expression in the pulmonary epithelium of the pregnant dam caused infiltration of her lungs with neutrophils and macrophages, and induced a mild systemic inflammatory reaction in the dam, whereas hIL-1β in her circulation was not detectable. The peripheral white cell count was higher in the blood of the hIL-1β–expressing dams than in control dams, and the expression of inflammatory genes, including 24p3, MMP-9, S100A8, and S100A9, was correspondingly increased in her blood. In addition, the level of the acute-phase protein SAA3 was increased in the liver of hIL-1β–expressing dams.
Besides being highly expressed in the lung, CCSP (also known as uteroglobin) is expressed at a low level in the uterine epithelium of pregnant and pseudopregnant rats and mice (25–27). Because the rat CCSP promoter drives the expression of hIL-1β in the present bi-TG mouse model, we studied the expression of hIL-1β in the uterus, and found that hIL-1β mRNA expression and protein production were induced in the uteri of pregnant bi-TG dams receiving doxycycline. This is the first study showing that the CCSP promoter drives transgene expression in the uterus. As expected (25), the uterine levels of hIL-1β were extremely low compared with the pulmonary levels of hIL-1β. The total amounts of hIL-1β in the pregnant uterus and in the adult lung were approximately 100 pg and 1,000 ng, respectively. However, because the expression of CCSP is restricted to the uterine endometrium (25), and only very low concentrations of IL-1β are required to elicit a biologic response (28), the levels of hIL-1β were sufficient to cause a mild infiltration of the uterine endometrium and the decidua basalis of the placenta of bi-TG dams with neutrophils. The numbers of macrophages in the endometrium or decidua basalis were not affected by hIL-1β production. Importantly, no neutrophils or macrophages were detected in the fetal membranes (yolk sac or amnion) of fetuses of either bi-TG or control dams receiving doxycycline. Thus, uterine hIL-1β production did not induce an inflammation in the fetal membranes, despite the fact that these tissues are adjacent to the uterine endometrium.
Preterm birth is the main risk factor for the development of BPD in newborn infants. Urogenital infections (e.g., chorioamnionitis, asymptomatic bacteriuria, and bacterial vaginosis) and infections or inflammatory illnesses at other sites (e.g., pneumonia, appendicitis, periodontal disease, and inflammatory bowel disease) have all been associated with preterm delivery (3, 8, 29). These conditions may initiate a systemic inflammation (30, 31) that may lead to preterm labor (32). Inflammatory cytokines, including IL-1β, can induce preterm delivery in experimental animals (33, 34). In the present study, no cases of preterm labor occurred in the mice, perhaps because of the mildness of the systemic and intrauterine inflammation and lack of inflammation in the fetal membranes. In addition, the FVB/N strain of mice seems to have a relatively high resistance to inflammation-induced preterm labor. A recent study demonstrated a low incidence of preterm labor in FVB/N mice injected with endotoxin compared with other strains of mice (35).
Maternal hIL-1β production preceding fetal hIL-1β production ameliorated alveolar septation and alveolar wall thinning in bi-TG pups. In addition, airway thickening and goblet cell hyperplasia caused by hIL-1β in infant mice were prevented by prior exposure to maternal inflammation. hIL-1β enhanced the expression of the chitinase-like lectins, Ym1 and Ym2, in the lungs of infant mice. However, the expression of these lectins was blunted if the mouse had been exposed to prior maternal hIL-1β production. Ym1 and Ym2 are markedly up-regulated in allergic airway inflammation, and may play a role in the development of asthma (36, 37). Treatment with antisense Ym1 RNA reduces airway responsiveness in a model of allergic asthma (37).
Maternal hIL-1β production preceding fetal hIL-1β production abolished the hIL-β–induced infiltration of the alveolar regions of the lungs of the offspring with neutrophils, and reduced the macrophage infiltration by 60%. Inflammation in and around the airways was also decreased in bi-TG offspring of bi-TG dams given doxycycline from E0. In contrast, inflammation in the lung was not suppressed in bi-TG pups of bi-TG dams given doxycycline from E15. In the former situation, but not in the latter, maternal hIL-1β production caused silencing of inflammatory genes in the lungs of the infant mice. The mRNA expression of mIL-1β, several neutrophil- and monocyte-attractant chemokines, SAA3, TLR2, and TLR4 was down-regulated in the lungs of hIL-1β–expressing newborns exposed to prior maternal inflammation compared with hIL-1β–expressing pups of control dams and to those of bi-TG dams given doxycycline from E15. Gene reprogramming to a silent phenotype, indicating tolerance to hIL-1β, may be a central mechanism by which hIL-1β–induced lung injury is inhibited in the pups born to dams producing hIL-1β from early gestation. To our knowledge, silencing of inflammatory genes in the offspring after chronic maternal inflammation has not been previously studied. Injecting endotoxin directly into the fetal circulation induces endotoxin tolerance in ovine fetuses, as seen by decreased hypotensive response and smaller elevation of blood IL-6 levels after subsequent injections (38). Similarly, cord monocytes isolated from preterm lambs injected with intra-amniotic endotoxin and thereafter challenged in vitro with endotoxin have a transient suppression of IL-6 production (39).
Silencing of acute proinflammatory genes is common to severe animal and human systemic inflammation with infection (40). Sustained silencing of inflammatory genes is observed in blood neutrophils and monocytes during systemic inflammation (40). Humans given intravenous endotoxin develop the silencing signature for a brief period, but in systemic inflammation, the silencing of expression of proinflammatory genes can persist for many days or even weeks (40). It is interesting that the silencing of expression of acute proinflammatory genes in the present model is still present postnatally about 4 weeks after the initiation of hIL-1β expression in the dam, and about 2 weeks after the fetuses themselves start to produce hIL-1β. It is unknown at present how long the silencing signature persists, and whether it is followed by a late proinflammatory response.
Both clinical and experimental evidence suggests that inflammation plays a role in the pathogenesis of BPD. The inflammatory response in the lungs of infants developing BPD is characterized by infiltration of the lungs with neutrophils and macrophages and increased production of various inflammatory cytokines and chemokines (41–43). Inflammation in the preterm lung can be caused by antenatal pulmonary or systemic infection in the fetus, or by postnatal events, such as trauma associated with resuscitation at birth, oxygen toxicity, volutrauma or barotrauma caused by ventilator therapy, or postnatal infection (41). The present study suggests that antenatal exposure to maternal inflammation may confer resistance to the fetal/newborn lung against inflammatory insults by down-regulating inflammatory genes and limiting the influx of inflammatory cells into the lung, and may thereby prevent the development of BPD in the newborn. On the other hand, silencing of inflammatory genes may render the infant vulnerable to bacterial infections. Decreased expression of inflammatory genes participates in immunodepression and predicts increased mortality in adults from sepsis (40).
Infections associated with preterm birth, such as chorioamnionitis, periodontitis, or asymptomatic bacteriuria, are often indolent and chronic (6), but may also be acute and fulminant. The fetal response to maternal inflammation is probably dependent on the duration, timing, type, and invasiveness of the inflammation. Differences in variables such as these may explain why some infants unpredictably develop BPD, whereas other infants born very early are spared from poor pulmonary outcome. Such differences may also serve to explain why studies examining the relationship of antenatal infection and BPD have found variable results. Although antenatal inflammation has been associated with the development of BPD in some studies (9, 44), other studies have not found such an association (10, 45, 46).
In summary, the present study demonstrates that maternal inflammation modifies the responses of the newborn to inflammatory insult in ways that are critically dependent on the timing of maternal inflammation with respect to the inflammation in the fetus. These results may have important clinical implications, and may help us to understand the complexity of the response of newborn infants to antenatal and postnatal inflammation and infection.
This work was supported by the Swedish Medical Research Council (K.B.), the Swedish Heart and Lung Foundation (K.B.), the Frimurare Barnhus Foundation (K.B.), the Swedish Government Grants for Medical Research (K.B.), and the Queen Silvia Children's Hospital Research Foundation (E.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0287OC on May 1, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 2.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8:73–81. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–612. [DOI] [PubMed] [Google Scholar]
- 5.Ghidini A, Jenkins CB, Spong CY, Pezzullo JC, Salafia CM, Eglinton GS. Elevated amniotic fluid interleukin-6 levels during the early second trimester are associated with greater risk of subsequent preterm delivery. Am J Reprod Immunol 1997;37:227–231. [DOI] [PubMed] [Google Scholar]
- 6.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–550. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Culhane JF. Preterm birth and periodontal disease. N Engl J Med 2006;355:1925–1927. [DOI] [PubMed] [Google Scholar]
- 8.Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med 2005;33:S390–S397. [DOI] [PubMed] [Google Scholar]
- 9.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–215. [PubMed] [Google Scholar]
- 10.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–808. [DOI] [PubMed] [Google Scholar]
- 11.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997;99:2992–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, Possmayer F, Hallman M, Ikegami M. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182:401–408. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–830. [DOI] [PubMed] [Google Scholar]
- 14.An H, Nishimaki S, Ohyama M, Haruki A, Naruto T, Kobayashi N, Sugai T, Kobayashi Y, Mori M, Seki K, et al. Interleukin-6, interleukin-8, and soluble tumor necrosis factor receptor-I in the cord blood as predictors of chronic lung disease in premature infants. Am J Obstet Gynecol 2004;191:1649–1654. [DOI] [PubMed] [Google Scholar]
- 15.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002;140:171–176. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–123. [DOI] [PubMed] [Google Scholar]
- 17.Rindfleisch MS, Hasday JD, Taciak V, Broderick K, Viscardi RM. Potential role of interleukin-1 in the development of bronchopulmonary dysplasia. J Interferon Cytokine Res 1996;16:365–373. [DOI] [PubMed] [Google Scholar]
- 18.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 2005;32:311–318. [DOI] [PubMed] [Google Scholar]
- 19.Bry K, Whitsett JA, Lappalainen U. IL-1β disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol 2007;36:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 21.Sisson TH, Hansen JM, Shah M, Hanson KE, Du M, Ling T, Simon RH, Christensen PJ. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am J Respir Cell Mol Biol 2006;34:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prophet EB, Mills B, Arrington JB, Sobin LH. Armed Forces Institute of Pathology—laboratory methods in histotechnology. Washington, DC: American Registry of Pathology; 1992.
- 24.Ashour K, Shan L, Lee JH, Schlicher W, Wada K, Wada E, Sunday ME. Bombesin inhibits alveolarization and promotes pulmonary fibrosis in newborn mice. Am J Respir Crit Care Med 2006;173:1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagen G, Wolf M, Katyal SL, Singh G, Beato M, Suske G. Tissue-specific expression, hormonal regulation and 5′-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res 1990;18:2939–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray MK, Wang G, Barrish J, Finegold MJ, DeMayo FJ. Immunohistochemical localization of mouse Clara cell 10-kD protein using antibodies raised against the recombinant protein. J Histochem Cytochem 1996;44:919–927. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocr Rev 2007;28:707–725. [DOI] [PubMed] [Google Scholar]
- 28.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996;87:2095–2147. [PubMed] [Google Scholar]
- 29.Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol 2002;97:641–648. [DOI] [PubMed] [Google Scholar]
- 30.Antunes G, Evans SA, Lordan JL, Frew AJ. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur Respir J 2002;20:990–995. [DOI] [PubMed] [Google Scholar]
- 31.Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler Thromb Vasc Biol 2007;27:1433–1439. [DOI] [PubMed] [Google Scholar]
- 32.El-Shazly S, Makhseed M, Azizieh F, Raghupathy R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol 2004;52:45–52. [DOI] [PubMed] [Google Scholar]
- 33.Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol 1993;168:1318–1322. [DOI] [PubMed] [Google Scholar]
- 34.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006;195:1578–1589. [DOI] [PubMed] [Google Scholar]
- 35.Salminen A, Paananen R, Vuolteenaho R, Metsola J, Ojaniemi M, Autio-Harmainen H, Hallman M. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res 2008;63:280–286. [DOI] [PubMed] [Google Scholar]
- 36.Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem 2001;276:41969–41976. [DOI] [PubMed] [Google Scholar]
- 37.Iwashita H, Morita S, Sagiya Y, Nakanishi A. Role of eosinophil chemotactic factor by T lymphocytes on airway hyperresponsiveness in a murine model of allergic asthma. Am J Respir Cell Mol Biol 2006;35:103–109. [DOI] [PubMed] [Google Scholar]
- 38.Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, Rees SM. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res 2002;52:941–949. [DOI] [PubMed] [Google Scholar]
- 39.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med 2005;171:73–77. [DOI] [PubMed] [Google Scholar]
- 40.McCall CE, Yoza BK. Gene silencing in severe systemic inflammation. Am J Respir Crit Care Med 2007;175:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 2006;11:354–362. [DOI] [PubMed] [Google Scholar]
- 42.Munshi UK, Niu JO, Siddiq MM, Parton LA. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol 1997;24:331–336. [DOI] [PubMed] [Google Scholar]
- 43.Baier RJ, Loggins J, Kruger TE. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of Ureaplasma urealyticum. J Investig Med 2001;49:362–369. [DOI] [PubMed] [Google Scholar]
- 44.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 2004;55:1009–1017. [DOI] [PubMed] [Google Scholar]
- 45.Akram Khan M, Kuzma-O'Reilly B, Brodsky NL, Bhandari V. Site-specific characteristics of infants developing bronchopulmonary dysplasia. J Perinatol 2006;26:428–435. [DOI] [PubMed] [Google Scholar]
- 46.Redline RW, Wilson-Costello D, Hack M. Placental and other perinatal risk factors for chronic lung disease in very low birth weight infants. Pediatr Res 2002;52:713–719. [DOI] [PubMed] [Google Scholar]