Abstract
Lumbar spinal fusions have been performed for spinal stability, pain relief and improved function in spinal stenosis, scoliosis, spinal fractures, infectious conditions and other lumbar spinal problems. The success of lumbar spinal fusion depends on multifactors, such as types of bone graft materials, levels and numbers of fusion, spinal instrumentation, electrical stimulation, smoking and some drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs). From January 2000 to December 2001, 88 consecutive patients, who were diagnosed with spinal stenosis or spondylolisthesis, were retrospectively enrolled in this study. One surgeon performed all 88 posterolateral spinal fusions with instrumentation and autoiliac bone graft. The patients were divided into two groups. The first group (n=30) was infused with ketorolac and fentanyl intravenously via patient controlled analgesia (PCA) postoperatively and the second group (n=58) was infused only with fentanyl. The spinal fusion rates and clinical outcomes of the two groups were compared. The incidence of incomplete union or nonunion was much higher in the ketorolac group, and the relative risk was approximately 6 times higher than control group (odds ratio: 5.64). The clinical outcomes, which were checked at least 1 year after surgery, showed strong correlations with the spinal fusion status. The control group (93.1%) showed significantly better clinical results than the ketorolac group (77.6%). Smoking had no effect on the spinal fusion outcome in this study. Even though the use of ketorolac after spinal fusion can reduce the need for morphine, thereby decreasing morphine related complications, ketorolac used via PCA at the immediate postoperative state inhibits spinal fusion resulting in a poorer clinical outcome. Therefore, NSAIDs such as ketorolac, should be avoided after posterolateral spinal fusion.
Keywords: Lumbar spine, spinal fusion, ketorolac
INTRODUCTION
Lumbar spinal fusions are widely performed for improving spinal stability and for pain relief. In addition, these procedures show improved functional outcomes in spinal stenosis, scoliosis, spinal fractures, infectious conditions and other lumbar spinal problems.1-4 The success of lumbar spinal fusion depends on many factors, such as the type of bone graft materials, the level and number of fusions, spinal instrumentation, electrical stimulation, smoking, bone morphogenic proteins and some drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs).3-8
Ketorolac (Toradol, Roche Laboratories, Nurley, NJ) is an NSAID, and has been largely used for postoperative pain control via patient controlled analgesia (PCA) after spinal fusion surgery in order to reduce the need for morphine. Ketorolac's role in PCA is increasing.9,10 Even though NSAIDs have no influence on normal bone homeostasis, they have been reported to be associated with a delayed fracture healing and the inhibition of heterotopic ossification after a total hip arthroplasty.11 In one animal study, indomethacin and ibuprofen were reported to inhibit spine bone union after spinal fusion surgery.12-14
However, there have been no reports on the influence of an intravenous ketorolac injection via PCA on spinal fusion. This study retrospectively compares the clinical outcomes and fusion rates of ketorolac intravenous injected patients via PCA after spinal fusion surgery with those not injected with ketorolac.
MATERIALS AND METHODS
The database of the Yonsei University Medical College Severance Hospital Orthopaedic Department was accessed to collect data on 88 consecutive patients who had an instrumented posterolateral spinal fusion of the low lumbar spine performed from January, 2000 to December, 2001 and who had been observed for a minimum of one year after surgery. All patients underwent posterolateral lumbar spine fusion procedures using pedicle screws and a rod system and an autologous iliac bone graft. All patients chose PCA for their postoperative pain control after the spinal fusion procedure. The PCAs of all patients were managed by an anesthesiologist. The patients pre-operative diagnoses were restricted to spinal stenosis and spondylolisthesis, because of the possible overuse of NSAIDs for postoperative pain control. Patients were excluded from this study if they had undergone a previous fusion procedure or if their treatment included additional procedures, such as lumbar interbody fusion or electrical stimulation. Cases in which there were complicated problems such as difficult operation techniques and problems associated with the fusion procedure itself also, were excluded. Patients were also excluded from the study based on the following criteria:15
Documented allergy to aspirin or other NSAIDs, morphine, meperidine
History of peptic ulcer disease, congestive heart failure, liver disease, bleeding disorder
Concurrent medications including warfarin, lithium, methotrexate
Hypoalbuminemia
The records of all 88 patients were examined for their demographic data, including age, gender, smoking history, diagnosis, level and the number of fusions, the type of spinal instrument used, the operation times, hospital days, and first postoperative walking day. The patients were divided into two groups. Group I was the ketorolac infused group, Group II was the control group, not given ketorolac. Patients in Group I were infused with ketorolac via PCA after the spinal fusion procedure. In the PCA, ketorolac 120 mg and fentanyl 900 µg were mixed and continuously infused intravenously for postoperative pain control. The PCA was removed approximately 3 days later. The patients in Group II were infused via PCA with only fentanyl 1200 µg. The PCA was also removed after approximately 3 days. Follow-up examinations were carried out at 3 months, 6 months, 1 year, and 2 years after surgery and at least a 1 year follow-up was done for all patients. At each visit, clinical symptoms were evaluated, a physical examination was performed and radiological assessments, such as the plain anteroposterior view and the lateral view and a dynamogram (flexion-extension view), were obtained.
The comparison between the two groups was evaluated according to two categories.
Radiologic assessments
At least 1 year after surgery, the plain anteroposterior views, the lateral views and the dynamograms were evaluated by two orthopaedic surgeons. Radiological fusion patterns were classified into one of the following four grades:16
Definitely solid: Solid, big trabeculated bilaterally
Possibly solid: Solid, big fusion mass unilaterally with a small fusion mass on contralateral aspect
Probably not solid: Small, thin fusion masses bilaterally with apparent crack
Definitely not solid (Pseudarthrosis): Graft resorption bilaterally or fusion mass with an obvious bilateral pseudarthrosis or breakage of hardware
Probably not solid and definitely not solid are considered nonunion.
Clinical assessments
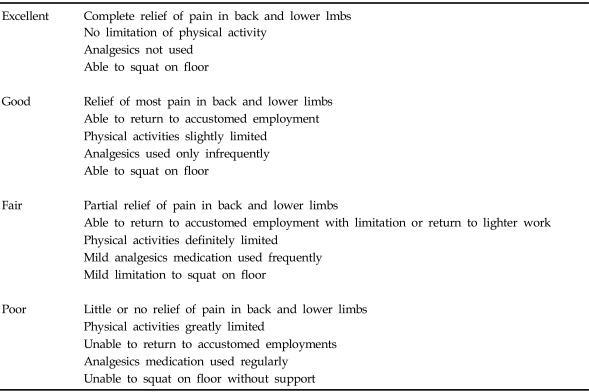
At least 1 year after surgery, the clinical outcomes were evaluated using the criteria reported by Kim and Kim (Table 1).17
Table 1.
Criteria for Clinical Outcomes
Statistical analysis
The differences between the two groups were analyzed using a Fisher's exact test. The differences were considered to be significant at p < 0.05.
RESULTS
Among all 88 patients, 30 patients (Group I) received ketorolac via PCA for approximately 3 days post surgery, and 58 patients (Group II) received no ketorolac.
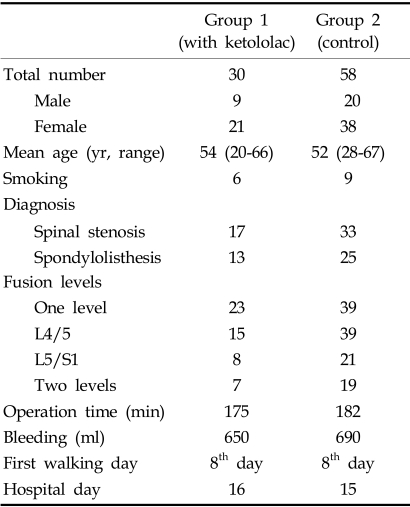
Demographic characteristics (Table 2)
Table 2.
Demographic Characteristics
There was no statically difference between the two groups.
Age and gender
The mean age of Group I and II was 54 (range, 20-66), and 52 (range, 28-67) respectively. There was no significant difference in the age distribution. Among the 88 patients, there were 29 males and 59 females. Group I consisted of 9 males and 21 females and Group II consisted of 20 males and 38 females.
Diagnosis
There were 50 spinal stenosis cases and 38 spondylolisthesis cases. Group I contained 17 spinal stenosis cases and 13 spondylolisthesis cases. Group II contained 33 spinal stenosis cases and 25 spondylolisthesis cases. There was no statistically significant difference in the diagnosis between the two groups.
Levels and numbers of fusion
A one-level fusion was performed in 62 cases; L5-S1 in 26 cases and the L4-5 was done in 36 cases. A two-level fusion was performed in 26 cases and all were fused from L4 - S1. In Group I, there were 23 one-level fusion cases and 7 two-level fusion cases. In Group II, there were 39 one-level fusion cases and 19 twolevel fusion cases. In Group I, there were 15 L4-L5 fusions, 8 L5-S1 fusions and 7 L4-S1 fusions. In Group II, there were 21 L4-L5 fusions, 18 L5-S1 fusions, and 19 L4-S1 fusions. There was no significant difference between the two groups.
Procedures of operation and rehabilitation
All operations were performed by the one author (HML). After decompression, a spinal segmental system was used and a posterolateral spinal fusion with autoiliac bone was performed on all patients. The types of spinal systems used were Moss-Miami systems in 56 patients and Synergy systems in 32 patients. The operation time was almost the same for each group; 175 minutes for Group I, 182 minutes for Group II. Blood loss through the procedures was about 650 ml in Group I and about 690 ml in Group II.
The same postoperative rehabilitation protocol was used in both groups. The patients were sitting up in bed for the first 3 days and out of bed with a back brace at 7 days after the operation. All patients were discharged from the hospital on approximately the 14th day post-operatively. All patients wore a back brace for 3 months.
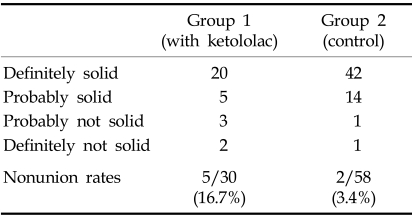
Radiologic results (Table 3)
Table 3.
Radiologic Results
Nonunion, which met the criteria of probably or definitely not solid, was identified in 5 out of the 30 patients (16.7%) who received ketorolac via PCA, postoperatively. In contrast, nonunion was identified in 2 out of the 58 patients (3.4%) who did not receive ketorolac. There was a statistically significant difference in the nonunion rate (p < 0.05) between each group; there was an approximately 6 times greater likelihood of developing a nonunion with a ketorolac PCA than without (odds ratio: 5.64). There was no significant difference in the nonunion rate between diagnoses (spinal stenosis and spodylolisthesis).
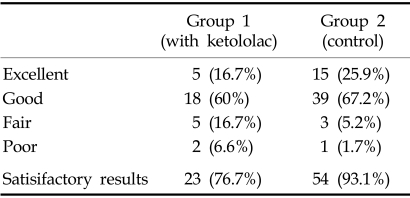
Clinical outcomes (Table 4)
Table 4.
Clinical Outcomes
In Group I, the clinical results were excellent in 5 patients (16.7%), good in 18 patients (60%), fair in 5 patients (16.7%), and poor in 2 patients (6.6%). In Group II, the clinical results were excellent in 15 patients (25.9%), good in 39 patients (67.2%), fair in 3 patients (5.2%), and poor in 1 patient (1.7%).
Therefore, satisfactory results were obtained in 23 patients (77%) in Group I and 54 patients (93.1%) in Group II. There was statistically a significant difference between the two groups (p < 0.05). There was high correlation between fusion and better clinical results.
DISCUSSION
Worldwide, NSAIDs are the most frequently prescribed medication for the treatment of many musculoskeletal diseases.18 Many NSAIDs are licensed for prescription use throughout the world. The mechanism through which NSAIDs exert their anti-inflammatory effect is the inhibition of the enzyme cyclo-oxygenase which is responsible for converting arachidonic acid to prostaglandin. NSAIDs also may exert their analgesic effects by inhibiting prostaglandin synthesis, because it is believed that the pain receptors are prostaglandin sensitive. Recently, it was reported that NSAIDs may use an alternative pathway to achieve their anti-inflammatory and analgesic effect, such as the inhibition of leukotriene production, a blockade of oxygen-free radical formation, and interference with a protein-protein interaction.18,19 In addition, NSAIDs have been shown to have a significant effect on bone metabolism.12-14 Even though NSAIDs cannot alter the normal bone homeostasis, they have been documented to inhibit fracture healing and decrease heterotopic ossification after a total hip arthroplasty. The mechanism of this action is believed to be due to various etiologies, including the inhibition of bone forming cells of the endosteal bone surfaces, a reduction in the immune and inflammatory response or the inhibition of prostaglandin synthesis.12,13 In an animal study, the earlier the indomethacin was received postoperatively, the greater its negative effect on spinal fusion,20 showing that this inhibitory effect is potentially time-dependent. Indomethacin appears to play a significant inhibitory role in the early phase of healing in the spinal fusion or fracture healing process because it inhibits the inflammatory reactions in the early inflammatory phase. However, initiating indomethacin treatment in the later phase of healing does not appear to significantly affect the fusion rates.20
Recently, there have been many studies on the specific inhibitors of cyclo-oxygenase, such as specific cyclooxygenase-2 inhibitors. Conventional NSAIDs exhibited inhibitory effects on both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). However, the newly developed NSAIDs, which are specific cyclooxygenase-2 (COX-2) inhibitors, have recently been introduced to treat musculoskeletal disorders. COX-1 has been reported to be a housekeeping enzyme that is constitutively expressed in virtually all tissues under basal conditions. COX-1 regulates the prostaglandins that have a homeostatic function, particularly in regards to gastrointestinal and renal functions as well as platelet aggregation. COX-2 expression is more highly regulated compared with COX-1. COX-2 expression is rapidly inducible, particularly in instances of tissue injury and inflammation.21-25 Specific COX-2 inhibitors primarily inhibit the production of prostaglandin E2, which is a prostaglandin believed to play an important role in the inflammatory cascade. In addition, specific COX-2 inhibitors are reported to have a less inhibitory effect on bone healing or bone union after a spinal fusion compared with conventional nonspecific NSAIDs both in vivo and vitro.25,26
Ketorolac is a NSAID that has been widely used for postoperative pain control. It plays an important role in decreasing the demand for morphine which has many potential complications, such as respiratory depression, addiction, nausea, vomiting, and urinary retention.9,10,19 Glassman et al. reported that the fusion rate of a ketorolac intramuscularly injected group was lower than a non-ketorolac injected group after a posterolateral spinal fusion procedure.14 In this study, the ketorolac group showed decreased fusion rates compared with the control group. The ketorolac group had a high relative risk of non fusion, approximately 6 times higher than the control group. Therefore, conventional NSAIDs such as ketorolac should be avoided after spinal fusion in order to avoid fusion failures. If it is necessary to use NSAIDs for postoperative pain control, specific COX-2 inhibitors are preferred after spine fusion.24-27
Cigarette smoking has also been correlated with poor outcomes in the surgical treatment of a lumbar spinal disorder and has been shown to inhibit the lumbar spinal fusion rate approximately 2 - 3 times than normal. The mechanism of inhibition of spinal fusion has been identified as the diminished revascularization of a cancellous bone graft.28 In this study, there were 15 patients who smoked before the spinal fusion, but there were no spinal nonunion cases in either the ketorolac injected group or non-ketorolac injected group, and no correlation could be found between the smoking and spinal fusion rate. We advised patients who smoked, to quit before their spinal fusion and remain smoke-free post-operatively, as smoking cessation before and after spinal fusion has been reported to have an improved outcome compared with the continuation of smoking in other studies.6,28
The clinical outcomes after posterolateral spinal fusion may vary. It has been estimated that 50% of patients with pseudarthrosis have no symptoms. There are some reports showing that there are no differences in the symptoms between complete fusion cases and pseudarthrosis cases. However, complete fusion cases have usually been reported to show better outcomes than those with pseudarthrosis.29,30 Similarly, in our study Group II which did not receive ketorolac showed better clinical outcomes than Group I and there was a strong correlation between the spinal fusion rates and the clinical outcome.
In conclusion, NSAIDs such as ketorolac can be used for postoperative pain control via PCA to reduce the need for morphine and avoid its complications. However, ketorolac used via PCA immediate after surgery, inhibits spinal fusion and shows worse clinical results. Therefore, NSAIDs such as ketorolac should be avoided after posterolateral spinal fusion.
Footnotes
This work was partly supported by the Brain Korea 21 Project for Medical Science, Yonsei University.
References
- 1.Moon MS, Ok IY, Lee KS, Yoon HY. A Clinical study of 52 cases of posterolaterally fused lumbar spine. J Korean Orthop Assoc. 1986;21:585–594. [Google Scholar]
- 2.Kwon H, Kim BK, Song JM, Shin BJ, Na SK. Comparative study of fusion rate in the thoracolumbar and lumbar posterolateral fusion using autograft or xenograft lubboc. J Korean Spine Surg. 1997;4:43–51. [Google Scholar]
- 3.Scott DB, Jeffery HS, William CH. An experimental lumbar intertransverse process spinal fusion modelradiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20:412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kim NH. Anterior interbody fusion in the treatment of the lumbar herniated nucleus pulposus. Yonsei Med J. 1999;40:256–264. doi: 10.3349/ymj.1999.40.3.256. [DOI] [PubMed] [Google Scholar]
- 5.Bassett CA, Creighton DK. A comparison of host response to cortical autograft and processed calf heterograft. J Bone Joint Surg. 1962;44A:842–846. [Google Scholar]
- 6.Toth JM, Seim HB, Schwardt JD, Humphrey WD, Wallskog JA, Turner AS. Direct current electrical stimulation increases the fusion rate of spinal fusion cages. Spine. 2000;25:2580–2587. doi: 10.1097/00007632-200010150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Martin GJ, Boden S, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketoloac, a nonsteroidal anti inflammatory drugs (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188–2196. doi: 10.1097/00007632-199911010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Brown CW, Orme TJ, Richardson HD. The rate of pseudoarthrosis in patients who are smokers and patients who are nonsmokers: A comparison study. Spine. 1986;11:942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Scott SR, Neil RC, Shari L, Margaret K, Charles SG. Dose-response of ketoloac as an adjunct to patient-controlled analgesia morphine in patients after spinal fusion surgery. Anesth Analg. 1998;87:98–102. doi: 10.1097/00000539-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez PJ, Tortosa JA, Martinez LJ, Perz FD. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesth Analg. 2001;92:1473–1476. doi: 10.1097/00000539-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Kjaersgaard-Anderson P, Schmidt SA. Total hip arthroplasty. The role of anti-inflammatory medications in the prevention of heterotopic ossification. Clin Orthop. 1991;263:78–86. [PubMed] [Google Scholar]
- 12.Lebwohl NH, Starr JK, Milne EL, Latta LL, Malini TI. Inhibitory effect of ibuprofen on spinal fusion in rabbits. Rosemont, IL: AAOS; 1994. p. 278. [Google Scholar]
- 13.Dimar JR, Ante WA, Zang YP, Glassman SD. The effects of nonsteroidal anti inflammatory drugs on posterior spinal fusions in rat. Spine. 1996;21:1870–1876. doi: 10.1097/00007632-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Glassman SD, Rose SM, Dimar JR, Puno RM, Campcell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drugs administration on spinal fusion. Spine. 1998;23:834–838. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Turner DM, Warson JS, Wirt TC, Scalley RD, Cochran RS, Miller KJ. The use of Ketololac in lumbar spine surgery: A cost benefit analysis. J Spinal Disord. 1995;8:206–212. doi: 10.1097/00002517-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lenke LG, Bridwell KH, BUllis DB, Betz RR, Baldus C, Schoenecker PL. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5:433–442. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kim NH, Lee JW. Anterior interbody fusion versus posterolateral fusion with transpedicular fixation for isthmic spondylolithesis. Spine. 1999;24:812–817. doi: 10.1097/00007632-199904150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Saag KG, Cowdery JS. Nonsteroidal anti-inflammatory drugs. Balancing benefits and risks. Spine. 1994;19:1530–1534. doi: 10.1097/00007632-199407000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Coloma M, White PF, Huber PJ, Kendall TW, Dullye KK, Duffy LL. The effect of ketorolac on recovery after anorectal surgery: Intravenous versus local administration. Anesth Analg. 2000;90:1107–1110. doi: 10.1097/00000539-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Daniel R, Jhon L, John R, Stephen L, Timothy K, Kim YJ, et al. Time-dependent inhibitory effects of indomethacin on spine fusion. J Bone Joint Sug. 2003;85:632–636. doi: 10.2106/00004623-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Goodman S, Ma T, Trindade M, Ikenoue T. Cox-2 selective NSAID decrease bone growth in vivo. J Orthop Res. 2002;20:1164–1172. doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 22.Long J, Lewis S, Kuklo T, Zhu Y, Riew KD. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg. 2002;84:1763–1768. doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Reuben SS. Considerations in the use of Cox-2 inhibitors in spinal fusion surgery: In response. Anesth Analg. 2001;93:803–804. doi: 10.1097/00000539-200109000-00056. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 25.Hiroshi M, Ryuichi S, Minoru D, Masahiro K, Kosaku M. The role of cyclooxygenase-2 in lumbar disc herniation. Spine. 2002;27:2477–2483. doi: 10.1097/00007632-200211150-00011. [DOI] [PubMed] [Google Scholar]
- 26.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture healing in the rat femur. J Bone Joint Surg. 2004;86A:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto H, Saura R, Doita M, Kurosaka M, Mizuno K. The role of cyclo oxygenase-2 in lumbar disc herniation. Spine. 2002;27:2477–2483. doi: 10.1097/00007632-200211150-00011. [DOI] [PubMed] [Google Scholar]
- 28.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation and spinal fusion. Spine. 2000;25:2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Depalma AF, Prabhaker M. Posterior-posterobilateral fusion of the lumbosacral spine. Clin Orthop. 1996;47:165–173. [PubMed] [Google Scholar]
- 30.Carpenter MC, Dietz MJW, Leng KYK, Hanscom DA, Wagner TA. Repair of a pseudoarthrosis of lumbar spine. A functional outcome study. J Bone Joint Surg. 1996;78A:712–720. doi: 10.2106/00004623-199605000-00011. [DOI] [PubMed] [Google Scholar]