Abstract
The lung is dynamically remodeled in response to injury, which alters extracellular matrix composition, and can lead to either healthy or impaired lung regeneration. To determine how changes in extracellular matrix can influence alveolar epithelial barrier function, we examined the expression and function of tight junction proteins by rat alveolar epithelial type II cells cultured on one of three different matrix components: type I collagen or fibronectin, matrix glycoproteins which are highly expressed in injured lungs, or laminin, a basement membrane matrix component. Of note, alveolar epithelial cells cultured for 2 days on fibronectin formed high-resistance barriers and showed continuous claudin-3 and claudin-18 localization to the plasma membrane, as opposed to cells cultured on either type I collagen or laminin, which had low resistance monolayers and had areas of cell–cell contact that were claudin deficient. The barrier formed by cells cultured on fibronectin also had preferential permeability to chloride as compared with sodium. Regardless of the initial matrix composition, alveolar epithelial cells cultured for 5 days formed high-resistance barriers, which correlated with increased claudin-18 localization to the plasma membrane and an increase in zonula occludens-1. Day 5 cells on laminin had significantly higher resistance than cells on either fibronectin or type I collagen. Thus, although alveolar epithelial cells on fibronectin formed rapid barriers, it was at the expense of producing an optimized barrier.
Keywords: tight junction, claudin, basement membrane, lung injury, fibronectin
CLINICAL RELEVANCE.
A fibronectin-enriched provisional matrix accumulates during acute lung injury. We found that, although fibronectin promoted rapid reformation of alveolar barriers, these were suboptimal as compared with barriers more slowly produced by cells on healthy matrix.
The alveolar epithelial barrier is critical to maintain optimal lung function. Failure of this barrier after injury contributes to flooding of the alveolar airspaces with protein-rich fluid, which significantly contributes to the severity of acute lung injury (1). The need for an intact alveolar epithelial barrier is such a critical requirement for resolution of acute lung injury that patient outcomes positively correlate with the ability to maintain a tight alveolar barrier (2, 3). Despite its importance, however, the mechanisms regulating alveolar barrier function remain incompletely characterized. In particular, little is known about the effect of cell microenvironment on regulating alveolar epithelial permeability.
Permeability between alveolar epithelial cells is controlled by tight junctions, which are located at cell–cell contacts, and consist of several different classes of transmembrane and peripheral proteins (4). Of these proteins, tight junction permeability is most directly regulated by claudins, a family of over two dozen tetraspan transmembrane proteins (5, 6). Tight junction permeability is also modulated by other proteins, including transmembrane proteins such as occludin and scaffold proteins, including zonula occludens (ZO)-1 and -2 (7–9). Depending on the profile of claudin expression, different epithelia will have different paracellular permeability characteristics (10).
Several studies have demonstrated that lung epithelia express multiple claudins, and that type II and type I alveolar epithelial cells express different claudins (11–14). The transition of primary alveolar epithelial cells from a type II cell phenotype to a type I cell phenotype is sensitive to their microenvironment (15–20). However, even in vivo, the distinction between type II and type I cells is not always clear cut, and transitional cells can be identified with characteristics of both cell types (21, 22). The microenvironment can also promote alveolar cells to undergo an epithelial-to-mesenchyme transition, which contributes to pulmonary fibrosis (23, 24). Consistent with this observed plasticity, alveolar epithelial cells cultured under different conditions show differences in expression and function of gap junction proteins (25–27). However, it remains to be determined how the cell microenvironment influences tight junction protein expression.
The extracellular matrix is a critical component of the cellular microenvironment. In the unstressed lung, alveolar cells rest on basement membranes composed of several matrix components, including laminin, type IV collagen, and nidogen (28, 29). In response to acute lung injury, lung extracellular matrix composition is dramatically altered (30). Specifically, the destruction of alveolar basement membranes induced during injury is followed by increased expression and deposition of fibronectin and type I collagen by alveolar epithelial cells and fibroblasts. Through integrin-mediated signaling, this fibronectin-enriched “provisional” matrix is thought to promote the re-epithelialization of denuded alveolar walls, stimulate vascularization, and provide a scaffold for the accumulation and organization of recruited inflammatory cells (31–33).
Although previous in vitro studies have demonstrated that fibronectin promotes wound healing by alveolar epithelial cells (34) and stimulates the ability of these cells to form a high-resistance tight junction barrier after 4 days in culture (35), the effect of the extracellular matrix on the molecular composition and ion permeability of tight junctions has not been determined. We used primary alveolar epithelial type II cells cultured on defined substrata composed of laminin, type I collagen, or fibronectin to determine how extracellular matrix composition affects claudin expression and, consequently, alters alveolar epithelial barrier function. We found that cells cultured on fibronectin for 2 days formed high-resistance monolayers, which were associated with claudin localization to the plasma membrane. By contrast, cells on either laminin or type I collagen did not exhibit significant barrier function. However, after 5 days in culture, cells on all three matrices formed high-resistance monolayers. Day 5 cells on laminin had the highest barrier function, whereas cells on type I collagen and fibronectin were equivalent, with respect to transepithelial resistance (TER) and claudin expression. Together, these observations suggest that fibronectin-rich matrices produced during the early stages of lung injury might promote the fast generation of epithelial cell barriers. However, by Day 5, these barriers were suboptimal as compared with barriers produced by cells cultured for 5 days on laminin, suggesting that the rapid formation of a high-resistance monolayer by cells after 2 days on fibronectin was at the expense of producing a more optimal alveolar epithelial barrier.
MATERIALS AND METHODS
Coating Transwell Permeable Supports with Matrix Components
Laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane (Sigma Chemicals, Inc., St. Louis, MO), rat tail type I collagen (Roche Diagnostics, Mannheim, Germany), and matrix fibronectin (Sigma Chemicals, Inc.) were dissolved in coating buffer (0.1 M NaHCO3 [pH 8.5]) at a concentration of 20 μg/ml (36). Transwells (12-well; Corning Costar, Lowell, MA) received 1 ml per well and were incubated at 4°C overnight. The wells were then washed twice with PBS (120 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate [pH 7.4]) and further incubated at 37°C for 2 hours in PBS containing 10 mg/ml BSA. Matrix-coated permeable supports were washed twice with PBS at room temperature before use. All experiments were performed on coated permeable supports except for cell spreading experiments, which were done using glass coverslips in 6-well tissue culture dishes (Nunc, Rochester, NY) that had been treated using 2 ml of matrix coating buffer, and then washed as described above.
Isolation and Culture of Rat Type II Alveolar Epithelial Cells
Animal protocols were reviewed and authorized by the Institutional Animal Care and Use Committee of Emory University. Sprague-Dawley rat type II alveolar epithelial cells were isolated from lungs lavaged and perfused with elastase using the method of Dobbs and colleagues (37), with modifications (26). For routine preparations, cells were biopanned with IgG-coated culture dishes to remove alveolar macrophages and other Fc receptor–expressing cells. Routine preparations routinely contained greater than 90–95% type II alveolar epithelial cells. Freshly isolated cells were cultured in Earle's MEM (Life Technologies, Rockville, MD) containing 10% FBS, 25 μg/ml gentamicin, and 0.25 μg/ml amphotericin B (Life Technologies) in coated Transwells at 5 × 105 cells/ml under conditions that promote differentiation toward a type I–like phenotype (17, 38).
For cell-spreading experiments, cells were cultured on matrix-coated glass cover slips at 104 cells/ml and analyzed after 2 days in culture by phase-contrast microscopy. Cell area was calculated using ImagePro (Media Cybernetics, Bethesda, MD), and represents the average from three independent cultures for laminin (31 cells), type I collagen (28 cells), or fibronectin (29 cells).
For some experiments, cells isolated from elastase-digested lungs were purified by fluorescence activated cell sorting. Macrophages were depleted using rat IgG/goat anti-rat magnetic beads and elution through a Large Cell Separation column (Miltenyi Biotec, Auburn, CA). Eluted cells were centrifuged and resuspended in flow sort buffer (2% FBS in PBS) at 1 × 106 cells/ml. Before sorting, the cells were labeled for 5 minutes at 37°C using a 1:1,000-fold dilution of Lysotracker red (Molecular Probes, Invitrogen, Carlsbad, CA), a vital dye that accumulated into type II cell lamellar bodies. Lysotracker red–positive cells were isolated using a FACSVantage flow cytometer (Becton Dickson, Franklin Lakes, NJ) based on their high side-scatter profile and far forward-scatter profile using the 633-nm argon laser excitation. Using this approach, greater than 95% of the sorted cells expressed surfactant proteins, based on immunofluorescence microscopy.
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described (12, 13). Unless otherwise stated, antibodies were from Invitrogen (Carlsbad, CA). Cy-3–conjugated anti-vimentin and FITC-conjugated anti–pan-cytokeratin were from Sigma Chemicals, Inc. After 2 or 5 days in culture, the cells were washed with PBS three times, fixed in MeOH/acetone 1:1 for 2 minutes at room temperature, washed three times with PBS, once with PBS plus 0.5% TX-100, then once with PBS plus 0.5% Triton X-100 plus 2% normal goat serum. Cells were incubated with primary anti-rabbit antibodies in PBS/GS for 1 hour, washed, incubated with Cy2-conjugated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) in PBS/GS, washed, and then mounted in Mowiol 4-88 under a glass coverslip. Cells were imaged by phase-contrast and fluorescence microscopy using an Olympus IX70 with a U-MWIBA filter pack (BP460–490, DM505, BA515–550) or U-MNG filter pack (BP530–550, DM570, BA590–800+) (Olympus, Center Valley, PA). Images were acquired using ImagePro software. Minimum and maximum intensity were adjusted for images in parallel so that the intensity scale remained linear to maximize dynamic range.
Immunoblot
After 2 or 5 days in culture, cells on permeable supports were harvested and lysed in triple-detergent lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% SDS, 1% nonidet P-40, 0.5% sodium deoxycholate, protease inhibitor cocktail tablets [Roche]). Protein concentration was determined for each sample using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Total protein (50 μg) per sample was diluted into SDS-PAGE sample buffer and resolved on a 10% gel. Proteins were transferred to Immobilon membranes using a Bio-Rad semidry transfer apparatus, and the membrane blocked with Blotto (nonfat dry milk in TBS-T [25 mM Tris, 140 mM NaCl, 3 mM KCl, 0.05% Tween-20 (pH 7.4)]). Blots were incubated overnight in Blotto containing rabbit anti-claudin or anti-occludin antibodies, as described previously here. Blots were washed, then incubated with horeseradish peroxidase–conjugated goat anti-rabbit IgG (Jackson Immunoresearch), washed, and detected using the ECL reagent (GE Healthsciences, Pittsburgh, PA). Relative protein expression was normalized to parallel blots probed for actin as a measure of total protein, and to the amount of protein expressed by Day 2 cells on laminin (defined as 1.0 for each protein examined).
Barrier Function Measurements
Transepithelial resistance (TER) of cells in medium cultured on permeable supports was measured using an Ohmmeter (World Precision Instruments, Sarasota, FL) as previously described (12, 13). For conductance and dilution potential measurements, we used an Ussing chamber (Warner Instruments, Holliston, MA), where current was clamped and voltage measured (VCC600; Physiologic Instruments, San Diego, CA) with cells in different saline solutions: NaCl (140 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes, pH 7.3), Lys-HCl (140 mM lysine-HCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes [pH 7.3]) or Na-Asp (140 mM Na-aspartate, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes [pH 7.3]) (39). For dilution potential measurements (39, 40), the medium on either the apical or the basal side of the Ussing chamber was replaced with saline solutions diluted either 1:1 or 1:3 with iso-osmolar mannitol solution (280 mM mannitol, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes [pH 7.3]). After 5-minute equilibration, the current was clamped to 0 amperes and voltage measured in millivolts. Dilution potential measurements were comparable, regardless of whether the apical or basolateral medium was changed. For instance, for NaAsp at 1:3 dilution, potential measurements were −6.8 (± 0.3) mV (apical) versus −6.0 (± 0.2) mV (basolateral) (n = 4) and for LysCl at 1:3 dilution, potential measurements were 6.5 (± 0.6) mV (apical) versus 6.4 (± 1.0) (basolateral) (n = 4). Given these observations, we combined measurements from apical and basolateral medium changes to calculate net dilution potentials.
RESULTS
Effect of Extracellular Matrix on Tight Junction Protein Expression
Previous studies have shown that fibronectin promotes wound healing by enhancing alveolar epithelial cell spreading (34). To confirm that freshly isolated type II alveolar epithelial cells are sensitive to extracellular matrix composition, we measured the ability of the cells to spread on glass coverslips coated with laminin, type I collagen, or fibronectin. Phase-contrast microscopy revealed that cells cultured at low density for 2 days on either laminin or type I collagen spread to an average cell area of 810 (± 430; n = 31) μm2 and 920 (± 460; n = 28) μm2, respectively (Figures 1A and 1B). In contrast, cells cultured for 2 days on fibronectin (Figure 1C) spread to an average area of 1,460 (± 540; n = 29) μm2, nearly twice as large as cells cultured on laminin or type I collagen. These results are consistent with measurements of the rate of wound healing, where alveolar epithelial cells cultured on fibronectin were able to close scrape wounds twice as fast as cells cultured on type I collagen (34). This also confirmed that alveolar epithelial cells were sensitive to extracellular matrix composition in our culture system.
Figure 1.
Fibronectin promotes spreading of primary alveolar epithelial cells. Freshly isolated type II cells were cultured on laminin (A), type I collagen (B), or fibronectin (C) at low density for 2 days, and then photographed by phase-contrast microscopy. Scale bar, 10 μm. (D) Based on average area per cell, cells on fibronectin spread to be nearly twice as large as cells on either laminin or type I collagen (*P < 0.001).
To test the effect of extracellular matrix on tight junction protein expression, we cultured freshly isolated type II alveolar epithelial cells on permeable supports coated with laminin, type I collagen, or fibronectin. Immunofluorescence microscopy revealed that cells cultured for 2 days on either laminin or type I collagen showed heterogeneous localization of claudin-18, where some cell–cell interfaces contained claudin-18 and several did not (Figure 2A). In contrast, claudin-18 in cells cultured for 2 days on fibronectin was more uniformly localized to cell–cell interfaces. Claudin-3 showed a similar pattern of continuous localization for cells on fibronectin, as opposed to cells on laminin or type I collagen, where several cell–cell interfaces lacked detectable claudin-3 (Figure 2B).
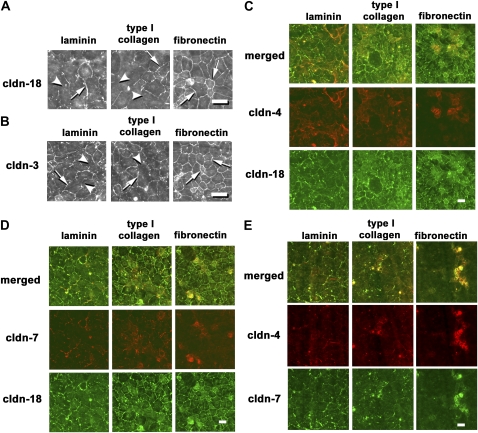
Figure 2.
Localization of claudins by cells cultured for 2 days on different extracellular matrices. Freshly isolated type II cells were cultured for 2 days on permeable supports coated with laminin, type I collagen, or fibronectin, and then fixed, permeabilized, and immunostained for claudin-18 (A), claudin-3 (B), claudin-18 and -4 (C), claudin-18 and -7 (D), or claudin-4 and -7 (E). Cells on laminin and type I collagen showed areas of cell–cell contact that lacked claudin-18 (arrowheads), as opposed to cells on fibronectin, which had a more uniform distribution of claudin-18 (arrows). Claudin-3 had a similar distribution. Claudin-4 and -7 colocalized on a subset of cells cultured on fibronectin (E). By contrast, expression of claudin-4 and -7 were more evenly distributed throughout cells cultured on either laminin or collagen. Scale bars, 30 μm (A and B) and 50 μm (C–E).
Two other claudins, claudin-4 and -7, showed a different pattern of localization. Cells that were cultured for 2 days on laminin or type I collagen showed significant colocalization of claudin-4 and -7 with claudin-18 (Figures 2C and 2D). By contrast, expression of claudin-4 and -7 were detected in a subset of cells cultured for 2 days on fibronectin. Furthermore, claudin-4 and -7 colocalized to the same subpopulation of Day 2 cells on fibronectin.
We then determined whether these differences persisted during time in culture. After 5 days in culture, claudin-18 localization was more uniform for cells, regardless of extracellular matrix composition (Figure 3A). Moreover, claudin-4 expression was more limited as compared with Day 2 cells (Figure 3B); however, claudin-7 was more broadly expressed (Figure 3C). In particular, cells cultured for 5 days on fibronectin showed prominent localization of claudin-7 to cell–cell interfaces.
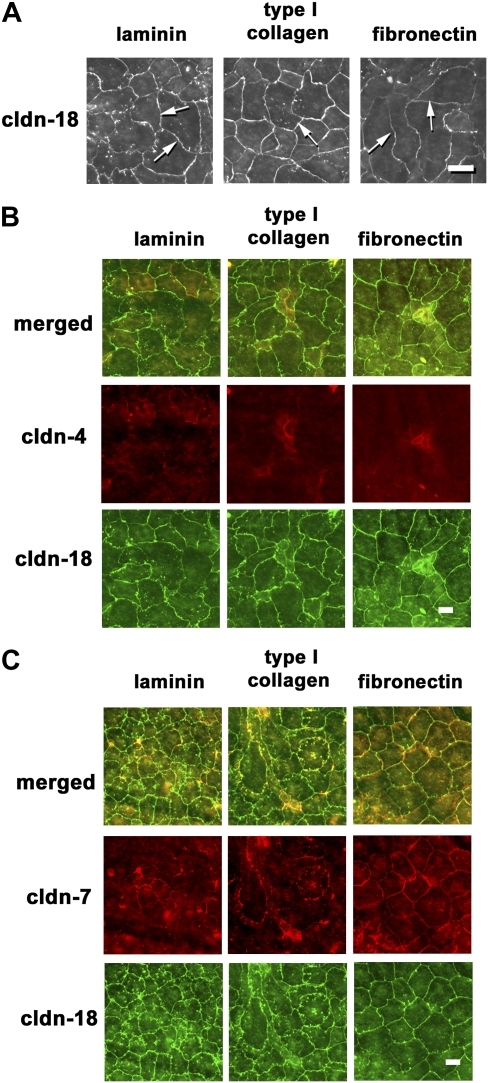
Figure 3.
Localization of claudins by cells cultured for 5 days on different extracellular matrices. Freshly isolated type II cells were cultured for 5 days on permeable supports coated with laminin, type I collagen, or fibronectin, and then fixed, permeabilized, and immunostained for claudin-18 (A), claudin-18 and -4 (B), or claudin-18 and -7 (C). Claudin-18 was continuous and localized to nearly all cell–cell interfaces (arrows), regardless of extracellular matrix. Claudin-4 localization was more limited. By contrast, claudin-7 was more uniformly localized throughout the monolayer for all three matrices tested. Scale bars, 30 μm (A) and 50 μm (B and C).
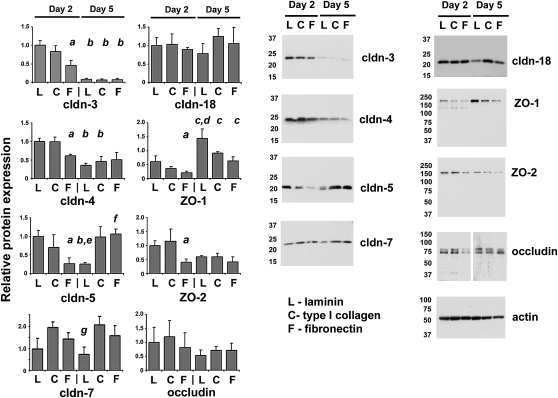
To determine whether matrix-dependent differences in claudin localization were related to differences in total protein, we examined tight junction proteins by immunoblot (Figure 4). In comparing Day 2 cells on different matrices, the protein content of claudin-3, -4, -5, -7, -18, ZO-1, ZO-2, and occludin was equivalent for cells on either laminin or type I collagen. However, cells cultured for 2 days on fibronectin had significantly lower claudin-3, -4, -5, ZO-1, and ZO-2 than Day 2 cells on laminin. Of note, total claudin-18 protein was comparable, regardless of the extracellular matrix composition, despite the differences in localization observed by immunofluorescence microscopy. In contrast, after 5 days in culture, cells on type I collagen and fibronectin had comparable tight junction protein content, and cells cultured for 5 days on laminin showed differences in tight junction protein expression. In particular, Day 5 cells on laminin had significantly less claudin-5 and -7, and more ZO-1, as compared with cells cultured for 5 days on either type I collagen or fibronectin. As was the case for Day 2 cells, claudin-18 was comparable for Day 5 cells, regardless of extracellular matrix.
Figure 4.
Tight junction protein expression by cells cultured for 2 or 5 days on different extracellular matrices. Freshly isolated type II cells were cultured on permeable supports coated with laminin (L), type I collagen (C), or fibronectin (F) for 2 or 5 days, and then harvested, resolved by SDS-PAGE, and levels of claudin-3, -4, -5, -7, -18, zonula occludens (ZO)-1, ZO-2, or occludin were determined by immunoblot. A parallel blot was labeled for actin to normalize to total sample protein content. Relative values for each were normalized to cells cultured for 2 days on laminin. Bar graphs reflect the average (±SEM) for triplicate independent measurements. aSignificantly lower than Day 2 cells on laminin (P < 0.04); bsignificantly lower than Day 2 samples on the same matrix (P < 0.04); csignificantly higher than Day 2 samples on the same matrix (P < 0.04); dsignificantly higher than Day 5 cells on either collagen or fibronectin (P < 0.03); esignificantly lower than Day 5 cells on either collagen or fibronectin (P < 0.05); fsignificantly higher than Day 2 cells on fibronectin (P < 0.05); gsignificantly lower than Day 5 cells on collagen (P < 0.05).
In comparing cells on the same matrix, the main changes in tight junction protein expression that occurred from Day 2 to Day 5 were a decrease in claudin-3 and an increase in ZO-1 (Figure 4). Claudin-4 expression also decreased from Day 2 to Day 5 for cells on laminin or type I collagen to a level comparable to cells cultured for 5 days on fibronectin. Interestingly, claudin-5 showed the most differences with time in culture in response to matrix composition, as it decreased from Day 2 to Day 5 for cells on laminin, remained unchanged for cells on type I collagen, and increased for cells on fibronectin.
Considering the complex differences in claudin expression exhibited by alveolar epithelial cells as a function of time in culture and extracellular matrix composition, we further characterized the cell cultures. Cells cultured for either 2 or 5 days on matrix-coated permeable supports were double labeled with cy3–anti-vimentin (a fibroblast marker) and FITC–anti-pan cytokeratin (an epithelial cell marker) (Figure 5). By this measure, the cells were predominantly epithelial. Some fibroblasts were also present, although they accounted for less than 10% of the cells present in the culture, and they did not significantly proliferate. Note also that the number of cells on fibronectin was significantly higher than the number of cells on either laminin or type I collagen. Greater cell number on fibrobectin is consistent with their ability to spread on the matrix, suggesting a greater affinity for fibronectin as compared with laminin or type I collagen.
Figure 5.
Fibronectin promotes alveolar epithelial cell attachment. (A) Freshly isolated type II cells were plated on permeable supports coated with laminin, type I collagen, or fibronectin and then cultured for 2 (top row) or 5 (bottom row) days, fixed, and then immunolabeled for cytokeratin (green) and vimentin (red) as markers for epithelial cells and fibroblasts, respectively. Note that there were relatively few fibroblasts (red cells) present in the preparations, regardless of matrix composition. (B) This was confirmed by quantitation, which showed that the vast majority of cells in the monolayers were epithelial. Note that there was a significantly higher number of cells attached to the fibronectin-coated wells as compared with the two other matrices (*P < 0.01, Day 2, < 0.03, Day 5). Scale bar, 30 μm.
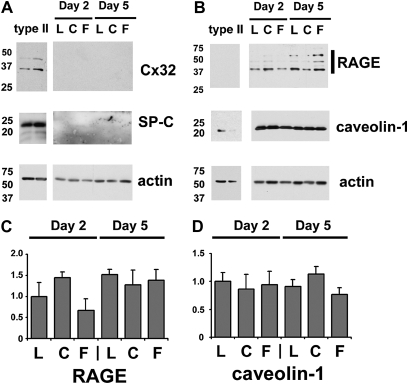
Changes in cell phenotype also were assessed using surfactant protein C (SP-C) and connexin32 as type II cell markers and receptor for advanced gylcosylation end products and caveolin-1 as type I cell markers (26, 41) (Figure 6). Under the culture conditions tested, neither SP-C or connexin32 was detectable by immunoblot, although it was present in control purified type II cells. By contrast, receptor for advanced gylcosylation end products and caveolin-1 were comparable for cells cultured on all three different matrices (Figures 6C and 6D). Thus, the differences observed for tight junction protein levels were not tightly linked to other markers for alveolar epithelial cell phenotype.
Figure 6.
Expression of type I cell markers by alveolar epithelial cells cultured on different matrices. (A) Freshly isolated type II cells were plated on laminin (L), type I collagen (C), or fibronectin (F), and then cultured for 2 or 5 days, then harvested and analyzed by immunoblot for expression of either type II cell markers ([A] connexin32 [Cx32], SP-C) or type I cell markers ([B] receptor for advanced gylcosylation end products [RAGE], caveolin). Flow-sorted type II cells were used as controls. A parallel blot was labeled for actin to normalize to total sample protein content. The type II cell markers were undetected in the cultured cells. Quantitative analysis of type I cell markers revealed that there were no significant differences in the amount of RAGE (C) or caveolin (D) expressed by the cells, regardless of extracellular matrix composition.
Effect of Extracellular Matrix on Barrier Function
To determine if the observed changes in tight junction protein content and localization were associated with alterations in alveolar epithelial barrier function, barrier function of alveolar epithelial cells on permeable supports coated with laminin, type I collagen, or fibronectin was measured. As shown in Figure 7A, cells cultured for 2 days on fibronectin formed a monolayer with a TER of 370 (± 70) Ohm·cm2 (n = 7); however, cells cultured for 2 days on either laminin or collagen had TER values of 21 (± 9) and 87 (± 13) Ohm·cm2, respectively. Thus, cells cultured on fibronectin were stimulated to form a high-resistance barrier as compared with cells on laminin or type I collagen. However, by Day 5, cells cultured on all three different matrices had formed high-resistance monolayers with TER values of at least 500 Ohm·cm2 (Figure 7B), suggesting that the fibronectin-dependent increase in TER was a transient phenomenon. In fact, cells cultured on laminin, representative of a healthy basement membrane matrix, had TER on Day 5 of 890 (± 31) Ohm·cm2 (n = 4), significantly higher than cells on either type I collagen (530 ± 63 Ohm·cm2) or fibronectin (520 ± 95 Ohm·cm2).
Figure 7.
Effect of extracellular matrix on barrier formation by alveolar epithelial cells in vitro. (A and B) Freshly isolated type II cells were plated on laminin, type I collagen, or fibronectin, cultured for 2 (A) or 5 (B) days, and then transepithelial resistance (TER) was measured. Shown are means (±SEM) for n = 6 independent experiments consisting of at least six Transwells. (A) On Day 2, only cells cultured on fibronectin showed a measurable increase in TER (*P < 0.05 versus laminin or collagen). (B) By Day 5, cells on all three matrices showed high TER values, suggesting the formation of tight junctions. Cells on laminin showed significantly higher TER than cells on collagen or fibronectin (*P < 0.05). (C and D) Freshly isolated type II cells were cultured on fibronectin-coated Transwells for either 2 (C) or 5 (D) days, and the barrier conductance was determined in Ringers saline (NaCl), lysine-HCl (Lys-HCl), or Na-aspartate (Na-Asp). For Day 2 cells, conductance in Na-Asp was significantly less than conductance in NaCl or Lys-HCl (*P < 0.01, n = 4 independent experiments), suggesting increased anion conductance. By Day 5, the conductance for all of the saline solutions tested was equivalent and significantly lower than the comparable conductance measurements for Day 2 (P < 0.01). (E–G) Dilution potential measurements were done as described in Materials and Methods using bath solutions containing 140 mM NaCl (E), Lys-Cl (F), or Na-Asp (G), and diluted either 1:1 or 1:3 with iso-osmotic mannitol solution to generate either 70 or 35 mM saline solutions. Voltage (mV) was measured with samples, where current was clamped to 0 amp. For NaCl, the dilution potential was significantly greater than zero (P < 0.01, n = 6 independent determinations).
Given differences in claudin expression and the precocious formation of a high-resistance barrier by cells on fibronectin, we analyzed transcellular ion conductance of cells on fibronectin in greater detail using buffers with different ion composition to determine the relative permeability of monolayers to sodium and chloride. Cells cultured for 2 days on fibronectin had significantly higher conductance for NaCl or Lys-Cl than Na-Asp, indicating higher chloride permeability as compared with sodium permeability. By contrast, cells cultured for 5 days on fibronectin had equivalent ion conductance for all three buffers that was significantly lower than the conductance measurements for Day 2 cells on fibronectin. The preferential transepithelial permeability of Day 2 cells for chloride was further confirmed by dilution potential measurements (Figure 7E), in which cells showed a positive change in voltage using NaCl buffer. Taken together, these results suggest that the barrier formed by cells cultured for 2 days on fibronectin had differential ion permeability as compared with the barrier formed by cells after 5 days on fibronectin, where sodium and chloride were equivalently regulated.
DISCUSSION
To explore the factors that control claudin expression and lung epithelial cell barrier function, we examined the effects of extracellular matrix on tight junction protein expression and function. We examined cells cultured on fibronectin and type I collagen, matrix components found at low levels in normal lungs, but which increase dramatically after injury. We also used laminin, which resembles a normal basement membrane. Primary rat alveolar epithelial type II cells cultured on fibronectin more rapidly developed a high-resistance monolayer as compared with cells on laminin or type I collagen. Although it was surprising that cells on fibronectin had the most rapid barrier formation, previous work by Sugahara and colleagues (35) showed a similar effect on alveolar epithelial cells, although the earliest time point they measured was after 4 days in culture. Here, we found that this effect occurred as early as 2 days after culture on fibronectin, and correlated with a continuous pattern of claudin-3 and -18 localization to cell–cell contacts, suggesting early formation of tight junctions. The rapid development of a high-resistance barrier was also linked to an enhanced capacity for alveolar epithelial cells to attach to fibronectin (Figures 1 and 5) as compared with the other two matrices, consistent with a previous study (34). Increased cell spreading could allow cells to more rapidly form contacts with one another and thus promote tight junction formation. Moreover, by promoting more efficient coverage of the extracellular substrate by cells, fibronectin can contribute to barrier function independently from the paracellular route.
Although alveolar epithelial cells on fibronectin quickly formed a tight barrier, their TER only increased an additional 40% by Day 5 (Figure 7). Strikingly, the TER of cells on laminin increased nearly 45-fold by Day 5, to a TER that was nearly twice the TER of Day 5 cells on fibronectin. Thus, despite the ability to form a rapid barrier, cells on fibronectin ultimately had suboptimal barrier function as compared with cells on laminin. In contrast to cells on laminin, cells cultured for 5 days on type I collagen were comparable to Day 5 cells on fibronectin, from the standpoint of both barrier function and tight junction protein expression. This difference in alveolar epithelial cell response is consistent with the concept of laminin as a healthy matrix versus type I collagen and fibronectin as matrix proteins associated with an injured lung.
Cells cultured for 5 days showed increased ZO-1 content as compared with Day 2 cells, regardless of the initial extracellular matrix. In fact, cells on laminin had the highest levels of ZO-1 expression, and formed the most restrictive barriers. This is significant, because ZO-1 is critical for proper insertion of claudins into functional tight junction strands (7). Also, in an HIV transgenic rat model, we found that impaired alveolar barrier function correlated with decreased ZO-1 expression, consistent with an important role for ZO-1 in alveolar epithelial tight junctions (42). However, ZO-1 expression is not the sole determinant of epithelial barrier function, as cells cultured for 2 days on fibronectin formed a high-resistance monolayer, yet had lower ZO-1 content than Day 2 cells on laminin or type I collagen.
Claudins also play key roles in regulating paracellular permeability. Of note, alveolar epithelial cells cultured on fibronectin had lower claudin-5 content after 2 days as compared with cells cultured on laminin or type I collagen (Figure 4). This observation is significant in light of previous studies showing that increased claudin-5 expression by alveolar epithelial cells induced by methanandamide or in response to chronic alcohol ingestion correlates with increased paracellular permeability (12, 14). Consistent with the hypothesis that claudin-5 reduces alveolar epithelial barrier function, cells cultured for 5 days on laminin had higher TER and lower claudin-5 content than cells cultured on either type I collagen or fibrnectin. Although several claudins in addition to claudin-5 have been found to reduce rather than promote barrier function in certain cell types (6, 10, 43), the molecular basis for this effect is currently unknown.
By Day 5, alveolar epithelial cells on type I collagen had barrier function and tight junction protein expression that were indistinguishable from cells on fibronectin. It is possible that the cells were either adapting to the culture conditions in general or were remodeling the extracellular matrix. Consistent with these possibilities, alveolar epithelial cells have the capacity to synthesize and degrade several extracellular matrix components, including laminin, collagen and fibronectin (44). However, in the absence of an ability to remodel the matrix to a more normal composition, it seems likely that the alveolar barrier of cells on fibronectin would remain suboptimal as compared with a barrier formed by cells on a healthy matrix.
If left unopposed, fibronectin-mediated events lead to excessive inflammation and fibroblast proliferation, resulting in permanent disruption of normal alveolar structure (45). This is likely to be the case for both acute and chronic forms of lung injury where fibronectin expression is increased (46, 47). Consistent with this, others have shown that animals deficient in the extra type III domain A fibronectin splice variant develop less fibrosis in response to bleomycin-induced lung injury (48). In addition to these effects of fibronectin during injury, there is emerging data that subtle remodeling of the alveolar basement membrane can also occur before acute lung injury. For instance, we have demonstrated that chronic alcohol abuse promotes fibronectin expression in lung both in vitro and in vivo, and is associated with increased severity of acute lung injury (47, 49). In the alcoholic lung, there is little or no histological disruption, despite a significant increase in fibronectin content and deficiency in alveolar barrier function (50, 51). Thus, an impaired alveolar barrier does not necessarily require the alveolus to be significantly damaged, because remodeling of the extracellular matrix may contribute to an exaggerated injury response (3).
Alveolar epithelial cells on fibronectin for 2 days formed a high-resistance barrier, yet did not have a balanced barrier to both sodium and chloride. Instead, the barrier showed preferential chloride permeability (Figure 7E). The increase in chloride permeability was diminished by Day 5. Given the heterogeneous pattern of claudins expressed by these cells, it is difficult to assign this effect to one or more specific claudins. However, the decrease in chloride permeability for cells cultured for 5 days on fibronectin correlated with an increase in claudin-7 localized to the plasma membrane, consistent with studies demonstrating that claudin-7 preferentially restricts chloride permeability (52).
Although the increased chloride permeability by Day 2 cells on fibronectin was modest, it could have a significant influence on pulmonary fluid balance if present throughout a large enough area of the lung. In fact, increased paracellular chloride permeability might be advantageous, because it could provide an additional pathway for counter-ion transport to support increased sodium transport, which, in turn, could promote lung fluid clearance (53). Thus, it is tempting to speculate that this process could occur in the injured lung, where a high level of fibronectin persists in the matrix. However, any putative benefit from an early increase in paracellular chloride diffusion would later be offset by an overall decrease in alveolar barrier function, as opposed to barriers formed on laminin. Such would be the case in a lung enriched for fibronectin, where our results predict that newly differentiating type II cells would be induced to form a premature, suboptimal alveolar barrier. This, in turn, is likely to render the lung more susceptible to a second hit, leading to a more severe acute injury response.
Acknowledgments
The authors thank Leslie Cunningham and Tekla Smith for critical reading of the manuscript.
This work was supported by Emory Alcohol and Lung Biology Center/National Institutes of Health (NIH) grant P50-AA013757 (J.R. and M.K.), Department of Defense grant DAMD17-02-1-0179 (J.R.), NIH grants K99-HL092226 (M.N.H.) and R01-HL083120 (M.K.), and in part by the Flow Cytometry Core Facility of the Emory University School of Medicine.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0270OC on May 7, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 3.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol 2007;292:L813–L823. [DOI] [PubMed] [Google Scholar]
- 4.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004;286:C1213–C1228. [DOI] [PubMed] [Google Scholar]
- 5.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006;68:403–429. [DOI] [PubMed] [Google Scholar]
- 6.Koval M. Claudins: key pieces in the tight junction puzzle. Cell Commun Adhes 2006;13:127–138. [DOI] [PubMed] [Google Scholar]
- 7.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006;126:741–754. [DOI] [PubMed] [Google Scholar]
- 8.Everett RS, Vanhook MK, Barozzi N, Toth I, Johnson LG. Specific modulation of airway epithelial tight junctions by apical application of an occludin peptide. Mol Pharmacol 2006;69:492–500. [DOI] [PubMed] [Google Scholar]
- 9.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 2005;288:C1231–C1241. [DOI] [PubMed] [Google Scholar]
- 10.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens 2007;16:459–464. [DOI] [PubMed] [Google Scholar]
- 11.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 2003;285:L1166–L1178. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 2003;29:62–70. [DOI] [PubMed] [Google Scholar]
- 13.Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L1266–L1273. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 2007;41:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S. Extracellular matrix–driven alveolar epithelial cell differentiation in vitro. Exp Lung Res 2005;31:461–482. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 2005;288:L179–L189. [DOI] [PubMed] [Google Scholar]
- 17.Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of T1alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol 1998;275:L155–L164. [DOI] [PubMed] [Google Scholar]
- 18.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol 2003;65:669–695. [DOI] [PubMed] [Google Scholar]
- 19.Shannon JM, Pan T, Nielsen LD, Edeen KE, Mason RJ. Lung fibroblasts improve differentiation of rat type II cells in primary culture. Am J Respir Cell Mol Biol 2001;24:235–244. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 2007;36:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC. Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 2005;289:L382–L390. [DOI] [PubMed] [Google Scholar]
- 22.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150. [DOI] [PubMed] [Google Scholar]
- 23.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol 2001;281:C1291–C1299. [DOI] [PubMed] [Google Scholar]
- 26.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol 1999;276:L825–L834. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Martinez-Williams C, Yellowley CE, Donahue HJ, Rannels DE. Connexin expression by alveolar epithelial cells is regulated by extracellular matrix. Am J Physiol Lung Cell Mol Physiol 2001;280:L191–L202. [DOI] [PubMed] [Google Scholar]
- 28.Crouch EC, Martin GR, Brody JS, Laurie GW. Basement membranes. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations. Philadelphia: Lippincott-Raven; 1997. pp. 769–791.
- 29.Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med 2008;34:631–639. [DOI] [PubMed] [Google Scholar]
- 30.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest 2004;113:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roman J. Extracellular matrix and lung inflammation. Immunol Res 1996;15:163–178. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Henke CA, Savik SK, Ingbar DH. Integrin mediation of alveolar epithelial cell migration on fibronectin and type I collagen. Am J Physiol 1997;273:L134–L141. [DOI] [PubMed] [Google Scholar]
- 33.Rickard KA, Taylor J, Rennard SI, Spurzem JR. Migration of bovine bronchial epithelial cells to extracellular matrix components. Am J Respir Cell Mol Biol 1993;8:63–68. [DOI] [PubMed] [Google Scholar]
- 34.Garat C, Kheradmand F, Albertine KH, Folkesson HG, Matthay MA. Soluble and insoluble fibronectin increases alveolar epithelial wound healing in vitro. Am J Physiol 1996;271:L844–L853. [DOI] [PubMed] [Google Scholar]
- 35.Sugahara K, Kiyota T, Clark RA, Mason RJ. The effect of fibronectin on cytoskeleton structure and transepithelial resistance of alveolar type II cells in primary culture. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;64:115–122. [DOI] [PubMed] [Google Scholar]
- 36.Han S, Ritzenthaler JD, Wingerd B, Rivera HN, Roman J. Extracellular matrix fibronectin increases prostaglandin E2 receptor subtype EP4 in lung carcinoma cells through multiple signaling pathways: the role of AP-2. J Biol Chem 2007;282:7961–7972. [DOI] [PubMed] [Google Scholar]
- 37.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986;134:141–145. [DOI] [PubMed] [Google Scholar]
- 38.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol 1997;273(2 Pt 1):L347–L354. [DOI] [PubMed] [Google Scholar]
- 39.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci 2005;118:2683–2693. [DOI] [PubMed] [Google Scholar]
- 40.Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol 2006;571:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 2004;31:309–316. [DOI] [PubMed] [Google Scholar]
- 42.Lassiter C, Fan X, Joshi PC, Jacob BA, Sutliff RL, Jones DP, Koval M, Guidot DM. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turksen K, Troy TC. Barriers built on claudins. J Cell Sci 2004;117:2435–2447. [DOI] [PubMed] [Google Scholar]
- 44.Dunsmore SE, Martinez-Williams C, Goodman RA, Rannels DE. Turnover of fibronectin and laminin by alveolar epithelial cells. Am J Physiol 1995;269:L766–L775. [DOI] [PubMed] [Google Scholar]
- 45.Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 1992;5:404–410. [PubMed] [Google Scholar]
- 46.Roman J, Ritzenthaler JD, Bechara R, Brown LA, Guidot D. Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase-dependent signals that activate CREB. Am J Physiol Lung Cell Mol Physiol 2005;288:L975–L987. [DOI] [PubMed] [Google Scholar]
- 47.Burnham EL, Moss M, Ritzenthaler JD, Roman J. Increased fibronectin expression in lung in the setting of chronic alcohol abuse. Alcohol Clin Exp Res 2007;31:675–683. [DOI] [PubMed] [Google Scholar]
- 48.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 2008;177:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown LA, Ritzenthaler JD, Guidot DM, Roman J. Alveolar type II cells from ethanol-fed rats produce a fibronectin-enriched extracellular matrix that promotes monocyte activation. Alcohol 2007;41:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 2004;170:188–194. [DOI] [PubMed] [Google Scholar]
- 51.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 2000;279:L127–L135. [DOI] [PubMed] [Google Scholar]
- 52.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl− permeability. Biochem Biophys Res Commun 2007;357:87–91. [DOI] [PubMed] [Google Scholar]
- 53.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 2009;71:403–423. [DOI] [PubMed] [Google Scholar]