Abstract
We have reported that moderate-intensity aerobic exercise training attenuates airway inflammation in mice sensitized/challenged with ovalbumin (OVA). The current study determined the effects of repeated bouts of aerobic exercise at a moderate intensity on airway hyperresponsiveness (AHR) in these mice. Mice were sensitized/challenged with OVA or saline and exercised at a moderate intensity 3 times/week for 4 weeks. At protocol completion, mice were analyzed for changes in AHR via mechanical ventilation. Results show that exercise decreased total lung resistance 60% in OVA-treated mice as compared with controls; exercise also decreased airway smooth muscle (ASM) thickness. In contrast, exercise increased circulating epinephrine levels 3-fold in saline- and OVA-treated mice. Because epinephrine binds β2-adrenergic receptors (AR), which facilitate bronchodilatation, the role of β2-AR in exercise-mediated improvements in AHR was examined. Application of the β2-AR antagonist butoxamine HCl blocked the effects of exercise on lung resistance in OVA-treated mice. In parallel, ASM cells were examined for changes in the protein expression of β2-AR and G-protein receptor kinase-2 (GRK-2); GRK-2 promotes β2-AR desensitization. Exercise had no effect on β2-AR expression in ASM cells of OVA-treated mice; however, exercise decreased GRK-2 expression by 50% as compared with controls. Exercise also decreased prostaglandin E2 (PGE2) production 5-fold, but had no effect on E prostanoid-1 (EP1) receptor expression within the lungs of OVA-treated mice; both PGE2 and the EP1 receptor have been implicated in β2-AR desensitization. Together, these data indicate that moderate-intensity aerobic exercise training attenuates AHR via a mechanism that involves β2-AR.
Keywords: asthma, airway hyperresponsiveness, exercise, β2-adrenergic receptor
CLINICAL RELEVANCE.
This is the first report to demonstrate that aerobic exercise at a moderate intensity attenuates airway hyperresponsiveness via a mechanism that involves the β2-adrenergic receptor. Because increased airway hyperresponsiveness is a hallmark of the asthmatic response, this study supports the use of moderate-intensity aerobic exercise as an adjunct therapy for the treatment of this chronic disease.
Allergic or atopic asthma, the most common form of asthma, is identified by the presence of characteristic clinical symptoms of wheezing, chest tightness, dyspnea and cough, and by the presence of reversible airway narrowing and/or airway hyperresponsiveness (AHR) to a variety of inhaled bronchoconstrictor stimuli. For the treatment of asthma-associated AHR, patients are typically administered inhaled, long-acting β2-agonists. β2-agonists bind to and activate β2-adrenergic receptors (β2-AR), which are expressed on a variety of cell types, including airway smooth muscle (ASM) cells, airway epithelial cells, and mast cells (1).
Several clinical studies suggest that continued aerobic exercise training improves the overall physical fitness and health of individuals with asthma and reduces their disease-related hospital admissions (2–4); these studies also observed improvements in exercise-induced bronchoconstriction after physical training. We have reported previously that moderate intensity aerobic exercise training reduces lung inflammatory responses in an ovalbumin (OVA)-driven mouse model of pulmonary inflammation (5, 6).
Repetitive exercise increases the levels of circulating catecholamines, including epinephrine (7). Epinephrine binds β2-AR in the airways to facilitate bronchodilatation and, thereby, functions as a β2-agonist. Upon binding β2-agonist, the β2-AR couples with the stimulatory G protein (Gs) to stimulate adenylate cyclase, cAMP production, and subsequent protein kinase A (PKA) activation. Activated PKA stimulates myosin light chain kinase and myosin light chain phosphatase; these events lead to decreased myosin light chain activity and smooth muscle tone (1). Desensitization occurs upon G-protein receptor kinase-2 (GRK-2)–mediated phosphorylation of β2-AR, which uncouples the receptor from the Gs-adenylated cyclase complex (8). The prostaglandin PGE2 may also regulate β2-AR desensitization indirectly via activation of the E prostanoid-1 (EP1) receptor. Upon activation, the EP1 receptor heterodimerizes with β2-AR, which impairs β2-AR coupling to the Gs-adenylated cyclase complex and, thereby, reduces β2-AR–stimulated cAMP production (9).
In the current study, we hypothesized that repetitive moderate-intensity aerobic exercise would attenuate asthma-associated AHR. To test this hypothesis, mice were sensitized and challenged with OVA or control saline and exercised on a motorized treadmill repetitively at a moderate intensity as reported previously (5, 6). At the conclusion of the protocol, changes in AHR were monitored upon exposure to increasing doses of methacholine, a bronchoconstrictor stimulus. In parallel, changes in circulating epinephrine levels, β2-AR expression, GRK-2 expression, ASM thickness, PGE2 content, and EP1 receptor expression were measured. Collectively, results presented herein indicate that moderate-intensity aerobic exercise attenuates AHR via a mechanism that involves β2-AR.
MATERIALS AND METHODS
Animals
Female BALB/cJ mice (3–5 wk old; The Jackson Laboratory, Bar Harbor, ME), a strain susceptible to OVA-induced IgE responses (10, 11), were used. Mice were housed in a pathogen-free containment facility and maintained in autoclaved Microisolator cages (Lab Products, Maywood, NJ). Mice were provided with food (Teklad, Madison, WI) and water ad libitum. Mice were allowed to acclimate to housing condition for 1 week before experimental manipulation. All animal treatments were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health recommended guidelines.
OVA Sensitization and Exercise Protocol
OVA sensitization and moderate-intensity aerobic exercise training were performed as described previously (5, 6). Briefly, sensitization (Days 0 and 14, intraperitoneally) and challenge (Days 21–25 and Day 28, via aerosolization) with OVA (Sigma Chemical, St. Louis, MO) or saline was performed (5). We have shown previously that the utilization of this OVA sensitization scheme in BALB/cJ mice initiates an airway inflammatory response and immunoglobulin profile that is consistent with those observed in asthma (5). Exercised mice underwent bouts of moderately-intense aerobic exercise on a motorized treadmill (Exer 6M; Columbus Instruments, Columbus, OH) at 1 hour after OVA treatment. Exercise bouts were performed three times per week for a total of 10 bouts; mice were exercised for 45 minutes at 13.5 m/minute (0% grade). As noted in our earlier study, previous reports have defined moderate-intensity aerobic exercise as brief (15- to 60-min) bouts of treadmill running at 50 to 75% maximum O2 consumption or approximately 15 to 22 m/minute (5, 12). All exercise bouts included brief warm-up and cool-down periods so that the total treadmill time was about 60 minutes.
Mechanical Ventilation
Mice were mechanically ventilated and challenged with increasing concentrations of methacholine as described previously (13). Briefly, 48 hours after the last exercise bout, mice were anesthetized with diazepam (17.5 mg/kg) and ketamine (450 mg/kg), and a tracheotomy tube (18G) was inserted and connected to the inspiratory and expiratory ports of a ventilator (Flexivent; SCIREQ, Montreal, PQ, Canada). Mice were ventilated at a rate of 160 breaths per minute at a tidal volume of 0.2 ml with a positive end-expiratory pressure of 2 to 4 cm H2O. Increasing concentrations of methacholine (0–50 mg/ml) were administered via aerosolization. From 20 seconds up to 3 minutes after each aerosol challenge, resistance (R) was recorded continuously; an average value of R was taken to express changes in murine airway function. For studies involving the β2-AR antagonist butoxamine HCL (Sigma), mice received a nebulization of either butoxamine HCL (200 mg/ml) or saline before each methacholine concentration as just described; R was recorded continuously.
Lung Fixation and ASM Thickness Measurements
Lungs were extracted and prepared for analysis as described previously (5, 6). Briefly, mice were injected with a lethal dose of ketamine/xylazine. The mice were treacheaotomized using an 18G catheter and the lungs were filled with 10% paraformyldehyde. Once the lungs were filled the trachea was tied off to prevent leakage of the paraformedyhyde using suture silk and lungs were removed from the body cavity. All excess tissue was clipped off and the lungs were suspended in a tube of 10% paraformeldehyde, embedded in paraffin, and then was sliced into 10-μm sections for staining. Paraffin tissue sections were rehydrated as described (5, 6). Rehydrated tissue was stained with a biotinylated primary antibody against α–smooth muscle actin (Dako, Carpinteria, CA) and subsequently probed with steptavidin–peroxidase. Staining was completed with incubation with 3,3′-diaminobenzadine (DAB) + substrate, resulting in a brown-colored precipitate at the antigen site. Slides were then visualized under a light field microscope.
For ASM thickness measurements, airways with a longitudinal diameter between 150 and 200 μm were identified and ASM thickness was measured in a blinded fashion. Specifically, a minimum of 10 airways with the required diameter per mouse were selected randomly and ASM thickness was measured on each airway at two sites 90 degrees apart using the imaging program Metamorph. Measuring two sites on each airway analyzed accounted for any differences in the plane of sectioning between airways.
Epinephrine Measurements
For measurement of circulating epinephrine levels, mice underwent cardiac puncture and blood was collected into EDTA-coated tubes (Becton Dickinson, Franklin Lakes, NJ) as described previously (5). Care was taken to ensure that the same individual handled all the mice in the study in the same manner to minimize the impact of killing on basal epinephrine measurements. From these blood samples, plasma was isolated and then stored at −20°C. Epinephrine levels were assayed using an epinephrine-specific ELISA kit (R&D Systems and Research Diagnostics, Minneapolis, MN, performed according to manufacturer's instructions). All plasma sampling was performed between 7:30 a.m. and 11:00 a.m.
ASM Isolation and Western Blot Analysis
Tracheas were removed, and the trachealis muscle was isolated and digested in a digestion buffer containing collagenase, soybean trypsin inhibitor, and elastase as described previously (14). The resulting cell suspension was filtered to remove debris and resuspended in Ham's F-12 medium (supplemented with 10% FBS). Isolated ex vivo ASM cells were immediately lysed and prepared for Western blot analysis as described previously (15). Equivalent amounts of protein lysates (20 μg/lane) were electrophoresed and transferred to PVDF membrane blots. Blots were blocked with 5% BSA-containing buffer and then stained with either a rabbit anti-mouse anti–β2-AR antibody (Abcam, Cambridge, MA), anti–GRK-2 antibody, or anti-EP1 receptor antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a goat–anti-rabbit IgG antibody conjugated to horseradish peroxidase (HRP). Immunoblots were developed using chemiluminescence and imaged using an Chemidoc XRS Imaging Station (Bio-Rad).
To verify ASM phenotype and to account for differences in lane loading, blots were stripped and re-probed with HRP-conjugated antibodies directed against the ASM marker α-smooth muscle actin (Santa Cruz). Re-probed blots were developed via chemiluminescence and analyzed as just described. Results were quantitated via densitometry.
Lavage Protein Analysis
Mice were lavaged as described previously (5, 6). Briefly, mice were lavaged with 1.0 ml of 0.9% saline, and bronchoalveolar lavage fluid (BALF) supernatant samples were analyzed for differences in PGE2 content via ELISA (R&D Systems).
Statistical Analysis
Data were analyzed using SPSS Version 11.0. Results are reported as group means ± SD. A one-way ANOVA determined differences among the group means and the Bonferroni post hoc analysis determined which group means differed significantly (at a level of P ≤ 0.05).
RESULTS
Moderate-Intensity Aerobic Exercise Decreases Airway Resistance in OVA-Treated Mice
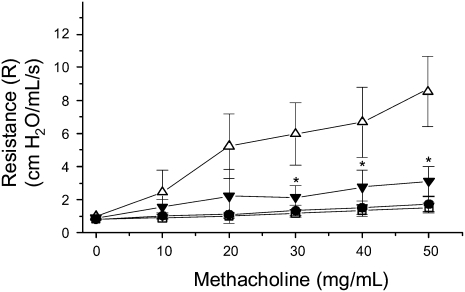
To determine the effect of a moderate-intensity aerobic exercise training on AHR in OVA-treated mice, mice were mechanically ventilated and challenged with increasing concentrations of methacholine (0–50 mg/ml); changes in resistance (R) were recorded continuously. Results shown in Figure 1 demonstrate that, at the highest methacholine dose, OVA-treated, sedentary (SO) mice exhibited a total lung resistance that was approximately 4-fold greater than saline-treated sedentary (S) and exercise (E) controls. In contrast, mice that were OVA-treated and exercised (EO) displayed a 65% reduction in total lung resistance as compared with OVA-treated, sedentary mice (SO); this level of resistance was equivalent to that observed in S and E control groups. Significant decreases in overall lung resistance were also observed in OVA-treated and exercised (EO) mice at intermediate methacholine doses (20 mg/ml, 40 mg/ml) as compared with sedentary controls (Figure 1).
Figure 1.
Moderate-intensity aerobic exercise attenuates airway hyperresponsiveness (AHR) in ovalbumin (OVA)-treated mice. Mice were OVA treated and exercised as described in Materials and Methods (open squares: sedentary, saline-treated; open circles: exercised, saline-treated; open triangles: sedentary, OVA-treated; solid triangles: exercised, OVA-treated). At the conclusion of the protocol, mice were assessed for changes in AHR via mechanical ventilation with methacholine challenge at increasing concentrations. Results are presented as total lung resistance (cm H2O/ml/s; *P < 0.02, as compared with sedentary, OVA-treated mice; n = 6–8 mice per group).
Aerobic Exercise at a Moderate Intensity Decreases ASM Thickness in OVA-Treated Mice
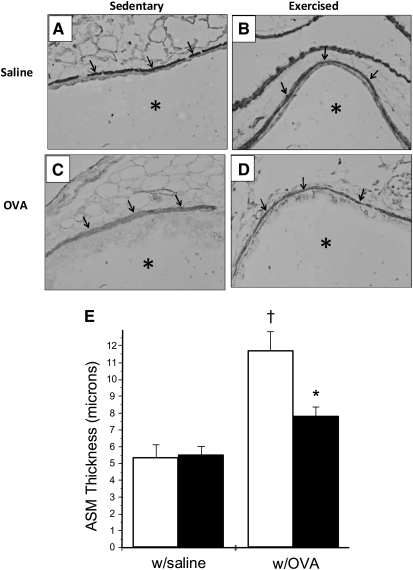
Increases in airway wall thickness have been implicated as an underlying mechanism in the initiation and/or exacerbation of AHR (16, 17). Because increases in ASM thickness contribute most to the increased airway wall thickness in individuals with asthma (17), the effect of moderate-intensity aerobic exercise on ASM thickness in OVA-treated mice was determined. To this end, lung tissues were harvested and stained with a biotinylated α-smooth muscle actin antibody and subsequently probed with steptavidin–peroxidase; tissues were then analyzed for changes in ASM thickness via Metamorph analysis. Data presented in Figure 2 demonstrate that OVA treatment of sedentary (SO) mice increased ASM thickness 2-fold as compared with saline-treated controls (S, E; Figures 2A, 2B, and 2E). Mice that were OVA-treated and exercised (EO), however, displayed a 35% reduction in ASM thickness as compared with sedentary controls (Figures 2C, 2D, and 2E); this level of thickness was equivalent to that observed in saline-treated control mice (Figures 2A, 2C, and 2E).
Figure 2.
Moderate-intensity aerobic exercise decreases airway smooth muscle (ASM) thickness in OVA-treated mice. Mice were OVA treated and exercised as described in Materials and Methods. At the conclusion of the protocol, whole lung tissues were harvested and prepared for peroxidase staining and ASM thickness measurements with MetaMorph software. (A–D) Representative images of lung tissue stained for α–smooth muscle actin and probed with peroxidase (×40 magnification). Arrows indicate ASM layer; asterisks indicate airway lumen. (E) Results of Metamorph analysis are presented as ASM thickness in microns (†P < 0.02, as compared with sedentary, saline-treated mice; *P < 0.02, as compared with sedentary, OVA-treated mice; n = 4–8 mice per group). Open bars, sedentary mice; solid bars, exercised mice.
Repeated Bouts of Aerobic Exercise at a Moderate Intensity Increase Circulating Epinephrine Levels
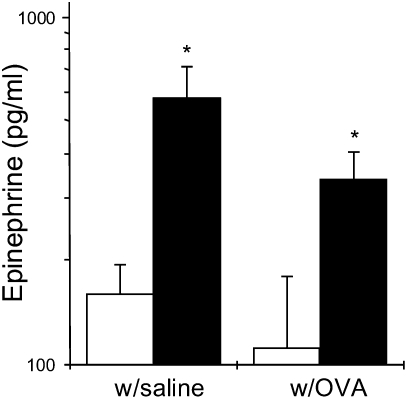
Because circulating epinephrine has been implicated in bronchodilatation (18), the effects of exercise on epinephrine production in OVA-treated mice was determined. At the conclusion of the exercise and OVA treatment protocol, mice were cardiac punctured and plasma was isolated; epinephrine levels were assessed via ELISA. As shown in Figure 3, epinephrine production was enhanced approximately 3-fold in both saline- (E) and OVA-treated, exercised (EO) mice as compared with sedentary controls (S, SO).
Figure 3.
Moderate-intensity aerobic exercise increases circulating epinephrine levels in saline- and OVA-treated mice. Mice were OVA treated and exercised as described in Materials and Methods. At the conclusion of the protocol, plasma was isolated from blood collected via cardiac puncture and then analyzed via ELISA for changes in epinephrine levels. Results are reported as pg/ml of epinephrine (*P < 0.05 as compared with sedentary, OVA-treated mice; n = 10 per group). Open bars, sedentary mice; solid bars, exercised mice.
Exercise-Mediated Improvement of AHR in OVA-Treated Mice Is Dependent upon β2-AR
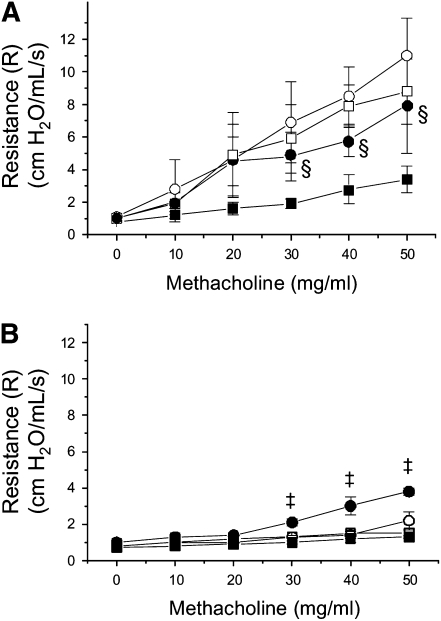
β2-AR expressed within the airways facilitates bronchodilatation (1, 8); therefore, the role of β2-AR in exercise-mediated attenuation of AHR in OVA-treated mice was examined. Mice were ventilated and challenged with methacholine at increasing concentrations as described above. Before each methacholine challenge, however, mice received a nebulization of either saline or the specific β2-AR antagonist butoxamine HCl. At the highest methacholine doses (30–50 mg/ml), treatment with the β2-AR antagonist increased total lung resistance significantly in mice that were OVA-treated and exercised (EO) as compared with controls (Figure 4A); increases in total lung resistance were also observed in exercised (E) mice that were treated with saline (Figure 4B). In contrast, treatment with the β2-AR antagonist had no significant effect on total lung resistance in sedentary (S, SO) mice (Figure 4).
Figure 4.
β2-AR antagonist blocks the effect of moderate intensity aerobic exercise on AHR in OVA-treated mice. Mice were OVA-treated and exercised as described in Materials and Methods. At the conclusion of the protocol, mice were assessed for changes in AHR upon challenge with methacholine at increasing concentrations. Before each methacholine challenge, mice received a nebulization of either butoxamine HCL or saline. Results for (A) OVA-treated and (B) saline-treated mice are presented as total lung resistance (cm H2O/ml/s; ‡P < 0.02 as compared with exercised, saline-treated [E]; §P < 0.02 as compared with EO, saline-treated; n = 6–8 mice per group). OVA- and saline-treated mice were prepared and analyzed in a paired fashion; results from paired experiments are presented on separate graphs for the sake of clarity. (A) Open squares: sedentary, OVA-treated—saline; open circles: sedentary, OVA-treated—β2-AR antagonist; solid squares: exercised, OVA-treated—saline; solid circles: exercised, OVA-treated—β2-AR antagonist. (B) Open squares: sedentary, saline-treated—saline; open circles: sedentary, saline-treated—β2-AR antagonist; solid squares: exercised, saline-treated—saline; solid circles: exercised, saline-treated—β2-AR antagonist.
Moderate-Intensity Aerobic Exercise Alters GRK-2, but Not β2-AR, Protein Expression in the Lungs of OVA-Treated Mice
β2-AR are expressed on a variety of cell types, including ASM cells, and have been shown to facilitate bronchodilatation (1). To determine the effect of moderate-intensity aerobic exercise training on β2-AR protein expression, ASM cells were harvested and prepared for Western blot analysis. Data presented in Figure 5A suggests that moderate-intensity aerobic exercise training does not alter β2-AR protein expression on ASM cells. OVA treatment also did not affect β2-AR expression in these cells (Figure 5A).
Figure 5.
Moderate-intensity aerobic exercise decreases protein expression of GRK-2, but not β2-AR pr EP1 receptor, in the ASM of OVA-treated mice. Mice were OVA-treated and exercised as described in Materials and Methods (S, sedentary, saline-treated; E, exercised, saline-treated; SO, sedentary, OVA-treated; EO, exercised, OVA-treated). At the conclusion of the protocol, ASM cells were harvested and prepared for Western blot analyses of (A) β2-AR (65 kD), GRK-2 (80 kD), and EP1 receptor (EP1 R; 32 kD) protein expression. (B) Densitometric analyses were performed; differences in lane loading were normalized via measurement of α-smooth muscle actin levels. Results are reported as fold difference in protein expression (†P < 0.02, as compared with sedentary, saline-treated controls; *P < 0.02, as compared with sedentary, OVA-treated mice; n = 4–7 mice per group). Open bars, sedentary mice; solid bars, exercised mice.
In parallel with analyses of β2-AR expression, studies also determined the effect of moderate-intensity aerobic exercise training on GRK-2 protein expression. GRK-2 phosphorylates β2-AR, which promotes desensitization of the receptor and, thereby, reduces its bronchodilatory actions (1, 8). Results shown in Figure 5B indicate that OVA treatment of sedentary (SO) mice increased GRK-2 expression approximately 4-fold above that of saline-treated (S, E) controls. In contrast, mice that were OVA-treated and exercised (EO) exhibited a 50% reduction in GRK-2 expression as compared with OVA-treated, sedentary mice; this level of GRK-2 expression was equivalent to that observed in saline-treated controls (S, E).
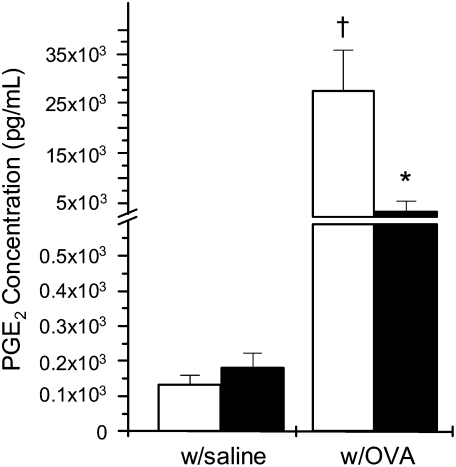
Moderate-Intensity Aerobic Exercise Attenuated PGE2 Levels but Not EP1 Receptor Expression within the Lungs of OVA-Treated Mice
Increases in PGE2 levels within the lung have been implicated in the initiation and/or exacerbation of AHR (19, 20). To determine the effect of moderate-intensity aerobic exercise on PGE2 levels within the lungs of OVA-treated mice, mice were subjected to bronchoalveolar lavage and the resulting BALF was analyzed for changes in PGE2 content. Results presented in Figure 6A demonstrate that OVA treatment of sedentary mice increased BALF PGE2 levels approximately 250-fold as compared with saline-treated sedentary or exercised controls. In contrast, mice that were OVA-treated and exercised displayed an 80% reduction in BALF PGE2 levels as compared with OVA-treated, sedentary mice (Figure 6A); however, these levels were significantly greater than those observed in saline-treated sedentary or exercised (S, E) controls.
Figure 6.
Moderate-intensity aerobic exercise attenuates PGE2 levels but not EP1 receptor expression in the lungs of OVA-treated mice. Mice were OVA-treated and exercised as described in Materials and Methods. At the conclusion of the protocol, mice were subjected to bronchoalveolar lavage (BAL); BAL fluid was analyzed for changes in PGE2 content via PGE2-specific ELISA (†P < 0.01 as compared with sedentary, saline-treated controls; *P < 0.01 as compared with sedentary, OVA-treated; n = 6–8 mice per group). Open bars, sedentary mice; solid bars, exercised mice.
PGE2 binds and activates the E prostanoid (EP) receptor-1, which is expressed on ASM cells. As described above, activation of the EP1 receptor permits its heterodimerization with β2-AR, which impairs β2-AR coupling to the Gs-adenylated cyclase complex and, thereby, reduces β2-AR–stimulated cAMP production (9). To determine the effect of exercise on EP1 receptor expression, ASM cells were harvested and prepared for Western blot analysis. Data presented in Figure 5 indicate that moderate-intensity aerobic exercise training does not alter EP1 receptor protein expression on ASM cells. OVA treatment also did not affect EP1 receptor expression in these cells (Figure 5).
DISCUSSION
We have reported previously that repeated bouts of aerobic exercise at a moderate intensity attenuate airway inflammation and remodeling in OVA-treated mice (5); our current findings extend and complement this original study. Specifically, in the current study, we report that repeated bouts of aerobic exercise at a moderate intensity decreased the following parameters as compared with sedentary controls: (1) total lung resistance, (2) ASM thickness, (3) GRK-2 protein expression in ASM cells, and (4) PGE2 production. In contrast, repeated bouts of exercise increased circulating levels of epinephrine in OVA-treated mice. Exercise had no affect on β2-AR or EP1 receptor expression in ASM cells. Treatment with a β2-AR antagonist, however, blocked the effects of exercise on total lung resistance in OVA-treated mice. Together, these results suggest that repeated bouts of moderate-intensity aerobic exercise improve AHR in OVA-treated mice via a mechanism that involves β2-AR.
In a separate study, we have demonstrated that a single bout of moderate-intensity aerobic exercise decreased airway inflammatory responses, but had no effect on airway remodeling or AHR (13). These seemingly contrasting results between our studies may be explained, in part, by the differences in the length of the exercise protocols. The effects of exercise on physiologic responses are dependent upon several variables, including the total duration of the exercise protocol (21–26). The mechanism that underlies such a disparity likely involves changes in the levels of circulating hormones, including catecholamines, that are released from the hypothalamic–pituitary–adrenal axis (HPA) upon exercise. In the current study, we demonstrate that repeated exercise bouts increase circulating epinephrine levels in both saline- and OVA-treated mice; epinephrine levels were elevated at 48 hours after exercise. Previous reports suggest that aerobic exercise training increases plasma concentrations of epinephrine and, moreover, that such increases are sustained at rest (7). Further, these reports indicate that post-exercise epinephrine concentrations are a consequence of increased secretion rather than accumulation (7). Importantly, Gilbert and colleagues have shown that repetitive exercise correlates positively with increased epinephrine levels and enhanced bronchodilatation in adults with asthma (18); epinephrine can function as a β2-AR agonist, which promotes brochodilitation. In light of these previous reports, we conclude that repeated bouts of exercise attenuated AHR in OVA-treated mice via a mechanism that may involve sustained increases in circulating epinephrine.
Because epinephrine can function as a β2-AR agonist, the role of β2-AR in exercise-mediated attenuation of AHR was determined. Data presented herein demonstrate that, in both saline- and OVA-treated mice, acute exposure to the specific β2-AR antagonist butoxamine HCl blocked the effects of exercise on total lung resistance in response to methacholine challenge. This observation argues for a role of β2-AR in exercise-mediated attenuation of AHR; however, no changes in β2-AR protein expression were observed in ASM. These results contrast with those of Lin and coworkers, which demonstrated that chronic administration of the β2-AR–selective antagonist ICI 118551 produced a bronchoprotective effect and an increase in β2-AR protein expression within the airways of OVA-treated mice (27). As such, the differences in the effect of β2-AR antagonist exposure on AHR between these studies are likely due to the level of β2-AR expression within the airways at the time of AHR measurements.
Upon activation, β2-AR facilitates bronchodilatation through coupling to the Gs-protein complex and subsequent decreases in myosin light chain activity and smooth muscle tone (1). Associated with β2-AR activation is the autoregulatory process of receptor desensitization that involves phosphorylation of β2-AR via GRK-2 and its subsequent uncoupling from the Gs–protein complex. GRK-mediated phosphorylation of β2-AR also facilitates the binding of β2-AR to β-arrestins, including βarr-1 and βarr-2, which promote its subsequent desensitization (28). In particular, Deshpande and colleagues have shown recently that βarr-2 serves as a selective regulator of β2-AR signaling and function in ASM (29). Interestingly, repeated bouts of exercise decreased GRK-2 protein expression in ASM. Mak and coworkers have indicated that decreasing the expression of GRK-2 within the airways may lessen β2-AR desensitization (30). This observation is supported by a recent study from Kong and colleagues, which reported that inhibition of endogenous GRK2 and GRK3 in ASM is associated with increases in β2-AR acute signaling (31). Together, these results indicate that repeated bouts of moderate-intensity aerobic exercise may attenuate AHR by decreasing GRK-2–mediated desensitization of β2-AR.
Thickening of the airway wall is an indicator of asthma severity (17). Factors that contribute to increased airway wall thickness in the asthmatic lung include epithelial thickening, reticular basement membrane thickening, increased extracellular matrix, and fibrosis; however, ASM hypertrophy and/or hyperplasia contributes most to overall airway wall thickening (17). Data presented herein demonstrate that, in OVA-treated mice, repeated bouts of aerobic exercise at a moderate intensity decreased ASM thickness to control levels; however, it is unclear whether exercise lessened ASM cell size and/or proliferation. Within the OVA-treated lung, exercise-mediated decreases in ASM thickness correlated positively with exercise-mediated attenuation of AHR. The relationship between increased airway wall/ASM thickness and AHR has been explored in both clinical and animal model studies. Several studies suggest that increases in total wall thickness are associated with increased AHR. For example, Boulet and coworkers reported a positive correlation between airway wall thickening and hyperresponsiveness to methacholine challenge in patients with asthma with airway obstruction (32). The findings presented in the current study support this observation. In contrast, Niimi and coworkers reported that there exists a negative correlation between airway wall thickness and AHR (33). These authors suggested that increased airway stiffness due to increased airway thickening may prevent ASM shortening and subsequent narrowing of the airway lumen.
Within the asthmatic lung and airways, PGE2 has been considered protective against allergen-induced AHR (reviewed in Ref. 20). In contrast, results presented herein suggest that increased PGE2 levels in the lungs of sedentary, OVA-treated mice do not protect against AHR. Specifically, results presented in Figure 6 show that exercise-mediated decreases in BALF PGE2 levels correlate with decreased total lung resistance in response to methacholine challenge. Our results, in part, support the observations of Mathe and Hedqvist, who reported that administration of PGE2 to individuals with asthma caused either bronchodilation or bronchoconstriction (34). Our results also support the findings of Pang and colleagues, who demonstrated that blockade of PGE2 production leads to partial reversal of β2-AR desensitization in the context of inflammation (35). PGE2 exerts its biological effects via binding and activating EP receptors, including the pro-bronchoconstrictive EP1 receptor (9). McGraw and colleagues have reported that activation of the EP1 receptor causes its heterodimerization with β2-AR, which impairs β2-AR coupling to the Gs-adenylated cyclase complex and, thereby, reduces β2-AR–stimulated cAMP production (9); therefore, we examined the effect of moderate-intensity aerobic exercise on EP1 receptor expression in these cells. Results presented in Figure 5 indicate that exercise has no affect on EP1 receptor expression in the ASM of saline- or OVA-treated mice. In light of these results, we conclude that decreases in PGE2 within the asthmatic airways may lessen PGE2-mediated EP1 receptor activation and subsequent decreases in β2-AR signaling.
Because of the potential for exercise-induced bronchospasm, we would like to note that none of the exercised mice exhibited labored or difficult breathing. Interestingly, these observations parallel the findings of previous clinical studies, which documented improvements in exercise-induced bronchoconstriction after physical training (36–40). Specifically, reports from Emtner and coworkers and from Henriksen and Nielsen each demonstrated that adults with mild–moderate asthma who participated in an aerobic exercise training program exhibited decreased exercise-induced bronchospasm (36, 37).
As noted above, the results of the current study demonstrate that repeated bouts of aerobic exercise at a moderate intensity attenuate asthma-associated AHR via a mechanism that involves β2-AR. Exercise-mediated decreases in GRK-2 protein expression, ASM thickness, and PGE2 levels within the OVA-treated lung combined with exercise-mediated increases in circulating epinephrine levels support this conclusion. We believe that this study builds upon our previous work and further demonstrates the potential of moderate-intensity aerobic exercise as a therapeutic intervention for the treatment of asthma-associated airway inflammation, attenuate disease progression, and decrease asthmatic symptomatology.
Acknowledgments
The authors thank Drs. Marcas Bamman, Ed Blalock, Mitch Olman, and Ed Postlethwait for helpful discussions. In addition, the authors thank Drs. Namasivayam Ambalavanan and Erik Schwiebert for technical assistance.
This work was supported by National Institutes of Health grants 1R01HL075465 (to L.M.S.) and 5T32HL007553 (to M.H.; trainee).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0038OC on May 7, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Yang I, Ng T, Molenaar P, Fong K. b2-adrenoreceptor polymorphisms and obstructive airway diseases: Important issues of study design. Clin Exp Pharmacol Physiol 2007;34:1029–1036. [DOI] [PubMed] [Google Scholar]
- 2.Satta A. Exercise training in asthma. J Sports Med Phys Fitness 2000;40:277–283. [PubMed] [Google Scholar]
- 3.Lucas S, Platts-Mills TA. Physical activity and exercise with asthma: relevance to etiology and treatment. J Allergy Clin Immunol 2005;115:928–934. [DOI] [PubMed] [Google Scholar]
- 4.Lucas S, Platts-Mills TA. Paediatric asthma and obesity. Paediatr Respir Rev 2006;7:233–238. [DOI] [PubMed] [Google Scholar]
- 5.Pastva A, Estell K, Schoeb TR, Atkinson TP, Schwiebert LM. Aerobic exercise attenuates airway inflammation in a mouse model of atopic asthma. J Immunol 2004;172:4520–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastva A, Estell K, Schoeb TR, Schwiebert L. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Behav Immun 2005;19:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med 2008;38:401–423. [DOI] [PubMed] [Google Scholar]
- 8.Mutlu G, Koch W, Factor P. Alveolar epithelial b2-adrenergic receptors. Am J Respir Crit Care Med 2004;170:1270–1275. [DOI] [PubMed] [Google Scholar]
- 9.McGraw DW, Mihlbacker K, Schwarb M, Rahman F, Small K, Almoosa K, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest 2006;116:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duguet A, Biyah K, Minshall E, Gomes R, Wang C, Taoudi-benchekroun M, Bates JHT, Eidelman DH. Bronchial responsiveness among inbred mouse strains. Am J Respir Crit Care Med 2000;161:839–848. [DOI] [PubMed] [Google Scholar]
- 11.Herz U, Lumpp U, Daser A, Gelfand EW, Renz H. Murine animal models to study the central role of T cells in immediate-type hypersensitivity responses. Adv Exp Med Biol 1996;409:25–32. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CB. Exercise in chronic pulmonary disease: limitations and rehabilitation. Med Sci Sports Exerc 2001;33:S643–S646. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt M, Creel A, Estell K, Davis I, Schwiebert L. Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am J Respir Cell Mol Biol 2009;40:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrani Y, Ammit AJ, Panettieri RA. Tumor necrosis factor receptor (TNFR) 1, but not TNFR2, mediates tumor necrosis factor-a-induced interleukin-6 and RANTES in human airway smooth muscle cells: role of p38 and p42/44 mitogen-activated protein kinases. Mol Pharmacol 2001;60:646–655. [PubMed] [Google Scholar]
- 15.Propst SM, Estell K, Schwiebert LM. CD40-mediated activation of NF-kB in airway epithelial cells. J Biol Chem 2002;277:37054–37063. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft D, Davis B. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol 2006;118:551–559. [DOI] [PubMed] [Google Scholar]
- 17.James A, Wenzel S. Clinical relevance of airway remodeling in airway diseases. Eur Respir J 2007;30:134–155. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert I, Lenner K, McFadden E. Sympathoadrenal response to repetitive exercise in normal and asthmatic subjects. J Appl Physiol 1988;64:2667–2674. [DOI] [PubMed] [Google Scholar]
- 19.McGraw DW, Elwing JM, Foget KM, Wang W, Glinka C, Mihlbachler KA, Rothernberg ME, Liggett SB. Crosstalk between G1 and Gq/Gs pathways in airay smoothg muscle regulates bronchial contractility and relaxation. J Clin Invest 2007;117:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vancheri C, Mastruzzo C, Sortino M, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol 2004;25:40–46. [DOI] [PubMed] [Google Scholar]
- 21.Ceddia MA, Voss EW, Woods JA. Intracellular mechanisms are responsible for the exhaustive exercise-induced suppression of macrophage antigen presentation. J Appl Physiol 2000;88:804–810. [DOI] [PubMed] [Google Scholar]
- 22.Nieman DC, Nehlsen-Cannarella SL. The immune response to exercise. Semin Hematol 1994;31:166–179. [PubMed] [Google Scholar]
- 23.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 2000;80:1055–1081. [DOI] [PubMed] [Google Scholar]
- 24.Smith LL, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D. Cytokines and cell adhesion molecules associated with high intensity eccentric exercise. Eur J Appl Physiol 2000;82:61–67. [DOI] [PubMed] [Google Scholar]
- 25.Su SH, Chen H, Jen CJ. Severe exercise enhances phagocytosis by murine bronchoalveolar macrophages. J Leuk Biol 2001;69:75–80. [PubMed] [Google Scholar]
- 26.Woods JA, Lu Q, Ceddia MA, Lowder T. Exercise-induced modulation of macrophage function. Immunol Cell Biol 2000;78:545–553. [DOI] [PubMed] [Google Scholar]
- 27.Lin R, Peng H, Nguyen L, Dudekula N, Shardonofsky F, Knoll B, Parra S, Bond R. Changes in b2-adrenoreceptor and other signaling proteins produced by chronic administration of ‘b-blockers’ in a murine asthma model. Pulm Pharm Therapeut. 2008;21:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penn R, Pascual R, Kim Y, Mundell S, Krymskaya V, Panettieri R, Benovic J. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem 2001;276:32648–32656. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande DA, Theriot B, Penn RB, Walker J. β-arrestins specifically constrain b2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J 2008;22:2134–2141. [DOI] [PMC free article] [PubMed]
- 30.Mak J, Hisada T, Salmon M, Barnes PJ, Chung K. Glucocorticoids reverse IL-1b-induced impairment of b-adrenoreceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br J Pharmacol 2002;135:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong K, Gandhi U, Martin T, Anz C, Yan H, Misior A, Pascual R, Deshpande D, Penn R. Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 2008;47:9279–9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulet L, Turcotte H, Hudon C, Carier G, Maltais F. Clinical, physiological, and radiological features of asthma with incomplete reversibility of airflow obstruction compared with those of COPD. Can Respir J 1998;5:270–277. [DOI] [PubMed] [Google Scholar]
- 33.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med 2003;168:983–988. [DOI] [PubMed] [Google Scholar]
- 34.Mathe A, Hedqvist P. Effect of prostaglandins F2 alpha and E2 on airway conductance in healthy subjects and asthmatic patients. Am Rev Respir Dis 1975;111:313–320. [DOI] [PubMed] [Google Scholar]
- 35.Pang L, Holland E, Know A. Role of cyclo-oxygenase-2 induction in interleukin-1beta induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br J Pharmacol 1998;125:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emtner M, Herala M, Stalenheim G. High-intensity physical training in adults with asthma: a 10-week rehabilitation program. Chest 1996;109:323–330. [DOI] [PubMed] [Google Scholar]
- 37.Henriksen JM, Nielsen TT. Effect of physical training on exercise-induced bronchoconstriction. Acta Paediatr Scand 1983;72:31–36. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto I, Araki H, Tsuda K, Odajima H, Nishima S, Higaki Y, Tanaka H, Tanaka M, Shindo M. Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax 1999;54:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaaban R, Leynaert B, Soussan D, Anto J, Chinn S, de Marco R, Garcia-Aymerich J, Heinrich J, Janson C, Jarvis D, et al. Physical activity and bronchial hyperresponsiveness: European Community Respiratory Health Survey II. Thorax 2007;62:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest 2000;118:1460–1469. [DOI] [PubMed] [Google Scholar]