Abstract
We previously proposed a model of surfactant protein (SP)–C biosynthesis in which internalization of the proprotein from the limiting membrane of the multivesicular body to internal vesicles represents a key step in the processing and secretion of SP-C. To test this hypothesis, alanine mutagenesis of the N-terminal propeptide of SP-C was performed. Adenoviruses encoding mutant proproteins were infected into type II cells isolated from Sftpc−/− mice, and media analyzed for secreted SP-C 24 hours after infection. Mutation of S12PPDYS17 completely blocked secretion of SP-C. PPDY (PY motif) has previously been shown to bind WW domains of neural precursor cell-expressed developmentally down-regulated (Nedd) 4-like E3 ubiquitin ligases. Purified recombinant glutathione S-transferase–SP-C propeptide (residues 1–35) bound recombinant Nedd4-2 strongly, and Nedd4 weakly; the S12PPDYS17mutation abrogated binding of SP-C to Nedd4-2. Immobilized recombinant Nedd4-2 WW domain captured SP-C proprotein from mouse type II cell lysates; in the reverse pulldown, endogenous SP-C in type II cells was captured by recombinant Nedd4-2. To determine if the interaction of Nedd4-2 and SP-C resulted in ubiquitination, the SP-C proprotein was immunoprecipitated from transiently transfected human embryonic kidney 293 cells, and analyzed by SDS-PAGE/Western blotting with ubiquitin antibody. Two ubiquitinated forms of SP-C were detected; ubiquitination was blocked by mutation of K6, but not K34, in the SP-C propeptide. Mutation of K6 also inhibited processing of SP-C proprotein to the mature peptide in human embryonic kidney 293 cells. Nedd4-2–mediated ubiquitination regulates lumenal relocation of SP-C, leading to processing and, ultimately, secretion of SP-C.
Keywords: E3 ligase, multivesicular body, PY motif, type II cell, ubiquitin
The conducting airways of the mammalian lung progressively branch and narrow in diameter, ultimately terminating in sac-like structures called alveoli. Gas exchange occurs across the alveolar epithelium, which is comprised predominantly of thin type I cells that share a basal lamina with underlying capillary endothelial cells to form the air–blood barrier. The epithelial surface of the air–blood barrier is coated with pulmonary surfactant, a phospholipid-rich film synthesized and secreted by alveolar type II epithelial cells. Surfactant reduces surface tension generated by hydrostatic forces at the epithelial–air interface, thereby preventing alveolar collapse and decreased gas exchange at end expiration. The formation, maintenance, and function of the surfactant film are facilitated by two hydrophobic peptides, surfactant protein (SP)-B and SP-C (1).
In the human lung, the SP-C gene (SFTPC) is expressed exclusively in alveolar type II epithelial cells (2). The two most common SFTPC alleles encode an SP-C proprotein (proSP-C) of 191 or 197 amino acids (3). Newly synthesized proSP-C is a type II integral membrane protein in which the NH2-terminal 35 residues are located in the cytoplasm, and residues 59–191/197 are located in the lumen of the endoplasmic reticulum (ER) (4, 5). The lumenal domain was recently shown to function as an intramolecular chaperone for the inherently unstable, valine-rich, α-helical transmembrane domain (6, 7); chaperone activity is presumably maintained until the helix is stabilized by palmitoylation of adjacent cysteine residues in the “cytosolic domain” (8). The cytosolic domain also encodes information that directs intracellular trafficking of proSP-C (4, 5). Proteolytic processing of proSP-C commences in the multivesicular body (MVB) by step-wise cleavage of the lumenal domain, followed by cleavage of the cytosolic domain, to generate the 35–amino acid mature peptide (residues 24–58 of proSP-C), consisting of the transmembrane domain and 12 extramembrane residues, derived from the cytosolic domain (4, 9). Fusion of an MVB with a lamellar body (LB) leads to incorporation of SP-C into surfactant membranes. The surfactant complex is stored in the form of bilayer membranes in LBs until being secreted into the alveolar airspaces.
We have previously proposed a model of SP-C biosynthesis in which internalization of proSP-C from the limiting membrane of the MVB to internal (intralumenal) vesicles represents a key step in the processing and secretion of the proprotein (2, 5). Proteases within the MVB lumen can access the lumenal domain, but not the cytosolic domain, of proSP-C. Inward vesiculation of the limiting membrane and pinching off of the nascent bud results in the formation of an intralumenal vesicle in which the cytosolic domain of proSP-C is located within the interior of the vesicle. SP-B–mediated lysis of lumenal vesicles permits access of proteases to the vesicle interior and completion of proSP-C processing: the role of SP-B in this process is supported by the documented membranolytic properties of the peptide (10, 11), and the finding that SP-C recovered from the airspaces of SP-B–deficient infants is extended by 12 amino acids at the N-terminal end (12, 13). In addition to proteolytic generation of the mature form of SP-C, internalization of proSP-C into the MVB lumen is likely required for secretion of this integral membrane protein. The current study was designed to identify molecular components involved in the relocation of proSP-C from the limiting membrane to intralumenal vesicles of the MVB.
MATERIALS AND METHODS
DNA Constructs
Adenoviral constructs, encoding mouse SP-C (residues 1–58), followed by a hemagglutinin (HA) tag (YPYDVPDYA), were generated as previously described (5). Alanine mutations were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Recombination and virus production were performed as described by Davis and colleagues (14). For studies in transiently transfected human embryonic kidney (HEK) 293 cells, human full-length wild-type (WT) proSP-C (SP-C1–197) was cloned into the expression vector pIRES2-EGFP (BD Biosciences, San Jose, CA) to permit expression of “untagged” proSP-C and cytosolic EGFP from a single bicistronic mRNA; mutations of the PY motif (P13 P D Y16) and potential ubiquitination sites (K6 and K34) were generated by site-directed mutagenesis of the WT proprotein in pIRES2-EGFP. Human WT proSP-C and a K6R mutant were also cloned into the expression vector pEGFP-C1 (BD Biosciences) for expression as green fluorescent protein (GFP)–SP-C fusion proteins in HEK293 cells. For pulldown experiments, sequences encoding the WW domains of mouse neural precursor cell-expressed developmentally down-regulated (Nedd) 4 (residues 238–492), Nedd4-2 (residues 74–460), WWP1 (residues 347–525), WWP2 (residues 305–477), and AIP4/itch (residues 288–471) were cloned in frame with an NH2-terminal HA tag and ligated into the Nbe1/Not1 sites of pcDNA3.1 (Invitrogen, Carlsbad, CA) for expression in HEK293 cells. Full-length human and mouse Nedd4.2 were also cloned into pIRES2-EGFP. Sequences encoding the cytosolic domain of mouse WT SP-C propeptide (residues 1–35) and a mutant cytosolic domain, in which residues 12–17 (SPPDYS) were replaced with alanine, were cloned into pET41c (EMD Chemicals, Gibbstown, NJ) for expression as glutathione S-transferase (GST)–SP-C fusion proteins in Escherichia coli and purified by glutathione affinity chromatography, as described by the manufacturer (Novagen).
Transfection and Cell Culture
Type II cells were isolated from 6-week-old female C57BL6 mice and cultured on Matrigel (BD Biosciences):rat tail collagen (70:30, vol/vol), as previously described (15). After 2 days of culture, cells were infected with adenoviral particles encoding SP-C constructs, exactly as previously described (16). For in vivo analyses of SP-C trafficking, WT and Sftpc−/− mice (in the FVB/N background) were intratracheally injected with adenoviral particles, as previously described (5). At 3 days after infection, mice were lavaged with saline and large aggregate (LA) surfactant isolated (17); LBs were prepared from lung tissue homogenates, as described by Osanai and colleagues (18). HEK293 cells (ATCC, Manassas, VA) were grown in Richter's medium (BioWhittaker, Walkersville, MD), as previously described (16). For transfection of SP-C constructs cloned into pIRES2-EGFP, pEGFP-C1, or pcDNA3.1, HEK293 cells were seeded at 3–5 × 105 cells/well in a six-well plate. After 24 hours, cells were transfected with 3 μl Lipofectamine 2000 (Invitrogen):0.25 μg DNA. For brefeldin A (BFA; Sigma-Aldrich, St. Louis, MO) experiments, cells were treated with 5 μg/ml BFA or methanol vehicle for 20 hours after transfection. For bafilomycin A1 experiments, cells were treated 100 nM bafilomycin A1 (EMD Chemicals) for 4 hours, and harvested 24 hours later for analysis.
Analyses of Cell Lysates
At 24 hours after transfection, secreted surfactant was recovered from media by centrifugation at 10,000 × g, 4°C for 5 minutes; surfactant pellets and cells were suspended in Laemmli sample buffer (Bio-Rad, Hercules, CA) and immediately sonicated. β-Mercaptoethanol was added to a final concentration of 1%, samples heated at 100°C for 4 minutes, and proteins separated by SDS-PAGE on 10–20% tricine gels (Invitrogen). Gels were electrophoretically transferred to 0.1-μm nitrocellulose membranes (ISC BioExpress, Kaysville, UT) and blocked with 5% nonfat milk. Western analyses were performed with rabbit polyclonal antibodies directed against the N-terminal peptide of proSP-C (13) (Seven Hills Bioreagents, Cincinnati, OH), the C-terminal peptide (lumenal domain) of proSP-C (6), the mature SP-C peptide (19) (Seven Hills Bioreagents), HA antibody (Santa Cruz Biotechnology, Santa Cruz, CA), green fluorescent protein (Molecular Probes, Carlsbad, CA), or mouse monoclonal actin antibody (Seven Hills Bioreagents). Antigen/antibody complexes were visualized using the SuperSignal West Pico detection system (Pierce, Rockford, IL), and membrane stripping was performed with the Restore Western Blot Stripping Buffer (Pierce).
To determine if SP-C was processed to the mature peptide in HEK293 cells, cell lysate was mixed with surfactant isolated from human bronchoalveolar lavage fluid (BALF) and subjected to SDS-PAGE/Western blotting with antibody directed against the mature SP-C peptide. For these experiments, deidentified BALF (collected from patients with alveolar proteinosis under a protocol approved by the University of Cincinnati College of Medicine institutional review board) was centrifuged for 30 minutes at 14,000 × g (4°C); the surfactant pellet was extensively diluted in Laemmli electrophoresis sample buffer (20) so that the SP-C signal in BALF was of similar intensity to that in transfected HEK293 cells.
Confocal and Electron Microscopy
For confocal microscopy, HEK293 cells were plated in poly-D-lysine–coated two-well chamber slides (Nalge Nunc International, Rochester, NY) at 3 × 105 cells/well. After 24 hours, cells were transiently transfected with SP-C constructs, and, 24 hours later, fixed with 4% paraformaldehyde for 15 minutes at room temperature. Fixed cells were permeablized for 10 minutes at room temperature with 0.2% Triton-X100 and 0.1% BSA in PBS. Permeabilized cells were blocked for 1 hour at room temperature with 0.1% BSA and 5% normal goat serum in 1× PBS. Cells were incubated overnight at 4°C with antibodies directed against mature SP-C and Lamp-1 (CD107A MAB; Fitzgerald, Concord, MA). After PBS washes, the cells were incubated with Alexa Fluor 633 anti-rabbit IgG, followed by incubation with Alexa Fluor 546 anti-mouse IgG (Molecular Probes). Slides were mounted using Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA), and fluorescence was visualized on a confocal microscope (LSM 510; Carl Zeiss Microimaging, Inc., Thornwood, NY).
For electron microscopy, HEK293 cells were fixed with 4% paraformaldehyde–lysine–sodium periodate, 0.1% glutaraldehyde, and 0.1% CaCl2 in 0.2 M Hepes (pH 7.2) for 1 hour at 4°C. Cells were cryoprotected with buffered polyvinylpyrrolidone/sucrose and processed for cryoultramicrotomy. Colocalization of mature SP-C and Lamp-2 was demonstrated using sequential double labeling. Localization of mature SP-C was detected using rabbit antisera directed against mature SP-C and incubated with 10 nm protein A gold, as previously described (21); Lamp-2 was detected using mouse monoclonal antibodies directed against human Lamp-2 (H4B4; Developmental Studies Hybridoma Bank, University of Iowa), incubated with rabbit anti-mouse IgG (1 μg/ml; MP Biomedical, Solon, OH), then visualized with 5 nm protein A gold. Electron micrographs were acquired at a magnification of 80,000× using a Hitachi H-7600 EM (Hitachi High Technologies America, Inc., Pleasanton, CA) equipped with a 2D kx2k AMT digital camera (Advanced Microscopy Techniques Corp., Danvers, MA). Electron micrographs were further adjusted for brightness and contrast using Adobe Photoshop (Adobe, San Jose, CA).
Pulldown Assays
HEK293 cells were transiently transfected with constructs encoding WW domains and harvested in 1 ml Nonidet P-40 lysis buffer (1% NP-40, 20 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1× protease inhibitor cocktail; Sigma-Aldrich) 48 hours later. Cell lysates were rotated at 4°C for 10 minutes, then centrifuged at 13,000 × g (4°C) for 10 minutes to remove insoluble particles. Rotation of cleared lysates at 4°C for 2 hours with 10 μg GST:SP-C fusion protein was followed by rotation for 30 minutes with 50 μl GST-Bind Resin (Novagen). The resin was washed four times with lysis buffer, and protein eluted with 2× reduced Laemmli buffer. Eluates were subjected to Western blot analysis with antibody directed against the WW domains of Nedd4 (Abcam, Cambridge, MA) that detects both Nedd4 and Nedd4-2.
For analyses of SP-C–Nedd4.2 interactions in type II epithelial cells, type II cells from WT and Sftpc−/− mice were harvested, washed twice with PBS, and resuspended in lysis buffer (20 mM Hepes [pH 7.6], 125 mM NaCl, 10% glycerol, 1% Trition X-100, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, with 1× protease inhibitors). Cells were rotated at 4°C overnight, sonicated briefly, and centrifuged 10,000 × g for 10 minutes Lysates were rotated overnight with 4 μl C-terminal SP-C antibody or recombinant Nedd4 or Nedd4.2 protein, followed by a 2-hour rotation with 50 μl trisacryl immobilized protein A (Pierce) and four 1-ml washes with lysis buffer. Proteins were eluted with 50 μl 2× reduced Laemmli buffer, followed by Western analysis with antibody directed against Nedd4 or the N-terminal propeptide of SP-C using the One-Step Complete Western Kit (GenScript, Piscataway, NJ).
To analyze ubiquitination of SP-C, HEK293 cells were cotransfected with SP-C constructs and full-length human or mouse Nedd4.2, or transfected with SP-C constructs alone. Cell lysates were prepared 48 hours after transfection, as described above, for HEK293 cells, immunoprecipitated with antibody directed against the C-terminal domain of proSP-C, and analyzed by SDS-PAGE/Western blotting with antibody that detects mono- and polyubiquitinated proteins (ENZO Life Sciences, Plymouth Meeting, PA).
Knockdown Experiments
Mouse type II cells were isolated and cultured as previously described here. siRNA (200 pmol) for Nedd4, Nedd4-2, or control siRNA (Invitrogen) were mixed with Sendai virus envelopes, according to the manufacturer's instructions (Cosmo Bio USA, Inc., Carlsbad, CA), and added to 1–2 × 106 type II cells on Day 2 of culture. Media were replaced on Days 3 and 4 of culture, and cells harvested on Day 5 using dispase (BD Biosciences), as previously described (15). RNA was isolated for first-strand cDNA synthesis, followed by real-time PCR to assess the efficiency of knockdown. Cell lysates were analyzed by SDS-PAGE/Western blotting with antibody directed against mature SP-C.
For analysis of SP-C processing in adult Nedd4-2−/− mice (22), lungs were homogenized in PBS containing protease inhibitor cocktail (Sigma-Aldrich). Equal amounts of protein from WT and knockout mice were analyzed by SDS-PAGE/Western blotting with antibody directed against mature SP-C and actin.
RESULTS
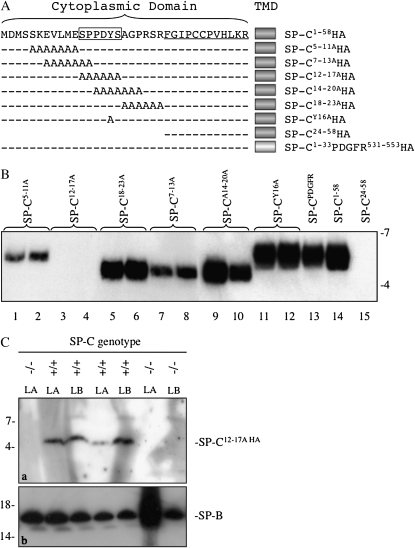
ProSP-C is a type II integral membrane protein in which 23 residues of the N-terminal propeptide and 12 residues of the mature peptide (underlined in Figure 1A) are located in the cytoplasm. Previous deletion analyses established that residues 1–23 were essential for secretion of SP-C (5). Scanning alanine mutagenesis was therefore performed in this region to identify candidate motifs that interact with trafficking machinery in the secretory pathway. An HA tag was added to the C terminus of each construct, because preliminary studies indicated that alanine mutagenesis destroyed antigenic epitopes for the antibody directed against the N-terminal region of proSP-C. Adenoviruses encoding mutant or control SP-CHA peptides (Figure 1A) were generated and used to infect type II epithelial cells isolated from Sftpc−/− mice (23, 24). Media were recovered 1 day after infection, surfactant pellets isolated by centrifugation, and secreted SP-CHA peptides identified by Western blotting with HA antibody (Figure 1B). Secretion of control SP-CHA peptides (Figure 1B, last three lanes) was similar to previous in vivo studies in Sftpc−/− mice (5), thus establishing the validity of the in vitro cell culture system: SP-C1–58HA (positive control) was readily detected in media, whereas SP-C24–58HA (negative control) was not (Figure 1B, lanes 14 and 15); further, substitution of the transmembrane domain of SP-C with the corresponding region from platelet-derived growth factor receptor (PDGFR) resulted in secretion of SP-CHA (Figure 1B, lane 13), as previously described (5). SP-C1–58HA was processed to a smaller peptide (relative electrophoretic mobility [Mr], ∼5 k) consistent with appropriate cleavage of the NH2-terminal propeptide. All mutant SP-CHA peptides, except SP-C12–17AHA, were detected in the media (Figure 1B). The electrophoretic mobility of SP-C18–23AHA, SP-C7–13AHA, and SP-C14–20AHA was increased relative to the other mutant and control peptides. In a study unrelated to the current work, we observed that mutation of proline residues in the cytosolic domain increased the electrophoretic mobility of SP-C (see Figure E1A in the online supplement). In two independent experiments in which each construct was analyzed in duplicate (results of one experiment are shown in Figure 1B), secretion of SP-C7–13AHA and SP-C5–11AHA was decreased compared with other SP-CHA peptides, and SP-C12–17AHA was not detectable. These results suggest that the 12SPPDYS17 (boxed region in Figure 1A) sequence contained a motif that was critical for secretion of SP-C; furthermore, mutations of S12 and/or P13 and S5KEVLME11, but not Y16, reduced secretion of SP-C by type II cells.
Figure 1.
Identification of a motif required for secretion of surfactant protein (SP)–C by type II epithelial cells. (A) Alanine mutagenesis of the NH2-terminal propeptide of SP-C was performed, and adenovirus encoding the mutated cytosolic domain, native transmembrane domain (TMD), and a C-terminal hemagglutinin (HA) tag was generated for each construct. Underlined residues constitute the cytosolic domain of the mature peptide; boxed residues include a PY motif (PPDY). (B) Mouse type II cells were isolated from Sftpc−/− mice, cultured for 2 days, then infected in duplicate with adenovirus encoding wild-type (WT) SP-C (SP-C1–58HA), SP-C carrying alanine substitutions in the propeptide (SP-CnHA), SP-C in which the TMD was replaced with the comparable domain from PDGFR (SP-C1–33 PDGFR531–553HA), or mature SP-C peptide (SP-C24–58HA). Media were collected 24 hours later, the surfactant pellet isolated by centrifugation, and secreted SP-C detected by SDS-PAGE/Western blotting with HA antibody. Mature SP-CHA migrated with relative electrophoretic mobility (Mr) of approximately 5 k or approximately 6 k. Representative of two experiments with each construct analyzed in duplicate. (C) WT (+/+) or Sftpc (−/−) mice were intratracheally infected with adenovirus encoding SP-C12–17AHA. At 3 days after infection, mice were lavaged and the large aggregate (LA) fraction of surfactant recovered; lamellar bodies (LBs) were isolated from lung homogenates by flotation on sucrose gradients. SP-C was detected by SDS-PAGE/Western blotting with HA antibody. (a) Mature SP-C12–17AHA, Mr approximately 5 k. (b) LA and LB fractions were immunoblotted for mature SP-B; Mr, approximately 16 k. Representative of two experiments (see Figure S1B).
The importance of the SPPDYS motif was confirmed by intratracheal infection of WT or Sftpc−/− mice with adenovirus encoding SP-C12–17AHA (Figure 1C). At 3 days after infection, mice were lavaged and the LA fraction of surfactant recovered; lung tissues were subsequently homogenized, and the LB fraction isolated. Western blotting with HA antibody detected SP-CHA in the LB and LA fractions of infected WT mice, confirming that the adenovirus encoded an SP-CHA peptide that was secreted in the presence of WT SP-C; furthermore, the peptide comigrated with SP-C24–58HA expressed in HEK293 cells (Figure E1B), demonstrating that SP-C12–17A was processed to the mature peptide in the presence of endogenous SP-C. In two independent experiments (Figure 1C and Figure E1B), SP-CHA was not detected in LB or LA fractions isolated from infected Sftpc−/− mice, whereas SP-B was readily detected (Figure 1C, panel b). Taken together, the results of in vitro and in vivo experiments indicate that the SPPDYS mutation prevents secretion of SP-C, but that this defect can be rescued, presumably via heterodimerization of WT and mutant SP-C (5, 25).
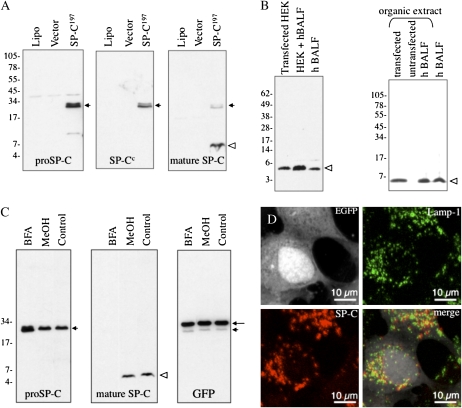
The SPPDYS sequence contains a PY motif (L/PP x Y, where x is any amino acid) that has been shown to interact with WW domains of the Nedd4 family of E3 ubiquitin ligases. This observation suggests that PPDY in the propeptide facilitated interaction with an E3 ligase that, in turn, catalyzed the transfer of ubiquitin to a critical lysine residue(s) involved in sorting of SP-C within the secretory pathway. Reduced secretion of SP-C5–11A suggests that K6 might be the target of an E3 ligase bound at the PPDY motif. To facilitate testing of these hypotheses, we developed an assay system in which: (1) processing of proSP-C could be analyzed without the need to generate adenovirus and transfect culture of type II cells (Figure 2); and (2) mutations of the PY motif could be assessed. A previous immunofluorescence study demonstrated that transient transfection of HEK293 cells with plasmid encoding human proSP-C resulted in sorting of proSP-C to the lysosome (26). Western blot analyses of cell lysates identified proSP-C (Figure 2A, short arrow) and a smaller peptide that was detected by antibody directed against the mature SP-C peptide (Figure 2A, open arrowhead) and comigrated with mature SP-C in human BALF (Figure 2B, left panel). The SP-C peptide in HEK293 cells also extracted into organic solvents, similar to mature SP-C in human BALF, confirming the hydrophobicity of the SP-C peptide in HEK293 cells (Figure 2B, right panel). Processing of the proprotein was inhibited by BFA (Figure 2C) or bafilomycin A1 (Figure 3C), indicating that maturation of proSP-C occurred in a post-Golgi, acidic compartment. Immunofluorescence microscopy demonstrated partial colocalization of mature SP-C peptide with punctate Lamp-1–positive structures, consistent with processing of proSP-C in the lysosome or late endosome/MVB (Figure 2D); localization of mature SP-C to lysosomes was confirmed by immunogold labeling (Figure E2). Collectively, these results suggest that transiently transfected HEK293 cells were an appropriate model to study the intracellular trafficking and processing of proSP-C to the mature peptide.
Figure 2.
Intracellular trafficking and processing of WT proSP-C in human embryonic kidney (HEK) 293 cells. (A) HEK293 cells were transfected with a construct encoding human WT proSP-C (SP-C1–197) cloned into pIRES2-EGFP. After 24 hours, lysates from cells transfected with lipofectamine alone (lipo), pIRES2-EGFP vector, or plasmid encoding SP-C1–197 were analyzed by SDS-PAGE/Western blotting with antibody directed against the NH2-terminal propeptide of proSP-C (proSP-C); blots were subsequently stripped and reprobed with antibodies directed against the lumenal domain of SP-C (SP-Cc) or the mature SP-C peptide. Short arrow indicates proSP-C; open arrowhead indicates mature SP-C. (B) Cell lysates from HEK293 cells transfected with SP-C1–197 (transfected HEK) were mixed with human bronchoalveolar lavage fluid (hBALF) and analyzed by SDS-PAGE, followed by Western blotting with antibody directed against mature SP-C peptide (left panel). In a separate experiment (right panel), HEK293 cells transfected with plasmid encoding SP-C1–197 (transfected), untransfected HEK293 cells (untransfected), or hBALF were extracted with chloroform/methanol, and the organic phase analyzed by Western blotting with antibody directed against the mature SP-C peptide. Open arrowhead indicates mature SP-C peptide in both panels. (C) HEK293 cells were transfected with SP-C1–197 cloned into pIRES2-EGFP, and treated with brefeldin A (5 μg/ml) or vehicle control (MeOH) for 20 hours. Cell lysates were prepared 24 hours after transfection, and analyzed by SDS-PAGE/Western blotting. Blots were serially probed with antibody directed against the N-terminal peptide of proSP-C, SP-C mature peptide, or GFP. Long arrow indicates GFP (encoded by the bicistronic pIRES2-EGFP vector); short arrowhead indicates proSP-C; and open arrowhead indicates mature peptide. (D) HEK293 cells were transfected with SP-C1–197 cloned into pIRES2-EGFP. Cells were permeabilized 24 hours after transfection, stained with antibodies directed against Lamp-1 or mature SP-C peptide, and analyzed by confocal microscopy. Upper left panel is signal from EGFP (encoded by the pIRES2 vector), which is expressed in the cytosol and delineates the boundary of the cell.
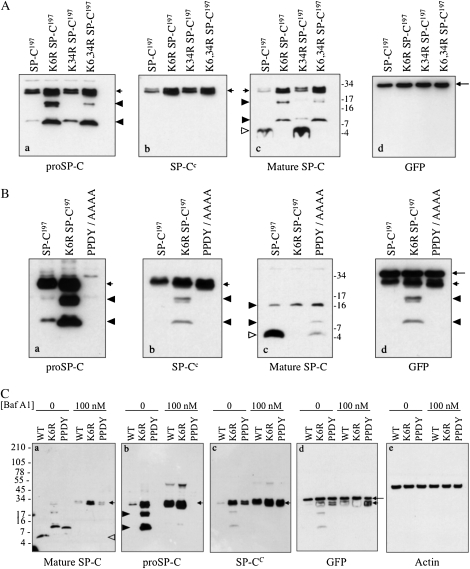
Figure 3.
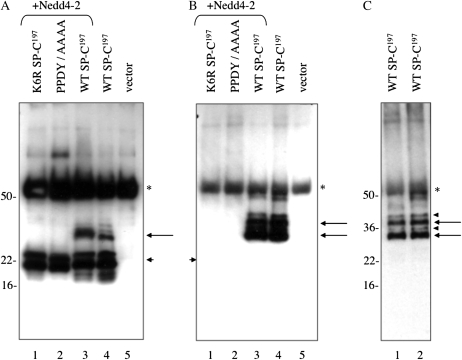
Effect of K6 and PY motif mutations on trafficking and processing of proSP-C. (A) HEK293 cells were transfected with WT proSP-C (SP-C1–197) or proSP-C in which K6 and/or K34 were mutated to arginine. Cell lysates were prepared 24 hours after transfection and analyzed by SDS-PAGE/Western blotting. Blots were serially probed with antibodies directed against proSP-C (a), the C-terminal, lumenal domain of proSP-C (b), mature SP-C (c), or GFP (d). Short arrows indicate proSP-C; open arrowhead indicates mature peptide; closed arrowheads indicate proSP-C processing intermediates; and long arrow indicates GFP (expressed from the pIRES2-EGFP vector). (B) HEK293 cells were transfected with WT proSP-C or proSP-C carrying a K6R mutation or PPDY/AAAA (PY motif) mutation. Cell lysates were prepared 24 hours after transfection, and analyzed by SDS-PAGE/Western blotting. Blots were serially probed with the indicated antibodies, as described for (A). Incomplete stripping between blots resulted in detection of proSP-C (short arrows), processing intermediates (closed arrowheads), in addition to GFP (long arrow) in (d). proSP-C carrying the PPDY/AAAA mutation was not detected by antibody directed against the N-terminal peptide of proSP-C ([a], short arrow), but was detected by antibody directed against the lumenal domain of proSP-C ([b], short arrow). (C) HEK293 cells were transfected as described in (B), and treated with bafilomycin A1 for 4 hours. Cell lysates were prepared 24 hours after transfection, and analyzed by SDS-PAGE/Western blotting. Blots were serially probed with the indicated antibodies. Short arrows indicate proSP-C; closed arrowheads indicate processing intermediates; open arrowhead indicates mature peptide; and long arrow indicates GFP. GFP expression (d) indicates equivalent expression of SP-C constructs; actin expression (e) indicates equivalent loading of samples on gel.
The cytosolic domain of proSP-C contains two potential ubiquitination targets: K6 and K34 (Figure 1A). Full-length human proSP-C constructs were generated in which K6 or K34 were individually mutated to arginine, or both K6 and K34 were mutated (K6, 34R). Constructs were transfected into HEK293 cells and cell lysates analyzed by Western blotting (Figure 3A). SP-C processing intermediates were detected by antibodies directed against the N-terminal propeptide (Figure 3A, panel a, closed arrowheads), but not the C-terminal peptide (Figure 3A, panel b), indicating that these peptides were derived from processing of the lumenal, C-terminal domain of proSP-C. The K6R and K6, K34R mutations, but not the K34R mutation, blocked processing of proSP-C to the mature peptide (Figure 3A, panel c, open arrowhead) leading to accumulation of both proprotein and processing intermediates (Figure 3A, panel a, lane 2). Interestingly, GFP–SP-C1−197 and GFP–K6R–SP-C1–197 fusion proteins were both fully processed to the mature peptide, suggesting that the GFP tag “rescued” the K6R mutation (see Figure E4 and Discussion section). For this reason, all studies in HEK293 cells were performed with untagged SP-C. These results implicate K6 as a critical residue in SP-C maturation. Mutation of PPDY to alanine also inhibited maturation of proSP-C, although, unlike the K6 mutation, a small amount of mature peptide was detected (Figure 3B, panel C). Bafilomycin A1 (Figure 3C) and BFA (Figure E3) completely blocked processing of WT proSP-C to mature peptide (Figure 3C, panel a), resulting in dramatic accumulation of proprotein (Figure 3C, panel b); processing of K6R and PPDY proprotein to intermediate forms (Figure 3C, panel a, lanes 1 and 2, closed arrowheads) was also blocked, suggesting that these maturation events also occurred in a post-Golgi, acidic compartment. Expression of cytosolic GFP from the bicistronic vector was unaffected by bafilomycin A1 (Figure 3C, panel d) or BFA (data not shown) treatment. Treatment with MG-132 did not cause accumulation of either WT or mutant proprotein (data not shown). Thus, the K6R and PPDY/AAAA mutations did not appear to cause protein misfolding and degradation via ER-associated degradation (ERAD), but rather prevented terminal processing of proSP-C to mature peptide late in the biosynthetic/endocytic pathway.
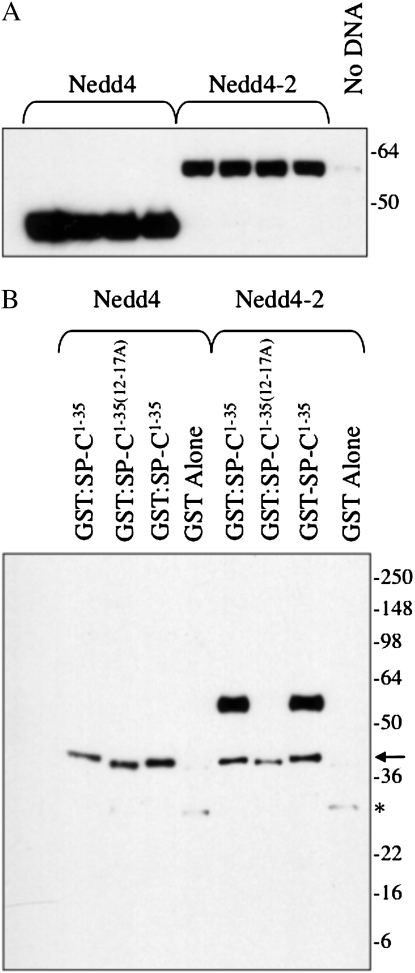
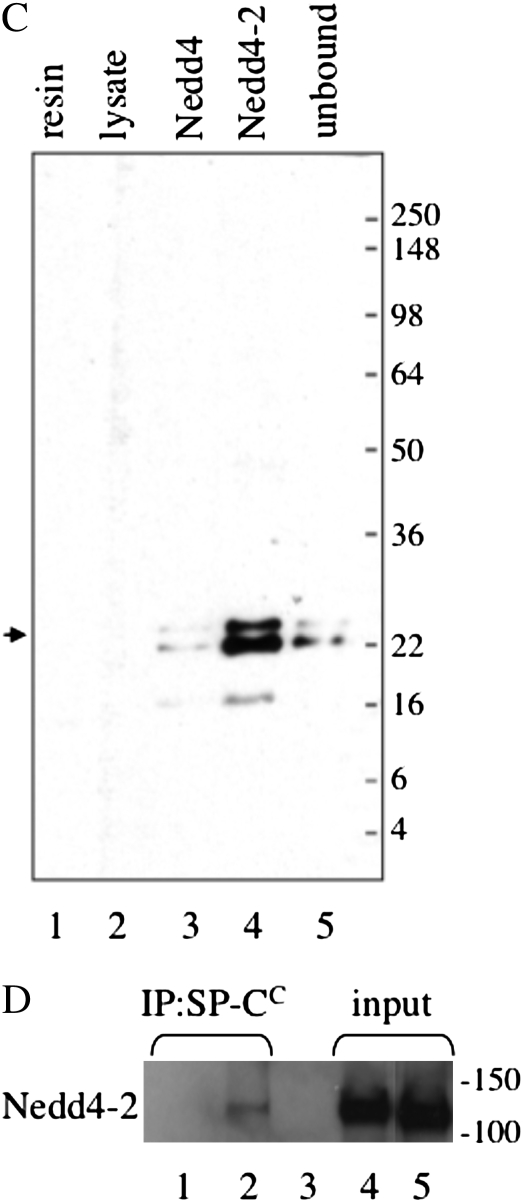
To determine if the PPDY sequence of pro SP-C could bind to a WW domain, constructs encoding the WW domains of Nedd4, Nedd4-2, WWP1, WWP2, and AIP4/itch were expressed in HEK293 cells (Figure 4A; only results for Nedd4-2 and Nedd4 are shown, because, like Nedd4, the other WW domains did not interact with SP-C in this assay). Cell lysates were then incubated with purified, recombinant GST–SP-C1–35, which included only the cytosolic domain of the proprotein in which the PY motif is located. Complexes were captured with glutathione agarose and analyzed by SDS-PAGE, followed by Western blotting with an antibody that detects the WW domains of both Nedd4 and Nedd4-2 (Figure 4B). GST–SP-C1–35 associated with the WW domains of Nedd4-2, but not Nedd4 or WW domains from other Nedd4-like E3 ligases. Recombinant GST–SP-C1–35(12–17A), in which the SPPDYS sequence was replaced with alanines, did not associate with Nedd4-2. Thus, Nedd4-2 bound to the PPDY motif of pro SP-C in the absence of any cross-linking reagent. To confirm the association between SP-C and Nedd4-2, the WW domains of mouse Nedd4 and Nedd4-2 were tagged with HA, expressed in HEK293 cells, and recovered from cell lysates by immunoprecipitation with HA antibody. Freshly prepared mouse type II cell lysate was then serially incubated with Nedd4 and Nedd4-2 immunoprecipitates (in the absence of cross-linking reagent), and the captured complexes analyzed by SDS-PAGE and Western blotting with proSP-C antibody (Figure 4C). Nedd4 associated weakly with the proSP-C, whereas Nedd4-2 bound most of the proprotein in the cell lysate, even though the lysate had been first incubated with Nedd4. Immobilized Nedd4-2 also captured an SP-C processing intermediate (Mr, ∼17 k) from the type II cell lysate. The reverse coprecipitation experiment was also performed (Figure 4D). ProSP-C was immunoprecipitated from mouse type II cell lysates (in the absence of cross-linking reagent) with antibody directed against the C-terminal, lumenal domain of proSP-C (to avoid blocking the N-terminal PY motif), and immunoprecipitates analyzed by SDS-PAGE/Western blotting with Nedd4 antibody. SP-C coprecipitated with Nedd4-2 in lysates prepared from WT type II cells (Figure 4D, lane 2), but not lysates of type II cells isolated from Sftpc−/− mice (Figure 4D, lane 1), although Nedd4-2 was clearly detected in type II cells from both WT and knockout mice (Figure 4D, lane 4). Pulldown of Nedd4-2 by SP-C (Figure 4D) was much less efficient than the reverse pulldown (Figure 4C), presumably because only a fraction of proSP-C is ubiquitinated at any one point in time.
Figure 4.
Neural precursor cell–expressed developmentally down-regulated (Nedd) 4-2 binds to the cytosolic domain of proSP-C. (A) Plasmids encoding the WW domains of mouse Nedd4 or Nedd4-2 were transfected into HEK293 cells. At 48 hours after transfection, cell lysates were prepared and analyzed by SDS-PAGE/Western blotting with antibody directed against the Nedd4 WW domain. (B) Cell lysates (seen in [A]) were incubated with purified, recombinant GST–SP-C1–35 encoding the cytosolic domain of WT proSP-C or GST–SP-C1–35(12–17A) in which the SPPDYS motif was mutated to alanine. Complexes were captured with glutathione agarose and analyzed by SDS-PAGE/Western blotting with WW domain antibody. Arrow and asterisk indicate cross-reactivity of antibody with GST–SP-C and GST, respectively. (C) Recombinant Nedd4 or Nedd4-2 domain was captured from transfected HEK293 cells (seen in [A]) with HA antibody conjugated to protein G sepharose. Freshly isolated mouse type II epithelial cells were lysed and serially incubated with protein G sepharose (lysate), Nedd4, and Nedd4-2. The unbound type II cell lysate (lane 5) and protein G sepharose complexes (lanes 1–4) were subsequently analyzed by SDS-PAGE/Western blotting with antibody directed against the N-terminal peptide of proSP-C. Small arrow indicates proSP-C. (D) Type II cells isolated from WT (lanes 2, 3, and 5) or Sftpc−/− mice (lanes 1 and 4) were lysed and immunoprecipitated with antibody directed against the C-terminal, lumenal domain of SP-C (lanes 1 and 2). Captured proSP-C complexes were analyzed by SDS-PAGE, followed by Western blotting with WW domain antibody (lanes 1 and 2). Lane 1, type II cell lysate from Sftpc−/− mice; lane 2, type II cell lysate from WT mice; lane 3, type II cell lysate from WT mice immunoprecipitated with protein G sepharose (no SP-C antibody); lane 4, input lysate from Sftpc−/− type II cells; lane 5, input lysate from WT type II cells.
Based on the results of the foregoing experiments (Figure 4), association of Nedd4-2 with the PY motif of proSP-C should lead to ubiquitination of K6. This hypothesis was tested by transient transfection of HEK293 cells with plasmids encoding WT, PPDY/AAAA, or K6R forms of proSP-C; cell lysates were subsequently immunoprecipitated for SP-C, and analyzed by SDS-PAGE and Western blotting with antibody directed against proSP-C or ubiquitin (Figure 5). SP-C antibody detected larger forms of the proprotein only after long exposure (Figure 5A, long arrow). Cotransfection of Nedd4-2 resulted in an increase in the amount of larger SP-C isoform (Figure 5A, lane 3) that was not detected when K6 or PPDY was mutated (Figure 5A, lanes 1 and 2). The K6R and PPDY/AAAA mutant proproteins differ from WT SP-C at only one and four residues, respectively, strongly supporting a specific modification of proSP-C at K6. Western blotting with ubiquitin antibody readily detected larger forms of proSP-C in two independent experiments (Figures 5B and 5C). Two major ubiquitinated SP-C isoforms were detected, corresponding to the lower band of the proprotein doublet (Figure 5C, long arrows); the upper band of the proprotein doublet was also ubiquitinated, but the signal was much less intense (Figure 5C, closed arrowhead). Overexposure of the membrane did not detect polyubiquitination of SP-C (Figure 5B). Given the decrease in electrophoretic mobility of the ubiquitinated SP-C isoforms (Mr, ∼30 k and 38 k), the absence of ubiquitinated SP-C in cells transfected with the K6R mutants, and the absence of polyubiquitination, we conclude that SP-C is mono- or biubiquitinated at K6.
Figure 5.
Ubiquitination of proSP-C. HEK293 cells were cotransfected with plasmids encoding WT (SP-C1–197) or mutant (K6R and PPDY/AAAA) proSP-C and plasmid encoding full-length human Nedd4-2 (+Nedd4-2). Cell lysates were prepared 24 hours after transfection and immunoprecipitated for proSP-C. Antigen–antibody complexes were captured by protein G sepharose and analyzed by SDS-PAGE/Western blotting with antibody directed against the N-terminal peptide of proSP-C (A), or antibody that detects mono- and polyubiquitinated proteins (B). Overexposure was required to detect ubiquitinated SP-C in (A) and (B) was deliberately overexposed to demonstrate absence of polyubiquitination. (C) The experiment was repeated, and lysates from cells transfected with WT SP-C were analyzed as in (B). A shorter exposure confirmed the presence of two major ubiquitinated forms of SP-C (long arrows) and two minor forms (arrowheads). Short arrows indicate proSP-C; *IgG detected by secondary antibody.
To determine if loss of Nedd4-2 expression would lead to decreased processing of proSP-C, type II cells were stably transfected with Nedd4-2, Nedd4, or control siRNA. Expression of Nedd4-2 and Nedd4 mRNAs was decreased by approximately 80% and 60%, respectively, relative to control siRNA; however, processing of proSP-C to mature peptide was not affected (data not shown). Subsequently, lungs from Nedd4-2−/− mice (22) were analyzed by SDS-PAGE/Western blotting. Processing of proSP-C to mature peptide was not affected, suggesting that Nedd4 or another E3 ligase compensates for loss of Nedd4-2 activity (data not shown).
DISCUSSION
The destination for newly synthesized membrane proteins in the secretory pathway is the plasma membrane or the limiting membrane of organelles and vesicles involved in secretion. The current study was designed to test the hypothesis that SP-C is diverted from the limiting membrane of carrier vesicles late in the biosynthetic/endocytic pathway to the lumen of the MVB, thus allowing processing of proSP-C and secretion of the mature peptide with surfactant phospholipids into the alveolar airspaces. Mutagenesis of a PY motif (P13PDY16) in the cytosolic domain of proSP-C blocked secretion of SP-C by type II epithelial cells. This finding led to the hypothesis that the PY motif mediated binding of an E3 ubiquitin ligase and monoubiquitination of pro SP-C; the latter event would result in internalization of proSP-C and processing to its mature peptide. Consistent with this model, processing of pro SP-C was blocked by mutation of the PY motif; furthermore, mutation of K6 blocked both ubiquitination of the proprotein and processing of proSP-C. The PY motif strongly interacted with Nedd4-2 and weakly bound Nedd4, implicating Nedd4-2 as the primary E3 ligase. Surprisingly, processing of proSP-C to mature peptide was essentially unaffected in Nedd4-2−/− mice. Nedd4 also bound the PY motif in SP-C, albeit more weakly than Nedd4-2, and may therefore compensate for loss of Nedd4-2; however, we cannot exclude the possibility that another Nedd4 family member fulfills this role. Overall, these results suggest that ubiquitination of the cytosolic propeptide of SP-C leads to internalization of the proprotein from the limiting membrane of the MVB to intralumenal vesicles, facilitating processing of proSP-C to its mature peptide.
The Nedd4 family of HECT (homologous to E6-AP COOH terminal)-type E3 ligases includes nine members that ubiquitinate a wide range of target proteins involved in multiple cellular processes (27–29). Family members are characterized by an NH2-terminal C2 domain that mediates calcium-dependent, membrane-binding, 2-4 WW domains involved in substrate recognition and binding, and a C-terminal catalytic HECT domain containing E3 ubiquitin ligase activity. WW domains bind proline-rich sequences with a preference for PPxY, where x is any amino acid. Recombinant protein encompassing the four WW domains of Nedd4-2 strongly bound the PY motif (PPDY) in the cytosolic domain of proSP-C in HEK293 and type II epithelial cells; other Nedd4 family members, including AIP4/itch, WWP1, and WWP2, did not interact with proSP-C. Secretion of SP-C was affected by sequences outside the core PY motif; in particular, residues immediately N-terminal to PPDY (SP-C7–13A). This finding is consistent with a recent report that interaction of the high-affinity WW domain (WW3) of Nedd4 with a PY motif (LPSY) in the target protein, commisureless, involved residues both N-terminal and C-terminal to the motif (30). Interestingly, mutation of Y16 in the SP-C PY motif had no effect on SP-C secretion, whereas mutation of S12 and/or P13 (S12 and P13 overlap in the 12–17 and 7–13 alanine mutants; Figure 1A) was associated with decreased SP-C secretion. These results raise the possibility that Nedd4-2 may also bind S12. Peptides containing phosphoserine followed by a proline have been shown to interact with the WW2 domain of Nedd4-2 (31). Consistent with this hypothesis, a small amount of proSP-C was completely processed to the mature peptide in transfected HEK293 cells when the PPDY motif was mutated to alanine (Figure 3B). Thus, there are two partially overlapping motifs in the cytosolic domain of proSP-C that may bind Nedd4-2, leading to ubiquitination of the propeptide.
It is important to note that these studies do not exclude the possibility that Nedd4-2 associates with proSP-C through an intermediary protein(s). However, given the presence of the PPDY motif in the cytosolic domain of proSP-C, the binding of WW domains from Nedd4-2, and the loss of WW domain binding after mutation of the PPDY motif, we believe that this is unlikely. It is also formally possible that Nedd4-2 binds directly to proSP-C, but that ubiquitination is mediated by association of another E3 ligase with Nedd4-2. Although we favor direct ubiquitination of proSP-C by Nedd4-2, we cannot exclude an indirect role of this ligase.
Mono- and multimonoubiquitination play an important role in sorting membrane proteins from the plasma membrane and Golgi to the lumen of the lysosome for degradation or function (e.g., hydrolase activation) (28, 29, 32). These sorting pathways converge at the MVB, where the ubiquitinated protein is recognized and internalized from the limiting membrane to intralumenal vesicles, an event regulated by interaction with ESCRT (endosomal sorting complexes required for transport) I–III (33). Fusion of an MVB with a lysosome results in delivery of intralumenal vesicles to the lumen of the lysosome and degradation of cargo. In specialized cells, sorting within the MVB is critical for biogenesis and/or function of lysosome-related organelles, such as melanosomes in melanocytes (34), secretory lysosomes in cells of hematopoietic origin (35), and LBs in pulmonary type II epithelial cells (36). Pre-melanosomal protein 17 (PMEL17) is a melanosomal membrane protein that forms the fibrillar infrastructure onto which melanin is deposited within the lumen of melanosomes (37). Unlike SP-C, relocation of PMEL17 from limiting membrane to lumen occurs via a ubiquitin-independent mechanism (38). Fas ligand is a type II membrane protein that is sorted to internal vesicles of secretory lysosomes in cytotoxic T lymphocytes and natural killer cells. Secretion of exosome-like vesicles bearing FasL leads to apoptosis of target cells expressing Fas. As for SP-C, lumenal relocation of FasL is essential for both secretion and function. Sorting of FasL is dependent on tyrosine phosphorylation, in addition to monoubiquitination of the cytosolic domain (39). Phosphorylation is mediated by binding of Src family tyrosine kinases to a protein-rich domain; the identity of the E3 ligase(s) involved in ubiquitination of FasL is not known. Although the cytosolic domain of SP-C contains a single tyrosine residue, in the context of the PY motif, mutation of this residue did not affect SP-C secretion. Thus, multiple mechanisms are used to effect relocation of membrane proteins to internal vesicles of lysosome-related organelles.
There are both advantages and limitations to the use of HEK293 cells for the study of SP-C trafficking/processing. The “SPPDYS” mutant was unstable and rapidly degraded in type II epithelial cells; in contrast, this mutant was very stable when expressed in HEK293 cells, allowing analysis of the PY motif. Rapid turnover of the SPPDYS mutant in type II cells, however, suggests that quality control of SP-C may be more stringent in type II cells than in HEK293 cells (40). We have previously identified several chaperones required for ERAD of disease-associated SP-C mutants in HEK293 cells (40); the results of the current study suggest that cell and/or substrate-specific chaperones may be required for SP-C ERAD. A second limitation of HEK293 cells is that SP-C is not secreted; thus, although trafficking/processing of SP-C can be evaluated in HEK293 cells, secretion must be analyzed in type II cells. Mutation of K6 in type II cells (i.e., the SP-C5–11HA mutant, Figure 1) diminished, but did not completely block secretion of SP-C by type II epithelial cells. This outcome may be related to conjugation of ubiquitin at the NH2-terminal residue of SP-C in type II cells, as previously reported for T cell antigen receptor and myoD (41, 42).
In the current study, we observed a clear effect of EGFP on the processing of SP-C in HEK293 cells. In the absence of any tag, mutation of K6 to arginine blocked ubiquitination and subsequent internalization/processing of the proprotein to the mature peptide (Figure 3C, panel c); however, when SP-C was fused to the C terminus of EGFP, the K6R mutant proprotein was processed to mature peptide exactly like WT SP-C (Figure E4). The most likely explanation for this outcome is that a residue in EGFP is ubiquitinated, leading to internalization and processing of proSP-C, even when K6 is mutated. Consistent with this hypothesis, the C-terminal residue of EGFP is lysine; therefore, in the EGFP/SP-CK6R fusion protein, a new ubiquitination target is introduced six residues upstream of K6 in SP-C (43, 44). Thus, although EGFP may be a useful tool for analyses of some aspects of SP-C trafficking, it is important to recognize the limitations of this approach.
In summary, the cytosolic domain of proSP-C contains a canonical PY motif that interacts with the WW domains of Nedd4-2, leading to ubiquitination at K6. Mutation of the PY motif blocked interaction of SP-C with Nedd4-2, and prevented both ubiquitination and processing of the proprotein to its mature peptide; similarly, mutation of K6 blocked ubiquitination and processing. We conclude that ubiquitination of the cytosolic domain of proSP-C is an important step in the relocation of the proprotein from the limiting membrane to internal vesicles of MVBs, where processing to mature peptide is completed. It is currently unclear which E3 ubiquitin ligase(s) can compensate for loss of Nedd4-2 expression; in addition, the enzyme involved in deubiquitination of proSP-C after internalization has yet to be identified.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the secretarial assistance of Ann Maher.
This work was supported by National Heart, Lung, and Blood Institute grants HL61646 and HL086492.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0058OC on May 7, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid–protein interactions. Biochim Biophys Acta 2008;1778:1676–1695. [DOI] [PubMed] [Google Scholar]
- 2.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol 2001;63:555–578. [DOI] [PubMed] [Google Scholar]
- 3.Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol 2004;66:601–623. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AL, Braidotti P, Pietra GG, Russo SJ, Kabore A, Wang WJ, Beers MF. Post-translational processing of surfactant protein-c proprotein: protein trafficking motifs in the NH2-terminal flanking domain are cleaved in late compartments. Am J Respir Cell Mol Biol 2001;24:253–263. [DOI] [PubMed] [Google Scholar]
- 5.Conkright JJ, Bridges JP, Na CL, Voorhout WF, Trapnell B, Glasser SW, Weaver TE. Secretion of surfactant protein C, an integral membrane protein, requires the N-terminal propeptide. J Biol Chem 2001;276:14658–14664. [DOI] [PubMed] [Google Scholar]
- 6.Johansson H, Nordling K, Weaver TE, Johansson J. The Brichos domain–containing C-terminal part of pro-surfactant protein C binds to an unfolded poly-val transmembrane segment. J Biol Chem 2006;281:21032–21039. [DOI] [PubMed] [Google Scholar]
- 7.Nerelius C, Martin E, Peng S, Gustafsson M, Nordling K, Weaver T, Johansson J. Mutations linked to interstitial lung disease can abrogate anti-amyloid function of prosurfactant protein C. Biochem J 2008;416:201–209. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson M, Griffiths WJ, Furusjo E, Johansson J. The palmitoyl groups of lung surfactant protein C reduce unfolding into a fibrillogenic intermediate. J Mol Biol 2001;310:937–950. [DOI] [PubMed] [Google Scholar]
- 9.Vorbroker DK, Voorhout WF, Weaver TE, Whitsett JA. Posttranslational processing of surfactant protein C in rat type II cell cells. Am J Physiol Lung Cell Mol Physiol 1995;269:L727–L733. [DOI] [PubMed] [Google Scholar]
- 10.Ryan MA, Qi XY, Serrano AG, Ikegami M, Perez-Gil J, Johansson J, Weaver TE. Mapping and analysis of the lytic and fusogenic domains of surfactant protein B. Biochemistry 2005;44:861–872. [DOI] [PubMed] [Google Scholar]
- 11.Shiffer K, Hawgood S, Duzgunes N, Goerke J. Interactions of the low molecular weight group of surfactant-associated proteins (SP 5–18) with pulmonary surfactant lipids. Biochemistry 1988;27:2689–2695. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ikegami M, Na CL, Hamvas A, Espinassous Q, Chaby R, Nogee LM, Weaver TE, Johansson J. N-terminally extended surfactant protein (SP) C isolated from SP-B–deficient children has reduced surface activity and inhibited lipopolysaccharide binding. Biochemistry 2004;43:3891–3898. [DOI] [PubMed] [Google Scholar]
- 13.Vorbroker DK, Profitt SA, Nogee LM, Whitsett JA. Aberrant processing of surfactant protein C (SP-C) in hereditary SP-B deficiency. Am J Physiol Lung Cell Mol Physiol 1995;268:L647–L656. [DOI] [PubMed] [Google Scholar]
- 14.Davis AR, Meyers K, Wilson JM. High throughput method for creating and screening recombinant adenoviruses. Gene Ther 1998;5:1148–1152. [DOI] [PubMed] [Google Scholar]
- 15.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 2002;283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 16.Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem 2003;278:52739–52746. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol Lung Cell Mol Physiol 1996;14:L650–L658. [DOI] [PubMed] [Google Scholar]
- 18.Osanai K, Mason RJ, Voelker DR. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am J Respir Cell Mol Biol 1998;19:929–935. [DOI] [PubMed] [Google Scholar]
- 19.Ross GF, Ikegami M, Steinhilber W, Jobe AH. Surfactant protein C in fetal and ventilated preterm rabbit lungs. Am J Physiol Lung Cell Mol Physiol 1999;277:L1104–L1108. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 21.Voorhout WF, Veenendaal T, Haagsman HP, Weaver TE, Whitsett JA, van Golde LMG, Geuze HJ. Intracellular processing of pulmonary surfactant protein-B in an endosomal/lysosomal compartment. Am J Physiol 1992;263:L479–L486. [DOI] [PubMed] [Google Scholar]
- 22.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 2008;295:F462–F470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasser SW, Burhans MS, Korfhagen TR, Na C-L, Sly PD, Ross GF, Ikegami M, Whitsett JA. Altered stability of pulmonary surfactant in SP-C–deficient mice. Proc Natl Acad Sci USA 2001;98:6366–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasser SW, Detmer EA, Ikegami M, Na C-L, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in SP-C gene targeted mice. J Biol Chem 2003;278:14291–14298. [DOI] [PubMed] [Google Scholar]
- 25.Wang WJ, Russo SJ, Mulugeta S, Beers MF. Biosynthesis of surfactant protein C (SP-C): sorting of SP-C proprotein involves homomeric association via a signal anchor domain. J Biol Chem 2002;277:19929–19937. [DOI] [PubMed] [Google Scholar]
- 26.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol 2006;172:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shearwin-Whyatt L, Dalton HE, Foot N, Kumar S. Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays 2006;28:617–628. [DOI] [PubMed] [Google Scholar]
- 28.d'Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 2005;6:429–441. (Review) [DOI] [PubMed] [Google Scholar]
- 29.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev 2006;86:669–707. [DOI] [PubMed] [Google Scholar]
- 30.Kanelis V, Bruce MC, Skrynnikov NR, Rotin D, Forman-Kay JD. Structural determinants for high-affinity binding in a Nedd4 WW3*domain-Comm PY motif complex. Structure 2006;14:543–553. [DOI] [PubMed] [Google Scholar]
- 31.Sudol M, Hunter T. NeW wrinkles for an old domain. Cell 2000;103:1001–1004. [DOI] [PubMed] [Google Scholar]
- 32.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol 2006;16:514–521. [DOI] [PubMed] [Google Scholar]
- 33.Babst M. A protein's final ESCRT. Traffic 2005;6:2–9. [DOI] [PubMed] [Google Scholar]
- 34.Raposo G, Marks MS. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic 2002;3:237–248. [DOI] [PubMed] [Google Scholar]
- 35.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol 2002;3:122–131. [DOI] [PubMed] [Google Scholar]
- 36.Weaver T, Na C, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 2002;13:263. [DOI] [PubMed] [Google Scholar]
- 37.Raposo G, Marks MS. Melanosomes—dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol 2007;8:786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain–dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell 2006;10:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuccato E, Blott EJ, Holt O, Sigismund S, Shaw M, Bossi G, Griffiths GM. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci 2007;120:191–199. [DOI] [PubMed] [Google Scholar]
- 40.Dong M, Bridges JP, Apsley K, Xu Y, Weaver TE. ERdj4 and ERdj5 are required for endoplasmic reticulum-associated protein degradation of misfolded surfactant protein C. Mol Biol Cell 2008;19:2620–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Kopito RR. The role of multiubiquitination in dislocation and degradation of the alpha subunit of the T cell antigen receptor. J Biol Chem 1999;274:36852–36858. [DOI] [PubMed] [Google Scholar]
- 42.Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J 1998;17:5964–5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baens M, Noels H, Broeckx V, Hagens S, Fevery S, Billiau AD, Vankelecom H, Marynen P. The dark side of EGFP: defective polyubiquitination. PLoS One 2006;1:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katayama H, Yamamoto A, Mizushima N, Yoshimori T, Miyawaki A. GFP-like proteins stably accumulate in lysosomes. Cell Struct Funct 2008;33:1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.