Abstract
In recent years the discovery of a number of major transporter proteins expressed in the liver and intestine specifically involved in bile acid transport has led to improved understanding of bile acid homeostasis and the enterohepatic circulation. Na+-dependent bile acid uptake from portal blood into the liver is mediated primarily by the Na+ taurocholate co-transporting polypeptide (NTCP), while secretion across the canalicular membrane into bile is carried out by the Bile salt export pump (BSEP). In the ileum, absorption of bile acids from the lumen into epithelial cells is mediated by the Apical Na+ bile salt transporter (ASBT), whereas exit into portal blood across the basolateral membrane is mediated by the Organic solute transporter α/ β () heterodimer. Regulation of transporter gene expression and function occurs at several different levels: In the nucleus, members of the nuclear receptor superfamily, regulated by bile acids and other ligands are primarily involved in controlling gene expression, while cell signaling events directly affect transporter function, and subcellular localization. Polymorphisms, dysfunction, and impaired adaptive responses of several of the bile acid transporters, e.g. BSEP and ASBT, results in liver and intestinal disease. Bile acid transporters are now understood to play central roles in driving bile flow, as well as adaptation to various pathological conditions, with complex regulation of activity and function in the nucleus, cytoplasm, and membrane.
Introduction on enterohepatic circulation and bile acid transport in normal physiology
The liver plays an essential role in removing endogenous and xenobiotic compounds from the body. Normal hepatobiliary secretion and enterohepatic circulation are required for the elimination of endogenous compounds such as cholesterol and bilirubin and their metabolites from the body, as well as the maintenance of lipid and bile acid homeostasis. In addition it provides the body’s primary means of eliminating toxic compounds such as drugs, carcinogens and potentially toxic metabolites of endogenous compounds (i.e., endobiotics). These xenobiotics and endobiotics, including bile acids, are taken up by the liver from portal blood and secreted into bile by distinct transport proteins with individualized substrate specificities and capacities located at the sinusoidal and canalicular membranes. At physiological pH, taurine and glycine conjugated bile acids exist in anionic form and are unable to cross membranes by diffusion, and thus, are completely dependent upon membrane transport proteins to enter or exit the hepatocyte. Moreover, since bile acids are the main solute in bile, their transport across the canalicular membrane provides the primary driving force for bile formation (Hofmann 1999; Hofmann 2007). In addition, bile acid secretion into bile occurs against a concentration gradient with very high concentration of bile acids in bile, requiring an active transport system. Thus, the creation, maintenance and regulation of bile formation rests mainly upon the functional competency of membrane transporters, with a central reliance upon the transport of bile acids.
After secretion into bile, bile acids, cholesterol and phospholipids are stored in the gallbladder as mixed micelles. Food entering the duodenum triggers the release of hormones that cause the gallbladder to contract, and delivery of bile from the gallbladder into the duodenum to mix with food and pancreatic juices. In the intestine, bile acids are essential for facilitation of absorption of lipids and cholesterol and lipid-soluble vitamins by incorporation into mixed micelles.
Within the intestinal lumen, a portion of the pool of primary bile acids is modified by intestinal bacteria into secondary bile acids, mainly by single or dual dehydroxylation reactions of the steroid nucleus. Deconjugation of the side chain linkage to taurine or glycine can also occur. Although some bile acids can be passively absorbed in the jejunum, most of the reabsorption, both primary and secondary, takes place in the terminal ileum where they are transported into these enterocytes, and then secreted into portal blood to return to the liver. In this first pass, 75-90% of bile acids are taken up by hepatocytes, completing one round of enterohepatic circulation (Hofmann 1999; Hofmann 2007). Bile acids make several rounds per day and in each tour, a small amount of bile acids is lost in the feces, which is replaced by neosynthesis in the liver via a complex negative feedback loop (see accompanying review by Graham et al in this issue).
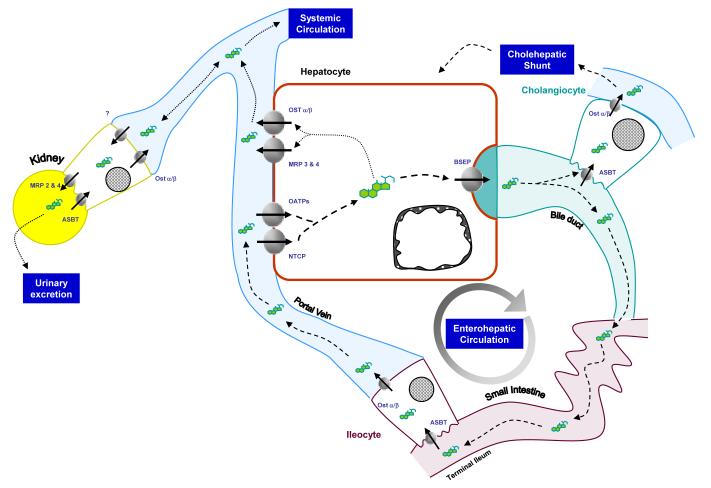
Over the past 5-10 years, much has been learned about the mechanisms of bile acid transport and homeostasis, mainly due to the identification and cloning of membrane transporter genes, and by new findings about the means of regulating their expression in health and disease. Interestingly, the layers of regulation extend from the nucleus, to cell signaling, to membrane transporter protein modification, all woven together in ways that modulate bile acid flux, with a strong overriding regulatory drive to maintain safe intracellular levels of bile acids. Many bile acid transporters have been discovered that are principally involved in maintaining the flow of the enterohepatic circulation, including transporters of the MRP (ABCC) and OATP (SLCO) families (see respective Chapters in this issue by Drs. Ishikawa and Hagenbuch). In this review, we will focus upon the function and regulation of the four main bile acid transporters: Bile Salt Export Pump (Bsep), Sodium Taurocholate Co-Transporting Polypeptide (Ntcp), Apical Sodium Bile salt Transporter (Asbt) and the heterodimeric Organic Solute Transporter α and β (Ostα/β) (Figure 1).
Figure 1. Roles for bile acid transporters in enterohepatic and systemic circulations.
Bile acids are taken up from the intestinal lumen by the apical sodium bile acid transporter (Asbt) in the terminal ileum. After intracellular transport in the ileocyte mediated by Intracellular bile acid binding protein (Ibabp, not shown), bile acids are excreted by the heterodimeric transporter Ostα/β into the portal circulation. Upon reaching the liver sinusoids, the majority of conjugated bile acids are taken up by hepatocytes via sodium dependent and independent mechanisms, mediated by Ntcp and Oatps, respectively. Intracellular transport to the canalicular membrane is mediated by several possible transport proteins (see text), and subsequently excreted across the canalicular membrane into bile by the bile salt export pump (Bsep). After secretion into bile, the majority of bile acids travel the bile ducts to reach the intestinal lumen. A small proportion of bile acids can be taken up by cholangiocytes lining the bile ducts via Asbt and secreted across the basolateral membrane back into circulation, destined for either reuptake by the liver, (cholehepatic shunting), or or systemic circulation. Under conditions of cholestasis, alternative routes for elimination are used exporting bile acids across the sinusoidal membrane into the systemic circulation via Mrp3, Mrp4 and Ostα/β, ultimately destined for urinary excretion via the kidney. For more information about Mrp and Oatp proteins, see accompanying reviews in this Issue. The various components of the enterohepatic and cholehepatic shunts are connected with dashed lines, while those routes for systemic circulation or urinary excretion are denoted by dotted lines.
2. Specific roles for NTCP, BSEP, ASBT, and OST α/β in bile acid transport
Bile acids in portal blood coming from the intestine are bound to albumin (Meier 1995) and released by conformational changes in albumin upon contact with the basolateral membrane (Horie et al. 1988; Meier 1995; Trauner and Boyer 2003). The majority of conjugated bile acids are taken up from portal/sinusoidal blood by hepatocytes via high-affinity, sodium dependent transport, mediated by the Sodium Taurocholate Co-Transporting Polypeptide NTCP (official nomenclature: SLC10A1) as well as by a subset of the family of organic anion transporters (OATP/SCLO) that mediate sodium independent uptake of amphipatic organic compounds including conjugated and unconjugated bile acids, (see review in this issue by Hagenbuch for a more detailed discussion of the OATP family, as well as references (Hagenbuch and Meier 2004; Kullak-Ublick et al. 2004; Meier and Stieger 2002). The relative proportion of Na+-dependent vs. Na+-independent uptake mechanisms is not fully defined, but the majority appears Na+-dependent with differences among species. In humans, it has been estimated that approximately >80% of conjugated bile acid uptake and <50% of unconjugated bile acids takes place via Na+-dependent mechanisms (Kullak-Ublick et al. 2004; Meier and Stieger 2002). In addition, there may be a potential role for the enzyme microsomal epoxide hydrolase (mEH) in hepatic Na+-dependent bile acid uptake (von Dippe et al. 1996; von Dippe et al. 2003) although this is controversial and its role is not fully defined. In transfected HepG2 cells mEH mediated uptake of bile acids (von Dippe et al. 2003.). A mutation in mEH has been found in a patient with hypercholanemia (Zhu et al. 2003) with normal levels of NTCP, but mEH-/- mice have normal bile acid homeostasis (Miyata et al. 1999). However these mice may not have a major phenotype, since it appears that glycoconjugated bile acids are the preferred substrates for mEH and most bile acids in mice are taurine conjugated. The ultimate contribution of mEH to Na+- dependent bile acid uptake is unknown.
Early studies using purified basolateral plasma membrane vesicles, isolated hepatocytes and perfused liver (Meier 1995) (Anwer and Hegner 1978) (Van Dyke et al. 1982), indicated that bile acid uptake is dependent upon both a Na+-gradient and an electrogenic mechanism (Bear et al. 1987); (Weinman et al. 1998). The high affinity uptake for conjugated bile acids such as taurocholate is driven by an inward Na+ gradient which is maintained by the activity of a Na+/K+ ATPase (Meier and Stieger 2002) (Trauner and Boyer 2003). The responsible transporter, NTCP was cloned from rat liver RNA by expression cloning (Hagenbuch et al. 1990; Hagenbuch et al. 1991). Furthermore, these authors showed that Na+-dependent bile acid transport was dependent upon full expression of Ntcp RNA (Hagenbuch et al. 1996). In a Xenopus Oocytes expression system, Na+-dependent bile acid uptake driven by expression of total rat liver RNA was inhibited by 95% after injecting Ntcp antisense RNA. The expression cloning of rat Ntcp was a landmark event in transporter research—it was the first hepatobiliary transporter gene cloned and ushered in the current era of molecular analysis of transporter biology. Ntcp is exclusively expressed in the liver at the basolateral membrane of hepatocytes (Hagenbuch and Dawson 2004) (Ananthanarayanan et al. 1994; Geyer et al. 2006) however appears present in rat pancreatic acinar cells, where it may be involved in clearing bile acids from pancreatic ducts (Kim et al. 2002).
Once taken up by the hepatocyte, the intracellular transport of bile acids is poorly understood, but appears to involve several proteins, including: Liver Fatty Acid Binding Protein, oxysterol binding proteins, glutathione transferases and hydroxysteroid dehydratases (Agellon and Torchia 2000).
Upon reaching the canalicular membrane, conjugated bile acids from the enterohepatic circulation join with newly synthesized bile acids for secretion into bile. Transport of bile acids across the canalicular membrane is the critical rate-limiting step of the enterohepatic circulation, as well as the main generator of bile flow. Secretion of bile acids into bile occurs against a steep concentration gradient: the concentration of bile acids in the canalicular lumen is 100-1000 fold higher than that found in the cytoplasm. (Meier and Stieger 2002; Trauner and Boyer 2003). Bile acid secretion is ATP-dependent and the majority is mediated by the bile salt export pump, Bsep (Abcb11/Spgp). In 1995, Childs et al (Childs et al. 1995) cloned a liver-enriched p-glycoprotein (pgp) gene, originally called sister of pgp (spgp), that was subsequently shown to mediate ATP-dependent bile acid transport (Childs et al. 1995; Childs et al. 1998; Gerloff et al. 1998) (Byrne et al. 2002) (Noe et al. 2002). This gene product was renamed to Bsep and is exclusively expressed in liver, at the apical membrane of hepatocytes (Gerloff et al. 1998).
Bile produced by the liver enters the intestine in the duodenum after being stored in the gall bladder, where further concentration of contents occurs. A small proportion of conjugated bile acids entering the intestinal lumen are deconjugated by the intestinal bacteria rendering them uncharged and with increased pKA. Reabsorption of these bile acids can take place by passive diffusion throughout the whole intestine (Krag and Phillips 1974; McClintock and Shiau 1983; Wilson and Treanor 1975) (Alrefai and Gill 2007) (Hagenbuch and Meier 2004; Hofmann 1999; Hofmann 2007). Alternatively bile acids may be dehydroxylated by the intestinal bacteria which increases the hydrophobicity and facilitates crossing the membrane passively. However this means of reabsorption represents only small part of the total bile acid uptake in intestine. The majority of the reabsorbed bile acids are conjugated and taken up in the terminal ileum (McClintock and Shiau 1983) via a highly efficient sodium dependent mechanism mediated by the apical sodium bile acid transporter Asbt (Slc10a2) (Wong et al. 1994). This transporter is expressed on the apical membrane of ileal intestinal epithelial cells. Asbt is also expressed in cholangiocytes and renal proximal tubular cells (Alpini et al. 1997; Christie et al. 1996; Lazaridis et al. 1997; Wong et al. 1994). In cholangiocytes Asbt may be involved in reabsorption of bile acids from bile, facilitating the “cholehepatic shunting” pathway. The exact physiological relevance of this shunt is not clear, but this pathway may play a role in the modification of bile in the bile ductules and may become more important under cholestatic conditions, providing a mechanism to attenuate high levels of intracellular bile acids, or help improve bile flow within the liver. Within the intestinal epithelia bile acids are transported by the intestinal bile acid binding protein IBABP (Crossman et al. 1994; Gong et al. 1994), after which the bile acids are secreted by several transport proteins including roles for Ostα and Ostβ. This heterodimeric transporter has been identified only recently as a solute transporter in skate liver (Wang et al. 2001). Soon afterwards the human and rodent homologs were identified (Seward et al. 2003). Human OSTα and OSTβ are expressed in a variety of tissues with the highest levels in liver, small intestine, colon, kidney, adrenal gland, testes, and ovary and lower levels in heart, lung, brain, pituitary, thyroid gland, uterus, prostate, mammary gland, and fat (Seward et al. 2003). In mouse, Ostα and Ostβ mRNAs were also abundantly expressed in the small intestine and kidney, but in contrast to human, the genes were expressed at very low levels in the liver (Dawson et al. 2005). At the protein level, localization was found at the basolateral membrane of ileal enterocytes, cholangiocytes and hepatocytes (Ballatori et al. 2005; Dawson et al. 2005; Seward et al. 2003). Ostα and Ostβ can only function as heterodimers, secreting bile acids back into the circulation, both in the intestine and in hepatocytes. Their role in the intestine is of importance under physiological circumstances, whereas in the hepatocytes they seem of more importance during cholestasis, when upregulation serves to protect the hepatocytes against high intracellular bile acids.
3. Substrate specificity
NTCP
NTCP mediates hepatic uptake of conjugated and unconjugated bile acids in a 1:2 stoichiometry with Na+ (Hagenbuch and Meier 1996) (Weinman 1997). It has the highest affinity for conjugated di- and trihydroxy bile acids (Meier et al. 1997), which is similar between human, mouse and rat NTCP/Ntcp, although human NTCP has a higher affinity for taurocholate (Hagenbuch and Meier 1994). In addition transport of estrogen conjugates such as estrone-3-sulfate (Craddock et al. 1998), as well as bromosulphothalein (BSP), DHEA’s and thyroid hormones (Meier et al. 1997) has been shown. Other studies showed that drugs covalently bound to taurocholate (Kullak-Ublick et al. 1997) and chenodeoxycholate-3-sulfate and taurolithocholate-3-sulfate are also substrates for NTCP, at least in vitro (Hata et al. 2003).(Geyer et al. 2006; Hagenbuch and Meier 1996; Pauli-Magnus and Meier 2006; Pauli-Magnus et al. 2005)
BSEP
Bsep mediates the excretion of mainly monovalent conjugated bile acids and has also been shown to have a low affinity for transporting certain drugs (Childs et al. 1998; Lecureur et al. 2000), but the physiological relevance for this is not clear. Bsep of all species has a poor affinity for unconjugated bile acids, with high affinity for conjugated bile acids in this order: taurochenodeoxycholate (TCDCA) over taurocholate (TCA) > taurodeoxycholate (TDCA) > glycocholate (GCA) (Byrne et al. 2002) (Gerloff et al. 1998; Noe et al. 2002) (Green et al. 2000) (Oude Elferink et al. 2006; Stieger et al. 2007; Suchy and Ananthanarayanan 2006). The poor affinity of BSEP for unconjugated bile acids is best exemplified in the patients who have a defect in bile acid conjugation, but when in the presence of normal BSEP protein, secrete very little unconjugated bile acids into bile (Carlton et al. 2003).
ASBT
Human ASBT transports both conjugated and unconjugated bile acids with preference for conjugated bile acids (both taurine and glycine) (Geyer et al. 2006) (Craddock et al. 1998; Hagenbuch and Meier 2004). Asbt has a higher affinity for dihydroxy bile acids which are more hydrophobic (such as CDCA and DCA) over trihydroxy bile acids like CA and TCA and GCA (Craddock et al. 1998). These same researchers reported a lower affinity for TDCA than for TCA. Bile acid transport by Asbt is, similar to Ntcp, sodium-dependent and electrogenic with 2:1 Na+/BA coupling stoichiometry (Weinman et al. 1998). The two genes are phylogenetically related, with similar membrane spanning structures (see below) and intron-exon organization (Cohn et al. 1995; Wong et al. 1994).
OSTα/β
The transport mediated by the heterodimeric transporter OSTα/β was found to be Na+-independent and saturable (Wang et al. 2001) (Seward et al. 2003). Transport by Ostα/β is via a facilitated diffusion mechanism, and therefore, in contrast to the other bile acid transporters in this review, can mediate either efflux or uptake of the substrates, depending on the electrochemical gradient (Ballatori et al. 2005). Substrates include the bile acid taurocholate as well as digoxin, DHEAs estrone-3-sulfate, prostaglandin E2 (Seward et al. 2003; Wang et al. 2001), as studied in Xenopus Oocytes expressing Ostα/β.
4. BA transporter gene and protein structure
NTCP
The human NTCP gene is located on chromosome14q24 and encodes for a 349 amino acid protein with an apparent mass of 56 kDa (Hagenbuch and Meier 1994). The Rat Ntcp gene is located on chromosome 6q24 and encodes for a 363 amino acid protein and has an apparent mass of 51kDa (Kullak-Ublick et al. 2000; Meier and Stieger 2002). It is N-glycosylated at two sites at amino acids N5 and N11 (Hagenbuch 1997). The mouse Ntcp gene is located on chromosome 12q D1 (Cohn et al. 1995), and similar to the rat Ntcp protein, mouse Ntcp is a 362 amino acid protein but exists in 2 isoforms, Ntcp1 and Ntcp2, differing in the C-terminal sequence (Cattori et al. 1999). Ntcp2 is produced by alternative splicing resulting in a truncated protein of 317 amino acids (Cattori et al. 1999) and is expressed at a much lower level than Ntcp1. Although both isoforms are albe to mediate taurocholate transport when expressed in oocytes (Cattori et al. 1999), the physiological relevance of the Ntcp2 isoform is not yet clear.
Initial studies suggested that NTCP had a predicted topology of either seven or nine transmembrane domains with the N-terminus extracellular and the C-terminal intracellular (Hagenbuch and Meier 1994; Hallen et al. 2002) (Mareninova et al. 2005). Earlier experimental data supported a model with nine transmembrane spanning, or seven transmembrane and two membrane-associated domains, (Hallen et al. 2002). However later data indicated that most likely NTCP has 7 transmembrane domains with two segments either forming a large extracellular loop, or are associated with the membrane (Mareninova et al. 2005).
BSEP
The Human BSEP gene maps to chromosome 2q24 (Childs et al. 1998) and results in a protein of 1321 amino acids and a molecular mass of 150–170 kDa (Byrne et al. 2002). Rat Bsep is 1321 amino acid protein of approximately 160 kD with 12 putative membrane spanning domains and four potential N-like glycosylation sites (Childs et al. 1998; Gerloff et al. 1998). Mouse Bsep is localized to a region on mouse chromosome 2 (Green et al. 2000). Mouse Bsep protein contains several phosphorylation sites appearing to be involved in regulation of bile salt transport function (see below) (Noe et al. 2001). Bsep is a member of the ATP-binding cassette (ABC) superfamily of transporters and consists of 12 transmembrane spanning domains determining the substrate specificity and two typical and highly conserved intracellular nucleotide-binding domains with Walker A and B motifs required for binding and hydrolysis of ATP (Meier and Stieger 2002) (Trauner and Boyer 2003). There is no evidence of BSEP splicing isoforms.
ASBT
The human ASBT gene is found on chromosome 13q33 (Wong et al. 1996) and on 16q12 and 8A1 for rat and mouse Asbt respectively, ASBT is a 48-kDa glycoprotein consisting of 348 amino acids. It shares characteristics with NTCP with respect to amino acid identity (35%) and membrane topology. Similar to NTCP, ASBT is a transmembrane protein containing an extracellular N-terminus and a cytoplasmic C-terminus. As for NTCP, the number of predicted ASBT transmembrane domains is either seven or nine (Geier et al. 2006; Hagenbuch and Dawson 2004), depending on the model. The most recent studies suggested that human ASBT is composed of seven transmembrane-spanning segments (Banerjee and Swaan 2006). In addition, the cytoplasmic tail of ASBT plays an important role in the sorting of ASBT in the apical membrane (Sun et al. 1998; Sun et al. 2003).
OSTα/β
The murine Ostα gene is located on chromosome 16B13 whereas murine Ostβ is mapped to chromosome 9C. The Ostα and Ostβ proteins are two distinct gene products with very different protein structures: Ostα is a 340 amino acid protein with seven transmembrane domains, whereas Ostβ is a 128-amino acid single transmembrane domain protein (Wang et al. 2001). Ostα and Ostβ need to be co-expressed to reach the membrane and function properly(Ballatori et al. 2005; Dawson et al. 2005).
5. Regulation of gene expression
Introduction to Nuclear Receptors
The expression of the bile acid transporter genes discussed in this paper is mainly regulated by the class II subfamily of the nuclear receptor (NR) superfamily. This superfamily comprises 48 ligand-regulatable transcription factors serving as intracellular receptors for endocrine hormones and endogenous and xenobiotic compounds (Karpen 2002) (Gronemeyer et al. 2004; Moore et al. 2006; Shulman and Mangelsdorf 2005; Zollner et al. 2006). The class II NR ligands are mostly endogenous lipophilic compounds such as cholesterol, lipids and bile acids and their metabolites, but also xenobiotic compounds such as rifampicin, St. John’s Wort, spironolactone. To regulate target gene expression, a heterodimeric complex of class II NR members is formed with the common binding partner Retinoid X Receptor (RXR) in order to bind to response elements present in promoter regions of target genes. Among the twelve Class II NRs, 6 of these play major roles in regulating bile acid transport genes: Retinoic Acid Receptor (RARα), Farnesoid X Receptor (FXR), Pregnane X Receptor (PXR), Peroxisome Proliferator -Activated Receptor-alpha (PPARα), Constitutive Androstane Receptor (CAR), Liver X Receptor-alpha (LXRα), and their common partner, RXR. Non-Class II NRs also participate in bile acid transporter gene regulation, including Hepatocyte nuclear factor-4alpha (HNF4α, Short Heterodimeric Partner (SHP), and Liver Receptor Homologue-1 (LRH-1), as well as other liver enriched transcription factors such as HNF1-α/β and HNF3β A number of recent reviews provide greater details of the mechanisms by which NR family members regulate and integrate gene expression and cell signaling. (Geier et al. 2007) (Alrefai and Gill 2007; Geier et al. 2006; Zollner et al. 2006).
NTCP
For NTCP, differential regulation between species exists with respect to the transcription factors that act on the NTCP promoter. Rat Ntcp expression is reduced by high concentrations of bile acids which is FXR mediated, however FXR does not act directly on the NTCP promoter but acts indirectly via activation of SHP, an inhibitory NR which in turn suppresses basal activation by RAR/RXR of rat Ntcp (Denson et al. 2001). During fasting NTCP is upregulated by HNF4α and PGC1α in rats (Dietrich et al. 2007), and this was suggested to provide a means of removing bile acids from the circulation to avoid signaling function in peripheral tissues.
In mice, Ntcp expression was decreased in mice fed a taurocholate containing diet (Sinal et al. 2000) (Wolters et al. 2002). Although no FXR/RXR binding site has been identified in the mouse Ntcp promoter, this does require FXR since this reduction was absent in Fxr-/- mice (Sinal et al. 2000); (Zollner et al. 2005). Basal Ntcp expression levels does not appear to be dependent on FXR since these were not changed in Fxr-/- mice (Sinal et al. 2000); (Zollner et al. 2005). Moreover basal Ntcp expression is not changed in Shp-/- mice (Kerr et al. 2002), and was still reduced when these mice were fed a CA diet (Wang et al. 2002). Therefore hepatic FXR and SHP do not seem to play an essential role in regulation of murine Ntcp by bile acids. Although not directly reported, it is quite possible that bile acids may primarily regulate murine hepatic Ntcp RNA expression via ileal FGF15, via hepatic activation of cell signaling expression (Inagaki et al. 2005). In addition, mouse Ntcp expression seems to be regulated mainly by HNF4α and HNF1α, although the relative contributions to overall Ntcp expression are not fully defined. Experimental data showing involvement of HNF4α and HNF1α in regulation of murine Ntcp expression include reduced Ntcp expression in both hepatocyte-specific Hnf4α-/- mice (Hayhurst et al. 2001) and in Tcf1-/- mice (the gene encoding the HNF1α protein) (Shih et al. 2001) and the presence of both HNF4α and HNF1α binding sites in the mouse Ntcp promoter (Jung et al. 2004). In addition HNF1α activity was reduced by bile acids via activation of FXR and induction of SHP, which in turn would interfere with HNF4α induced HNF1α activation subsequently reduced Ntcp expression (Jung et al. 2004; Jung and Kullak-Ublick 2003).
Two other liver enriched transcription factors have been reported to regulate NTCP expression. In rat, the homeobox gene Hex activated the NTCP promoter via an upstream response element (Denson et al. 2000), whereas HNF3β (or Foxa2) may act as a negative regulator of Ntcp, since overexpression of HNF3β in mice resulted in a decrease of Ntcp (Rausa et al. 2000). The binding site for HNF3β is conserved between rat, mouse and human NTCP (Jung et al. 2004; Shiao et al. 2000), but so far a physiological role has not been determined. Human NTCP is also regulated by members of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors (Jung et al. 2004; Shiao et al. 2000). In addition, the glucocorticoid receptor regulates the expression of human NTCP in a ligand dependent manner (Eloranta et al. 2006). This activation by GR was blocked by FXR induced expression of SHP, providing a negative feedback mechanism by bile acids on their uptake, but activation of NTCP by GR was increased in the presence of the coactivator PCG-1α (Eloranta et al. 2006).
A potential GR binding site was also identified in mouse Ntcp, (Cheng et al. 2007) and indeed, Dexamethasone induced Ntcp through activation of GR. Dexamethasone is also a ligand for PXR, and the effect of Dexamethasone on Ntcp is absent in Pxr-/- mice. However no PXR binding site in the mouse Ntcp promoter has been found. Moreover PCN showed no effect on Ntcp expression, suggesting PXR does not play a role in regulation of murine Ntcp expression. Estradiol reduced Ntcp RNA (Lee et al. 2000), which similar to regulation of Bsep by estradiol, was absent in Erα-/- mice (Yamamoto et al. 2006), indicating a role for ERα.
In HepG2 cells, NTCP expression was responsive to cholesterol treatment (Dias and Ribeiro 2007) however this could be either a direct or indirect effect since neither the transcription factors nor the mechanism involved were investigated, but may involve a role for Sterol regulatory element binding protein-2 (SREBP2), another cholesterol sensing transcription factor. Basal Ntcp gene expression is also influenced by gender: Ntcp protein and mRNA are expressed at higher levels in female murine and human liver tissue (Cheng et al. 2007). In contrast, in rats ntcp expression is higher in males (Simon et al. 2004). The difference in mice starts to become apparent 45 days after birth not due not to sex hormones, but rather to inhibitory effects of sex-specific growth hormone secretion patterns. The effect of growth hormone on Ntcp with respect to gender differential expression is mainly mediated by Stat5a and Stat5b (Clodfelter et al. 2006; Clodfelter et al. 2007). A potential Stat5b element in promoters of mouse and human NTCP has been identified (Cheng et al. 2007). In rat the Ntcp gene was upregulated by prolactin which was mediated by direct Stat5 binding to the Ntcp promoter (Ganguly et al. 1997). Finally, inflammation suppresses Ntcp expression rapidly and profoundly, mostly at the transcriptional level (Trauner et al. 1998; Zollner et al. 2006)
BSEP
The expression of the Bsep/BSEP gene is highly regulated by the heterodimer FXR/RXR in both human and rodents. Bile acids have been identified as ligands for FXR (Makishima et al. 1999) (Parks et al. 1999; Wang et al. 1999), resulting in a feedforward regulation of Bsep by the substrates it transports. By increasing Bsep RNA expression as a result of elevated levels of intracellular bile acids, increased efflux of bile acids from the hepatocytes results in a return to normal levels of intracellular bile acids. Physiological dependency on FXR for regulation of Bsep gene expression was confirmed in Fxr-/- mice which showed markedly reduced basal expression levels of Bsep (Sinal et al. 2000), along with a complete lack of induction by bile acids (Sinal et al. 2000; Wolters et al. 2002).
Recently hepatocyte-specific Fxr-/- mice and intestinal epithelial cell-specific Fxr-/- mice were created (Kim et al. 2007) and the contribution of FXR in these organs to Bsep expression was studied. Only the hepatocyte-specific absence of Fxr affected bile acid regulation of Bsep, whereas in the intestine-specific Fxr-/- mice the hepatic FXR-dependent regulation of Bsep was intact. Although Bsep expression is directly regulated by FXR in the hepatocytes, Bsep expression was decreased in livers of hepatocyte-specific Lrh1-/- mice (Mataki et al. 2007), although there may be other explanations, such as a reduction in the bile acid pool size. In addition Bsep expression was decreased in mice overexpressing human SHP specifically in the hepatocytes (Boulias et al. 2005), again probably indirectly due to decreased bile acid pool.
Hydrophobic bile acids are better ligands for FXR than hydrophilic bile acids with CDCA and CA being the most potent FXR agonists (Makishima et al. 1999; Parks et al. 1999; Wang et al. 1999). In contrast, in vitro studies showed the hydrophobic bile acid Litocholic acid antagonizes CDCA-induced Bsep expression as well as activation by the synthetic FXR ligand GW4064 (Yu et al. 2002). In addition, the synthetic RXRα ligand LG268 antagonized CDCA-induced FXR activity on the human BSEP promoter in vitro (Kassam et al. 2003). Other in vitro studies showed that the coactivators CARM1 and PRMT1 are required for full activation of FXR by bile acids to increase BSEP expression (Ananthanarayanan et al. 2004) (Rizzo et al. 2005), suggesting that there are multiple layers of complexity to the overall contributions of regulation of Bsep/BSEP RNA expression.
In addition to being regulated by bile acids, the FXR/RXR binding site identified in the rat minimal Bsep promoter is inhibited by rifampicin and estradiol and activated by tamoxifen, which are ligands for PXR and ER (Gerloff et al. 2002). Estradiol treatment of mice reduced Bsep RNA levels (Lee et al. 2000), which appears to be ERα dependent (Yamamoto et al. 2006). Although the mechanism is not fully known this could potential be explained by the reduced bile acid synthesis due to reduced expression of Cyp7a1, Cyp7b1, Cyp8b1 in mice treated with ethinyl estradiol, EE2. Further upstream in the Bsep promoter several other potential sites were identified, such as NF-1 and an overlapping SP-1/ HNF3β binding sites so far not shown to be functional. In addition MyoD was suggested to potentially have a repressive effect on the rat Bsep promoter (Gerloff et al. 2002).
Early observations reported spironolactone to increase bile flow in rodents (Klaassen 1974; von Bergmann et al. 1974). Later spironolactone was identified as a ligand for PXR, the Glucocorticoid Receptor (GR) and for the Mineralocorticoid Receptor (MR). Recently spironolactone was shown to induce Bsep expression (Cheng et al. 2007) potentially explaining the increase bile flow induced by this compound. Basal Bsep expression levels were lower in Pxr-/- mice compared to wild-type mice and spironolactone induced Bsep expression was absent, indicating the involvement of Pxr. Potential PXR response elements in mouse and human bsep/BSEP promoters were identified, confirming potential direct regulation of Bsep/BSEP expression by PXR. However, treatment of mice with the typical murine PXR ligand PCN did not affect Bsep expression. However another study did show that PXR was required for upregulation of mouse Bsep by PCN and RU486 (Teng and Piquette-Miller 2005). In addition potential GR elements were found in the murine Bsep promoter, but appear not to be functional since activation of GR with dexamethasone treatment did not affect Bsep expression (Cheng et al. 2007). Finally, there is substantial inflammation-mediated suppression of Bsep RNA, apparently via reduction in the amount and function relevant transcriptional regulators(Geier et al. 2006; Zollner et al. 2006) All together, ligand and NR regulation of Bsep gene expression is complex, and points to this gene as a critical component of the hepatocytes response to a variety of disease states and xenobiotics.
ASBT
Similar to Ntcp, differential regulation of Asbt exists between species. In rat Asbt expression in the ileum was not affected by reduced amounts of bile acids present in the intestinal lumen (Arrese et al. 1998). In mice, however, Asbt is under negative feedback regulation by bile salts concluded from experiments in mice with increased secretion of biliary bile acids as a result of overexpression of hepatic Bsep (Figge et al. 2004). Bile acid feeding decreased Asbt levels (Chen et al. 2003; Zollner et al. 2006), which was absent in Fxr-/- mice. In vitro studies confirmed that mouse but not rat Asbt is regulated by the FXR/SHP/LRH-1 pathway (Chen et al. 2003), however FXR appears not to be required for basal Asbt expression since Fxr-/- mice did not show a change in expression of Asbt (Sinal et al. 2000).
For human ASBT, a different mechanism of regulation by bile acids has been found, such that bile acids activate FXR, which in turn activates SHP but this represses RXR-RAR induced activation of Asbt (Neimark et al. 2004). In addition, the human Asbt gene was responsive to the RXR ligand, 9-cis retinoic acid. In rabbits bile acid mediated downregulation of Asbt was shown to be mediated by the FXR-SHP-FTF cascade (Li et al. 2005).
Besides regulation by bile acids through FXR, Asbt is regulated by multiple other transcription factors, including other nuclear receptors, one of which is the Glucocorticoid Receptor. Human intestinal ASBT expression was increased by GR and its synthetic ligand dexamethasone (Jung et al. 2004) and the human ASBT promoter contains 2 GR response elements (IR-3) which were induced by dexamethasone-activated GR (Jung et al. 2004). Similarly, an increase in Asbt in response to glucocortiocoids in rat and rabbit was shown (Nowicki et al. 1997), suggesting also in other species Asbt may be regulated by GR and its ligands.
Rat and human Asbt/ASBT are also regulated by vitamin D3 (Chen et al. 2006) via a Vitamin D Receptor (VDR) response element. Activation resulted in increased ileal bile acid transport in mice, indicating there might be a physiological role for VDR in intestinal bile acid uptake. Interestingly the VDR is also activated by certain bile acids, such as lithocholic acid and its conjugated forms (Makishima et al. 2002) but its role in regulating Asbt gene expression is unknown. In addition a functional PPARα/RXR DR-1 binding site was identified in human ABST, resulting in increased promoter activity and upregulated ASBT expression in a cholangiocytes cell line (Jung et al. 2002).
In mice Asbt appears to be regulated by HNF1α as well, since the expression was reduced to absence in intestine and kidney of Tcf (HNF1α)-/- mice (Shih et al. 2001). In addition basal promoter activity of human ASBT was dependent on HNF1α (Jung et al. 2002). In human intestinal epithelial cells the addition of 25-OH-cholesterol repressed ASBT promoter activity, RNA expression levels and transport activity of ASBT protein (Alrefai et al. 2005). No direct regulation by the cholesterol sensor LXRα has been shown, but rather the downregulation of ASBT by cholesterol metabolites required both the presence of SREBP2, another transcription factor sensing cholesterol levels, and HNF1α, the latter directly interacting with the ASBT promoter, whereas SREBP2 did not (Thomas et al. 2006). The exact mechanism as to how SREBP2 and HNF1α mediate repression of the ASBT gene is not yet clear. SREBP2 did not bind directly to the ASBT promoter. In addition, the HNF1α promoter was insensitive to regulation by cholesterol, excluding the possibility of SREBP2 binding to HNF1α but suggesting a cooperative mechanism of potential SREBP2 and HNF1α interaction. The physiological relevance of cholesterol regulating ASBT levels and transport activity may be considered an adaptive response to systemic high cholesterol levels leading to the inhibition of intestinal bile acid absorption and resulting in the reduction in bile acids entering the liver. This in turn would relieve the suppression of bile acid synthesis by bile acids and increase in the metabolism of cholesterol into bile acids, resulting in reduction of high cholesterol levels.
Finally, the Asbt/ASBT gene in both rodents and human is downregulated by activation of AP-1, in particular by the component protein c-Fos, in response to inflammatory cytokines, such as IL-1β and TNFα in CaCo2 and IEC6 cells (Chen et al. 2001; Chen et al. 2002; Neimark et al. 2006). Another very recent study indicates that the human ASBT gene can be activated by bile acids on the AP-1 site, via the EGF-receptor and ERK1/2 activation (Duane et al. 2007).
Octα/β
Little is known of the regulation of expression of Ostα and Ostβ genes, due mainly to their recent discovery. In humans, OSTα/β have higher expression in liver compared to the intestine, whereas in mice, they are very low expressed in the liver with much higher expression levels in the intestine (Ballatori et al. 2005; Seward et al. 2003) (Boyer et al. 2006).
Both genes are regulated by bile acids via the FXR/RXR heterodimer, which is conserved in human (Landrier et al. 2006) and mouse (Frankenberg et al. 2006). Ostα and Ostβ were shown to be responsive to a CA containing diet but not to UDCA diet, with a complete lack of response in Fxr-/- mice, indicating that indeed FXR is required for this increase (Zollner et al. 2006) in both liver and intestine. OSTα/β expression was also increased ex vivo in human ileal biopsies exposed to CDCA (Landrier et al. 2006). Increased Ostα/β promoter activity induced by ligand activated FXR indicates a feedforward regulation exists by bile acids inducing their own clearance by upregulating Ostα/β expression, similar to Bsep. Reduced expression of Ostα/β was observed in the ileum of Asbt-/- mice, whereas the expression was increased in the cecum and proximal colon (Dawson et al. 2005). This could be explained by a reduction in ileal bile acid uptake in these Asbt-/- mice, resulting in decreased activation of FXR and as a consequence the increased concentration of bile acids in the colon and cecum may increase the activation of FXR and increasing the expression of Ostα/β. In addition LRH-1 binding sites in both mouse and human OSTα/β have been found (Frankenberg et al. 2006) indicating a more complex regulation in which bile acids can also indirectly inhibit Ostα/β via activation of FXR and SHP-mediated inhibition of LRH-1.
Another recent study identified an LXR activation site for mouse Ostα/β (Okuwaki et al. 2007), which overlapped with the FXR/RXR site. The activation of Ostα but not Ostβ by ligand activated LXR may be enhanced by HNF4α but no physiological relevant function has been associated with it yet. In addition to regulation by nuclear receptors, ileal Ostα/β expression was dependent on c-fos in a mouse model of indomethacin induced intestinal inflammation (Neimark et al. 2006).
6. Post-translational regulation
Besides regulation at the gene expression level which results in delayed effects, a more rapid regulation of transporter function can be obtained by actions on the protein itself. These post-translational modifications include phosphorylation/dephosphorylation of the transport proteins, increasing or decreasing the amount of protein on the membrane by insertion or retrieval of vesicles to or from the basolateral or apical membranes to submembrane, intracellular pools. Activation of several signaling pathways has been shown to be involved in bile acid uptake and bile formation. These signaling pathways include intracellular cAMP, cGMP, Ca2+ increase, PKC activation, PI3K and MAPK activation (Anwer 2004; Bouscarel et al. 1999)
NTCP
Initial studies showed that cAMP stimulates bile acid uptake in hepatocytes (Botham and Suckling 1986) (Grune et al. 1993). This was later shown to be due to effects on NTCP transport activity: increased cAMP resulted in a cAMP-dependent increase in vesicular trafficking of Ntcp from an intracellular pool to the basolateral membrane, resulting in an increased presence of NTCP (Dranoff et al. 1999; Mukhopadhayay et al. 1997). Trafficking of these NTCP containing vesicles was dependent on actin skeleton/microfilaments (Dranoff et al. 1999) (Webster and Anwer 1999). In addition PI3K and PKCζ signaling are required for the vesicle transport of NTCP to and from the basolateral membrane (Webster and Anwer 1999; Webster et al. 2000).
NTCP is a serine/threonine phosphorylated protein and is de-phosphorylated in response to cAMP, which leads to increased retention of NTCP in the membrane (Mukhopadhyay et al. 1998; Mukhopadhyay et al. 1998). This dephosphorylation is mediated by protein phosphatase 2B (PP2B), which is a calcium/calmodulin-dependent serine and threonine protein phosphatase (Webster et al. 2002). PP2B requires calcium for its activity and an increase of intracellular Ca2+ levels was shown to be induced by cAMP (Webster et al. 2002). The effect of cAMP on increased Ntcp activity may be resulting from dephosphorylation of Ntcp at S226 (Anwer et al. 2005) leading to increased translocation of the protein to the membrane. Dephosphorylation retains Ntcp in the membrane and prevents its removal, resulting in higher levels on the membrane and increased bile acid uptake activity. The mechanism that seemed to emerge from this is the following: cAMP increases intracellular Ca2+, activating PP2B, which in turn dephosphorylates NTCP, facilitating its translocation to the membrane. The latter is dependent upon PI3K. In addition phosphorylation of 2 tyrosine residues on the cytoplasmic tail of rat NTCP appear to be involved in targeting to the basolateral membrane (Sun et al. 2001). Finally, Ntcp is degraded via the ubiqutination/proteasomal pathway (Kuhlkamp et al. 2005).
BSEP
Similar to Ntcp, Bsep is recruited to the apical membrane from intracellular sub-apical vesicles in response to several stimuli, including cAMP and PI3K-dependent signaling, hypo-osmolarality induced cell swelling, and certain bile acids (Kipp et al. 2001; Misra et al. 1998; Schmitt et al. 2001; Suchy and Ananthanarayanan 2006). Newly synthesized Bsep directly targeted from Golgi to canalicular membranes via subcanalicular pools (Kipp and Arias 2000; Kipp et al. 2001). The insertion and retrieval of Bsep in and from the canalicular membrane appears to be dependent on the microtubule cytoskeleton (Boyer and Soroka 1995; Gatmaitan et al. 1997) as well as on PI3K signaling (Misra et al. 1998) and on PI3K generated lipid products, such as PI3,4P2 (Misra et al. 1999). In addition, radixin, a protein involved in cross-linking the plasma membrane and intracellular actin filaments is required for maintaining Bsep in the plasma membrane (Wang et al. 2006).
Cyclic AMP stimulates canalicular secretion of bile acids (Hayakawa et al. 1990) and induced a 3-fold increase of Bsep in the canalicular membrane, which was partially dependent on microtubules (Gatmaitan et al. 1997; Kipp et al. 2001). In addition, Bsep is recruited to the canalicular membrane in response to the bile acid taurocholate, which is dependent on microtubules (Gatmaitan et al. 1997), although others found this to be independent of cAMP (Misra et al. 1998; Misra et al. 2003).Cell swelling also stimulates biliary bile acid secretion and translocation of Bsep to the canalicular membrane (Schmitt et al. 2001) and also activates PI3K (Krause et al. 1996) suggesting that PI3K signaling is involved in cell swelling induced translocation of Bsep. Regulation of trafficking of rat Bsep to the canalicular membrane is regulated by PKC and p38 MAPK kinase signaling (Kubitz et al. 2004). Phosphorylation of murine Bsep was mediated by PKCα (Noe et al. 2001), and enhances its affinity for substrates, whereas phosphorylation of rat Bsep by PKC led to retrieval of Bsep from the membrane in HepG2 cells (Kubitz et al. 2004).
ASBT
Only a few studies have reported on posttranslational regulation of Asbt and almost all of these are in cholangiocytes. It is currently unknown if similar modifications occur in the intestinal epithelial cells. Similar to Ntcp and Bsep, Asbt seems to be residing in subapical vesicles, for a fast response to stimuli to go to the membrane. In rat cholangiocytes, secretin increased the activity of Asbt by shuttling the protein from subapical endosomes to apical membrane and thereby increasing the expression at the surface membrane (Alpini et al. 2005). Under basal conditions Asbt is ubiquitinated and degraded by the proteasome (Xia et al. 2004). In response to the cytokine Il-1β, Asbt is phosphorylated which resulted in increased proteasomal degradation.
OSTα/β
Data on posttranslational modifications of Ostα and Ostβ are beginning to emerge. Two recent studies reported on trafficking to of the Ostα and Ostβ to the membrane. For human OSTα and OSTβ, in vitro studies showed a protein-protein interaction needs to exist between the two partners before translocation to the membrane can occur (Ballatori et al. 2005; Sun et al. 2007), with an important role for the N-terminal part of hOSTα in this interaction (Sun et al. 2007). In mice (Dawson et al. 2005), co-expression of both Ostα and Ostβ was required for the synthesis of a mature glycosylated Ostα protein. In addition both Ostα and Ostβ were required to be expressed for their appearance at the membrane, whereas expression of either one of the proteins results in degradation (Li et al. 2007). This was further confirmed in Ostα-/- mice, where Ostβ RNA was expressed, but not the protein, indicating that Ostα is required for Ostβ protein stability (Li et al. 2007) and Ostβ was suggested to function as a chaperone and as a regulator of the transport activity of the dimer.
6. Regulation of transport activity
In addition to gene and protein regulation, a direct effect of compounds on the transport activity of the respective protein is an effective manner of influencing transport (Anwer 2004; Meier and Stieger 2002)
NTCP
Transport activity of Ntcp is sodium dependent, and acts via an electrogenic mechanism, with a 2:1 stoichiometry with Na+. Certain drugs act as inhibitors of Ntcp transport, These are cyclosporine, R- and S-proranolol, BSP, furosemide, DIDS, 17-b-eostradiol-3-sulfate, tauro-lithocholate-3-sulfate (Hata et al. 2003; Kim et al. 1999; Kramer et al. 1999).
BSEP
Bsep protein transport activity is inhibited by glybenclamide and troglitazone by direct binding of these compounds to Bsep (Byrne et al. 2002; Funk et al. 2001) and cause drug induced cholestasis. In addition, Estradiol-17b-glucuronide, cyclosporine A, rifampicin and bosentan inhibit Bsep transporter function (Stieger et al. 2000) (Bohme et al. 1994) (Fattinger et al. 2001) Also internalization of membrane vesicles induced by taurolithocholate is involved in drug-induced cholestasis (Crocenzi et al. 2003; Crocenzi et al. 2003).
ASBT
Asbt transport function is inhibited by BSP, and cyclosporine (Baringhaus et al. 1999). Several chemical Asbt specific inhibitors have been developed: dimeric bile acid analogues, which bind to Asbt but cannot be transported (Kramer and Wess 1996; Wess et al. 1994) (Baringhaus et al. 1999). A different class of inhibitors consists of benzothiazepine derivatives, benzothiepine derivatives, and naphtol derivatives, (Kramer et al. 1999) (Tollefson et al. 2000) (Huang et al. 2005; Kurata et al. 2004; Root et al. 2002; West et al. 2002). Inhibition of Asbt by chemical inhibitors would be beneficial in reducing serum cholesterol due to increased bile acid synthesis as result of decreased absorption.
OSTα/β
For Ostα/β transport function in rodents no inhibitory compounds have been identified yet. However, skate Ostα/β mediated TCA transport is inhibited by hydrophobic sulfated bile acids (Wang et al. 2001) steroids such as estrone sulfate and spironolactone as well as by sulfobromophtalein and indomethacin.
8. Roles in diseases
Genetic defects, mutations and polymorphisms (see Table)
Table.
| Transporter | Tissue/Cell type |
Cellular location |
Major role | Regulation in human disease |
Human SNPs, polymorphisms & mutations |
|---|---|---|---|---|---|
| Bsep | Hepatocyte | Canalicular membrane |
ATP- dependent bile acid efflux |
Suppressed in sepsis and cholestasis |
Strautnieks et al. 1998 Jansen et al. 1999 Saito et al 2002 Wang et al 2002 Pauli-Magnus et al 2004 Plass et al 2004 Van Mil et al 2004 Hayashi et al 2005 Noe et al 2005 Lang et al 2006 Meier et al 2006 Kagawa et al 2007 Lam et al 2007 Lang et al 2007 Stieger et al 2007 |
| Ntcp | Hepatocyte | Sinusoidal membrane |
Na+ dependent bile acid uptake |
Suppressed in sepsis & cholestasis |
Ho et al 2004 Geyer et al 2007 |
| Asbt | Enterocyte, cholangiocyte, kidney |
Apical membrane |
Na+ dependent bile acid uptake |
Unknown |
Oelkers et al 1997 Geyer et al 2007 |
| Ostα/β | Hepatocyte, enterocyte, cholangiocyte, kidney |
Sinusoidal membrane |
bile acid efflux by facilitated diffusion |
Upregulated in liver in late stage PBC |
None described |
NTCP (SLC10A1)
No major diseases have been associated with defects in the NTCP gene, however several ethnicity dependent polymorphisms have been identified as well as marked inter-personal variations in human NTCP RNA expression levels (Ho et al. 2004) (Kullak-Ublick et al. 1997). These low frequency polymorphisms were associated with reduced membrane localization and with decreased to near absence of transport activity of bile acids and were suggested to potentially play role in development of hypercholanemia. Unfortunately it was not possible to relate these polymorphisms to clinical phenotypes, because of anonymity of the DNA samples. Moreover Ntcp-deficient mice have not been generated yet.
BSEP (ABCB11)
Mutations in the BSEP gene lead to the inherited cholestatic disorder Progressive Familial Intrahepatic Cholestasis type 2 or PFIC2 (Jansen et al. 1999; Oude Elferink et al. 2006; Strautnieks et al. 1998). This liver disease has is characterized by severe jaundice, hepatomegaly, failure to thrive and pruritus from infancy onset, whereas liver histology shows portal inflammation and giant-cell hepatitis (Bezerra and Balistreri 2000). In addition, high bile acids and aminotransferases are detected in serum, however a clinical hallmark of this disease presents with normal gamma glutamyl transpeptidase levels. The disease has a rapid progressive course and generally leads to cirrhosis and liver failure with a need for liver transplantation within the first decade of life. A number of different mutations have been found in the BSEP gene. Some of these mutations have been functionally analyzed in vitro. Most of these showed reduced transport activity, as a result of in decreased protein expression, altered membrane targeting, increased degradation by the proteasome (Hayashi et al. 2005; Kagawa et al. 2007; Wang et al. 2002), whereas others were functional but were unstable due to defective glycosylation (Plass et al. 2004). In addition a milder form of PFIC2 exists, BRIC2 (van Mil et al. 2004) (Noe et al. 2005) (Lam et al. 2007) (Kagawa et al. 2007), in which recurrent episodes of cholestasis occur and is associated with gallstone formation (van Mil et al. 2004).
In contrast to the human disease, mice lacking the Bsep gene have only mild, nonprogressive, but persistent intrahepatic cholestasis (Wang et al. 2001). This could be explained by the fact that the bile acid pool in mice is much more hydrophilic compared to the human bile acid pool, and that the mouse liver has more active detoxifying pathways than human. Indeed, when Bsep-/- mice were fed a diet containing CA to increase the hydrophobicity of the murine bile salt pool, severe cholestasis was induced (Wang et al. 2003).
Although no human gene defects have been identified that are associated with increased Bsep activity, in mice overexpression of hepatic Bsep (Figge et al. 2004), resulted in reduced hepatic steatosis when placed on a high cholesterol, high fat and cholic acid containing diet (Figge et al. 2004).
A number of studies have identified polymorphisms and sequence variabilities in the human ABCB11 gene (Pauli-Magnus et al. 2004) (Stieger et al. 2007). Interindividual variability for basal BSEP protein levels was identified in a study of 110 healthy liver tissue samples from an overall white population, but no correlation between expression level and cholestasis markers was found (Meier et al. 2006), however, a specific SNP (presence of C-allele at position 1457) in the ABCB11 gene tended to be associated with low BSEP protein expression levels. 86 polymorphisms were found in a population of Caucasians, Koreans and African-Americans. The polymorphisms were found in exons, introns as well as in 5’flanking regions. Most variants were population specific, with the largest variation found in the African-American group, but no functionality of the protein was determined for any of the variants (Lang et al. 2006). In addition, 82 polymorphisms were identified in a Japanese population (Saito et al. 2002) but again this was not associated with functionality studies of the variants. Polymorphisms in BSEP have also been associated with intrahepatic cholestasis of pregnancy (Keitel et al. 2006; Pauli-Magnus et al. 2004) and drug-induced cholestasis (Lang et al. 2007).
ASBT (SLC10A2)
A mutation in Asbt was identified in and shown to be directly responsible for primary bile acid malabsorption (PBAM) (Oelkers et al. 1997), which is a disease associated with interrupted enterohepatic circulation, infantile diarrhea, fat malabsorption and reduced level of plasma cholesterol (Balistreri et al. 1983) (Heubi et al. 1982; Thaysen and Pedersen 1973). A point mutation in the human ASBT gene, resulting in lack of taurocholate transport was identified in a patient with Crohn’s disease (Wong et al. 1995), although its relationship to Crohn’s Disease is uncertain.
In addition several other mutations and polymorphisms have been identified in ASBT/Asbt which reduced bile acid transport activity (Geyer et al. 2006). Consistent with the human phenotype, mice lacking the Asbt gene show profound intestinal bile acid malabsorption and an increase in elimination fecal bile acid (Dawson et al. 2003) as well as a slight increase in plasma cholesterol levels, confirming that Asbt is the major mechanism of intestinal bile acid absorption.
OSTα/β
No diseases, mutations or polymorphisms have been reported for Ostα/β; however in mouse models of cholestasis changes in gene expression have been shown suggesting alterations in function. Recently Ostα-/- mice were generated but no phenotype was described other than lack of both Ostα and Ostβ proteins and growth retardation, but no explanation was provided (Li et al. 2007)..
Besides genetic defects the expression bile acid transporters have been shown to be changed in several other liver diseases, such as inflammation induced cholestasis, obstructive cholestasis, and cholestasis of pregnancy, mainly due to effects of these pathological states on the function of nuclear receptors regulating the expression of bile acid transporter genes (reviews by (Arrese 2006; Geier et al. 2006; Geier et al. 2007). This will be discussed more extensively in the chapter by Graham of this issue.
Summary.
The genes responsible for bile acid transport have been only recently discovered, but their substrate specificities, and main mechanisms of functional regulation are beginning to be understood. Among the main points are the high functionality and regulatability of these genes, especially in response to cholestasis, the narrow substrate specificities, and the regulation at transcriptional and post-transcriptional levels. Moreover, human diseases result when the function of some of these genes, i.e. BSEP and ASBT is reduced, either by genetic mutations, or external regulation by inflammation or exogenous compounds. All together, we are currently at a point where we now have a broad view of the landscape of how bile acid transporters function in the cell (see Figure 1), and how this may be targeted for regulation in various disease states—both from a pathogenic view, as well as a therapeutic one.
Abbreviations used in this review
- Asbt/Slc10a2
Apical sodium bile salt transporter
- Bsep/Abcb11/Spgp
Bile salt export pump
- BSP
bromosulphothalein
- cAMP
cyclic Adenosine-monophosphate
- CAR
Constitutive androstane receptor
- ER
Estrogen receptor
- FXR
Farnesoid X receptor
- GCA
glycocholate
- GR
Glucocorticoid receptor
- HNF
Hepatocyte nuclear factor
- LRH-1
Liver receptor homologue-1
- LXR
Liver X receptor
- NR
nuclear receptor
- Ntcp/Slc10a1
Sodium taurocholate co-transporting polypeptide
- mEH
microsomal epoxide hydrolase
- OATP/SLCO
Organic anion transport polypeptide
- Ostα/β
Organic solute transporter α and β
- PI3K
Phosphoinositol-3-kinase
- PKC
Protein kinase C
- PP2B
Protein phosphatase 2B
- PPAR
Peroxisome proliferator-activated receptor
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- PXR
Pregnane X receptor
- RAR
Retinoic acid receptor
- RXR
Retinoid X receptor
- SHP
Short heterodimeric partner
- SREBP2
Sterol regulatory element binding protein-2
- TCA
taurocholate
- TCDCA
taurochenodeoxycholate
- TDCA
taurodeoxycholate
- UDCA
Ursodeoxycholic acid
- VDR
Vitamin D receptor
References
- Agellon LB, Torchia EC. Intracellular transport of bile acids. Biochim Biophys Acta. 2000;1486(1):198–209. doi: 10.1016/s1388-1981(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41(5):1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113(5):1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24(10):1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- Alrefai WA, Sarwar Z, Tyagi S, Saksena S, Dudeja PK, Gill RK. Cholesterol modulates human intestinal sodium-dependent bile acid transporter. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G978–985. doi: 10.1152/ajpgi.00379.2004. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Li S, Balasubramaniyan N, Suchy FJ, Walsh MJ. Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J Biol Chem. 2004;279(52):54348–54357. doi: 10.1074/jbc.M410021200. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Ng OC, Boyer JL, Suchy FJ. Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am J Physiol. 1994;267(4 Pt 1):G637–643. doi: 10.1152/ajpgi.1994.267.4.G637. [DOI] [PubMed] [Google Scholar]
- Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39(3):581–590. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem. 2005;280(39):33687–33692. doi: 10.1074/jbc.M502151200. [DOI] [PubMed] [Google Scholar]
- Anwer MS, Hegner D. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe Seylers Z Physiol Chem. 1978;359(2):181–192. [PubMed] [Google Scholar]
- Arrese M. Cholestasis during pregnancy: rare hepatic diseases unmasked by pregnancy. Ann Hepatol. 2006;5(3):216–218. [PubMed] [Google Scholar]
- Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28(4):1081–1087. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- Balistreri WF, Heubi JE, Suchy FJ. Bile acid metabolism: relationship of bile acid malabsorption and diarrhea. J Pediatr Gastroenterol Nutr. 1983;2(1):105–121. [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42(6):1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Swaan PW. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry. 2006;45(3):943–953. doi: 10.1021/bi052202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na(+)/bile acid cotransporter. J Lipid Res. 1999;40(12):2158–2168. [PubMed] [Google Scholar]
- Bear CE, Davison JS, Shaffer EA. Sodium-dependent taurocholate uptake by isolated rat hepatocytes occurs through an electrogenic mechanism. Biochim Biophys Acta. 1987;903(2):388–394. doi: 10.1016/0005-2736(87)90230-6. [DOI] [PubMed] [Google Scholar]
- Bezerra JA, Balistreri WF. The unique nature of the pediatric liver. Clin Liver Dis. 2000;4(4):xi–xv. doi: 10.1016/s1089-3261(05)70138-0. [DOI] [PubMed] [Google Scholar]
- Bohme M, Muller M, Leier I, Jedlitschky G, Keppler D. Cholestasis caused by inhibition of the adenosine triphosphate-dependent bile salt transport in rat liver. Gastroenterology. 1994;107(1):255–265. doi: 10.1016/0016-5085(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Botham KM, Suckling KE. The effect of dibutyryl cyclic AMP on the uptake of taurocholic acid by isolated rat liver cells. Biochim Biophys Acta. 1986;883(1):26–32. doi: 10.1016/0304-4165(86)90130-3. [DOI] [PubMed] [Google Scholar]
- Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. Embo J. 2005;24(14):2624–2633. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouscarel B, Kroll SD, Fromm H. Signal transduction and hepatocellular bile acid transport: cross talk between bile acids and second messengers. Gastroenterology. 1999;117(2):433–452. doi: 10.1053/gast.1999.0029900433. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Soroka CJ. Vesicle targeting to the apical domain regulates bile excretory function in isolated rat hepatocyte couplets. Gastroenterology. 1995;109(5):1600–1611. doi: 10.1016/0016-5085(95)90649-5. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1124–1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123(5):1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34(1):91–96. doi: 10.1038/ng1147. others. [DOI] [PubMed] [Google Scholar]
- Cattori V, Eckhardt U, Hagenbuch B. Molecular cloning and functional characterization of two alternatively spliced Ntcp isoforms from mouse liver1. Biochim Biophys Acta. 1999;445(1):154–159. doi: 10.1016/s0167-4781(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Chen F, Ma L, Al-Ansari N, Shneider B. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J Biol Chem. 2001;276(42):38703–38714. doi: 10.1074/jbc.M104511200. [DOI] [PubMed] [Google Scholar]
- Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278(22):19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- Chen F, Ma L, Sartor RB, Li F, Xiong H, Sun AQ, Shneider B. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology. 2002;123(6):2005–2016. doi: 10.1053/gast.2002.37055. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen F, Liu S, Glaeser H, Dawson PA, Hofmann AF, Kim RB, Shneider BL, Pang KS. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol Pharmacol. 2006;69(6):1913–1923. doi: 10.1124/mol.105.020792. [DOI] [PubMed] [Google Scholar]
- Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74(11):1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer Res. 1995;55(10):2029–2034. [PubMed] [Google Scholar]
- Childs S, Yeh RL, Hui D, Ling V. Taxol resistance mediated by transfection of the liver-specific sister gene of P-glycoprotein. Cancer Res. 1998;58(18):4160–4167. [PubMed] [Google Scholar]
- Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271(2 Pt 1):G377–385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, Waxman DJ. Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics. 2007;31(1):63–74. doi: 10.1152/physiolgenomics.00055.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MA, Rounds DJ, Karpen SJ, Ananthanarayanan M, Suchy FJ. Assignment of a rat liver Na+/bile acid cotransporter gene to chromosome 6q24. Mamm Genome. 1995;6(1):60. doi: 10.1007/BF00350902. [DOI] [PubMed] [Google Scholar]
- Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274(1 Pt 1):G157–169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Mottino AD, Pozzi EJ Sanchez, Pellegrino JM, Garay EA Rodriguez, Milkiewicz P, Vore M, Coleman R, Roma MG. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52(8):1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocenzi FA, Pozzi EJ Sanchez, Pellegrino JM, Garay EA Rodriguez, Mottino AD, Roma MG. Preventive effect of silymarin against taurolithocholate-induced cholestasis in the rat. Biochem Pharmacol. 2003;66(2):355–364. doi: 10.1016/s0006-2952(03)00253-3. [DOI] [PubMed] [Google Scholar]
- Crossman MW, Hauft SM, Gordon JI. The mouse ileal lipid-binding protein gene: a model for studying axial patterning during gut morphogenesis. J Cell Biol. 1994;126(6):1547–1564. doi: 10.1083/jcb.126.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278(36):33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280(8):6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson LA, Karpen SJ, Bogue CW, Jacobs HC. Divergent homeobox gene hex regulates promoter of the Na(+)-dependent bile acid cotransporter. Am J Physiol Gastrointest Liver Physiol. 2000;279(2):G347–355. doi: 10.1152/ajpgi.2000.279.2.G347. [DOI] [PubMed] [Google Scholar]
- Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121(1):140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- Dias V, Ribeiro V. The expression of the solute carriers NTCP and OCT-1 is regulated by cholesterol in HepG2 cells. Fundam Clin Pharmacol. 2007;21(4):445–450. doi: 10.1111/j.1472-8206.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Martin IV, Porn AC, Voigt S, Gartung C, Trautwein C, Geier A. Fasting induces basolateral uptake transporters of the SLC family in the liver via HNF4alpha and PGC1alpha. Am J Physiol Gastrointest Liver Physiol. 2007;293(3):G585–590. doi: 10.1152/ajpgi.00175.2007. [DOI] [PubMed] [Google Scholar]
- Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, Karpen SJ, Nathanson MH. Short-term regulation of bile acid uptake by microfilament-dependent translocation of rat ntcp to the plasma membrane. Hepatology. 1999;30(1):223–229. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- Duane WC, Xiong W, Wolvers J. Effects of bile acids on expression of the human apical sodium dependent bile acid transporter gene. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbalip.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol. 2006;20(1):65–79. doi: 10.1210/me.2005-0159. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69(4):223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- Figge A, Lammert F, Paigen B, Henkel A, Matern S, Korstanje R, Shneider BL, Chen F, Stoltenberg E, Spatz K. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J Biol Chem. 2004;279(4):2790–2799. doi: 10.1074/jbc.M307363200. others. [DOI] [PubMed] [Google Scholar]
- Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G912–922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001;167(1):83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- Ganguly TC, O’Brien ML, Karpen SJ, Hyde JF, Suchy FJ, Vore M. Regulation of the rat liver sodium-dependent bile acid cotransporter gene by prolactin. Mediation of transcriptional activation by Stat5. J Clin Invest. 1997;99(12):2906–2914. doi: 10.1172/JCI119485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatmaitan ZC, Nies AT, Arias IM. Regulation and translocation of ATP-dependent apical membrane proteins in rat liver. Am J Physiol. 1997;272(5 Pt 1):G1041–1049. doi: 10.1152/ajpgi.1997.272.5.G1041. [DOI] [PubMed] [Google Scholar]
- Geier A, Fickert P, Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(10):574–585. doi: 10.1038/ncpgasthep0602. [DOI] [PubMed] [Google Scholar]
- Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773(3):283–308. doi: 10.1016/j.bbamcr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. Eur J Biochem. 2002;269(14):3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273(16):10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(6):413–431. doi: 10.1007/s00210-006-0043-8. [DOI] [PubMed] [Google Scholar]
- Gong YZ, Everett ET, Schwartz DA, Norris JS, Wilson FA. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc Natl Acad Sci U S A. 1994;91(11):4741–4745. doi: 10.1073/pnas.91.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241(1):117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3(11):950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- Grune S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem. 1993;268(24):17734–17741. [PubMed] [Google Scholar]
- Hagenbuch B. Molecular properties of hepatic uptake systems for bile acids and organic anions. J Membr Biol. 1997;160(1):1–8. doi: 10.1007/s002329900290. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447(5):566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Lubbert H, Stieger B, Meier PJ. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990;265(10):5357–5360. [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93(3):1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin Liver Dis. 1996;16(2):129–136. doi: 10.1055/s-2007-1007226. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Scharschmidt BF, Meier PJ. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem J. 1996;316(Pt 3):901–904. doi: 10.1042/bj3160901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B, Foguet M, Lubbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991;88(23):10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen S, Mareninova O, Branden M, Sachs G. Organization of the membrane domain of the human liver sodium/bile acid cotransporter. Biochemistry. 2002;41(23):7253–7266. doi: 10.1021/bi012152s. [DOI] [PubMed] [Google Scholar]
- Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G829–839. doi: 10.1152/ajpgi.00352.2002. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Bruck R, Ng OC, Boyer JL. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990;259(5 Pt 1):G727–735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41(4):916–924. doi: 10.1002/hep.20627. [DOI] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21(4):1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubi JE, Balistreri WF, Fondacaro JD, Partin JC, Schubert WK. Primary bile acid malabsorption: defective in vitro ileal active bile acid transport. Gastroenterology. 1982;83(4):804–811. [PubMed] [Google Scholar]
- Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279(8):7213–7222. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159(22):2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6(1):15–27. [PubMed] [Google Scholar]
- Horie T, Mizuma T, Kasai S, Awazu S. Conformational change in plasma albumin due to interaction with isolated rat hepatocyte. Am J Physiol. 1988;254(4 Pt 1):G465–470. doi: 10.1152/ajpgi.1988.254.4.G465. [DOI] [PubMed] [Google Scholar]
- Huang HC, Tremont SJ, Lee LF, Keller BT, Carpenter AJ, Wang CC, Banerjee SC, Both SR, Fletcher T, Garland DJ. Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 2) J Med Chem. 2005;48(18):5853–5868. doi: 10.1021/jm0402162. others. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. others. [DOI] [PubMed] [Google Scholar]
- Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117(6):1370–1379. doi: 10.1016/s0016-5085(99)70287-8. others. [DOI] [PubMed] [Google Scholar]
- Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53(1):78–84. doi: 10.1136/gut.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Fried M, Kullak-Ublick GA. Human apical sodium-dependent bile salt transporter gene (SLC10A2) is regulated by the peroxisome proliferator-activated receptor alpha. J Biol Chem. 2002;277(34):30559–30566. doi: 10.1074/jbc.M203511200. [DOI] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G752–761. doi: 10.1152/ajpgi.00456.2003. [DOI] [PubMed] [Google Scholar]
- Jung D, Kullak-Ublick GA. Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology. 2003;37(3):622–631. doi: 10.1053/jhep.2003.50100. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Watanabe N, Mochizuki K, Numari A, Ikeno Y, Itoh J, Tanaka H, Arias IM, Mine T. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- Karpen SJ. Nuclear receptor regulation of hepatic function. J Hepatol. 2002;36(6):832–850. doi: 10.1016/s0168-8278(02)00129-0. [DOI] [PubMed] [Google Scholar]
- Kassam A, Miao B, Young PR, Mukherjee R. Retinoid X receptor (RXR) agonist-induced antagonism of farnesoid X receptor (FXR) activity due to absence of coactivator recruitment and decreased DNA binding. J Biol Chem. 2003;278(12):10028–10032. doi: 10.1074/jbc.M208312200. [DOI] [PubMed] [Google Scholar]
- Keitel V, Vogt C, Haussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology. 2006;131(2):624–629. doi: 10.1053/j.gastro.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kerr TA, Saeki S, Schneider M, Schaefer K, Berdy S, Redder T, Shan B, Russell DW, Schwarz M. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell. 2002;2(6):713–720. doi: 10.1016/s1534-5807(02)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007 doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 2002;122(7):1941–1953. doi: 10.1053/gast.2002.33617. [DOI] [PubMed] [Google Scholar]
- Kim RB, Leake B, Cvetkovic M, Roden MM, Nadeau J, Walubo A, Wilkinson GR. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J Pharmacol Exp Ther. 1999;291(3):1204–1209. [PubMed] [Google Scholar]
- Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275(21):15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276(10):7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of cardiac glycosides. J Pharmacol Exp Ther. 1974;191(2):201–211. [PubMed] [Google Scholar]
- Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53(6):1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40(9):1604–1617. [PubMed] [Google Scholar]
- Kramer W, Wess G. Bile acid transport systems as pharmaceutical targets. Eur J Clin Invest. 1996;26(9):715–732. doi: 10.1111/j.1365-2362.1996.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Krause U, Rider MH, Hue L. Protein kinase signaling pathway triggered by cell swelling and involved in the activation of glycogen synthase and acetyl-CoA carboxylase in isolated rat hepatocytes. J Biol Chem. 1996;271(28):16668–16673. doi: 10.1074/jbc.271.28.16668. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Saha N, Kuhlkamp T, Dutta S, vom Dahl S, Wettstein M, Haussinger D. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279(11):10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Sutfels G, Kuhlkamp T, Kolling R, Haussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126(2):541–553. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kuhlkamp T, Keitel V, Helmer A, Haussinger D, Kubitz R. Degradation of the sodium taurocholate cotransporting polypeptide (NTCP) by the ubiquitin-proteasome system. Biol Chem. 2005;386(10):1065–1074. doi: 10.1515/BC.2005.122. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Glasa J, Boker C, Oswald M, Grutzner U, Hagenbuch B, Stieger B, Meier PJ, Beuers U, Kramer W. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology. 1997;113(4):1295–1305. doi: 10.1053/gast.1997.v113.pm9322525. others. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20(3):273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126(1):322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Kurata H, Suzuki S, Ohhata Y, Ikeda T, Hasegawa T, Kitayama K, Inaba T, Kono K, Kohama T. A novel class of apical sodium-dependent bile acid transporter inhibitors: the amphiphilic 4-oxo-1-phenyl-1,4-dihydroquinoline derivatives. Bioorg Med Chem Lett. 2004;14(5):1183–1186. doi: 10.1016/j.bmcl.2003.12.063. [DOI] [PubMed] [Google Scholar]
- Lam P, Pearson CL, Soroka CJ, Xu S, Mennone A, Boyer JL. Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J Physiol Cell Physiol. 2007;293(5):C1709–1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G476–485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17(1):47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- Lang T, Haberl M, Jung D, Drescher A, Schlagenhaufer R, Keil A, Mornhinweg E, Stieger B, Kullak-Ublick GA, Kerb R. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11) Drug Metab Dispos. 2006;34(9):1582–1599. doi: 10.1124/dmd.105.008854. [DOI] [PubMed] [Google Scholar]
- Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100(11):2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol. 2000;57(1):24–35. [PubMed] [Google Scholar]
- Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118(1):163–172. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- Li H, Chen F, Shang Q, Pan L, Shneider BL, Chiang JY, Forman BM, Ananthanarayanan M, Tint GS, Salen G. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am J Physiol Gastrointest Liver Physiol. 2005;288(1):G60–66. doi: 10.1152/ajpgi.00170.2004. others. [DOI] [PubMed] [Google Scholar]
- Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem J. 2007;407(3):363–372. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Mareninova O, Shin JM, Vagin O, Turdikulova S, Hallen S, Sachs G. Topography of the membrane domain of the liver Na+-dependent bile acid transporter. Biochemistry. 2005;44(42):13702–13712. doi: 10.1021/bi051291x. [DOI] [PubMed] [Google Scholar]
- Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of the Liver Receptor Homolog 1. Mol Cell Biol. 2007 doi: 10.1128/MCB.00852-07. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock C, Shiau YF. Jejunum is more important than terminal ileum for taurocholate absorption in rats. Am J Physiol. 1983;244(5):G507–514. doi: 10.1152/ajpgi.1983.244.5.G507. [DOI] [PubMed] [Google Scholar]
- Meier PJ. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am J Physiol. 1995;269(6 Pt 1):G801–812. doi: 10.1152/ajpgi.1995.269.6.G801. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26(6):1667–1677. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Stieger B. Bile salt transporters. Annu. Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44(1):62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Gatmaitan Z, Varticovski L, Arias IM. The role of phosphoinositide 3-kinase in taurocholate-induced trafficking of ATP-dependent canalicular transporters in rat liver. J Biol Chem. 1998;273(41):26638–26644. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Varticovski L, Arias IM. Phosphoinositide 3-kinase lipid products regulate ATP-dependent transport by sister of P-glycoprotein and multidrug resistance associated protein 2 in bile canalicular membrane vesicles. Proc Natl Acad Sci U S A. 1999;96(10):5814–5819. doi: 10.1073/pnas.96.10.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G316–324. doi: 10.1152/ajpgi.00048.2003. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kudo G, Lee YH, Yang TJ, Gelboin HV, Fernandez-Salguero P, Kimura S, Gonzalez FJ. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J Biol Chem. 1999;274(34):23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA. International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol Rev. 2006;58(4):742–759. doi: 10.1124/pr.58.4.6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol. 1997;273(4 Pt 1):G842–848. doi: 10.1152/ajpgi.1997.273.4.G842. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology. 1998;28(6):1629–1636. doi: 10.1002/hep.510280624. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Webster CR, Anwer MS. Role of protein phosphatases in cyclic AMP-mediated stimulation of hepatic Na+/taurocholate cotransport. J Biol Chem. 1998;273(45):30039–30045. doi: 10.1074/jbc.273.45.30039. [DOI] [PubMed] [Google Scholar]
- Neimark E, Chen F, Li X, Magid MS, Alasio TM, Frankenberg T, Sinha J, Dawson PA, Shneider BL. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology. 2006;131(2):554–567. doi: 10.1053/j.gastro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40(1):149–156. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33(5):1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, Mullhaupt B, Meier PJ, Pauli-Magnus C. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43(3):536–543. doi: 10.1016/j.jhep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Noe J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123(5):1659–1666. doi: 10.1053/gast.2002.36587. [DOI] [PubMed] [Google Scholar]
- Nowicki MJ, Shneider BL, Paul JM, Heubi JE. Glucocorticoids upregulate taurocholate transport by ileal brush-border membrane. Am J Physiol. 1997;273(1 Pt 1):G197–203. doi: 10.1152/ajpgi.1997.273.1.G197. [DOI] [PubMed] [Google Scholar]
- Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99(8):1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuwaki M, Takada T, Iwayanagi Y, Koh S, Kariya Y, Fujii H, Suzuki H. LXR alpha transactivates mouse organic solute transporter alpha and beta via IR-1 elements shared with FXR. Pharm Res. 2007;24(2):390–398. doi: 10.1007/s11095-006-9163-6. [DOI] [PubMed] [Google Scholar]
- Elferink RP Oude, Paulusma CC, Groen AK. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 2006;130(3):908–925. doi: 10.1053/j.gastro.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–1368. doi: 10.1126/science.284.5418.1365. others. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Kerb R, Fattinger K, Lang T, Anwald B, Kullak-Ublick GA, Beuers U, Meier PJ. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39(3):779–791. doi: 10.1002/hep.20159. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14(2):91–102. doi: 10.1097/00008571-200402000-00003. others. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44(4):778–787. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43(2):342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Plass JR, Mol O, Heegsma J, Geuken M, de Bruin J, Elling G, Muller M, Faber KN, Jansen PL. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J Hepatol. 2004;40(1):24–30. doi: 10.1016/s0168-8278(03)00483-5. [DOI] [PubMed] [Google Scholar]
- Rausa FM, Tan Y, Zhou H, Yoo KW, Stolz DB, Watkins SC, Franks RR, Unterman TG, Costa RH. Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol Cell Biol. 2000;20(21):8264–8282. doi: 10.1128/mcb.20.21.8264-8282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Renga B, Antonelli E, Passeri D, Pellicciari R, Fiorucci S. The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol Pharmacol. 2005;68(2):551–558. doi: 10.1124/mol.105.012104. [DOI] [PubMed] [Google Scholar]
- Root C, Smith CD, Sundseth SS, Pink HM, Wilson JG, Lewis MC. Ileal bile acid transporter inhibition, CYP7A1 induction, and antilipemic action of 264W94. J Lipid Res. 2002;43(8):1320–1330. [PubMed] [Google Scholar]
- Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y. Three hundred twenty-six genetic variations in genes encoding nine members of ATP-binding cassette, subfamily B (ABCB/MDR/TAP), in the Japanese population. J Hum Genet. 2002;47(1):38–50. doi: 10.1007/s10038-002-8653-6. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Kubitz R, Lizun S, Wettstein M, Haussinger D. Regulation of the dynamic localization of the rat Bsep gene-encoded bile salt export pump by anisoosmolarity. Hepatology. 2001;33(3):509–518. doi: 10.1053/jhep.2001.22648. [DOI] [PubMed] [Google Scholar]
- Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278(30):27473–27482. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- Shiao T, Iwahashi M, Fortune J, Quattrochi L, Bowman S, Wick M, Qadri I, Simon FR. Structural and functional characterization of liver cell-specific activity of the human sodium/taurocholate cotransporter. Genomics. 2000;69(2):203–213. doi: 10.1006/geno.2000.6329. [DOI] [PubMed] [Google Scholar]
- Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet. 2001;27(4):375–382. doi: 10.1038/86871. others. [DOI] [PubMed] [Google Scholar]
- Shulman AI, Mangelsdorf DJ. Retinoid x receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353(6):604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- Simon FR, Fortune J, Iwahashi M, Qadri I, Sutherland E. Multihormonal regulation of hepatic sinusoidal Ntcp gene expression. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G782–794. doi: 10.1152/ajpgi.00379.2003. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118(2):422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453(5):611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20(3):233–238. doi: 10.1038/3034. others. [DOI] [PubMed] [Google Scholar]
- Suchy FJ, Ananthanarayanan M. Bile salt excretory pump: biology and pathobiology. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S10–16. doi: 10.1097/01.mpg.0000226385.71859.5f. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Ananthanarayanan M, Soroka CJ, Thevananther S, Shneider BL, Suchy FJ. Sorting of rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am J Physiol. 1998;275(5 Pt 1):G1045–1055. doi: 10.1152/ajpgi.1998.275.5.G1045. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Arrese MA, Zeng L, Swaby I, Zhou MM, Suchy FJ. The rat liver Na(+)/bile acid cotransporter. Importance of the cytoplasmic tail to function and plasma membrane targeting. J Biol Chem. 2001;276(9):6825–6833. doi: 10.1074/jbc.M008797200. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Balasubramaniyan N, Xu K, Liu CJ, Ponamgi VM, Liu H, Suchy FJ. Protein-protein interactions and membrane localization of the human organic solute transporter. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1586–1593. doi: 10.1152/ajpgi.00457.2006. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Salkar R, Sachchidanand, Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem. 2003;278(6):4000–4009. doi: 10.1074/jbc.M207163200. [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312(2):841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- Thaysen EH, Pedersen L. Diarrhoea associated with idiopathic bile acid malabsorption. Fact or fantasy? Dan Med Bull. 1973;20(6):174–177. [PubMed] [Google Scholar]
- Thomas C, Landrier JF, Gaillard D, Grober J, Monnot MC, Athias A, Besnard P. Cholesterol dependent downregulation of mouse and human apical sodium dependent bile acid transporter (ASBT) gene expression: molecular mechanism and physiological consequences. Gut. 2006;55(9):1321–1331. doi: 10.1136/gut.2005.085555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson MB, Vernier WF, Huang HC, Chen FP, Reinhard EJ, Beaudry J, Keller BT, Reitz DB. A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 2,3-disubstituted-4-phenylquinolines. Bioorg Med Chem Lett. 2000;10(3):277–279. doi: 10.1016/s0960-894x(99)00683-6. [DOI] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest. 1998;101(10):2092–2100. doi: 10.1172/JCI1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Van Dyke RW, Stephens JE, Scharschmidt BF. Bile acid transport in cultured rat hepatocytes. Am J Physiol. 1982;243(6):G484–492. doi: 10.1152/ajpgi.1982.243.6.G484. [DOI] [PubMed] [Google Scholar]
- van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127(2):379–384. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- von Bergmann K, Schwarz HP, Paumgartner G. Proceedings: Increased cholesterol saturation in rat bile induced by spironolactone and pregnenolone-16alpha-carbonitrile. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(0suppl 282):R288. [PubMed] [Google Scholar]
- von Dippe P, Amoui M, Stellwagen RH, Levy D. The functional expression of sodium-dependent bile acid transport in Madin-Darby canine kidney cells transfected with the cDNA for microsomal epoxide hydrolase. J Biol Chem. 1996;271(30):18176–18180. doi: 10.1074/jbc.271.30.18176. [DOI] [PubMed] [Google Scholar]
- von Dippe P, Zhu QS, Levy D. Cell surface expression and bile acid transport function of one topological form of m-epoxide hydrolase. Biochem Biophys Res Commun. 2003;309(4):804–809. doi: 10.1016/j.bbrc.2003.08.074. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2(6):721–731. doi: 10.1016/s1534-5807(02)00187-9. others. [DOI] [PubMed] [Google Scholar]
- Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest. 2002;110(7):965–972. doi: 10.1172/JCI15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38(6):1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98(4):2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A. 2001;98(16):9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131(3):878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CR, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277(6 Pt 1):G1165–1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- Webster CR, Blanch C, Anwer MS. Role of PP2B in cAMP-induced dephosphorylation and translocation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G44–50. doi: 10.1152/ajpgi.00530.2001. [DOI] [PubMed] [Google Scholar]
- Webster CR, Blanch CJ, Phillips J, Anwer MS. Cell swelling-induced translocation of rat liver Na(+)/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem. 2000;275(38):29754–29760. doi: 10.1074/jbc.M002831200. [DOI] [PubMed] [Google Scholar]
- Weinman SA. Electrogenicity of Na(+)-coupled bile acid transporters. Yale J Biol Med. 1997;70(4):331–340. [PMC free article] [PubMed] [Google Scholar]
- Weinman SA, Carruth MW, Dawson PA. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J Biol Chem. 1998;273(52):34691–34695. doi: 10.1074/jbc.273.52.34691. [DOI] [PubMed] [Google Scholar]
- Wess G, Kramer W, Enhsen A, Glombik H, Baringhaus KH, Boger G, Urmann M, Bock K, Kleine H, Neckermann G. Specific inhibitors of ileal bile acid transport. J Med Chem. 1994;37(7):873–875. doi: 10.1021/jm00033a001. others. [DOI] [PubMed] [Google Scholar]
- West KL, Ramjiganesh T, Roy S, Keller BT, Fernandez ML. 1-[4-[4[(4R,5R)-3,3-Dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy -1,1-dioxido-1-benzothiepin-5-yl]phenoxy]butyl]-4-aza-1-azoniabicyclo[2.2. 2]octane methanesulfonate (SC-435), an ileal apical sodium-codependent bile acid transporter inhibitor alters hepatic cholesterol metabolism and lowers plasma low-density lipoprotein-cholesterol concentrations in guinea pigs. J Pharmacol Exp Ther. 2002;303(1):293–299. doi: 10.1124/jpet.102.038711. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Treanor LL. Characterization of the passive and active transport mechanisms for bile acid uptake into rat isolated intestinal epithelial cells. Biochim Biophys Acta. 1975;406(2):280–293. doi: 10.1016/0005-2736(75)90010-3. [DOI] [PubMed] [Google Scholar]
- Wolters H, Elzinga BM, Baller JF, Boverhof R, Schwarz M, Stieger B, Verkade HJ, Kuipers F. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J Hepatol. 2002;37(5):556–563. doi: 10.1016/s0168-8278(02)00247-7. [DOI] [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269(2):1340–1347. [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270(45):27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- Wong MH, Rao PN, Pettenati MJ, Dawson PA. Localization of the ileal sodium-bile acid cotransporter gene (SLC10A2) to human chromosome 13q33. Genomics. 1996;33(3):538–540. doi: 10.1006/geno.1996.0233. [DOI] [PubMed] [Google Scholar]
- Xia X, Roundtree M, Merikhi A, Lu X, Shentu S, Lesage G. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J Biol Chem. 2004;279(43):44931–44937. doi: 10.1074/jbc.M400969200. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006;281(24):16625–16631. doi: 10.1074/jbc.M602723200. [DOI] [PubMed] [Google Scholar]
- Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277(35):31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- Zhu QS, Xing W, Qian B, von Dippe P, Shneider BL, Fox VL, Levy D. Inhibition of human m-epoxide hydrolase gene expression in a case of hypercholanemia. Biochim Biophys Acta. 2003;1638(3):208–216. doi: 10.1016/s0925-4439(03)00085-1. [DOI] [PubMed] [Google Scholar]
- Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3(3):231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G798–805. doi: 10.1152/ajpgi.00319.2004. others. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G923–932. doi: 10.1152/ajpgi.00490.2005. others. [DOI] [PubMed] [Google Scholar]