Abstract
Common iatrogenic procedures can result in translocation of the human pathogenic fungus Candida albicans from mucosal surfaces to the bloodstream. Subsequent disseminated candidiasis and infection of deep-seated organs may occur if the fungus is not eliminated by blood cells. In these cases, fungal cells adhere to the endothelial cells of blood vessels, penetrate through endothelial layers, and invade deeper tissue. In this scenario, endothelial adhesion events must occur during circulation under conditions of physiological blood pressure. To investigate the fungal and host factors which contribute to this essential step of disseminated candidiasis, we have developed an in vitro circulatory C. albicans-endothelium interaction model. We demonstrate that both C. albicans yeast and hyphae can adhere under flow at a pressure similar to capillary blood pressure. Serum factors significantly enhanced the adhesion potential of viable but not killed C. albicans cells to endothelial cells. During circulation, C. albicans cells produced hyphae and the adhesion potential first increased, then decreased with time. We provide evidence that a specific temporal event in the yeast-to-hyphal transition, regulated by the G1 cyclin Hgc1, is critical for C. albicans-endothelium adhesion during circulation.
Candida albicans is one of only a few fungal species which belong to the normal microbial flora of human beings and, under normal circumstances, exists as a commensal of the skin, gastrointestinal tract, oral cavity, or vagina. Alterations in the host environment, however, can result in the transition from a commensal to a pathogenic relationship. Even relatively mild immune suppression or antibiotic treatment can result in mucosal infections, and these superficial infections are extremely common (24). Candida species are also the most frequent cause of invasive fungal infections in humans, and C. albicans accounts for around 50% of disseminated candidiasis (23). These infections are extremely serious, with attributable mortality rates of 40 to 50%, even with first-line antifungal therapy. Although severe immune suppression—in particular defects in innate immunity, such as neutropenia—is associated with disseminated candidiasis, the major risk factors are common iatrogenic procedures and/or nosocomial conditions such as placement of a central venous catheter and disruption of normal skin barriers or gut mucosa.
In these situations, C. albicans can gain access to the bloodstream and, from there, disseminate throughout the body and colonize organs, which may ultimately result in sepsis and multiorgan failure. In order to exit the bloodstream and infect internal organs, however, the fungus must first adhere to and traverse the endothelial lining of blood vessels. Although this critical step in disseminated candidiasis has been the subject of several studies (reviewed in reference 13), the detailed mechanisms underlying it remain poorly understood, and it is likely that C. albicans-endothelium adhesion is mediated by numerous different host and fungal activities. While mostly uncharacterized at the molecular level, C. albicans has been shown to possess integrin-like molecules which mediate the adhesion of yeast cells to the endothelium (15). In addition, the hydrophobicity of the yeast cell surface was also demonstrated to influence adhesion under conditions which mimic the physical pressure of the circulatory system (11) and the glycosylation state of cell wall proteins is likely to play a major role, as a pmt6Δ mutant with defective O-glycosylation of secreted proteins displays attenuated endothelial adhesion (26).
The genome of C. albicans contains numerous genes encoding both putative and characterized adhesins (6, 21, 25). Of these, only a small number have been tested for involvement in endothelial interactions and only certain members of the ALS gene family have been demonstrated to play a role in endothelial attachment events. Als2 and Als3 represent multifunctional adhesins with roles in adherence to both endothelial and epithelial cells, while Als1, Als4, and Als9 appear to specifically mediate adhesion to endothelial cells (30, 31).
The aims of this study were to develop a circulatory blood vessel model and to characterize factors necessary for C. albicans-endothelium adhesion under physical pressure. A similar model has recently been described by Grubb et al. (14). These authors utilized a novel flow system to determine the relative adhesiveness of different C. albicans morphologies to endothelial cells. The authors found that yeast cells were more adherent under conditions of shear stress, which mimic the physical environment of postcapillary venules.
The experimental design of the current study, however, features several differences. Most importantly, we have developed a circulation system, as opposed to linear perfusion, which permitted fungal adaptation within the system and allowed us to monitor morphological and adhesion kinetics during circulation. Furthermore, we have used a pressure which is similar to that found in capillary networks, have quantified the orientation of fungal hyphae relative to flow, and have analyzed the importance of fungal viability, the role of serum factors, and the importance of hypha-associated genes by using mutants lacking regulators of morphogenesis. Similar to Grubb et al. (14), we found that C. albicans yeast and hyphae can rapidly adhere under flow. However, we also found that an adaptation event associated with the yeast-to-hypha transition can greatly enhance C. albicans-endothelium adhesion during circulation. In fact, C. albicans adhered most efficiently at a distinct stage during dimorphism. Furthermore, we found that C. albicans can adhere under relatively high pressure, above 3 dynes/cm2, and that serum factors are important for this process. Finally, we provide molecular evidence that adhesion to endothelial cells under these conditions requires hyphal formation and is specifically mediated by the G1 cyclin encoded by HGC1.
MATERIALS AND METHODS
C. albicans strains and culture conditions.
Unless otherwise stated, experiments were performed with the C. albicans wild-type clinical isolate SC5314. An hgc1Δ strain (hgc1Δ::ARG4/hgc1Δ::HIS1) and an HGC1-complemented strain were kindly provided by Yue Wang (32). A ras1Δ strain (ras1Δ::hisG/ras1Δ::hph) was kindly provided by Gerald Fink (9). To produce hgc1Δ and ras1Δ URA3 isogenic strains, URA3 was integrated at the RP10 locus using the CIp10 plasmid (20). CAI4+CIp10 was used as a wild-type comparison for experiments involving mutants. Strains were maintained on YPD agar plates and grown overnight in liquid YPD with shaking for use in experiments. For killing, yeast cells were washed twice in PBS and incubated at room temperature, with shaking in phosphate-buffered saline (PBS) containing 0.1% thimerosal for 3 h; cells were then washed three times in PBS before being used in experiments. Killing was confirmed by plating potential CFU onto YPD agar plates and incubating for 2 days at 37°C.
Unidirectional flow system.
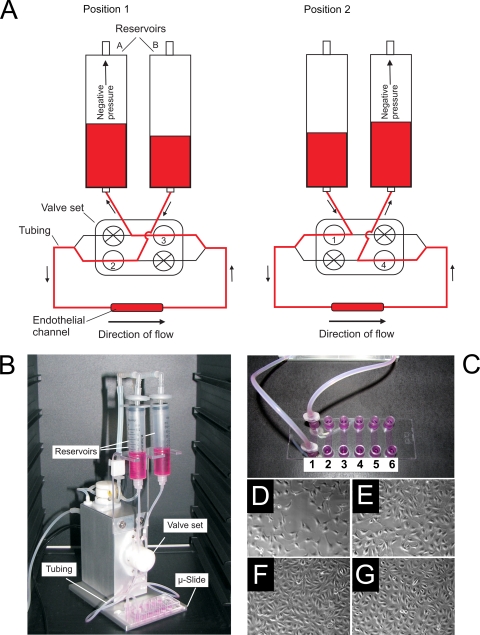
The ibidi pump system (ibidi—Integrated BioDiagnostics, Germany) was used for perfusing C. albicans cells through an endothelial channel. The general features of the system are shown in Fig. 1. The system comprises a fluidic unit, which can be placed in an incubator, and an air pressure pump, both of which are computer controlled. The fluidic unit holds the perfusion set (Fig. 1A and B), which consists of two reservoirs connected to opposite ends of the endothelial channel via silicone tubing (0.8-mm inner diameter). The silicone tubes from each reservoir branch above the valve set and rejoin with a branch of the opposing tube below the valve set (Fig. 1A). Within the valve set, each of the four silicone tube branches can be independently opened and closed. This setup (Fig. 1A) allows continuous unidirectional flow of medium through the endothelial channel: in position 1, valves 2 and 3 are open and negative pressure from the air pump is generated in reservoir A; in position 2, valves 1 and 4 are open and negative pressure is generated in reservoir B. Simultaneous switching of air pressure and valve sets between positions 1 and 2 generates medium movement through the tubing (arrows, Fig. 1A) and maintains continuous unidirectional flow through the endothelial channel. For standard experiments, the system was programmed to generate −13-mbar pressure and switch between positions 1 and 2 at 45-s intervals, resulting in 3.28 dynes/cm2 system pressure and medium flow of 2 ml/min.
Fig. 1.
The circulatory model. (A) Schematic representation of the circulatory model. Air pressure in the reservoirs and opening/closing of the valve set are controlled via an air pump and computer software. Note that in both position 1 and position 2 unidirectional flow through an endothelial channel is maintained. (B) Picture of the circulatory model (ibidi pump system) with reservoirs, valve set, tubing, and μ-Slide labeled. (C) Closeup picture of the μ-Slide. Note that each μ-Slide houses six independent channels, which can be seeded with endothelial cells and connected to the circulatory model via Luer adaptors. (D to G) Micrograph images of the development of endothelial cells within a μ-Slide channel, 1 (D), 2 (E), and 3 (F) days postseeding and following 20 min under flow (G). Note that monolayers are confluent by 3 days postincubation and that 20 min flow does not overtly effect the morphology of the endothelial monolayer.
Adhesion assay.
Media and the perfusion set were equilibrated overnight at 37°C, 5% CO2. Eleven milliliters of Dulbecco modified Eagle medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS) unless stated otherwise, was added to the perfusion set reservoirs (Fig. 1A), and air bubbles were removed. Single C. albicans yeast cells (from 30°C overnight YPD cultures, washed three times with PBS) were added to the reservoirs to a final concentration of 5 × 105 cells/ml (unless otherwise stated) and circulated for 3 min to allow equilibration. The perfusion set was then joined to endothelial channel 1 via the Luer connections (Fig. 1C) and incubated under flow conditions (flow, 0 to 20 min); at the same time, a 70-μl sample from the perfusion system was added to endothelial channel 2 and incubated under static conditions (static, 0 to 20 min). After 20 min incubation, the Luer connections were switched to channel 3 (flow, 20 to 40 min), a 70-μl sample from the perfusion system was added to channel 4 (static, 20 to 40 min), and channels 1 and 2 were immediately flush-washed three times with PBS and flush-fixed three times with Histofix (Roth). This process was repeated (with fresh endothelial channels) at 20-min intervals for the duration of the circulation experiments.
Endothelial cells.
Human umbilical vein endothelial cells (HUVEC) (ATCC CRL-1730; LGC Standards, Promocell) were routinely passaged in Dulbecco modified Eagle medium (DMEM) supplemented with heat-inactivated (56°C for 10 min) fetal bovine serum (FBS) at a final concentration of 10%. To generate endothelial channels for use in adhesion experiments, μ-Slides (ibiTreat μ-Slide-VI 0.4; ibidi—Integrated BioDiagnostics, Germany) were used. Each μ-Slide (Fig. 1C) contains six independent channels (0.4 × 3.8 × 17 mm) with female Luer adapters at the ends of each channel for connection to the pump system (see below). The base of each channel is tissue culture-treated plastic to allow cell cultivation. Each channel was seeded with 1.2 × 104 endothelial cells, 3 days prior to experiments, with media replaced daily to achieve confluent endothelial monolayers. Figure 1D to G shows the development of endothelia over this 3-day period and after exposure to flow conditions.
Adhesion kinetics determination.
Following fixation, endothelial channels were flushed three timed with PBS, flushed twice with calcofluor solution (10 μg/ml calcofluor white in 100 mM Tris, pH 9.5), incubated with calcofluor solution for 25 min in darkness at room temperature, and washed three times with water, and aqueous mounting solution (Sigma) was added for visualization by fluorescence microscopy. For each endothelial channel, 50 to 70 random 200-μm2 frames (covering representative areas of the channel) were scored for adherent C. albicans cells and adhesion kinetics—defined as the number of cells adhering per mm2 per min—were determined.
Germ tube orientation.
Around 80 random cells, which had adhered to the endothelial channel between 40 and 60 min, were scored for orientation. Briefly, the direction of flow was considered 0° and the angle of germ tubes to the direction of flow was measured. For example, a germ tube oriented exactly against the direction of flow was scored as 180°. As a control, the orientation of cells adhered to a static endothelial channel was determined in the same way.
Statistical analysis.
Samples were compared by a two-tailed, type 3 Student t test, and differences were considered significant when P was ≤0.05. For determining population enrichment (see Fig. 5), an F-test was applied and considered significant when P was ≤0.05.
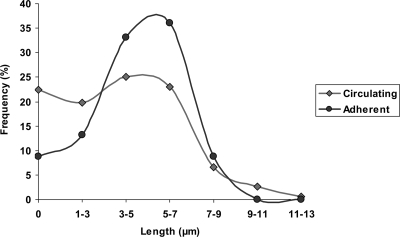
Fig. 5.
Morphological distribution. The morphology of C. albicans cells in circulation at 60 min and of cells which had adhered to the endothelial monolayer between 40 and 60 min was determined. At least 130 cells, over two independent experiments, for each condition were scored. Data represent the percentage of indicated germ tube length. Note that germ tubes of 3 to 7 μm in length adhered to the endothelium under flow at higher frequency. The variance of germ tube length of endothelium-adherent cells was significantly lower than that for circulating cells (P < 0.0001, by F-test, P = 0.0024 upon exclusion of zero [yeast] values).
RESULTS
Endothelial adhesion of circulating C. albicans cells.
Although a number of studies have addressed the mechanisms of C. albicans-endothelium adhesion, few have considered one of the major physical parameters of the circulatory system: circulation itself. We therefore developed an in vitro circulatory model for analyzing C. albicans-endothelium interactions under conditions of physiological flow. The system (for a detailed description, see Materials and Methods and Fig. 1) allowed circulation of C. albicans cells and perfusion through an endothelial channel at flow rates (3.28 dynes/cm2, 2 ml/min) similar to those found in capillary networks (27). First we determined whether C. albicans cells were capable of adhering to endothelial cells under conditions of flow in standard cell culture medium (DMEM with 10% FBS). Under this condition, we observed rapid adhesion of fungal cells to the endothelium within the first 20 min of perfusion; however, following longer incubation times we observed high degrees of fungus-fungus cell clumping on the endothelium (data not shown).
In order to focus our analysis on C. albicans-endothelium interaction, rather than fungal aggregation events, we designed an adhesion assay where C. albicans cells were circulated in the system from time point zero until the end of the experiment; the circulating cells were perfused through an endothelial channel for 20 min, and the circulating cells were consecutively switched to a fresh endothelial channel at 20-min intervals. Each adhesion kinetics data point displayed in Fig. 2, 4, 7, and 8 therefore represents the kinetics of adhesion to an endothelial channel within 20 min.
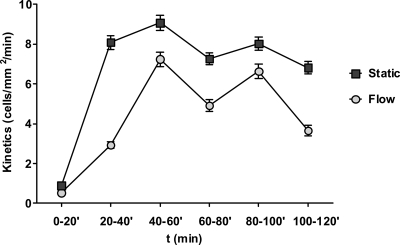
Fig. 2.
Adhesion dynamics of circulating C. albicans. C. albicans cells were circulated for 120 min, and the kinetics of cells adhering to endothelial channels (per mm2 per min) were determined for each 20-min interval. As a control (Static), cells taken from the perfusion system at the indicated time points were applied to a static endothelial monolayer for 20 min. The x axis represents the time of circulation, and each data point is the kinetics of adhesion within 20 min of exposure to a sterile endothelial monolayer. Data points are the means of results of three independent experiments, and error bars indicate the standard errors of the means. Note that adhesion under flow conditions reach maximum kinetics between 40 and 60 min.
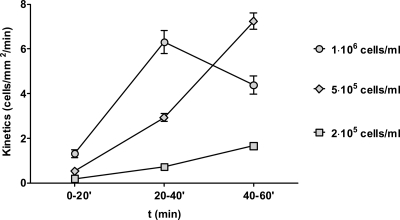
Fig. 4.
Adhesion is dose dependent. C. albicans cells were inoculated into the perfusion system at either 2 × 105, 5 × 105, or 1 × 106 cells/ml and circulated for 60 min. Adhesion kinetics were determined for 0 to 20, 20 to 40, and 40 to 60 min of perfusion. Data represent the means of results of at least two independent experiments, and error bars indicate the standard errors of the means between experiments.
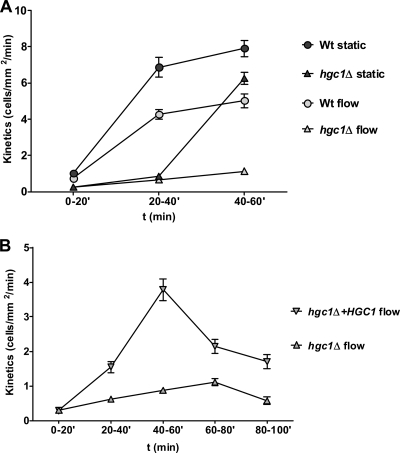
Fig. 7.
Circulatory adhesion is HGC1 dependent. (A) Wild-type CAI4+CIp10 (Wt) or hgc1Δ homozygous mutant strains were circulated for 60 min, and the adhesion kinetics occurring between 0 and 20, 20 and 40, and 40 and 60 min were determined. At 20 to 40 and 40 to 60 min, the adhesion kinetics of hgc1Δ were significantly lower than those of the Wt (P < 0.0001). The adhesion kinetics of cells taken from the perfusion system at the indicated time points and applied to a static endothelial monolayer are included as a control. Note that between 40 and 60 min, hgc1Δ adheres to the static endothelium at a rate similar to that of the Wt (P = 0.51) but not under conditions of flow. Data points are the means of results of at least two independent experiments, and error bars indicate the standard errors of the means. (B) hgc1Δ and hgc1Δ+HGC1 strains were circulated for 100 min, and adhesion kinetics were determined for 0 to 20, 20 to 40, 40 to 60, 60 to 80, and 80 to 100 min. At all time points except for 0 to 20 min, hgc1Δ adhered significantly less than hgc1Δ+HGC1 (P < 0.0001). Data points are the means of results of at least two independent experiments, and error bars indicate the standard errors of the means.
Fig. 8.
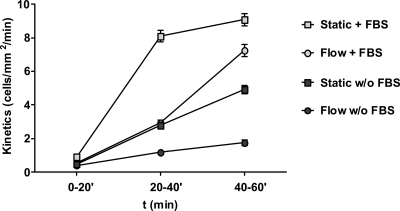
Effect of serum on adhesion. C. albicans cells were circulated for 60 min in either DMEM (w/o FBS) or DMEM supplemented with 10% fetal bovine serum (+ FBS), and the adhesion kinetics occurring between 0 and 20, 20 and 40, and 40 and 60 min were determined. As a (Static) control, cells from the perfusion system, taken at the indicated time points, were applied to a static endothelial monolayer for the 20-min intervals. Data points are the means of results of at least two independent experiments, and error bars indicate the standard errors of the means.
In the first 20 min of circulation, C. albicans yeast cells bound to the endothelial channel at a frequency of 0.47 cells/mm2/min. Between 20 and 40 min, the kinetics of C. albicans adhesion rose significantly to 2.9 cells/mm2/min and further still between 40 and 60 min of circulation to 6.8 cells/mm2/min (Fig. 2). Following 60 min of circulation, C. albicans adhesion had reached maximum kinetics and decreased thereafter. We therefore focused our studies on adhesion events within the first 60 min of circulation. Within this period, 70 to 80% of yeast cells germinated but did not produce extensive hyphae of more than 12 μm in length. During the course of the experiment, the numbers of circulating cells dropped over time (Fig. 3) as a result of adhesion to endothelia and, presumably, to other components of the system.
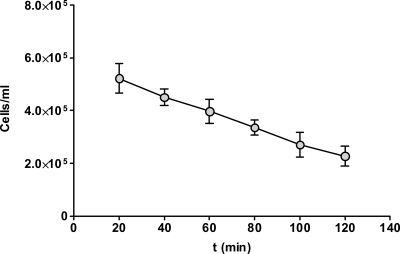
Fig. 3.
Population dynamics of circulating cells. The concentration of circulating C. albicans cells in the system was determined every 20 min by counting using a hemocytometer. Data represent the means of results of at least two independent experiments, and error bars indicate the standard errors of the means between experiments.
Although circulating cells were visible by inverse microscopy in the endothelial channel within seconds of turning on the pump system, it was possible that the lower adhesion rates at earlier time points were due to lower numbers of cells reaching the endothelial channel. We therefore set the perfusion system with cells inoculated into only one of the perfusion reservoirs and ran the pump for 5 min. Following this incubation, 10% of the total cells had migrated to the second perfusion reservoir, suggesting that C. albicans cell density equilibrium within the endothelial channel is reached within seconds (data not shown).
Adhesion is dose dependent.
The outcome and pathogenesis of virtually all microbial infections are dose dependent. We therefore determined the adhesion kinetics of C. albicans in our circulatory endothelial model at two additional cell densities: 2 × 105 and 1 × 106 cells/ml (Fig. 4). At the lower cell concentration of 2 × 105 cells/ml, adhesion kinetics rose significantly with increasing circulation time; however, between 20 to 40 min and 40 to 60 min, adhesion with 5 × 105 cells/ml was significantly higher than with 2 × 105 cells/ml (P = 0.003 and 0.0002, respectively). At the higher concentration of 1 × 106 cells/ml, adhesion kinetics between 0 to 20 min and 20 to 40 min were significantly higher than with 5 × 105 cells/ml (P < 0.0001 in both cases); however, between 40 and 60 min with 1 × 106 cells/ml, adhesion kinetics dropped off, suggesting that, following germ tube formation, at higher cell densities (∼106 cells/ml), adhesion dose dependency breaks down. We therefore concluded that 5 × 105 cells/ml is an appropriate cell density for flow adhesion experiments in our model.
The significant increase in adhesion kinetics within the first hour of circulation showed that C. albicans cells were becoming more adherent over time. We hypothesized that this observed increase in binding kinetics could be due to (i) germ tube formation, (ii) expression of adhesion factors, or (iii) host components.
Germ tube formation and circulatory adhesion.
As shown in Fig. 2, the dynamics of C. albicans-endothelium adhesion changed considerably over time. In our model, inoculated yeast cells always adhered relatively poorly. This is in agreement with the results of Grubb et al. (14), who found that yeast adhesion at pressures over 3 dynes/cm2 was also low. Strikingly, we observed a dramatic increase in adhesion kinetics during the first hour of circulation. Because experiments were performed in cell culture medium containing 10% serum at 37°C and 5% CO2, C. albicans cells rapidly underwent morphological conversion (70 to 80% germ tube formation within 60 min). There was a strong correlation between early germ tube formation and increased adhesion kinetics (maximum adhesion kinetics were always reached between 40 and 60 min); moreover, a significant (P < 0.0001) drop in adhesion was observed following more advanced stages of hyphal formation (>1 h), which could not be solely accounted for by decreasing cell numbers (see Discussion). These data may suggest that there exists an optimal stage of hyphal development for endothelial adhesion during circulatory dissemination. To investigate this hypothesis further, the morphology of C. albicans cells circulated within the system for 60 min was compared to that of C. albicans cells which had undergone adhesion to the endothelium under flow conditions between 40 and 60 min. Although contact with endothelium would induce hyphal development, this short 20-min incubation is insufficient for germ tube formation to manifest or for substantial germ tube elongation to occur. First, the gross morphology of circulating versus adherent cells was determined. Of the fungal cells circulating within the system, 77.6% had undergone the yeast-to-hyphal transition. Of the fungal cells which had adhered under flow, 91.2% had undergone the yeast-to-hyphal transition. Next, the length of circulating germ tubes was compared to that of germ tubes which had adhered to the endothelium under flow. Although the absolute mean length of circulating germ tubes was quite similar to that of adherent cells (both around 4.8 μm), the standard deviation of circulating germ tube length was much greater than that for adherent cells (2.17 versus 1.65). Figure 5 shows the distributions of germ tube length in the two populations. The variance of germ tube length of the circulating cell population was significantly higher (P < 0.0001 by F-test). The higher numbers of yeast cells (germ tube length = 0) in circulation versus endothelium-adherent cells may skew statistical analysis. The F-test was therefore repeated with only germ tubes (yeast cells excluded) from the two populations. Even upon exclusion of yeast cells, the P value remained low (0.0024), indicating that the distribution of germ tube length in the circulating population is significantly higher than in endothelium-adherent cells: in other words, germ tubes of 3 to 7 μm in length were significantly enriched in the population of cells which had adhered to the endothelium under flow. Indeed, the tighter Gaussian distribution of adherent cells suggests that there exists a critical length for optimal endothelial adhesion under flow.
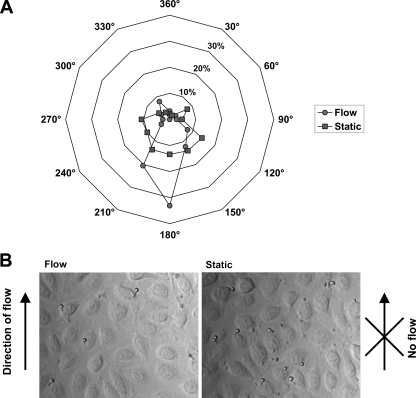
Because of the observed relationship between initial germ tube formation and increased endothelial adhesion kinetics, we decided to further investigate the role of the yeast-to-hyphal transition in this process. We therefore sought to determine which element of the germ tube mediated adhesion of C. albicans to the endothelium during circulation. We reasoned that if mother cells represented the major foci of adhesion, the majority of germ tubes would adhere with the mother cell directed against the direction of flow; conversely, if the germ tube tip was the major point of adhesion, germ tubes should adhere in the opposite direction, with the mother cell aligned with the direction of flow; finally, if adhesion was not mediated by any one particular element of the fungal cell, germ tubes would be oriented in a random fashion. We therefore measured the angles of 80 random germ tubes (which had adhered to the endothelial channel between 40 and 60 min from six frames over two independent experiments) to the direction of flow (Fig. 6). As a control, the angle of cells, which had adhered under static conditions, was determined against an arbitrary vector. Germ tubes which had adhered under static conditions were directed in a random fashion on the monolayer, with a slight tendency toward a certain orientation (120 to 270°); however, this mild orientation tendency was not relative to the direction of flow, as no flow was present in the static control. It is possible that the application of cells via flushing from one reservoir to the other (see Materials and Methods) accounts for this mild orientation tendency. On the other hand, under conditions of flow, the majority of cells were clearly oriented with the germ tube pointing against the direction of flow.
Fig. 6.
Germ tube orientation. (A) The orientation of germ tubes adhering to the endothelium between 40 and 60 min of circulation with the direction of flow as the comparative vector was determined and displayed as a radar plot. The orientations of germ tubes from the same population incubated on a static endothelial monolayer are included as a control. (B) Micrograph images (40× magnification) of cells which had undergone adhesion to an endothelial monolayer under flow (left panel) or under static conditions (right panel). Note that circulating germ tubes adhere with the mother cells aligned with the direction of flow, suggesting germ tube tip anchor events.
Although we cannot exclude the possibility that the observed adhesion orientation is partly due to the direction in which the germ tubes move through the endothelial channel, these data suggest that it is the germ tube tip, and not the mother cell, which acts as the main anchor site during circulatory C. albicans-endothelium adhesion events.
Hgc1 specifically mediates circulatory endothelial adhesion.
The correlation between initial germ tube formation and circulatory adhesion suggested that an early adaptation event, associated with morphological conversion, promoted adhesion. This could be due to increased expression of adhesion factors or to a physical aspect of the early germ tube. Unsurprisingly, we found that deletion of a central regulatory GTPase encoded by RAS1—which prevents hyphal formation and disrupts numerous biological processes, including the expression of pathogenicity factors (9, 17)—significantly reduced adhesion under both circulatory and static conditions (data not shown).
To more effectively dissect the role of hyphal formation itself, rather than the global effects elicited by deletion of a central regulator such as RAS1, we analyzed the effect of deletion of HGC1 on circulatory adhesion. HGC1 codes for a G1 cyclin, which is required for true hyphal formation but which is dispensable for the expression of distinct hypha-associated genes and adhesins. Zheng et al. (32) first showed that pseudohyphal cells of an hgc1Δ mutant displayed wild-type expression of HWP1, HYR1, and ECE1. More recently, Almeida et al. (1) performed genomewide transcriptional profiling of hgc1Δ, demonstrating wild-type expression levels of many hypha-associated genes, including the genes encoding a central transcriptional regulator of cellular morphology, Ume6 (2), and a major endothelial adhesion factor, Als3 (30). Moreover, Almeida et al. (1) also provided cellular evidence not only that ALS3 is transcribed in mutants lacking HGC1 but that the gene product is also localized to and functional at the cell surface of hgc1Δ cells.
We hypothesized that if the physical act of germ tube formation per se was dispensable for C. albicans-endothelium interactions, the hgc1Δ mutant should be comparable to the wild type in our endothelium adhesion model, due to the normal expression of hypha-associated genes and adhesion factors.
As shown in Fig. 7A, under static conditions, hgc1Δ cells (hgc1Δ+CIP10) adhered poorly up until 40 min. However, between 40 and 60 min, hgc1Δ cells underwent substantial adhesion at a rate similar (P = 0.51) to that of the wild-type control (CAI4+CIp10). This demonstrates that hgc1Δ cells retain adhesive potential, although somewhat delayed, under static conditions, despite failing to form germ tubes. Remarkably, during circulation, the same hgc1Δ cells were unable to adhere under flow (Fig. 7A). As these results suggested a specific role for Hgc1 in adhesion under conditions of physiological flow, we tested whether reconstitution of hgc1Δ with a wild-type copy would restore adhesion. In addition, we extended the circulation time to 100 min to determine whether hgc1Δ cells were simply delayed in their ability to undergo endothelial adhesion under flow. As shown in Fig. 7B, adhesion kinetics of hgc1Δ+HGC1 cells to the endothelium under flow increased within the first hour, reaching maximum kinetics between 40 to 60 min and declining thereafter. hgc1Δ+HGC1 cells reached maximum adhesion kinetics similar to those of the wild type, CAI4+CIp10 (Fig. 7A); furthermore, the significant drop in adhesion kinetics following over 1 h of circulation is similar to that observed for wild-type C. albicans cells (Fig. 2). On the other hand, hgc1Δ cells again adhered poorly to the endothelium under flow. hgc1Δ adhesion levels were not enhanced by extended circulation time, suggesting that Hgc1 is a critical factor for endothelial adhesion during circulation and hgc1Δ cells are not simply delayed in adhesive potential.
These data demonstrate a specific role for Hgc1 in mediating endothelial adhesion under conditions of physiological flow and suggest that a physical aspect of emerging germ tubes is specifically required for adhesion in our circulatory model.
Serum factors enhance adhesion of viable but not killed cells to endothelial cells under flow.
To avoid inadvertently stressing endothelial cells, experiments were performed in a standard cell culture medium (DMEM containing 10% heat-inactivated serum). However, we reasoned that serum might influence the adhesion of C. albicans to endothelial cells. For example, serum components may coat the surface of fungal or endothelial cells; this could either enhance or reduce adhesion by providing bridging molecules or by blocking adhesin-ligand interactions. Furthermore, serum may directly influence C. albicans signaling pathways and/or gene expression. We therefore determined the adhesion kinetics of C. albicans to endothelial cells in DMEM (cell culture medium) in the absence of fetal bovine serum (FBS) (Fig. 8). In the first 20 min of circulation, the numbers of C. albicans-endothelium adhesion events were similar in the absence and presence of serum (0.38 ± 0.086 and 0.47 ± 0.214 cells/mm2/min, respectively) and we did not observe any statistically significant differences. However, between 20 to 40 and 40 to 60 min of circulation, adhesion kinetics in the presence of serum rose significantly to 2.9 and 6.8 cells/mm2/min, respectively, while in the absence of serum, adhesion events remained relatively low at 1.21 (20 to 40 min) and 1.75 (40 to 60 min) cells/mm2/min. It should be noted that in the absence of serum, adhesion kinetics still did significantly increase between 0 to 20 and 40 to 60 min of circulation, indicating the presence of serum-independent adhesion mechanisms. This demonstrates that the presence of serum significantly enhances the adhesion potential of C. albicans to endothelial cells.
Interestingly, the adhesion kinetics profile of circulating C. albicans cells in the presence of serum was almost identical to that of static phase adhesion in the absence of serum. This demonstrates that serum permits adhesion under conditions of physiological flow (such as in blood vessels) at levels similar to those in static environments (or environments with considerably lower rates of movement) in the absence of serum (such as in other host niches).
To test whether the observed serum-enhanced adhesion was directly mediated by serum coating or by serum-induced activity of fungal cells, C. albicans yeast cells were treated with thimerosal, which kills the cells but, importantly, does not cause gross alterations to molecular structures on the fungal cell wall (in contrast to killing with heat or UV light). Thimerosal-treated cells were incubated for 30 min in 50% serum and either perfused through the circulatory endothelial model or incubated on a static endothelial monolayer for a further 30 min in DMEM without FBS; killed cells in PBS were used as a control. In addition, we used either heat-treated serum or native serum. Interestingly, killed yeast cells, irrespective of serum treatment, were unable to adhere to endothelial cells either in circulation or under static conditions (data not shown). This suggests that serum factors are not directly responsible for endothelial adhesion. In contrast, an activity of living fungal cells is required for endothelial adhesion in our model.
DISCUSSION
Circulatory adhesion and morphogenesis.
Adhesion to and subsequent invasion of the endothelial lining of blood vessels by C. albicans are prerequisites for the establishment of deep-seated infections following candidemia. Given the importance of this event in the development of disseminated candidiasis, a large number of studies have examined the mechanisms of C. albicans-endothelium interactions (reviewed in reference 13). However, the circulatory system within the human body, where C. albicans encounters luminal endothelia, is a dynamic environment and static experimental systems fail to consider a major physical environmental factor: the pressure of the bloodstream and consequent circulation of fungal cells. To simulate these physical parameters, we developed an in vitro system for examining fungus-endothelium interactions under conditions of physical pressure similar to those found in the human bloodstream. To our knowledge, only two previous reports on C. albicans-endothelium interactions have used pump systems to mimic blood pressure and flow (11, 14). Grubb et al. (14) employed a linear flow system whereby C. albicans cells are held in an input reservoir suspended in medium and perfused over an endothelial monolayer and nonadherent cells flow out as waste. In the present study, however, C. albicans yeast cells are inoculated once at time point zero and the same starting population of cells circulate through the system, experiencing transient contacts with the endothelium, and are exposed to endothelium-secreted factors for the duration of the experiment. The fact that we observed only infrequent adhesion of yeast cells to the endothelium was probably due to the relatively high pressure used in the present study (3.28 dynes/cm2). We chose this pressure to reflect that of the capillaries of the human circulatory system, which has been reported to be in the range of 0.5 to 23 dynes/cm2 (27). Grubb et al. (14) reported little to no adhesion of yeast cells at pressures above 3 dynes/cm2. Another possibility is the endothelial cell type used: in our study we performed adhesion assays with human umbilical vein endothelial cells whereas Grubb et al. used an HMEC-1 human microvascular cell line.
One feature of our system is that the absolute number of fungal cells in circulation decreases over time (Fig. 3). This may be a disadvantage; however, it reflects the in vivo situation, following intravenous murine infections, where fungi are removed from circulation within a short time (19). In fact, C. albicans cells are cleared even faster from the murine bloodstream than in our circulatory model, probably due to the larger surface area of mouse endothelium compared to the (51.3-mm2) endothelial channel used in this study. Despite this relative decrease in circulating cells, due to adhesion to endothelial cells and presumably other components of the system, the kinetics of C. albicans-endothelium adhesion increased dramatically and significantly during the first hour of circulation (Fig. 2) and correlated with the emergence of germ tubes. However, following the first hour of circulation, adhesion kinetics decreased significantly and then fluctuated. This decrease in adhesion can be explained in part by the falling numbers of circulating fungal cells, but this is, very likely, not the only explanation for declining adhesion. The number of circulating cells between the 40-to-60-min and 60-to-80-min time points dropped by only 15%, while the kinetics of adhesion fell by over 30%. Interestingly, Grubb et al. (14) have recently reported the surprising finding that under conditions of physiological flow, similar to that used in our model, C. albicans hyphae were less adherent than other morphologies. Concurrently, the decline in adhesion that we observed was also associated with the onset of more advanced germ tube formation, following over 1 h of incubation—the same incubation time used by Grubb et al. to generate hyphae (14). This suggested that there may exist a critical stage in germ tube formation (∼40 to 60 min post-hyphal induction), which dramatically increases the adhesion of C. albicans to endothelial cells. This hypothesis was supported by morphological analysis of C. albicans cells in circulation versus those which had undergone endothelial adhesion under flow. As expected, the population of adherent cells contained less yeast than the population of circulating cells, demonstrating that germ tubes are more adherent than yeast cells under this condition of physiological flow (Fig. 5). Interestingly, while the length of germ tubes in circulation formed a broad, near-Gaussian distribution with high deviation, representing a quite heterogeneous mixture of germ tube lengths, germ tubes of 3 to 7 μm in length were significantly enriched in the population of cells which had undergone endothelial adhesion under flow. This finding may also explain why we observed fluctuations in adhesion kinetics following 1 h of circulation: the subsequent spike in adhesion kinetics between 80 and 100 min (Fig. 2) may represent the “second wave” of optimally adherent cells, that is, germ tubes which germinated at a lower rate and therefore required more time to reach the correct size. Taken together, these data provide evidence that there exists a critical germ tube length for optimal endothelial adhesion under flow conditions used in this study. This phenomenon may be mediated by physical aspects of germ tubes and hyphae. Because the hyphal tip appears to be the major anchor for adhesion under conditions of flow (reference 14 and this study), it is likely that longer hyphae experience greater levels of shear stress and, therefore, are more readily swept away by the circulation following initial tip-endothelium contact. Alternatively, the smaller volume and surface area of shorter germ tubes (compared to those of longer hyphal morphologies) may result in a higher concentration of adhesion factors on the cell surface.
Although morphogenic conversion has long been proposed as a virulence factor, its exact role in the pathogenicity of C. albicans has remained somewhat contentious (7, 12, 29). The controversy stemmed mainly from the lack of direct molecular evidence demonstrating a requirement of hyphal formation for pathogenicity; mutants with defects in hyphal formation (e.g., ras1Δ, efg1Δ/cph1Δ) also displayed defects in the expression of other virulence traits (8, 16, 17, 18, 22). The role of morphogenesis was further confounded by the discovery that C. albicans locked in a filamentous form, via deletion of TUP1 (5), was also avirulent (3).
However, the identification of Hgc1 (32) strengthened the opinion that the yeast-to-hyphal transition may be a bona fide virulence factor. HGC1 encodes a hypha-specific G1 cyclin-related protein and is required for hyphal morphogenesis. However, deletion of this gene does not overtly alter the expression of a number of hypha-associated genes, including HWP1, HYR1, ECE1, ALS3, and UME6 (1, 32). We took advantage of the specific morphological defect of hgc1Δ in this study, reasoning that if the increased adhesion kinetics that we observed were solely due to increased expression of adhesion factors, hgc1Δ would behave similarly to wild-type cells, whereas, if a physical aspect of emerging hyphae was required for optimal adhesion, hgc1Δ would be defective. In line with this, hgc1Δ was capable of adhesion to endothelial cells under static conditions between 40 and 60 min, suggesting that hgc1Δ cells retain adhesive potential, presumably due to the expression of relevant adhesion factors. Strikingly, deletion of HGC1 prohibited endothelial adhesion during circulation. This provides molecular evidence that a physical aspect of the emerging germ tube (possibly involving the spatial localization of adhesion factors) specifically mediates endothelial adhesion under conditions of flow such as those found in the capillary networks.
Taken together, these data suggest that the yeast-to-hyphal transition, like other biological processes, is highly dynamic, and it would appear that distinct stages of this morphological transition are associated with particular phenotypic traits. For example, in this study we show that early-stage germ tubes have greater endothelial adhesion potential than both yeast and more advanced hyphal morphologies.
Host components.
Experiments were performed in cell culture medium (DMEM) supplemented with fetal bovine serum (our standard medium for maintaining mammalian cells) to avoid inadvertently stressing the endothelial cells. However, in the host, the “medium” in which C. albicans is circulated is blood. Although further detailed studies are required to elucidate the complex interactions between C. albicans, endothelium, and the components of blood, we began by dissecting the role of blood serum on adhesion. The adhesion rates in the absence and presence of serum either during circulation or under static conditions were determined. Interestingly, the presence of serum permitted circulatory adhesion at rates almost identical to that of static cells in the absence of serum. We propose that C. albicans requires a certain level of adhesiveness to attach to host surfaces in the various niches it occupies in the human body. For example, in niches such as the oral cavity or intestine, where shear stress is relatively low, C. albicans can successfully colonize by attaching to host cells—either directly or via other members of the microbial flora. On the other hand, it would appear that C. albicans may take advantage of the presence of serum components in the circulation to reach similar rates of adhesion at much higher levels of shear stress. In vivo, endothelial adhesion by fungal cells may also be mediated by binding extracellular matrix proteins. Interestingly, killed yeast cells were unable to undergo endothelial adhesion in our model, independent of serum treatment. This result suggests that serum-enhanced adhesion of living cells is probably not mediated solely by coating of the cells but rather relies on fungal activity, possibly by triggering distinct signaling cascades and/or activating the expression of adhesion-associated genes (28).
In vivo relevance.
In the present study, yeast cells were used to inoculate our circulatory endothelial model. At least four conceivable routes exist for C. albicans yeast cells to reach the circulatory system: (i) hyphae penetrate host tissue, and apical bud formation occurs upon access to the bloodstream; (ii) epidermal disruption via severe burns or the insertion of medical devices such as catheters permits migration from the skin to the blood; (iii) abdominal surgery or polytrauma provides a route from the gastrointestinal tract to the bloodstream (23); and (iv) dispersal events from biofilms growing on indwelling catheters directly seed the bloodstream (4). Following exposure to human blood in vitro, an interesting phenomenon occurs: around half the population of C. albicans rapidly form germ tubes, while the others remain in the yeast morphology (10). Such morphological divergence may provide C. albicans with an effective dissemination strategy for multiple organ colonization: for example, seeding of yeast cells from a central venous catheter-associated biofilm and subsequent heterogeneous morphological conversion could result in a split population of early germ tubes capable of endothelial adhesion in capillary networks, as described in this study, and yeast cells capable of continued dissemination before adhering to postcapillary venules, as described previously (14). Such a split population dynamic not only would impact dissemination/adhesion kinetics but may also play a role in immune evasion by influencing recognition and phagocytosis by immune cells, although further studies are required to elucidate the delicate interplay of C. albicans-immune system interactions in the context of the physical environment of the human host.
In summary, this work demonstrates that circulating C. albicans cells undergo a morphology-associated adaptation event, which allows optimal adhesion to endothelial cells at capillary pressure. Furthermore, we provide evidence that a specific stage of the yeast-to-hyphal transition—mediated by the G1 cyclin, Hgc1—is critical for this interaction.
ACKNOWLEDGMENTS
D.W. is the grateful recipient of an EU Intra-European Fellowship (grant number PIEF-GA-2008-219406).
We thank Yue Wang for providing hgc1Δ and hgc1Δ+HGC1 strains, Gerald Fink for providing a ras1Δ strain, Hans-Martin Dahse and Peter Zipfel for providing endothelial cells, Ricardo Almeida and Sascha Brunke for interesting discussions on data interpretation, and Ilse Jacobsen for critical reading of the manuscript.
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Almeida R. S., Brunke S., Albrecht A., Thewes S., Laue M., Edwards J. E., Filler S. G., Hube B. 2008. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 4:e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee M., Thompson D. S., Lazzell A., Carlisle P. L., Pierce C., Monteagudo C., Lopez-Ribot J. L., Kadosh D. 2008. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol. Biol. Cell 19:1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendel C. M., Hess D. J., Garni R. M., Henry-Stanley M., Wells C. L. 2003. Comparative virulence of Candida albicans yeast and filamentous forms in orally and intravenously inoculated mice. Crit. Care Med. 31:501–507 [DOI] [PubMed] [Google Scholar]
- 4.Blankenship J. R., Mitchell A. P. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594 [DOI] [PubMed] [Google Scholar]
- 5.Braun B. R., Johnson A. D. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105–109 [DOI] [PubMed] [Google Scholar]
- 6.Chaffin W. L. 2008. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 72:495–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert E. E., Sheth C. C., Mühlschlegel F. A. 2007. Regulation of morphogenesis in Candida species, p. 263–291Ind'Enfert C., Hube B. (ed.), Candida comparative and functional genetics Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 8.Felk A., Kretschmar M., Albrecht A., Schaller M., Beinhauer S., Nichterlein T., Sanglard D., Korting H. C., Schafer W., Hube B. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Q., Summers E., Guo B., Fink G. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fradin C., Kretschmar M., Nichterlein T., Gaillardin C., d'Enfert C., Hube B. 2003. Stage-specific gene expression of Candida albicans in human blood. Mol. Microbiol. 47:1523–1543 [DOI] [PubMed] [Google Scholar]
- 11.Glee P. M., Cutler J. E., Benson E. E., Bargatze R. F., Hazen K. C. 2001. Inhibition of hydrophobic protein-mediated Candida albicans attachment to endothelial cells during physiologic shear flow. Infect. Immun. 69:2815–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gow N. A., Brown A. J., Odds F. C. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366–371 [DOI] [PubMed] [Google Scholar]
- 13.Grubb S. E., Murdoch C., Sudbery P. E., Saville S. P., Lopez-Ribot J. L., Thornhill M. H. 2008. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect. Immun. 76:4370–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubb S. E., Murdoch C., Sudbery P. E., Saville S. P., Lopez-Ribot J. L., Thornhill M. H. 2009. Adhesion of Candida albicans to endothelial cells under physiological conditions of flow. Infect. Immun. 77:3872–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. 1991. Molecular mimicry in Candida albicans.Role of an integrin analogue in adhesion of the yeast to human endothelium. J. Clin. Invest. 87:1896–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumamoto C. A., Vinces M. D. 2005. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 7:1546–1554 [DOI] [PubMed] [Google Scholar]
- 17.Leberer E., Harcus D., Dignard D., Johnson L., Ushinsky S., Thomas D. Y., Schroppel K. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42:673–687 [DOI] [PubMed] [Google Scholar]
- 18.Lo H. J., Kohler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G. R. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949 [DOI] [PubMed] [Google Scholar]
- 19.MacCallum D. M., Odds F. C. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48:151–161 [DOI] [PubMed] [Google Scholar]
- 20.Murad A. M., Lee P. R., Broadbent I. D., Barelle C. J., Brown A. J. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325–327 [DOI] [PubMed] [Google Scholar]
- 21.Nather K., Munro C. A. 2008. Generating cell surface diversity in Candida albicans and other fungal pathogens. FEMS Microbiol. Lett. 285:137–145 [DOI] [PubMed] [Google Scholar]
- 22.Park H., Myers C. L., Sheppard D. C., Phan Q. T., Sanchez A. A., Edwards J. E., Jr., Filler S. G. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell. Microbiol. 7:499–510 [DOI] [PubMed] [Google Scholar]
- 23.Perlroth J., Choi B., Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45:321–346 [DOI] [PubMed] [Google Scholar]
- 24.Ruhnke M. 2002. Skin and mucous membrane infections, p. 307–322 InCalderone R. A. (ed.), Candida and candidiasis ASM Press, Washington, DC [Google Scholar]
- 25.Sundstrom P. 1999. Adhesins in Candida albicans. Curr. Opin. Microbiol. 2:353–357 [DOI] [PubMed] [Google Scholar]
- 26.Timpel C., Zink S., Strahl-Bolsinger S., Schroppel K., Ernst J. 2000. Morphogenesis, adhesive properties, and antifungal resistance depend on the Pmt6 protein mannosyltransferase in the fungal pathogen Candida albicans. J. Bacteriol. 182:3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walshe T. E., Ferguson G., Connell P., O'Brien C., Cahill P. A. 2005. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro coculture model of the retinal vasculature. Invest. Ophthalmol. Vis. Sci. 46:375–382 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Xu X. L. 2008. Bacterial peptidoglycan-derived molecules activate Candida albicans hyphal growth. Commun. Integr. Biol. 1:137–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteway M., Bachewich C. 2007. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 61:529–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X., Oh S. H., Cheng G., Green C. B., Nuessen J. A., Yeater K., Leng R. P., Brown A. J., Hoyer L. L. 2004. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology (Reading, Engl.) 150:2415–2428 [DOI] [PubMed] [Google Scholar]
- 31.Zhao X., Oh S. H., Yeater K. M., Hoyer L. L. 2005. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology (Reading, Engl.). 151:1619–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng X., Wang Y., Wang Y. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 23:1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]