Abstract
Transcriptional silencing by trans inactivation can contribute to the regulation of gene expression in eukaryotic cells. In the human intestinal protozoan parasite Entamoeba histolytica, trans inactivation of the amoebapore-A gene (AP-A) was recently achieved by episomal transfection of E. histolytica trophozoites with the plasmid psAP1. The mechanism of AP-A trans inactivation is largely unknown, though it was suggested that a partial short interspersed transposable element (SINE) is required. By systematic assessment of various E. histolytica isolates transfected with psAP1 derivates, trans inactivation of AP-A was restricted to the strain HM-1:IMSS (2411) but could not be achieved in other standard laboratory strains. Importantly, sequences of an E. histolytica tRNA array that were located on psAP1 in close proximity to the AP-A upstream region and comprising the glutamic acid (TTC) (E) and tyrosine (GTA) (Y) tRNA genes were indispensable for AP-A silencing. In contrast to the case described in previous reports, SINE was not required for AP-A trans inactivation. AP-A expression could be regained in silenced cells by episomal transfection under the control of a heterologous E. histolytica promoter, opening a way toward future silencing of individual genes of interest in E. histolytica. Our results indicate that tRNA gene-mediated silencing is not restricted to Saccharomyces cerevisiae.
The formation of heterochromatin is one of several hierarchical levels in the regulation of gene expression in eukaryotic cells (reviewed in reference 40). The expression of genes can be repressed by histone modifications shifting the chromatin state to heterochromatin. Telomeric, centromeric, and repetitive sequences are part of the heterochromatin (15). Heterochromatin formation and the architecture of the nucleus are closely interrelated. Some domains of eukaryotic cell nuclei are transcriptionally active, and others, such as the nuclear periphery, which is often organized as heterochromatin, are silent. The position of a gene within the nucleus can determine its expression state, also known as position effect. For a gene to become silenced, it is sufficient for it to be located in close proximity to the periphery of the nucleus (3).
Shifting of a gene locus into the state of inactive chromatin can be mediated by DNA elements, which are not part of the gene locus (in trans). Such events of trans inactivation (also termed transvection) in several eukaryotic organisms have been described, but the mechanism is poorly understood. It was shown that in mice, contact with centromeric heterochromatin can result in spreading of chromatin in trans (7, 14). trans inactivation of the Drosophila melanogaster brown locus is mediated by a large insertion of heterochromatin in one allele that silences a second allele as well (10, 35). Genes close to the brown locus are also silenced. trans inactivation appears to be enhanced by a stretch of endogenous sequence of about 300 bp near the heterochromatin insertion. The 300-bp element contains a bidirectional promoter and potential binding sites for silencing proteins that may influence heterochromatin formation (34).
Several sequence elements and proteins have been identified as being involved in heterochromatin silencing (8, 31). The HM mating-type loci in Saccharomyces cerevisiae contain silencer elements, termed E and I. Some silencer elements have been described to be functional as autonomous replication sequences, and some autonomous replication sequences can function as silencers (13, 16). tRNA genes have been shown to exhibit opposing effects at the silent HM loci in S. cerevisiae. Depending on the mating type, they can act as boundary elements that block the spread of heterochromatin (30) or as transcriptional repressors of genes transcribed by polymerase II (21, 24, 37). This mechanism is referred to as tRNA gene-mediated gene silencing (tgm). tgm depends on nucleolar localization and active transcription of the tRNA (41).
The human intestinal protozoan parasite Entamoeba histolytica is poorly studied with respect to gene expression and regulatory mechanisms. Although the principal nuclear organization has not been characterized in detail, certain characteristics that are distinct for this organism have been reported. These includes the presence of ribosomal DNA (rDNA) on episomes that are located in the vicinity of the nuclear membrane (20, 43). In addition, unique arrangements of tRNA genes have been described previously (9). tRNA genes are organized as multiple tandem-array units, which are spaced by tandem repeats of AT-rich sequences. There exists evidence that these arrays are located at chromosome ends and may be functionally equivalent to telomeres, which are absent in E. histolytica (28). It was suggested that tRNA arrays play a role in the structural organization of the nucleus (9).
In order to develop a tool to shut down gene expression in E. histolytica, we systematically assessed trans inactivation of the amoebapore-A (AP-A) gene. AP-A constitutes one of the main pathogenicity factors of this parasite and is involved in host tissue destruction (25). We followed up on a previous study reporting successful silencing of the endogenous AP-A gene after transfection with the plasmid psAP1 bearing an AP-A expression cassette (5). The 5′ region of the AP-A cassette was required for silencing, whereas the AP-A open reading frame (ORF) and the 3′ end were not. An RNA interference-based mechanism was highly unlikely, since small interfering RNAs were not detected (2). However, it was suggested that the presence of a 140-nucleotide fragment, representing part of a short interspersed transposable element (SINE) together with an adjacent thymidine-rich (T-rich) region on the plasmid is required to induce silencing (2). The endogenous, complete SINE is located approximately 300 bp upstream of the AP-A open reading frame in the reverse orientation. Whether SINE can indeed mechanistically underlie silencing is under debate. Recently, trans inactivation of further genes in E. histolytica was achieved but only in cells in which AP-A was already silenced (4, 6, 32). Here, we report for the first time that AP-A expression can be reconstituted in AP-A-deficient amoebae, opening a way toward silencing of genes of interest without additional AP-A deficiencies. We also show that SINE and the T-rich region are dispensable for AP-A trans inactivation. Instead, the trans inactivation property could be assigned to two elements present in close proximity on psAP1, namely, an array of the E. histolytica Glu(TTC) (E) and Tyr(GTA) (Y) tRNA genes and a 213-nucleotide fragment of the AP-A 5′ region. trans inactivation was dependent on the presence, but not the orientation, of the two elements. To our knowledge this is the first report of tRNA gene-mediated transcriptional repression of gene expression in trans.
MATERIALS AND METHODS
Strain and culture conditions.
Entamoeba histolytica trophozoites were cultured axenically in plastic culture flasks with TYI-S-33 medium (11). The culture medium was changed every other day. Single-cell cloning of amoebae was performed by limiting dilution in 24-well culture plates under anaerobic conditions by using Anaerocult A (Merck). Harvesting of cultured amoebae was carried out with cells in the late logarithmic phase of growth by chilling on ice for 10 min and sedimentation at 430 × g at 4°C for 5 min.
Plasmid construction and transfection.
Plasmid psAP1Δ2600 was generated by removing a 2,618-bp XbaI fragment from silencing plasmid psAP1 (5). psAP1Δ2600, which lacks the glutamic acid (TTC) and tyrosine (GTA) (EY) tRNA array, a partial tetracycline (Tet) repressor sequence, and part of the E. histolytica actin gene 3′ sequence that is flanking the neomycin resistance gene, was used as backbone for generating deletion mutant plasmids. PCR-amplified fragments bearing different fragments of the EY tRNA array (Table 1; see Fig. 4) were cloned into the TOPO TA vector (Invitrogen), sequenced, and directionally subcloned into the XbaI and NotI restriction sites of psAP1Δ2600. Cloning of the terminator site into psAP1 was achieved by oligonucleotide cloning into the BamHI/NotI site. Two plasmids were used as target vectors to generate mutants with mutations and deletions of the AP-A 5′ end. In both psAP1 and psAP1Δ2600 EY600, the AP-A cassette was removed by digestion with SacII, and the vector was religated. Subsequently, PCR-amplified AP-A 5′ fragments (Table 1) were cloned into the NotI restriction site. pNAP-A was derived from the E. histolytica transfection vector pN (neocassette) (19) by introducing the AP-A cassette into the HindIII-XbaI sites. The plasmid pNinAP-A was derived from the pN vector bearing the Lec485 promoter and the ehrpl27a intron upstream of the AP-A ORF. The intron was cloned into the KpnI-BamHI site of pN, and an NheI site was introduced. The AP-A ORF was added by using the BamHI-NheI site. All plasmids were verified by molecular sequencing. Transfections were performed as described previously (19). psAP1 background cells (AP-A-silent cells) were grown without the neomycin analogue G418 for at least 4 months, and G418 sensitivity was verified before transfection. Drug selection was started 48 h after transfection by using 10 μg/ml of G418. Total RNA was harvested at least 3 weeks posttransfection. The presence or absence of the transfected plasmid was analyzed by PCR.
Table 1.
Oligonucleotides used for PCR amplification, oligonucleotide cloning, mutagenesis, and quantitative RT-PCR
| Target/constructa | Oligonucleotide | Sequenceb | Orientationc |
|---|---|---|---|
| EY1000 | OH68 | attctagaTTTTATACTAATAGATAGG | S |
| OH71 | atgcggccgcTAGTGGATCCGACCAACCGG | AS | |
| EY600 | OH69 | attctagaTATAGTCATGGTAAATCC | S |
| OH71 | atgcggccgcTAGTGGATCCGACCAACCGG | AS | |
| Y200 | OH70 | attctagaGAATAGAAACATATATAAGCAC | S |
| OH71 | atgcggccgcTAGTGGATCCGACCAACCGG | AS | |
| E800 | OH68 | attctagaTTTTATACTAATAGATAGG | S |
| OH72 | atgcggccgcCCTTTTATATTTCTATATGTGC | AS | |
| E500 | OH68 | attctagaTTTTATACTAATAGATAGG | S |
| OH73 | atgcggccgcGCTAAAAAATTCCATCGCCGGG | AS | |
| Terminator in psAP1 | OH80 | gatccTTTTTTTTgc | S |
| OH81 | ggccgcAAAAAAAAg | AS | |
| E500Δ terminator | OH68 | attctagaTTTTATACTAATAGATAGG | S |
| OH79 | atgcggccgcTTCCATCGCCGGGAATCGAACCC | AS | |
| pNAP-A EY1000 | OH76 | atctcgagggtacccgggatccGACCAACCGGATTCGAACCAGTGACC | S |
| OH74 | attctagaTAGTGGATCCGACCAACCGG | AS | |
| pNinAP-A | OH13 | atgctagcACAAACAATCATGAAAGCC | S |
| OH14 | atggatccTTAGCAAGCATGAATCTTAGC | AS | |
| AP-A 5′ | OH88 | atgcggccgcGATTGTTTGTAAGATATG | S |
| OH89 | atgcggccgcCTTGCTGCACCCTTTG | AS | |
| AP-A 5′ ΔSINE | OH101 | atgcggccgctTTTATTATTTAAAAAACAAAATAG | S |
| OH89 | atgcggccgcCTTGCTGCACCCTTTG | AS | |
| AP-A 5′ Δ240 | OH116 | atgcggccgcTTTAATAAATATTAAAAGAGAAG | S |
| OH89 | atgcggccgcCTTGCTGCACCCTTTG | AS | |
| AP-A 5′ Δ260 | OH117 | atgcggccgcGAGAAGAAATGAAATAATCA | S |
| OH89 | atgcggccgcCTTGCTGCACCCTTTG | AS | |
| AP-A for qPCR | ap-a s30 | CACTAAGGGAGCTGATAAAGTAAAAGATTA | S |
| ap-a as25 | TCCAAAATCAAGAACTTTAGTGCAA | AS | |
| Actin gene for qPCR | act s28 | TGTAGATAATGGATCAGGAATGTGTAAA | S |
| act as23 | CAATGGATGGGAATACAGCTCTT | AS | |
| Y tag1 for mutagenesis | OH227 | CTCAGTTGGTAGAGCGGATGACTGTAGATGTAGAATAGAATTCATTAGGTCACTGGTTCGA | S |
| OH228 | TCGAACCAGTGACCTAATGAATTCTATTCTACATCTACAGTCATCCGCTCTACCAACTGAG | AS | |
| Y mutA | OH238 | CCGACCATAGCTCAGTTTCTAGAGCGGATGACTG | S |
| OH239 | CAGTCATCCGCTCTAGAAACTGAGCTATGGTCGG | AS | |
| Y tag1 for qPCR | OH225 | CTCAGTTGGTAGAGCGGAT | S |
| Y | OH226 | TCCGACCAACCGGATTC | AS |
| Y mutB1 for qPCR | OH245 | TCCGACCAACCGGACTC | AS |
Δ240, deletion of 240 nucleotides; Δ260, deletion of 260 nucleotides; Y mutA and Y mutB1, Y gene mutants (see Fig. 5 legend).
Lowercase letters represent restriction sites for subsequent cloning into the corresponding vectors for site-specific mutagenesis.
S, sense; AS, antisense.
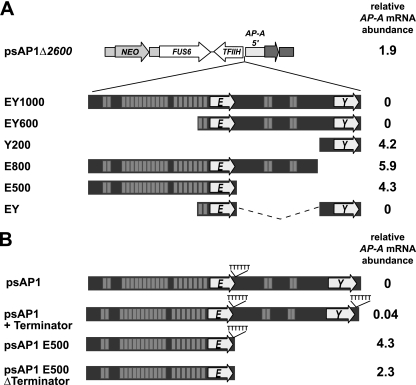
Fig. 4.
Sequence motifs within the EY tRNA array are required for AP-A trans inactivation. (A) Transfection plasmid psAP1Δ2600, incapable of mediating trans inactivation of AP-A, was used as a backbone to introduce various modifications of the 1.0-kb EY tRNA array as indicated. Glutamic acid (E) and tyrosine (Y) tRNAs are represented by arrows, and AT-rich repeat elements are shown as dashes. NEO, neomycin resistance gene. (B) AP-A mRNA abundance was measured by qRT-PCR with primers specific for AP-A or actin and the ΔΔCT method for quantification. To the right, AP-A mRNA abundances relative to that of wild-type cells (set at 1) are given. The relevance of polythymidine stretches (TTTTT) adjacent to the tRNA sequences was investigated. Note that trans inactivation was observed only with constructs containing both tRNA sequences but was independent of the presence of poly(T) stretches or repeat elements.
tRNA mutagenesis.
Site-directed mutagenesis was carried out using the Expand long-template PCR system (Roche) by using specific sense and antisense primers encompassing the nucleotides to be mutated (Table 1). The plasmid EY1000 was used as the template (30 ng). Amplification conditions were 30 cycles of 1 min at 95°C, 1 min at 50°C, and 10 min at 65°C. Restriction digestion was performed with DpnI at 37°C. A 7-μl volume of the reaction mixture was used for transformation into Escherichia coli DH5α cells. The relevant fragments were validated by molecular sequencing and subcloned into psAP1 with XmaI and BamHI.
Northern blotting.
RNA was isolated from cultured amoebae by using RNeasy (Qiagen). For Northern blotting, 10 μg of total RNA was separated on agarose gels and transferred to nylon membranes. Blots were hybridized with radiolabeled DNA probes. Hybridizations were performed in Dig Easy Hyb (Roche) at 42°C. Blots were washed with 2× and 1× SSC (1× SSC is 150 mM NaCl, 15 mM sodium citrate, pH 7) at 55°C.
Immunoblotting of trophozoite extract.
Frozen trophozoites (4.5 × 109 cells) were thawed in the presence of the proteinase inhibitor E-64 [100 μM, trans-epoxysuccinyl-l-leucylamino-(4-guanodino)butane]. The cells were frozen and thawed in three cycles in dry ice and stored at −70°C. Tricine-SDS-PAGE was performed under reducing conditions by using 13% separation gels (36). Subsequent immunoblotting was carried out as described previously (25). Briefly, wet blotting was used with 25 mM Tris, 192 mM glycine, 1.3 mM SDS (pH 8.3)–20% methanol as the transfer buffer onto a nitrocellulose membrane (BA83 Protan; Schleicher and Schuell). Antisera to amoebapores A and B were kindly provided by Matthias Leippe and used in dilutions of 1:300 and 1:5,000, respectively (25). Detection was carried out using luminol (Sigma) and para-hydroxycoumaric acid in the presence of H2O2.
RT-PCR and quantitative PCR.
DNase digestion and subsequent cDNA synthesis were carried out in duplicate for each sample by using 1 μg of RNA with the QuantiTect reverse transcription kit (Qiagen). Amplification was performed in a Rotor-Gene (Corbett) instrument with the QuantiTect SYBR green kit (Qiagen) by using 1 μl of the cDNA and E. histolytica AP-A, actin, or tRNA gene primers (Table 1). Amplification conditions were as follows: 50 cycles of 15 s at 95°C, 20 s at 65°C, 20 s at 68°C, and an adjacent melting step (42 to 95°C). The amount of AP-A relative to actin RNA was quantified using the ΔΔCT method provided in Rotor-Gene software 6.0.14 (27). All quantitative reverse transcription-PCR (qRT-PCR) experiments were performed at least in triplicate.
RESULTS
Transcriptional silencing of the E. histolytica amoebapore-A gene is restricted to a particular amoeba strain.
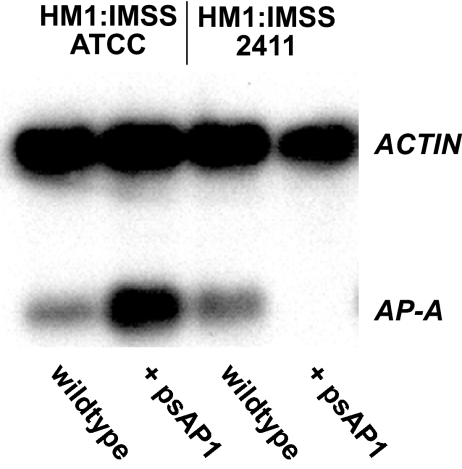
In order to examine whether transcriptional silencing of the amoebapore-A gene(AP-A) can be achieved in all E. histolytica strains or whether it is restricted to particular cell lines, various amoeba isolates were transfected with the silencing plasmid psAP1 as reported previously (5). Northern blot analysis revealed that AP-A trans inactivation was achieved only in strain HM-1:IMSS (2411) (Fig. 1), kindly provided by David Mirelman, but not in other standard laboratory strains from the American Type Culture Collection (ATCC), such as another HM-1:IMSS strain (no. 30459), 200:NIH (no. 30458), and HK9 (no. 30015). In contrast, transfection of psAP1 into amoeba isolates other than HM-1:IMSS (2411) resulted in a substantial increase of AP-A mRNA abundance relative to that of mock-transfected cells (Fig. 1). PCR analysis of the various amoeba isolates by using highly polymorphic markers suitable for subtyping individual strains (1) showed identical DNA patterns between the ATCC HM-1:IMSS strain and HM-1:IMSS (2411), indicating that the two strains are indeed closely related (data not shown). In addition, sequencing of the entire AP-A locus did not reveal any differences between the isolates. We note that AP-A trans inactivation in HM-1:IMSS (2411) was not detectable before day 18 posttransfection. Interestingly, trans inactivation did not require long-term selection for the transfected plasmid. AP-A trans inactivation was obtained even after selection for less than a week and subsequent growth without further addition of G418, suggesting that psAP1 initially triggers trans inactivation but is not required to further maintain it. Transinactivated cells revealed growth kinetics similar to those of wild-type cells. In particular, AP-A-silenced amoebae did not have any growth advantage compared to nontransfected or mock-transfected cells. Consistent with previous observations (5), psAP1-transfected amoebae had not only AP-A but also AP-B silenced (Fig. 2).
Fig. 1.
trans inactivation of AP-A in the E. histolytica isolate HM-1:IMSS. The abundance of AP-A mRNA was analyzed in various E. histolytica isolates transfected with plasmid psAP1. Shown is an autoradiogram of a Northern blot from gel-separated total RNA isolated from nontransfected (wild-type) or psAP1-transfected amoebae of HM-1:IMSS (2411) or the ATCC HM-1:IMSS strain. The blot was sequentially hybridized with radiolabeled AP-A and E. histolytica ACTIN gene probes. Note that trans inactivation was achieved in HM-1:IMSS (2411) but not in other E. histolytica isolates.
Fig. 2.
Reconstitution of AP-A expression in AP-A trans inactivated cells by episomal transfection with plasmid pNinAP-A. (A) Schematic depiction of the expression vector comprising the neomycin resistance gene (NEO) under the control of E. histolytica actin (ACT) gene 5′ and 3′ sequences and the AP-A open reading frame (AP-A) under the control of E. histolytica lectin gene 5′ (LEC 5′) and E. histolytica actin gene 3′ (ACT 3′) sequences. The lectin gene promoter and the AP-A open reading frame are separated by an intron from the gene encoding E. histolytica ribosomal protein L27a. (B) Immunoblot analysis of E. histolytica extracts from various HM-1:IMSS (2411) transfectants tested with antisera against E. histolytica AP-A or AP-B. As a control, a parallel blot was developed with antiserum against E. histolytica superoxide dismutase (SOD). Extracts were obtained from the following transfectants: wild-type cells transfected with pN (neocassette vector) and selected with G418; wild-type cells transfected with psAP1; AP-A-silenced cells grown for several months without G418 and subsequently transfected with pNinAP-A and selected again with G418 (pNinAP-A+G418) or without G418 selection (pNinAP-A-G418); and wild-type cells transfected with psAP1Δ2600. Note that reconstitution of expression was achieved when selective pressure was maintained after transfection with pNinAP-A.
AP-A expression can be reconstituted in AP-A-trans inactivated cells by episomal transfection.
To investigate whether AP-A expression can be reconstituted in AP-A-transinactivated cells, an episomal transfection vector, pNinAP-A, was constructed. Transfection with this plasmid induces G418 resistance in E. histolytica trophozoites and contains the ORF of AP-A, flanked by the E. histolytica lectin gene promoter, an intron from a gene encoding an E. histolytica ribosomal protein (rpl27a), and actin 3′ sequences (Fig. 2A). The lectin gene promoter and the AP-A ORF are separated by an intron from the gene encoding an E. histolytica ribosomal protein (rpl27a). Transfection of pNinAP-A into AP-A-trans inactivated amoebae and subsequent selection with G418 led to significant expression of AP-A similar to that of wild-type cells. AP-A expression was dependent on the persistent presence of the expression plasmid, as omission of G418 selection resulted in a gradual loss of AP-A mRNA over time. Expression of amoebapore in pNinAP-A-transfected cells was restricted to AP-A, and expression of AP-B was not restored (Fig. 2). This intron from rpl27a was previously used in reporter gene experiments and seemed to have enhancer properties (unpublished data). Plasmid constructs containing no intron did not yield any AP-A reconstitution.
An E. histolytica tRNA array is required for AP-A trans inactivation.
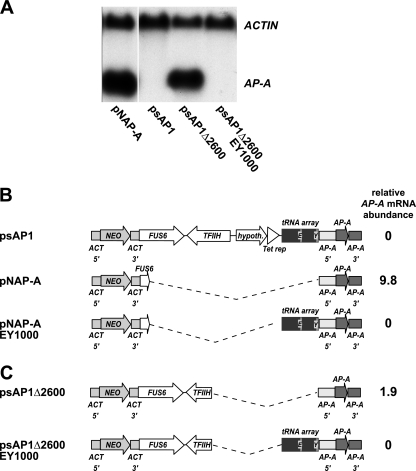
Initial transfection experiments using plasmid pNAP-A, which contains some sequence motifs similar to those of psAP1, increased AP-A mRNA expression but failed to induce AP-A trans inactivation in all amoeba strains examined, including HM-1:IMSS (2411) (Fig. 3A). Sequence comparison indicated that the two plasmids contain the same AP-A genomic fragment as well as the neomycin phosphotransferase gene under the control of E. histolytica actin gene 5′ and 3′ sequences. However, compared with pNAP-A, psAP1 contained an extended actin 3′ sequence bearing additional E. histolytica genes (FUS1 and TFIIH) and a partial bacterial Tet repressor ORF followed by a stretch of amoebic sequence previously designated ARS. The latter has been shown to exhibit functional properties of autonomous replication sequences when transferred to a yeast artificial chromosome (29). However, careful analysis of the E. histolytica ARS revealed that it represents a tRNA array (9), comprising the glutamic acid (TTC) and tyrosine (GTA) tRNAs, separated by repetitive AT-rich elements (Fig. 3). To determine which of the psAP1-specific sequences are responsible for AP-A trans inactivation, a 2.6-kb fragment comprising part of the extended actin gene 3′ sequence and the bacterial Tet repressor ORF as well as the amoeba EY tRNA array was deleted (Fig. 3B). The resulting plasmid, psAP1Δ2600, was incapable of inducing trans inactivation of AP-A (Fig. 3A). Instead, psAP1Δ2600 transfection led to AP-A mRNA overexpression, as observed after transfection with pNAP-A (data not shown). However, when the 1.0-kb EY tRNA array sequence was reintroduced into psAP1Δ2600 or pNAP-A, both plasmids acquired trans inactivation capacity. The introduction of other E. histolytica tRNA arrays, such as LS or SPPCK (9), into psAP1Δ2600 did not induce AP-A trans inactivation (data not shown), suggesting that trans inactivation may be restricted to the EY tRNA sequence.
Fig. 3.
An E. histolytica tRNA array is required to mediate trans inactivation of AP-A. (A) Northern blot analysis of gel-separated total RNA isolated from various amoeba transfectants as indicated. The blot was hybridized with radiolabeled ACTIN and AP-A gene probes. (B and C) Schematic depiction of sequence elements present on psAP1 and on various deletion constructs used for transfection. Compared to psAP1, plasmids pNAP-A and psAP1Δ2600 bear deletions of 3.2 and 2.6 kb, respectively, as indicated by the dotted lines. Transfection plasmids pNAP-AEY1000 and psAP1Δ2600EY1000 represent pNAP-A and psAP1Δ2600, respectively, in which the 1.0-kb EY tRNA array has been introduced. AP-A mRNA abundance was measured by qRT-PCR with primers specific for the E. histolytica AP-A and actin (ACT) genes, and the ΔΔCT method was used for quantification. The mRNA abundance of wild-type HM-1:IMSS (2411) amoebae was set at 1. A value higher than 1 indicates overexpression, while a value of 0 indicates that AP-A was trans inactivated. Note that only those transfection plasmids containing the EY tRNA array have AP-A-transinactivating capacity. NEO, neomycin resistance gene. hypoth., hypothetical protein.
Glutamic acid (TTC) and tyrosine (GTA) tRNA genes are sufficient to mediate AP-A trans inactivation.
In order to determine whether the complete tRNA array is required to mediate AP-A trans inactivation, a series of 5′ and 3′ deletion constructs was generated (Fig. 4A). In addition, the relevance of poly(T) stretches situated 3′ of amoeba tRNAs was investigated (Fig. 4B), and they most likely represent terminator sequences for polymerase III (Pol III) transcription as in other organisms. qRT-PCR analysis of amoebae transfected with the various constructs indicated that AP-A trans inactivation is independent of the presence or absence of the repeat elements or the poly(T) terminator sequences but requires the presence of both tRNA genes (Fig. 4). The orientation of the tRNA genes is not dependent on function (unpublished data).
Polymerase III transcription of the tRNATyr gene is not required for trans inactivation.
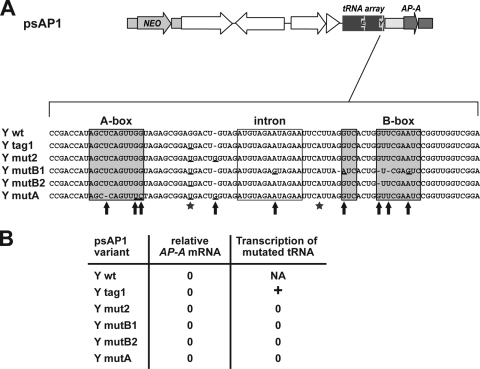
To investigate whether polymerase III-dependent transcription of the episomal tRNAs is required for trans inactivation, a series of mutations was introduced into the tRNATyr gene of psAP1 (Fig. 5A). Interestingly, though some of the mutations had severe effects on the internal promoter regions and for tRNA transcription, none of the mutations altered AP-A trans inactivation capacity of the plasmid (Fig. 5B).
Fig. 5.
The presence but not transcription of the tRNA on psAP1 is required for AP-A trans inactivation. (A) Schematic depiction of the relevant regions of psAP1 and location of various mutations introduced into the tyrosine (Y) tRNA gene to test the importance of the respective residues for the trans inactivation capacity of psAP1. Residues deviating from the wild-type sequence (Y wt) are underlined (Y mut). (B) Presence or absence of AP-A mRNA and tRNA transcripts after transfection with psAP1 containing mutations within the tyrosine tRNA gene. The abundances of AP-A mRNA and transcribed mutated tRNA relative to that of the wild type (set to 1), as determined by qRT-PCR, are given. Note that mutated tRNA was detectable only when mutations indicated by asterisks in panel A were introduced (Y tag1) but not when bearing mutations marked by arrows in that panel. The latter primarily comprised mutations located within the control region (A-box and B-box), which are likely to affect binding capacity of the polymerase III transcriptional machinery. However, none of the mutations affected AP-A trans inactivation capacity, indicating that the presence of the tRNA gene, but not its transcription or binding of the transcriptional machinery, is required for trans inactivation. NA, not applicable.
SINE is dispensable for AP-A trans inactivation.
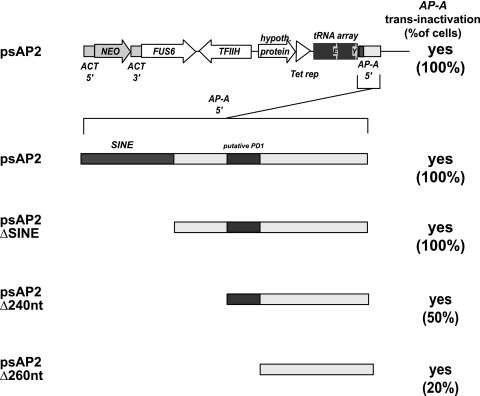
A SINE is located 5′ of the AP-A locus in the E. histolytica genome and thus is partially present on the psAP1 plasmid at the 5′ flanking region of AP-A. It has been suggested that the partial SINE sequence is required for the trans inactivation capacity of psAP1 (2). To further explore the characteristics of the 5′ flanking sequence required for AP-A trans inactivation, several 5′ deletions in the AP-A upstream region were performed in psAP1 plasmids (Fig. 6). Surprisingly, qRT-PCR analysis after transfection of these constructs revealed that the deletion of SINE did not change the trans inactivation property of psAP1. Even constructs containing only 213 bp of the AP-A upstream region were capable of trans inactivation AP-A in transfected amoebae. However, shortening of the AP-A upstream region reduced the trans inactivation efficacy of the plasmid. As determined by single-cell cloning of transfected cells and subsequent qRT-PCT analysis, depending on the length of the 5′ flanking sequence, only about 20 to 50% of the cell clones were affected by AP-A trans inactivation. Thus, silencing became evident only after single-cell cloning but was not obvious in transfected bulk cultures.
Fig. 6.
SINE and a putative PD1 element are dispensable for AP-A trans inactivation. The silencing plasmid psAP2 containing largely the same sequence elements as psAP1 but lacking the AP-A coding and 3′ flanking sequences was used to analyze the requirement for SINE and the putative PD1 element for AP-A trans inactivation. Various 5′ deletion mutant forms of the AP-A upstream region were generated and substituted for the AP-A 5′ region in psAP2 as indicated. Even the minimal fragment tested (psAP2Δ260nt) comprising 213 bp of the AP-A upstream region but lacking SINE as well as PD1 mediated trans inactivation. Note that in contrast to the longer fragments, which silenced AP-A in 100% of transfected amoebae, deletion plasmids psAP2Δ240nt and psAP2Δ260nt induced trans inactivation efficiency in only 50% and 20% of transfected cells, respectively. NEO, neomycin resistance gene.
DISCUSSION
To gain insight into the molecular mechanism of trans inactivation of AP-A in E. histolytica following transfection with the plasmid psAP1, we have identified two factors that are indispensable for AP-A transcriptional silencing. First, AP-A silencing is restricted to a particular cell line, and second, two specific tRNA genes are required on the plasmid in close proximity upstream of the AP-A promoter. In contrast to the case described by a previous report (2), a SINE motif located upstream of the AP-A promoter was not required. This discrepancy may be explained by our finding that shortening of the AP-A upstream region (e.g., by deletion of SINE) significantly reduces trans inactivation efficacy, as indicated by a reduced number of cells with an AP-A-silenced phenotype, which became evident only after single-cell cloning of transfected amoebae. The fact that the length of the AP-A upstream region plays a role for the trans inactivation capacity suggests that a base-pairing mechanism may be involved in E. histolytica trans inactivation.
Two sequence elements are required for trans inactivation, namely, the tyrosine and glutamic acid tRNA genes and about 200 bp of the AP-A 5′ region. These two elements operate in concerted action, as the EY tRNAs alone or in close proximity to other promoters cannot transinactivate, and neither does the AP-A promoter alone or in close proximity to other tRNA genes.
Two mechanisms found in other eukaryotes and which may play a role for the trans inactivation of AP-A in E. histolytica have been described previously. These are heterochromatic silencing in trans (transvection), as found in various organisms (12, 26, 33), and tgm, so far described exclusively for S. cerevisiae for tyrosine, leucine, and threonine (21). Both mechanisms depend on the nuclear architecture, and for both, it is likely that they play a role in E. histolytica transcriptional silencing. This does not exclude the possibility that other yet unknown factors may also participate in the silencing mechanism.
Chromatin structure.
Attempts to localize tRNA genes by fluorescence in situ hybridization (FISH) experiments turned out to be difficult with E. histolytica (43). Hybridization was successful with a single EY tRNA probe (called M11) but was unsuccessful in colocalization experiments using rDNA and tDNA probes simultaneously.
Nevertheless, several observations support the concept that the AP-A locus and the plasmid psAP1 localize to the heterochromatic periphery. First, Anbar et al. showed that in AP-A-silenced cells, the AP-A gene is no longer associated with the methylated K4 residue of histone H3 (2). Second, some ARS sequences, such as the yeast 2μ origin, have been localized to heterochromatin and can act as silencers (16). The EY tRNA array has also been shown to have properties of autonomous replication sequences, at least when transferred to S. cerevisiae (29). Finally, the nuclear periphery, a heterochromatic region where telomeres are localized in other organisms, has been suggested to play a role in the silencing mechanism (39). As telomeres are absent in E. histolytica, it is believed that tRNA arrays are located at the end of the amoeba chromosomes, presumably at the nuclear periphery, and thus may play a structural role similar to that of telomeres (28). The organization of tRNA genes in E. histolytica is exceptional in that they form long arrays consisting of hundreds of tRNA sequences and AT-rich repeat elements (9). Hence, tRNA genes are organized as natural clusters in E. histolytica in contrast to S. cerevisiae, in which the spatially clustered tRNAs upon Pol III transcription have been shown to be colocalized at the nucleolus (reviewed in reference 17). Likewise, the nucleolus in E. histolytica is localized at the periphery, as demonstrated with rDNA episomes (20, 43) and recently with fibrillarin (23). With these results taken together, we propose that tRNA gene arrays and the tRNA genes of the plasmid might be colocalized at the nuclear periphery in E. histolytica. Further studies to understand the architecture of the amoebae nucleus are necessary to finally prove this hypothesis.
A tgm-like mechanism.
The fact that AP-A trans inactivation depends on the presence of tRNA gene sequences suggests a tgm-related mechanism, which has been recognized so far exclusively in S. cerevisiae. For S. cerevisiae, Pol III transcription of tRNAs has been described as a central step of tgm required for their spatial clustering (18). However, in contrast to the case in S. cerevisiae, tgm in E. histolytica does not appear to depend on transcription or binding of the polymerase III machinery, as none of the mutations introduced into the two regulatory elements of the amoeba tyrosine tRNA gene did abolish the trans inactivation capacity of the transfected plasmid. If transcription or Pol III binding of tRNA genes were the cause for silencing, any mutations that cause loss of transcription or Pol III binding in one gene would yield the same effect as the deletion of that gene. However, tyrosine tRNA gene mutations did not even moderately impair trans inactivation, while its deletion completely abolished any silencing capacity. We suggest that binding of the transcriptional machinery (or transcription) may not be required for spatial clustering in E. histolytica, because amoeba tRNA genes are already “clustered” due to the fact that they are arranged in long tandem-repeat arrays (9).
In the search for a putative silencer element, a PD1 homologue was identified in the AP-A upstream region (42). However, deletion or mutation of this element did not interfere with the trans inactivation capacity of psAP1 (Fig. 6). As no other sequence similarities to known silencer elements have been identified, silencer elements are either not present or not well conserved between E. histolytica and other eukaryotes. The latter explanation seems more likely, as the molecular mechanisms of heterochromatin formation and gene silencing appear to be only weakly conserved in E. histolytica. For many of the proteins known to be involved in heterochromatin formation or silencing, there is no evidence of their existence in the genome of E. histolytica (data not shown).
There remain questions about the specific properties of HM-1:IMSS (2411) that enable transcriptional silencing in this particular strain but not in others. So far, various attempts to identify strain-specific differences have been made. These include PCR-based strain typing and sequencing of the AP-A locus and the tRNA array as well as microarray analyses using panels of selected amoeba genes (4, 22, 38) or a panel comprising protein-coding genes of the entire E. histolytica genome (D. Mirelman, personal communication). However, none of these approaches resolved the problem, suggesting that an epigenetic phenomenon or a mutation may affect any yet unknown gene involved in silencing.
Future comparative genome sequencing may help to decipher the molecular differences between the strains, thus providing further insights into the mechanism of gene silencing in E. histolytica.
Another important result of this study is that AP-A expression can be restored in AP-A-silenced amoeba by episomal transfections using the AP-A open reading frame under the control of a heterologous amoeba promoter including an intron with enhancer properties. This AP-A add-back mutant led to an abundance of AP-A similar to that of nonsilenced wild-type cells. This is in contrast to a study in which reconstitution of AP-A expression could not be achieved by episomal transfection (5), likely due to differences in the transfection vectors. Irrespectively, reexpression of genes by episomal transfection is a crucial advancement toward more sophisticated investigations of amoeba protein function, cell biology, and pathogenicity, as targeted silencing of genes of interest currently can be achieved only in amoebae in which AP-A is additionally silenced (4, 6, 22). However, AP-A-deficient cells are highly impaired in pathogenicity and therefore not suitable for the investigation of other pathogenicity factors.
ACKNOWLEDGMENTS
We thank David Mirelman for helpful discussions and for providing the psAP1 plasmid and HM-1:IMSS (2411) cells. We also thank Matthias Leippe for anti-AP-A and anti-AP-B antisera, and we thank Susann Ofori, Fabian Peters, Nicola Porter, and Heidrun von Thien for skillful technical assistance.
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Ali I. K., Zaki M., Clark C. G. 2005. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J. Clin. Microbiol. 43:5842–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anbar M., Bracha R., Nuchamowitz Y., Li Y., Florentin A., Mirelman D. 2005. Involvement of a short interspersed element in epigenetic transcriptional silencing of the amoebapore gene in Entamoeba histolytica. Eukaryot. Cell 4:1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrulis E. D., Neiman A. M., Zappulla D. C., Sternglanz R. 1998. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 394:592–595 [DOI] [PubMed] [Google Scholar]
- 4.Bracha R., Nuchamowitz Y., Anbar M., Mirelman D. 2006. Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica. PLoS Pathog. 2:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracha R., Nuchamowitz Y., Mirelman D. 2003. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot. Cell 2:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracha R., Nuchamowitz Y., Wender N., Mirelman D. 2007. Transcriptional gene silencing reveals two distinct groups of Entamoeba histolytica Gal/GalNAc-lectin light subunits. Eukaryot. Cell 6:1758–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown K. E., Guest S. S., Smale S. T., Hahm K., Merkenschlager M., Fisher A. G. 1997. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell 91:845–854 [DOI] [PubMed] [Google Scholar]
- 8.Buck S. W., Sandmeier J. J., Smith J. S. 2002. RNA polymerase I propagates unidirectional spreading of rDNA silent chromatin. Cell 111:1003–1014 [DOI] [PubMed] [Google Scholar]
- 9.Clark C. G., Ali I. K., Zaki M., Loftus B. J., Hall N. 2006. Unique organisation of tRNA genes in Entamoeba histolytica. Mol. Biochem. Parasitol. 146:24–29 [DOI] [PubMed] [Google Scholar]
- 10.Csink A. K., Henikoff S. 1996. Genetic modification of heterochromatic association and nuclear organization in Drosophila. Nature 381:529–531 [DOI] [PubMed] [Google Scholar]
- 11.Diamond L. S., Harlow D. R., Cunnick C. C. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431–432 [DOI] [PubMed] [Google Scholar]
- 12.Duncan I. W. 2002. Transvection effects in Drosophila. Annu. Rev. Genet. 36:521–556 [DOI] [PubMed] [Google Scholar]
- 13.Ehrenhofer-Murray A. E., Gossen M., Pak D. T., Botchan M. R., Rine J. 1995. Separation of origin recognition complex functions by cross-species complementation. Science 270:1671–1674 [DOI] [PubMed] [Google Scholar]
- 14.Fisher A. G., Merkenschlager M. 2002. Gene silencing, cell fate and nuclear organisation. Curr. Opin. Genet. Dev. 12:193–197 [DOI] [PubMed] [Google Scholar]
- 15.Funabiki H., Hagan I., Uzawa S., Yanagida M. 1993. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 121:961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünweller A., Ehrenhofer-Murray A. E. 2002. A novel yeast silencer. the 2mu origin of Saccharomyces cerevisiae has HST3-, MIG1- and SIR-dependent silencing activity. Genetics 162:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeusler R. A., Engelke D. R. 2006. Spatial organization of transcription by RNA polymerase III. Nucleic Acids Res. 34:4826–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeusler R. A., Pratt-Hyatt M., Good P. D., Gipson T. A., Engelke D. R. 2008. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 22:2204–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamann L., Nickel R., Tannich E. 1995. Transfection and continuous expression of heterologous genes in the protozoan parasite Entamoeba histolytica. Proc. Natl. Acad. Sci. U. S. A. 92:8975–8979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber M., Koller B., Gitler C., Mirelman D., Revel M., Rozenblatt S., Garfinkel L. 1989. Entamoeba histolytica ribosomal RNA genes are carried on palindromic circular DNA molecules. Mol. Biochem. Parasitol. 32:285–296 [DOI] [PubMed] [Google Scholar]
- 21.Hull M. W., Erickson J., Johnston M., Engelke D. R. 1994. tRNA genes as transcriptional repressor elements. Mol. Cell. Biol. 14:1266–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irmer H., Tillack M., Biller L., Handal G., Leippe M., Roeder T., Tannich E., Bruchhaus I. 2009. Major cysteine peptidases of Entamoeba histolytica are required for aggregation and digestion of erythrocytes but are dispensable for phagocytosis and cytopathogenicity. Mol. Microbiol. 72:658–667 [DOI] [PubMed] [Google Scholar]
- 23.Jhingan G. D., Panigrahi S. K., Bhattacharya A., Bhattacharya S. 2009. The nucleolus in Entamoeba histolytica and Entamoeba invadens is located at the nuclear periphery. Mol. Biochem. Parasitol. 167:72–80 [DOI] [PubMed] [Google Scholar]
- 24.Kendall A., Hull M. W., Bertrand E., Good P. D., Singer R. H., Engelke D. R. 2000. A CBF5 mutation that disrupts nucleolar localization of early tRNA biosynthesis in yeast also suppresses tRNA gene-mediated transcriptional silencing. Proc. Natl. Acad. Sci. U. S. A. 97:13108–13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leippe M., Ebel S., Schoenberger O. L., Horstmann R. D., Muller-Eberhard H. J. 1991. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. U. S. A. 88:7659–7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H., Huang J., Wang J., Jiang S., Bailey A. S., Goldman D. C., Welcker M., Bedell V., Slovak M. L., Clurman B., et al. 2008. Transvection mediated by the translocated cyclin D1 locus in mantle cell lymphoma. J. Exp. Med. 205:1843–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 28.Loftus B., Anderson I., Davies R., Alsmark U. C., Samuelson J., Amedeo P., Roncaglia P., Berriman M., Hirt R. P., Mann B. J., et al. 2005. The genome of the protist parasite Entamoeba histolytica. Nature 433:865–868 [DOI] [PubMed] [Google Scholar]
- 29.Lohia A., Haider N., Biswas B. B. 1990. Characterisation of a repetitive DNA family from Entamoeba histolytica containing Saccharomyces cerevisiae ARS consensus sequences. Gene 96:197–203 [DOI] [PubMed] [Google Scholar]
- 30.Loo S., Rine J. 1995. Silencing and heritable domains of gene expression. Annu. Rev. Cell Dev. Biol. 11:519–548 [DOI] [PubMed] [Google Scholar]
- 31.Marcand S., Buck S. W., Moretti P., Gilson E., Shore D. 1996. Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev. 10:1297–1309 [DOI] [PubMed] [Google Scholar]
- 32.Mirelman D., Anbar M., Bracha R. 2008. Trophozoites of Entamoeba histolytica epigenetically silenced in several genes are virulence-attenuated. Parasite 15:266–274 [DOI] [PubMed] [Google Scholar]
- 33.Rassoulzadegan M., Magliano M., Cuzin F. 2002. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 21:440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sage B. T., Jones J. L., Holmes A. L., Wu M. D., Csink A. K. 2005. Sequence elements in cis influence heterochromatic silencing in trans. Mol. Cell. Biol. 25:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sass G. L., Henikoff S. 1999. Pairing-dependent mislocalization of a Drosophila brown gene reporter to a heterochromatic environment. Genetics 152:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schägger H., Aquila H., Von Jagow G. 1988. Coomassie blue-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for direct visualization of polypeptides during electrophoresis. Anal. Biochem. 173:201–205 [DOI] [PubMed] [Google Scholar]
- 37.Simms T. A., Miller E. C., Buisson N. P., Jambunathan N., Donze D. 2004. The Saccharomyces cerevisiae TRT2 tRNAThr gene upstream of STE6 is a barrier to repression in MATalpha cells and exerts a potential tRNA position effect in MATa cells. Nucleic Acids Res. 32:5206–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tillack M., Biller L., Irmer H., Freitas M., Gomes M. A., Tannich E., Bruchhaus I. 2007. The Entamoeba histolytica genome: primary structure and expression of proteolytic enzymes. BMC Genomics 8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin B. D., Meister P., Gasser S. M. 2009. The nuclear envelope—a scaffold for silencing? Curr. Opin. Genet. Dev. 19:180–186 [DOI] [PubMed] [Google Scholar]
- 40.van Driel R., Fransz P. F., Verschure P. J. 2003. The eukaryotic genome: a system regulated at different hierarchical levels. J. Cell Sci. 116:4067–4075 [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Haeusler R. A., Good P. D., Thompson M., Nagar S., Engelke D. R. 2005. Silencing near tRNA genes requires nucleolar localization. J. Biol. Chem. 280:8637–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissman J. D., Singer D. S. 1991. A complex regulatory DNA element associated with a major histocompatibility complex class I gene consists of both a silencer and an enhancer. Mol. Cell. Biol. 11:4217–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willhoeft U., Tannich E. 2000. Fluorescence microscopy and fluorescence in situ hybridization of Entamoeba histolytica nuclei to analyse mitosis and the localization of repetitive DNA. Mol. Biochem. Parasitol. 105:291–296 [DOI] [PubMed] [Google Scholar]