Abstract
Background
Increased left atrial diameter (LAD) is associated with elevated risk of atrial fibrillation (AF) and cardiovascular disease. Information is limited regarding the short- or long-term correlates of LAD.
Methods and Results
We evaluated clinical correlates of LAD over a 16-year period in 4,403 Framingham Study participants (mean age 45 years, 52% women; median observations/participant=3) using multi-level modeling. We related age, sex, body mass index (BMI), systolic and diastolic blood pressure (BP), diabetes and anti-hypertensive treatment to LAD. Sex-specific growth curves for LAD were estimated for individuals with low, intermediate and high risk factor burden. We also related risk factors to changes in LAD over a 4-year period in 3,365 participants.
Age, male sex (+3.83 mm compared to women), greater BMI, higher systolic BP (0.24 mm per 10 mmHg increment), and anti-hypertensive treatment (+0.54 mm) were associated positively with LAD (p<0.001). Men had a greater increase in LAD with BMI than women (+2.02 vs. +1.77 mm in women, per 5-unit increment) and individuals on anti-hypertensive treatment experienced a greater increase in LAD with age (0.95 vs. 0.63 mm per 10–year age increment). Overall, greater risk factor burden was positively associated with LAD. These risk factors were also associated positively with 4-year change in LAD (p<0.001).
Conclusions
Our longitudinal study of a large community-based sample identified higher BP and greater BMI as key modifiable correlates of LAD, suggesting that maintaining optimal levels of these risk factors over the life course may prevent atrial remodeling and AF.
Keywords: left atrial diameter, atrial enlargement, serial measurements, epidemiology, multi-level modeling, echocardiography
Introduction
Atrial fibrillation (AF) is the most common sustained dysrhythmia, affecting over 2 million people in the United States.1 Preventing AF is a priority given the high lifetime risk for developing the condition, the projected increase in population burden, and the substantial morbidity and mortality associated with the disease.2 Identification and characterization of intermediate phenotypes for AF may identify high risk individuals prior to disease onset.2,3
Atrial remodeling, a process characterized by atrial structural and electrophysiologic changes, plays a central role in AF initiation and maintenance.2 Increased LAD is an echocardiographic marker of atrial remodeling and has well-established associations with the incidence of AF, heart failure, stroke, and with all-cause mortality.3-7 LA enlargement is often seen in association with left ventricular dysfunction and valvular heart disease, and in these settings reflects chronic LA volume or pressure overload.6,8 Whereas many risk factors have been identified for increased LAD and for AF in cross-sectional studies, little is known about the clinical determinants of longitudinal changes in LAD over the adult life course.9
We hypothesized that clinical factors associated with greater LAD in cross-sectional studies and with incident AF prospectively (e.g., age, sex, adiposity, systolic and diastolic blood pressure [BP], diabetes, and treatment with anti-hypertensive medication)9-15 are also key correlates long-term tracking of LAD during adulthood. We also postulated that cumulative risk factor burden would influence LAD both at baseline and its tracking over the adult life course. We tested these hypotheses by evaluating the clinical correlates of LAD in a large community-based sample that underwent serial echocardiography over a 16-year period spanning young- to mid-adulthood. We also examined the relation between these correlates and short-term (4 years) change in LAD.
Methods
Study sample
The study sample was comprised of 4,403 Framingham Offspring Study participants. The design of the Framingham Offspring Study has been described previously.16 In brief, participants are evaluated at the Heart Study approximately every 4-8 years. Each Heart Study visit includes a physician-administered medical history and physical examination, anthropometry, and laboratory evaluation of standard risk factors.
For the present investigation we focused on attendees at examination cycles 2 (1979-1982), 4 (1987-1990), 5 (1991-1995) and 6 (1996-1998) at which the participants underwent routine echocardiography (see below). Echocardiographic observations were excluded from this analysis for the following reasons: missing LAD (3,706 observations); age <25 or ≥75 years at any of the eligible examinations (303 observations); prevalent AF (195 observations), myocardial infarction or heart failure (437 observations), or valvular disease (174 observations) at these examinations. All observations with missing covariates (84 observations) were also excluded, leaving a total of 13,293 echocardiographic observations. Due to the nature of statistical modeling, although 3,706 echocardiographic observations were missing, only 30 unique participants with missing LAD at each of the eligible examinations were excluded from the overall analysis. Participants were considered to have valvular heart disease if either a grade 3/6 or higher systolic murmur or any diastolic murmur was auscultated by the Heart Study physician. Criteria used for defining myocardial infarction and heart failure have been described previously.17 AF was determined from 12-lead electrocardiograms (ECGs) obtained at each examination, and by reviewing ECGs obtained from hospitalization records or physician office visits.18 Study participants provided written informed consent, and the study protocol was approved by the Institutional Review Board at the Boston University Medical Center.
Echocardiographic examinations
Each participant completed a maximum of four sets of echocardiographic measurements at examination cycles 2, 4, 5, and 6, yielding a total of 13,293 observations (Figure 1) for the longitudinal analyses (median number of observations per participant =3). Although echocardiographic equipment differed across examinations, all echocardiograms were performed by experienced technicians and evaluated by trained technicians or cardiologists using a standardized protocol. LAD was determined from M-mode echocardiograms in accordance with the American Society of Echocardiography guidelines using a leading edge–to–leading edge technique, measuring the maximal distance between the posterior aortic root wall and the posterior LA wall at end-systole.19 Although data on the longitudinal reproducibility of LAD measurements using different echocardiographic equipment are lacking, excellent interobserver and intraobserver reproducibility for M-mode measurements of LAD have been reported in cross-sectional studies using these methods,20 including at the Framingham Heart Study.21
Figure 1.
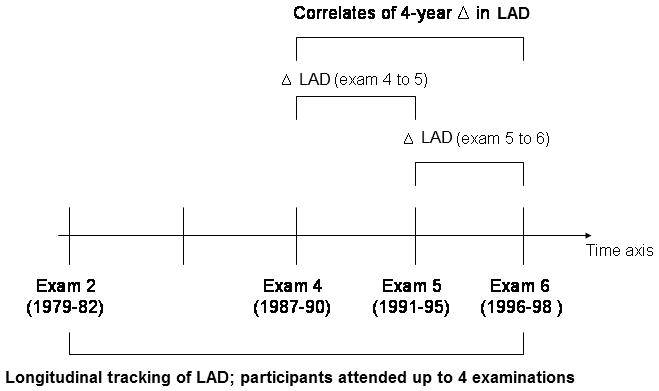
Overview of study design. Δ indicates change; LAD, left atrial dimension
Analyses of short-term LAD change focused on 3,365 participants who attended two consecutive Heart Study examinations at which echocardiography was performed (Figure 1). Whereas our longitudinal analyses focused on absolute LAD as the outcome variable, our short-term analyses examined change in LAD (regression coefficients for risk factors in the two sets of analyses are not directly comparable). Data from pairs of examination cycles (4, 5) and (5, 6) were pooled in order to maximize the number of observations available for short-term analyses (5,933 echocardiographic observations).
Statistical analyses
Multi-level modeling
Multi-level statistical modeling allows for the analysis of data that vary both within and between participants in a longitudinal study with serial multiple observations on individuals (Figure 1). This approach has the advantage of accommodating study participants with missing data at one or more examinations, thereby increasing the number of observations available for analyses and enhancing statistical power to detect key correlates of LAD. The method also allows for variable time intervals between serial examinations.
Growth Curves relating LAD to Clinical Covariates
Using a multi-level statistical model that adjusted for relatedness of individuals (SAS PROC MIXED with a “random statement;” using an unstructured correlation matrix), we related LAD (dependent variable) to the following risk factors (independent variables): age, sex, adiposity (BMI), systolic BP, diastolic BP, use of anti-hypertensive medications, and diabetes. These clinical variables were chosen a priori on the basis of their cross-sectional association with LA enlargement and AF and their association with LAD was examined using a direct entry model-building approach.9,22-29 Values for covariates were obtained from examinations corresponding to LAD assessment. Data are presented as increment in LAD per unit-increment in continuous covariates.
The association of baseline LAD with follow-up LAD was determined by relating tertiles of baseline LAD to mean LAD change over 16-years. The examination cycle was included as a variable in our analyses in order to adjust for differences in LAD related to changes in echocardiographic instrumentation. Non-linear effects of age also were examined, but were not statistically significant. Interaction terms for each covariate with age and sex were investigated using multivariable models.
Growth Curves for LAD based on Risk Factor Burden
To graphically illustrate the effect of age on LAD, 3 groups (low, intermediate, and high risk factor burden) were created for men and women on the basis of their risk factor profile, and growth curves for LAD were generated adjusting for other variables in our random effects model (Figure 2A and 2B). Since BP and BMI emerged as the key correlates, levels of these risk factors were used to define the 3 groups: women and men with a BMI of 25 kg/m2 with normal BP (defined as having a BP of 113/73, median BP for normotensive participants) and not receiving anti-hypertensive medications] were considered to have a low risk factor burden; individuals with a BMI of 27.5 kg/m2 and pre-hypertension (defined as having a BP of 133/86, median BP for pre-hypertensive participants) and not receiving anti-hypertensive medications were considered to have an intermediate risk factor burden; those with a BMI of 30 kg/m2 and hypertension (defined as having a BP of 146/91, median BP for hypertensive participants) were categorized as having a high risk factor burden. Thus, the median BP values used for plotting growth curves were determined from participants with BP values falling in normal, pre-hypertensive, or hypertensive ranges.
Figure 2.
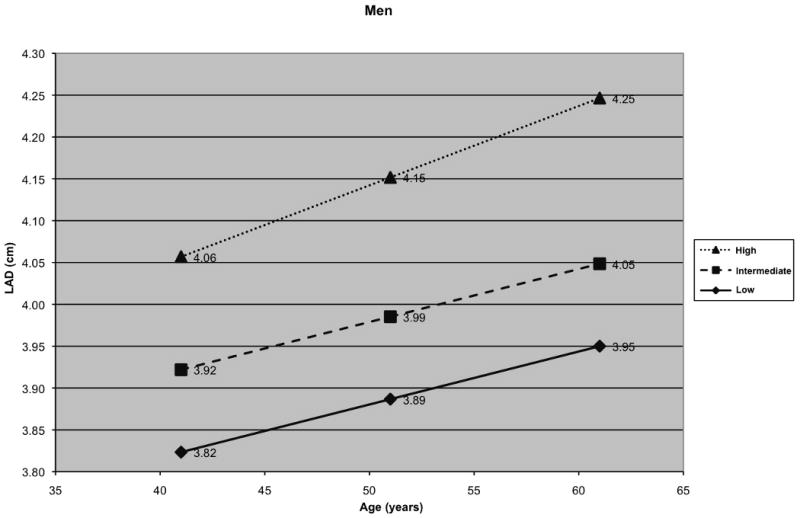
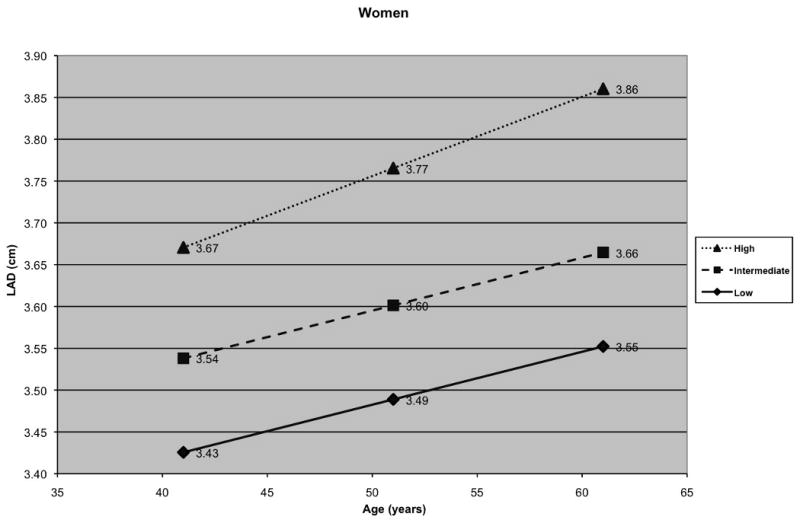
A) Long-term tracking of LA size in men and B) Long-term tracking of LA size in women with low, intermediate and high risk factor profiles (women and men with a low risk factor profile - black diamond; women and men with an intermediate risk factor profile - black square; women and men with a high risk factor profile - black triangle)
Analyses of short-term changes in LAD
Generalized estimating equations were used to evaluate the clinical correlates of changes in LAD over the short-term (4 year mean follow-up). Multivariable models adjusted for the same set of covariates used in the long-term analyses. Interaction terms examined in long-term analyses were also examined in short-term analyses.
A two-sided p of <0.05 indicated statistical significance. The authors had full access to the data and take responsibility for its integrity. All authors have read the manuscript as written and agreed with submission in its current form.
Results
The baseline clinical and echocardiographic characteristics of the study samples are shown in Table 1.
Table 1.
Clinical and echocardiographic characteristics of the study samples used to characterize clinical correlates of short-term (mean follow-up 4 years) change in and long-term (maximum 16 year-period) tracking of LA size
| Sample for correlates of long-term LA size tracking | Sample for correlates of short-term LA size change | |||
|---|---|---|---|---|
| Variable | Men (n=2102) | Women (n=2301) | Men (n=1559) | Women (n=1806) |
| Clinical features* | ||||
| Age, years | 46±10 | 45±10 | 51±10 | 51±10 |
| Systolic BP, mm Hg | 127±16 | 119±17 | 129±17 | 124±19 |
| Diastolic BP, mm Hg | 81±9 | 76±9 | 81±10 | 76±10 |
| Use of anti-hypertensives, % | 11.7 | 9.7 | 18.2 | 14.3 |
| Hypertension, % | 30.3 | 19.8 | 39.0 | 29.0 |
| Height, cm | 175±7 | 161±6 | 175±7 | 162±6 |
| Weight, kg | 83±13 | 65±14 | 86±13 | 68±14 |
| BMI, kg/m2 | 27.1±3.8 | 25.2±5.1 | 27.8±3.9 | 26.1±5.4 |
| Diabetes, % | 6.7 | 3.3 | 7.8 | 4.9 |
| Echocardiographic features | ||||
| Baseline LA size, mm | 39.82±4.42 | 34.81±4.29 | 39.56±4.74 | 35.43±4.69 |
| Follow-up LA size, mm | N/A | N/A | 40.26±4.76 | 35.67±4.74 |
Values are mean ± SD or percentages. Abbreviations: BP, blood pressure; LA, left atrial; anti-hypertensives = anti-hypertensive medications; N/A= not applicable. For the sample evaluating long-term tracking of LA size, characteristics are from the first eligible examination.
Values for covariates were obtained from the first available examination for each participant
Clinical correlates of long-term LAD tracking
Age, male sex, BMI, systolic BP, and use of anti-hypertensive medications were positively related to LAD, whereas diastolic BP was inversely related (Table 2). When pulse pressure was substituted for systolic and diastolic BP, LAD was positively related to pulse pressure (0.48 mm increase per 20 mmHg increment in pulse pressure, p< 0.0001). Statistically significant interactions were observed for sex and BMI, for age and anti-hypertensive medication use, and for sex and diastolic BP. Higher BMI was associated with a greater increment in LAD in men compared to women (p=0.009). Lower diastolic BP was associated with a greater increment in LAD in men compared to women (p=0.009). A greater increment in LAD was noted with advancing age in participants receiving anti-hypertensive medications compared to those not on anti-hypertensive treatment (p=0.003).
Table 2.
Clinical correlates of longitudinal tracking of LA size over a 16-year period
| Predictor variable | Effect on LA size (mm) | 95% Confidence Interval |
|---|---|---|
| Male sex* | 3.83 | (3.62,4.04) |
| Age (10 year increase; Use of anti-hypertensive medications) | 0.95 | (0.74,1.15) |
| Age (10 year increase; No use of anti-hypertensive medications) | 0.63 | (0.55,0.72) |
| BMI (5 units increase, men) | 2.02 | (1.87,2.18) |
| BMI (5 units increase, women) | 1.77 | (1.66,1.88) |
| Systolic BP (10 mm Hg increase) | 0.24 | (0.18,0.30) |
| Diastolic BP (10 mm Hg increase, men) | -0.39 | (-0.52,-0.26) |
| Diastolic BP (10 mm Hg increase, women) | -0.19 | (-0.31,-0.06) |
| Use of anti-hypertensive medications** | 0.54 | (0.29,0.78) |
The table shows the change in LA size per increment of the predictor variable as indicated. There was a significant interaction of age and use of anti-hypertensive medications, diastolic BP and sex, as well as BMI and sex. Therefore, the effects of age, diastolic BP and BMI are provided in the appropriate subgroups (men and women and use and non-use of anti-hypertensive medications).
The effect of male sex (as compared to female sex) is for participants with an age of 45 years (mean age of all participants at all exams), diastolic BP of 80 mm Hg and a BMI of 25 kg/m2, due to the inclusion of sex in significant interaction terms.
The effect of use of anti-hypertensive medications is for participants aged 45 years.
Baseline LAD was inversely related to long-term change in LAD. Compared to participants in the highest tertile of baseline LAD (LAD >39.5 mm), participants in the second tertile (34.5 mm ≤LAD ≤39.5 mm) and lowest tertile (LAD <34.5 mm) experienced a 1.9 mm and 4.5 mm greater change in mean LAD over time, respectively (p<0.001 for both).
We did not observe a significant association of diabetes with long-term tracking of LAD. Given the strong association between increased BMI and diabetes, we suspected that inclusion of BMI in our long-term models confounded the association between diabetes and LAD. After exclusion of BMI as a covariate in the models, multivariable modeling allowed for detection of a statistically significant positive association of diabetes with LAD (p<0.0001). When analyses were repeated incorporating body surface area instead of BMI as a measure of body size in multivariable models, we observed risk factor associations with LAD that were quite consistent with those noted for models with BMI (data not shown).
Impact of risk factor burden on LAD over adulthood
When compared to participants with intermediate levels of BP and BMI, participants with higher BP and BMI had a larger baseline LAD and a greater LAD over the 16-year follow-up period (Figure 2). Individuals with an optimal level of BP and BMI had smaller LAD at baseline and experienced lesser increments in LAD on follow-up relative to the other 2 groups. Figure 2 illustrates a step-wise increase in LAD at baseline and on follow-up with increasing BP and BMI, i.e. the change in LAD from low to intermediate group, or from intermediate to higher risk factor burden group, was approximately equivalent to that noted for twenty years of aging.
Clinical correlates of short-term changes in LAD
Analyses identified male sex, age, BMI, systolic BP and use of anti-hypertensive medications as key clinical correlates of short-term changes in LAD (Table 3). In addition, baseline LAD was inversely associated with short-term changes in LAD. The statistical interaction term (male*diabetes) was associated positively with LAD change, indicating that men with diabetes had greater increments in LAD compared to men without diabetes (β=3.29 and β =2.34, respectively). The significance of the male*diabetes interaction term also suggests that men with diabetes had greater increments in LAD compared to women with diabetes (β=0.66 and β =-0.28, respectively). Neither diastolic BP nor the interaction terms sex*BMI, sex*diastolic BP, or age*use of anti-hypertensive medications (observed in long-term analyses) demonstrated statistically significant associations in analyses of short-term changes in LAD.
Table 3.
Clinical correlates of short-term changes in LA size over a 4-year period
| Predictor variable | Change in LA Size (mm) | 95% Confidence Interval |
|---|---|---|
| Male sex (with diabetes)* | 3.29 | (2.55,4.03) |
| Male sex (without diabetes) | 2.34 | (2.12,2.57) |
| BMI (5 units increase) | 0.97 | (0.85,1.09) |
| Systolic BP (10 mm Hg increase) | 0.10 | (0.04,0.16) |
| Use of anti-hypertensive medications | 0.64 | (0.34,0.93) |
| Age (10 year increase) | 0.41 | (0.29,0.53) |
| Baseline LA size | -5.85 | (-6.13,-5.56) |
| Diabetes (men) | 0.66 | (0.24,1.08) |
| Diabetes (women) | -0.28 | (-0.86,0.29) |
The table shows the change in LA size per increment of the predictor variable as indicated.
Discussion
Increased LAD is associated with increased risk of AF (new-onset or recurrent), other cardiovascular disease and mortality.3-7,30-32 Therefore, an understanding of tracking of LAD over the adult life course in relation to known risk factors would be useful.
Principal findings
Age, male sex, higher BMI, higher systolic and lower diastolic BP, and use of anti-hypertensive medications were associated with greater LAD at baseline and over a 16-year follow-up period. Analyses of short-term change in LAD identified a similar set of risk factors. Important interactions between sex and diastolic BP, sex and BMI, as well as age and use of anti-hypertensive medications were noted in the long-term analysis. Men experienced a greater degree of LA enlargement over time with higher BMI levels and with lower diastolic BP (compared to women). Participants receiving anti-hypertensive medications also had a greater increase in LAD with increasing age compared to those not on anti-hypertensive treatment. Higher BMI and BP during mid-life were associated with greater LAD over time. Of note, we did not find an independent association of diabetes with long-term tracking of LAD in the present investigation, although an association of diabetes with change in LAD was observed in men in the short-term analyses. The inverse relation of baseline measurements to LAD on follow-up in both long- and short-term analyses likely reflects in part the phenomenon of ‘regression to the mean’ that is well known in longitudinal epidemiological investigations with serial measurements of select variables.
Comparison with the published literature
Relation of age to LAD
Autopsy, echocardiographic and radiographic studies have shown that advancing age is associated with increasing LAD.33-38 These changes have been attributed in part to age-associated alterations in myocardial (atrial) tissue composition.39 Recent analyses using volumetric atrial data suggest that atrial enlargement may not be a part of the ‘normal’ aging process per se, but may be the consequence of a greater burden of risk factors that accompanies aging.40 Our data suggest that age is a significant correlate of short- and long-term LAD, even in individuals with a low risk factor burden (Figure 2, panels A and B). However, risk factor burden strongly influenced LAD at baseline and during follow-up. As Figure 2 shows, the increase in LAD noted with change in risk factor burden group from low to intermediate, or from intermediate to high, was equivalent to approximately twenty years of aging. Of note, individuals receiving anti-hypertensive medications had higher LAD at baseline, and the effect of age on LAD was more pronounced in those receiving treatment for hypertension. This is likely due to the observation that people who receive anti-hypertensive medications typically have a greater chronicity and severity of BP elevation. Our findings suggest that hypertension plays a key role in determining baseline LAD and mediating LA enlargement during adulthood.
Relation of sex to LAD
Men in our sample had higher LAD at baseline and on short- and long-term follow-up after accounting for several covariates, including measures of body size. These observations are consistent with some investigations that reported sex-related differences in LA volumes.41 Absolute risk of AF is higher in men compared to women,42 and we speculate that this may be related in part to availability of a greater amount of atrial myocardium that serves as a substrate for the dysrhythmia. However, our findings differ from some previous cross-sectional studies that have reported that sex-related differences in LAD are nearly or completely accounted for by variation in body size among men and women.23,41,43
Relation of adiposity (BMI) to LAD
Several cross-sectional studies have demonstrated that obesity and greater BMI are important correlates of larger LAD (including LA volume).39 Higher BMI is also an important predictor of AF incidence in longitudinal studies.44-46 In the present study, we confirm the cross-sectional association noted above using a longitudinal design. The mechanisms by which increased BMI promotes LA enlargement may relate to hemodynamic changes (including increased intravascular volume and cardiac output as well as increased stroke volume) in addition to metabolic changes (including insulin resistance).24 We noted modest sex-related difference in the relations of BMI and LAD on longitudinal analyses, with a steeper slope noted in men. These observations are consistent with a previous cross-sectional study from our group that noted that a SD increment in BMI resulted in a 10% greater increment in LAD in men (compared to women).37 Overall, these findings are also consistent with sex-related differences in cardiac remodeling responses to volume-overload states.47 Men experience greater LV dilation in response to hemodynamic changes, and it is conceivable that this response pattern extends to LAD.
Relation of systolic BP and pulse pressure to LAD
Systolic BP and pulse pressure have been shown to be associated with greater LAD in a previous cross-sectional report from the Framingham Study,36 and with greater risk of AF prospectively.15, 37 Increased cardiac pulsatile load, as indicated by a higher pulse-pressure, promotes LA enlargement and is a key mediator of increased AF risk, especially in older adults. Our analyses are consistent with these observations. Of note, we noticed a stronger association of lower diastolic BP with LAD in men (compared to women) in our longitudinal analyses. Additional studies are warranted to confirm this latter finding, which may suggest a greater impact of pulsatile hemodynamics on LAD in men.
Relation of diabetes to LAD
Although we observed a statistical interaction between diabetes and male sex in our short-term analysis, diabetes was not independently associated with long-term tracking of LAD. Although our study conflicts with prior work showing an association between diabetes and increased LAD,25 it is noteworthy that the association between diabetes and LAD in that study was markedly attenuated by adjustment for BMI and rendered statistically non-significant after adjustment for history of AF. Since higher BMI is a strong risk factor for diabetes and diabetes may be along the causal pathway from BMI to greater LAD, adjustment for BMI in our long-term analyses may result in an underestimation of the potential contribution of diabetes to LAD.
Strengths and limitations
The strengths of the present investigation include the use of multi-level modeling in a large community-based sample with multiple serial echocardiographic observations, and a comprehensive evaluation of both short-term change in and long-term tracking of LAD.
Our study has important limitations. First, the observational nature of our study precludes causal inferences. Second, while M-mode based measurement protocols for LAD were consistent over the long study period, changes in echocardiographic instrumentation raise issues of comparability across examinations. However, any differences in LAD across examinations would likely have resulted in random misclassification and would bias toward the null hypothesis of no association of LAD with clinical covariates studied. In addition, we adjusted for ‘examination cycle’ in our analyses to account for changes in instrumentation. Third, the anteroposterior LA dimension provided by M-mode and used for the determination of LAD in our analyses may not reflect true atrial size.48 Although the LA generally enlarges in a spherical fashion, symmetrical enlargement does not always occur, and thus volumetric assessment of LA size is a more accurate measure of atrial size.49, 50 M-mode based LA assessment is, however, a well-studied and valid measure of LA size19 and we were limited by the availability of only M-mode data in the Framingham Study at the earlier examination cycles. Fourth, we excluded individuals with valve disease defined on the basis of physical examination, which may not be as sensitive as Doppler echocardiographic assessment (the latter was not available at earlier examinations). It is likely that some individuals in our sample had valve disease, which could increase LAD. Fifth, 30 participants were excluded on the basis of missing LAD measurements. These participants were on older and were more likely to be hypertensive (higher systolic BP level, and using anti-hypertensive medications) than participants included in our study sample. It is possible that exclusion of these participants influenced our findings. Sixth, participants in the Framingham Offspring cohort are middle-aged to elderly and largely white. The generalizability of our findings to other age or racial groups is unknown.
Conclusion
Given the increasing prevalence of AF in the United States and worldwide, it is important to identify and characterize important precursors, such as increased LAD. Our longitudinal investigation of a large community-based sample identified higher BP and BMI as key risk factors for short- and long-term changes in LAD. Indeed, individuals with a higher risk factor burden had greater LAD equivalent to 20-years of aging, relative to those with optimal risk factors. These data, while observational, are consistent with the notion that maintenance of optimal levels of BP and BMI over the life course may be critical for preventing increases in LA size that accompany the aging process, and may aid the prevention of AF itself.
Acknowledgments
None.
Sources of Funding: This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195), 6R01-NS 17950, 2 K24 HL04334, RO1HL080124 (RSV); 1R01HL092577(EJB).
Footnotes
Conflict of Interest Disclosures: none
Commentary: Preventing atrial fibrillation (AF) is a public health priority in light of the high lifetime risk for this condition, the projected increase in population burden, and the substantial morbidity and mortality associated with the disease. Increased left atrial diameter (LAD), a marker of left atrial remodeling, is associated with elevated risk of AF (new-onset and recurrent). This association has led to the hypothesis that increased LAD may represent an intermediate phenotype in the progression from risk factors to AF, especially in older individuals. Information is limited, however, on short- and long-term clinical correlates of LAD over the adult life course. In this investigation of a large, community-based sample, we used multi-level modeling to evaluate correlates of LAD over a 16-year period, and also related these risk factors to short-term change in LAD (over a 4-year period). We identified higher blood pressure and greater body mass index as key correlates of both short-term LAD change and long-term tracking of LAD. Using sex-specific growth curves for LAD, we also observed that LAD at baseline and over time was positively associated with greater risk factor burden. The results of our study suggest that maintenance of optimal levels of blood pressure and body mass during adulthood may be critical for preventing atrial remodeling and AF.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen JA, Kurl S, Eranen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 6.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102:70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, Dahlof B, Devereux RB. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739–743. doi: 10.1161/hy0302.105683. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 11.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64:477–482. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Amodeo C, Ventura HO, Messerli FH. Left atrial abnormalities indicating diastolic ventricular dysfunction in cardiopathy of obesity. Chest. 1987;92:1042–1046. doi: 10.1378/chest.92.6.1042. [DOI] [PubMed] [Google Scholar]
- 13.Thomas MC, Dublin S, Kaplan RC, Glazer NL, Lumley T, Longstreth WT, Jr, Smith NL, Psaty BM, Siscovick DS, Heckbert SR. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens. 2008;21:1111–1116. doi: 10.1038/ajh.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Sorlie P. Cardiovascular diseases and death following myocardial infarction and angina pectoris: Framingham study 20-year follow-up. In: Kannel WB, Gordon T, editors. The Framingham Study: an Epidemiologic Investigation of Cardiovascular Disease. Washington, D.C.: Government Printing Office; 1977. pp. 77–1247. [Google Scholar]
- 18.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 20.Wade MR, Chandraratna PA, Reid CL, Lin SL, Rahimtoola SH. Accuracy of nondirected and directed M-mode echocardiography as an estimate of left atrial size. Am J Cardiol. 1987;60:1208–1211. doi: 10.1016/0002-9149(87)90434-6. [DOI] [PubMed] [Google Scholar]
- 21.Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D'Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PW, Vasan RS. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J. 2004;25:523–530. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Hirschler V, Acebo HL, Fernandez GB, de Lujan Calcagno M, Gonzalez C, Jadzinsky M. Influence of obesity and insulin resistance on left atrial size in children. Pediatric diabetes. 2006;7:39–44. doi: 10.1111/j.1399-543X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 23.Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20-70 years. Influence of age, sex and body surface area. J Int Med. 1989;225:111–115. doi: 10.1111/j.1365-2796.1989.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 25.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial) Am J Cardiol. 2008;101:634–638. doi: 10.1016/j.amjcard.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Heckbert SR, Wiggins KL, Glazer NL, Dublin S, Psaty BM, Smith NL, Longstreth WT, Jr, Lumley T. Antihypertensive Treatment With ACE Inhibitors or beta-Blockers and Risk of Incident Atrial Fibrillation in a General Hypertensive Population. Am J Hypertens. 2009;22:538–544. doi: 10.1038/ajh.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind L, Berne C, Andren B, Lithell H. Relationship between diastolic hypertension and myocardial morphology and function in elderly males with diabetes mellitus. Diabetologia. 1996;39:1603–1606. doi: 10.1007/s001250050621. [DOI] [PubMed] [Google Scholar]
- 29.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D'Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Int Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 31.Wang YC, Lin LC, Lin MS, Lai LP, Hwang JJ, Tseng YZ, Tseng CD, Lin JL. Identification of good responders to rhythm control of paroxysmal and persistent atrial fibrillation by transthoracic and transesophageal echocardiography. Cardiology. 2005;104:202–209. doi: 10.1159/000088174. [DOI] [PubMed] [Google Scholar]
- 32.Volgman AS, Soble JS, Neumann A, Mukhtar KN, Iftikhar F, Vallesteros A, Liebson PR. Effect of left atrial size on recurrence of atrial fibrillation after electrical cardioversion: atrial dimension versus volume. Am J Card Imag. 1996;10:261–265. [PubMed] [Google Scholar]
- 33.Triposkiadis F, Tentolouris K, Androulakis A, Trikas A, Toutouzas K, Kyriakidis M, Gialafos J, Toutouzas P. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr. 1995;8:801–809. doi: 10.1016/s0894-7317(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 34.Gardin JM, Henry WL, Savage DD, Ware JH, Burn C, Borer JS. Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J Clin Ultrasound. 1979;7:439–447. doi: 10.1002/jcu.1870070606. [DOI] [PubMed] [Google Scholar]
- 35.Lie JT, Hammond PI. Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin Proc. 1988;63:552–564. doi: 10.1016/s0025-6196(12)64885-x. [DOI] [PubMed] [Google Scholar]
- 36.Pomerance A. Cardiac pathology in the elderly. Cardiovascular clinics. 1981;12:9–54. [PubMed] [Google Scholar]
- 37.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–1160. doi: 10.1161/01.hyp.25.6.1155. [DOI] [PubMed] [Google Scholar]
- 38.Pan NH, Tsao HM, Chang NC, Chen YJ, Chen SA. Aging dilates atrium and pulmonary veins: implications for the genesis of atrial fibrillation. Chest. 2008;33:1. 190–196. doi: 10.1378/chest.07-1769. [DOI] [PubMed] [Google Scholar]
- 39.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 40.Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–1635. doi: 10.1016/s0735-1097(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 41.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 42.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Nikitin NP, Witte KK, Thackray SD, Goodge LJ, Clark AL, Cleland JG. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 44.Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, Leonetti F. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes res. 2002;10:767–773. doi: 10.1038/oby.2002.104. [DOI] [PubMed] [Google Scholar]
- 45.Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM, Smith NL, Heckbert SR. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166:2322–2328. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 46.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 47.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail. 2002;8:101–107. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- 48.Echocardiography. Philadelphia: Lea and Febiger; 1994. [Google Scholar]
- 49.Lemire F, Tajik AJ, Hagler DJ. Asymmetric left atrial enlargement; an echocardiographic observation. Chest. 1976;69:779–781. doi: 10.1378/chest.69.6.779. [DOI] [PubMed] [Google Scholar]
- 50.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]


