Abstract
Objective
To test the hypothesis that perianal electrical stimulation in chronic spinal cord injured (SCI) cats could induce frequency dependent inhibitory or excitatory reflex bladder responses.
Methods
The experiments were conducted after at least 4–5 weeks following spinal cord transection at the T9–T10 level. Electrical stimulation was applied via a pair of hook electrodes to the perianal skin area in 3 awake female chronic SCI cats. A double lumen balloon catheter was inserted through the urethra into the bladder to monitor bladder pressure and infuse saline (2–4 ml/min).
Results
Under isovolumetric conditions electrical perianal stimulation at frequencies between 3 Hz and 10 Hz significantly inhibited large amplitude reflex bladder activity induced by bladder distension above the micturition volume threshold. However, stimulation at frequencies between 20 Hz and 50 Hz induced large amplitude bladder contractions when bladder volume was below the micturition volume threshold. Inhibitory stimulation (7 Hz) significantly increased bladder capacity 40±10% when it was applied continuously during cystometrograms (CMG). The optimal excitatory stimulation (30 Hz) induced large amplitude (greater than 25 cm H2O), long duration (greater than 20 sec) bladder contractions at a wide range of bladder volumes (10–90% of bladder capacity).
Conclusions
This study revealed that activation of pudendal afferent fibers by perianal electrical stimulation could induce frequency dependent reflex bladder responses in awake chronic SCI cats, indicating that a possible non-invasive treatment based on perianal electrical stimulation could be developed to restore both continence and micturition functions for SCI people.
Keywords: perianal skin, bladder, electrical stimulation, spinal cord injury, cat
INTRODUCTION
Spinal cord injury (SCI) at suprasacral levels induces severe lower urinary tract dysfunctions including: 1. detrusor sphincter dyssynergia (DSD) which results in large residual urine volume in the bladder and high voiding pressure; and 2. detrusor overactivity leading to frequent episodes of incontinence. This study focused on investigating suprasacral SCI instead of sacral SCI that could result in detrusor hypocontractility or flaccidity. Current treatments for bladder dysfunctions after suprasaral SCI have only limited success [1]. Many persons with SCI use intermittent self catheterization to empty their bladders, which is often combined with medication to inhibit detrusor overactivity [2]. If this treatment is not successful, a chronic indwelling catheter will be used to manage the bladder. Intermittent catheterization or a chronic indwelling catheter can cause frequent bladder infections [2]. Restoration of both storage and emptying functions without catheterization is difficult and may require major invasive spinal surgery [3]. The management of bladder function after SCI is a challenging task, because it requires inhibition of detrusor overactivity during urine storage and induction of a large amplitude bladder contraction to empty the bladder [4]. An effective, non-invasive method that is able to either inhibit the bladder or induce a bladder contraction will significantly improve the clinical management of bladder function after SCI.
Bladder inhibition can be elicited in both humans and animals with an intact spinal cord or after chronic SCI by electrical stimulation of the pudendal nerve or its branches (dorsal penile/clitoris nerve) or by intravaginal stimulation at frequencies below 10 Hz [5–18]. Stimulation at 10–15 Hz of the sacral roots in people with SCI also inhibits detrusor overactivity [19–22]. It is assumed that this inhibition is due partially to activation of the large diameter pudendal afferents in the sacral dorsal roots. Recent studies in spinal intact, acute SCI [23], and chronic SCI cats [24] revealed that the pudendal-to-bladder reflex could be either inhibitory or excitatory depending on the frequency of pudendal nerve stimulation. It is inhibitory at a stimulation frequency below 10 Hz, but becomes excitatory at a frequency between 20 Hz and 40 Hz. Our recent study [25] in awake, chronic SCI cats further demonstrated that electrical perigenital stimulation could activate a branch of the pudendal nerve to induce either an inhibitory or an excitatory spinal reflex to the bladder at different stimulation frequencies. Since the pudendal nerve also innervates the muscle and skin in the perianal area, we explored in this study if electrical stimulation applied on the perianal skin area could also activate either an inhibitory or an excitatory spinal reflex to the bladder depending on stimulation frequency.
Electrical stimulation applied by ring electrodes located on anal plug can induce contraction of the pelvic floor muscles and inhibition of detrusor overactivity [26]. This technique has been recommended for stress and urge urinary incontinence [27]. However, intra-anal stimulation produced some problems. For example, the anal plug electrodes had to be removed every 2–3 h to pass flatus. In addition, many patients could not use these electrodes because of pain and discomfort or because of a stenotic or closed anus due to previous injury or surgery. In order to provide a solution for these problems, the effect of electrical stimulation of the perianal skin on bladder function was investigated [28]. It was found that perianal electrical stimulation elicited a suppression of detrusor activity and caused poststimulation improvement in frequency, urgency, and incontinence. In this study we further characterized the spinal reflexes from the perianal skin area to the bladder using awake chronic SCI cats.
METHODS
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Spinal cord transection
Three female cats (2.8 – 3.4 kg) were spinalized under isoflurane anesthesia using aseptic surgical techniques. After performing a dorsal laminectomy at T9–T10 vertebral level, a local anesthetic (lidocaine 1%) was applied to the surface of the spinal cord and then injected into the cord through the dura. The spinal cord was then cut completely, and a piece of gel foam was placed between the cut ends (usually a separation of 2–3 mm). The muscle and skin were then sutured. After full recovery from anesthesia the animal was returned to its cage. Following spinal transection, the bladder was emptied daily by manual expression. If manual expression was not successful, a sterile catheter (3.5 F) was inserted through the urethra to empty the bladder. Ketaprofen (2 mg/kg twice a day for 3 days) and antibiotics (Clavamox, 15–20 mg/kg for 7 days) were given following surgery. Experiments to determine the properties of perianal-to-bladder spinal reflex were conducted after at least 4–5 weeks following spinal cord transaction.
Experimental setup
A sterile double lumen balloon catheter (7 F) was inserted through the urethra into the bladder of the chronic SCI cats without anesthesia. The balloon was distended by 2 ml of air and then positioned at the bladder neck by gently pulling the catheter back. The balloon prevented leakage of the fluid from the bladder. One lumen of the catheter was connected to a pump to infuse the bladder with sterile saline at a rate of 2 ml/min, and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. A pair of sterilized hook electrodes (made from 23G needles) was attached to the skin (about 1 mm penetration into the skin with 2–4 mm contact) on the left and right sides of the anus approximately 1–1.5 cm from the anal opening. Due to the complete spinal transection, the animals did not sense either bladder catheterization or electrical stimulation. During the experiment (usually 4–5 hours) the animals rested comfortably in a padded animal transport carrier. Since the animal was free to move in the carrier, bladder pressure recordings that were disrupted by the animal’s movements were discarded. At the end of the experiment the catheter was withdrawn and the electrodes were detached. After each experiment the animal was given 150 mg/kg of ampicillin subcutaneously. Multiple experiments were repeated on the same animal on different days.
Stimulation protocol
Uniphasic pulses (0.2 ms pulse width) of different intensities (1–30 V) and frequencies (0.5–50 Hz) were delivered to the perianal skin area via the attached electrodes using a stimulator (Grass Medical Instruments, S88) with a stimulus isolator (Grass Medical Instruments, SIU5).
In the first group of experiments, the bladder was infused to one of the two different volumes: (1) a volume slightly above the micturition threshold to induce large amplitude (greater than 25 cm H2O) rhythmic bladder contractions (see Fig. 1A); or (2) a volume slightly below the micturition threshold so that no large amplitude rhythmic bladder contractions occurred (see Fig. 3A). During rhythmic bladder contractions, electrical perianal stimulation was applied in order to determine the effective stimulation parameters to inhibit the bladder. The stimulation duration was longer than the period of at least 2 rhythmic bladder contractions in order to confirm the inhibitory effect. The effective stimulation parameters to induce bladder contractions were determined when bladder volume was low and large amplitude rhythmic contractions were absent. Stimulation duration of 30–50 seconds was used so that a full bladder contraction response could be induced which included the peak of the contraction and the gradual return of bladder pressure to the baseline.
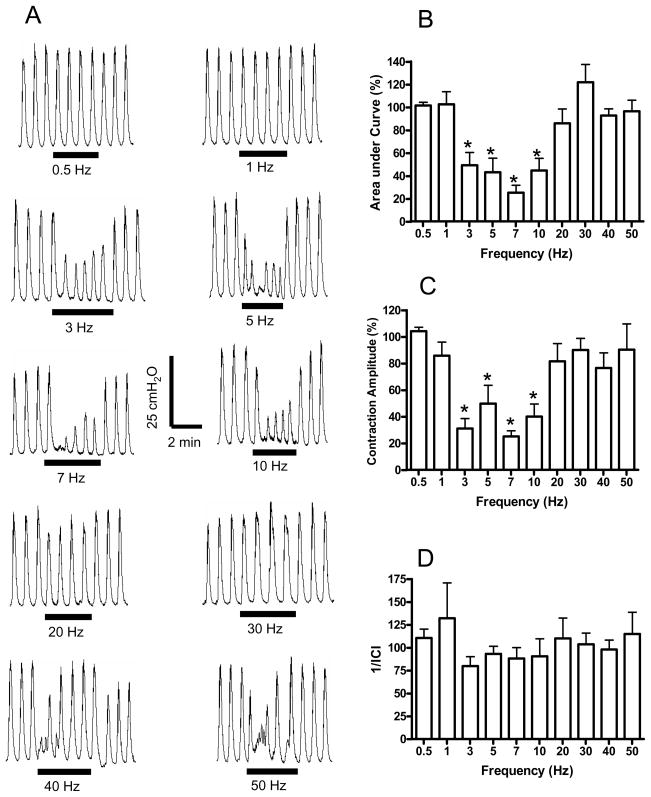
Fig. 1.
Frequency dependent inhibition of rhythmic bladder activity induced by electrical perianal stimulation under isovolumetric conditions. A. Effect on bladder pressure recordings at different stimulation frequencies. The black bars under bladder pressure recordings mark the stimulation duration. B. Area under bladder pressure curve during stimulation. C. Average bladder contraction amplitude during stimulation. D. The inverse of inter-contraction interval (1/ICI) during stimulation. Bladder responses during stimulation were normalized to the responses before stimulation in B–D. Stimulation: 30 V in A, but 8–30 V in B–D; 0.2 ms pulse width. * indicates statistical significance (P<0.05). N = 6 (2 tests on each cat).
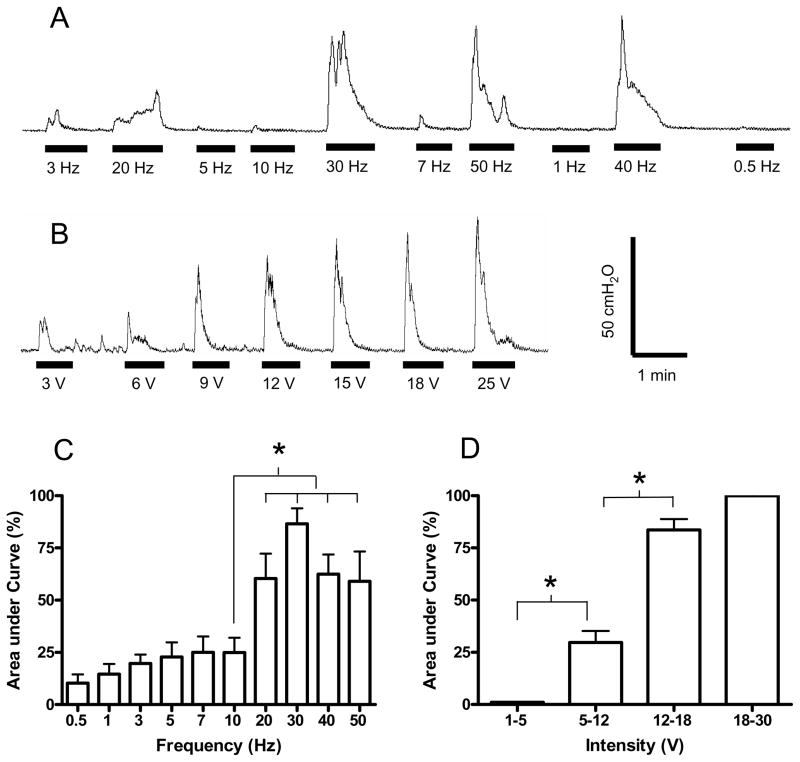
Fig. 3.
Bladder contraction induced by electrical perianal stimulation at different frequencies (A) or at different intensities (B). The area under bladder pressure curve is dependent on both stimulation frequency (C) and intensity (D). Stimulation: 15 V in A, but 10–30 V in C; 30 Hz in B and D; 0.2 ms pulse width. The black bars under bladder pressure traces mark the stimulation duration. The responses were normalized to the maximal response during each trial in C–D. * indicates statistical significance (P<0.05). N = 6 (2 tests on each cat). Data in A and B are from different animals.
In the second group of experiments, the most effective stimulation parameters to inhibit the bladder (7 Hz) identified in the first group of experiments were further tested during a slow infusion of the bladder (i.e. during a cystometrogram-CMG, see Fig. 4A). The CMG was always performed with an initially empty bladder. First, control CMGs were repeated 2–3 times without stimulation to obtain the control values and evaluate the reproducibility. Then, inhibitory perianal stimulation was applied during the CMG to quantify the inhibitory effect by measuring the change in bladder volume to induce the first large amplitude reflex contraction (i.e. bladder capacity). Stimulation and infusion were stopped when the first micturition contraction occurred which was defined as the first large amplitude (greater than 25 cm H2O), long duration (greater than 20 sec) reflex bladder contraction that was accompanied by hindlimb stepping movements. Previous studies [24,29] showed that hindlimb stepping movement was a useful marker for the occurrence of a micturition reflex in awake chronic SCI cats. Bladder capacity is defined as the bladder volume threshold during a CMG which evokes the first micturition contraction. The bladder was emptied after each CMG and a 5–10 minute waiting period was allowed between CMGs for the bladder reflexes to recover.
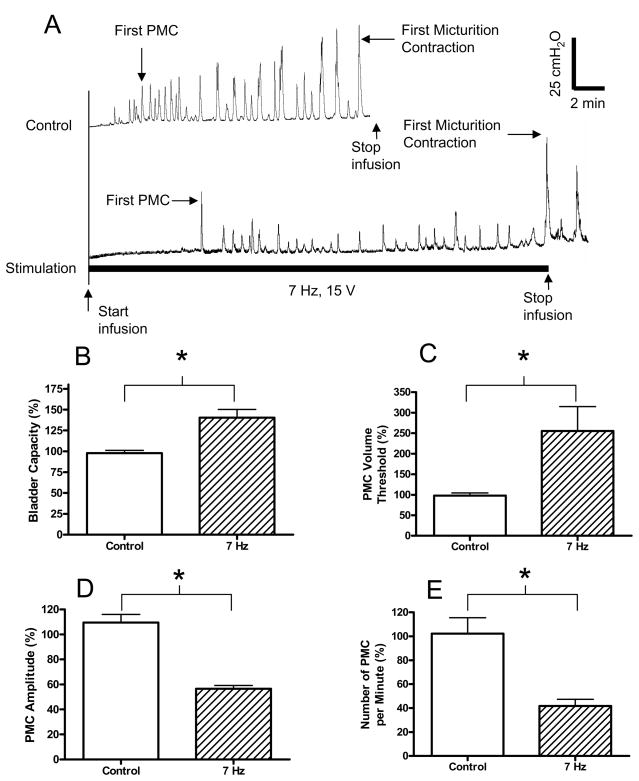
Fig. 4.
Inhibitory effect of electrical perianal stimulation on bladder activity during cystometrogram (CMG). A. Bladder pressure traces. The initial empty bladder was infused with saline at 4 ml/min. Control: no stimulation. Stimulation: 7 Hz; 15 V; 0.2 ms pulse width. The black bar under bladder pressure trace marks the stimulation duration. B. Bladder capacity was significantly increased by electrical perianal stimulation at 7 Hz. C. The volume threshold to induce the first pre-micturition contraction (PMC) was also significantly increased. D–E. PMC amplitude and number of PMCs per minute were decreased significantly. Responses in B–E were normalized to the measurements during first control CMG. Stimulation in B–E: 8–30 V; 0.2 ms pulse width. * indicates statistical significance (P<0.05). N = 9 (3 tests on each cat).
In the third group of experiments, the ability of the excitatory electrical stimulation (30 Hz) to induce bladder contractions at different bladder volumes was further evaluated. A short burst (30–50 sec) of stimulus pulses was applied during a CMG after infusion of saline in 4–8 ml increments.
Data analysis
For the rhythmic bladder activity, the area under bladder pressure curve, the inter-contraction interval (ICI), and the average bladder contraction amplitude were measured during the electrical stimulation and were normalized to the measurements during the same time period prior to the stimulation. The contraction frequency is represented as 1/ICI because ICI is an infinite value when complete bladder inhibition occurs. For the bladder contractions induced by electrical stimulation at a bladder volume below capacity, the areas under the induced bladder pressure curves were measured and normalized to the maximal measurement during each experimental trial. During CMGs small amplitude (10–25 cm H2O), short duration (less than 20 sec) pre-micturition contractions (PMCs) occurred prior to the large amplitude micturition contraction in chronic SCI cats [24]. PMCs indicate the bladder overactivity due to chronic SCI that plays a role in frequent incontinence after SCI. For the CMG recordings, the bladder capacity, the volume threshold to induce the first PMC, the amplitude and the number of PMCs per minute were measured and normalized to the measurements during the first control CMG. For the bladder contractions induced by electrical perianal stimulation at different bladder volumes during a CMG the amplitude of the contractions were measured. The tested bladder volumes were normalized to the bladder capacity, and then they were grouped in bins with every 10% increase of the bladder volume. The normalized data from different experiments are presented as mean ± SEM. Both one sample Student t-test and paired Student t-test were used to detect statistical significance (P<0.05). Linear regression analysis (95% confidence interval) and ANOVA analysis were used to determine whether the amplitude of bladder contractions induced by perianal stimulation was increased as the bladder volume increased.
RESULTS
1. Inhibitory perianal-to-bladder spinal reflex
The inhibitory effect of electrical perianal stimulation on rhythmic bladder activity in awake chronic SCI cats was dependent on stimulation frequency. Fig. 1A shows an example of electrical perianal stimulation at different frequencies (0.5–50 Hz) inhibiting rhythmic bladder contractions. The different stimulation frequencies were applied in a random order, but they are shown in ascending order in Fig. 1A for clarity. The inhibitory effect on rhythmic bladder activity was obvious (P<0.05) between 3 Hz and 10 Hz as either a decrease in the contraction amplitude or a reduction in the area under bladder pressure curve (Figs.1A and B). The stimulation significantly (P<0.05) decreased the average contraction amplitude during the stimulation at 3–10 Hz (Fig. 1C), but the frequency of bladder rhythmic contractions was not changed significantly (P>0.05, Fig. 1D).
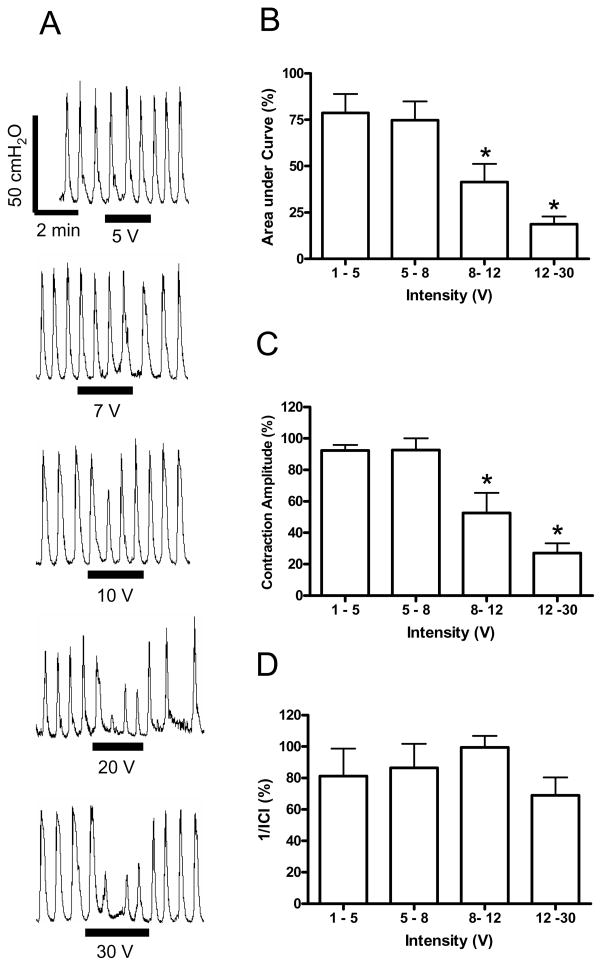
The inhibitory effect on rhythmic bladder activity was also dependent on stimulation intensity. Fig. 2A shows an example of inhibition of rhythmic bladder contractions at different intensities (5–30 V) using a frequency of 7 Hz. The different stimulation intensities were applied in a random order, but they are shown in ascending order in Fig. 2A for clarity. At stimulation intensity above 8 V, the electrical perianal stimulation significantly (P<0.05) reduced the area under the bladder contraction curve during the stimulation compared to the bladder activity prior to the stimulation (Fig. 2B). The stimulation also significantly (P<0.05) decreased the average contraction amplitude during the stimulation at an intensity above 8 V (Fig. 2C), but the frequency of bladder contractions was not changed significantly (P>0.05, Fig. 2D).
Fig. 2.
Intensity dependent inhibition of rhythmic bladder activity induced by electrical perianal stimulation under isovolumetric conditions. A. Effect on bladder pressure recordings at different intensities. The black bars under bladder pressure recordings mark the stimulation duration. B. Area under bladder pressure curve. C. Average bladder contraction amplitude. D. The inverse of inter-contraction interval (1/ICI). Bladder responses during stimulation were normalized to the responses before stimulation in B–D. Stimulation: 7 Hz; 0.2 ms pulse width. * indicates statistical significance (P<0.05). N = 6 (2 tests on each cat).
2. Excitatory perianal-to-bladder spinal reflex
The perianal-to-bladder reflex in awake chronic SCI cats could also be excitatory depending on the electrical stimulation frequency and intensity. Fig. 3A–B shows examples of bladder contractions induced by electrical perianal stimulation at different frequencies and intensities when bladder volume was below its capacity. Large amplitude (greater than 25 cm H2O), long duration (greater than 20 sec) bladder contractions were induced by stimulation at a frequency between 20 Hz and 50 Hz (Fig. 3A). This excitatory effect was enhanced as the stimulation intensity increased (Fig. 3B). Electrical stimulation at 30 Hz was optimal to induce bladder contractions since it produced the largest area under the bladder contraction curve. The effect of 30 Hz was significantly (P<0.05) larger than the responses produced at a frequency of 10 Hz (see Fig. 3C). In order for 30 Hz stimulation to induce a large bladder contraction greater than 75% of the maximal response, the required stimulation intensity was above 12 V (Fig. 3D).
3. Micturition volume threshold modulated by perianal stimulation
The threshold bladder volume (i.e. bladder capacity) to induce a micturition reflex contraction in awake chronic SCI cats was significantly increased by the inhibitory electrical perianal stimulation at 7 Hz. Fig. 4A shows an example of repeated CMG recordings. Compared to the first control CMG, the 7 Hz stimulation delayed the occurrence of both the first micturition contraction and the first pre-micturition contraction (PMC) (Fig. 1A). The bladder capacity was significantly (P<0.05) increased to 140±10% of the control capacity (Fig. 4B) and the volume threshold to induce the first PMC was significantly (P<0.05) increased to 255±60% of the control value (Fig. 4C). The average amplitude and the frequency of the PMCs were also significantly (P<0.05) decreased to 56±3% and 41±5% of the control value (Fig. 4D–E). The amplitude of the first micturition contraction was not influenced by the inhibitory 7 Hz stimulation (see Fig. 4A).
4. Independence of excitatory perianal-to-bladder spinal reflex on bladder volume
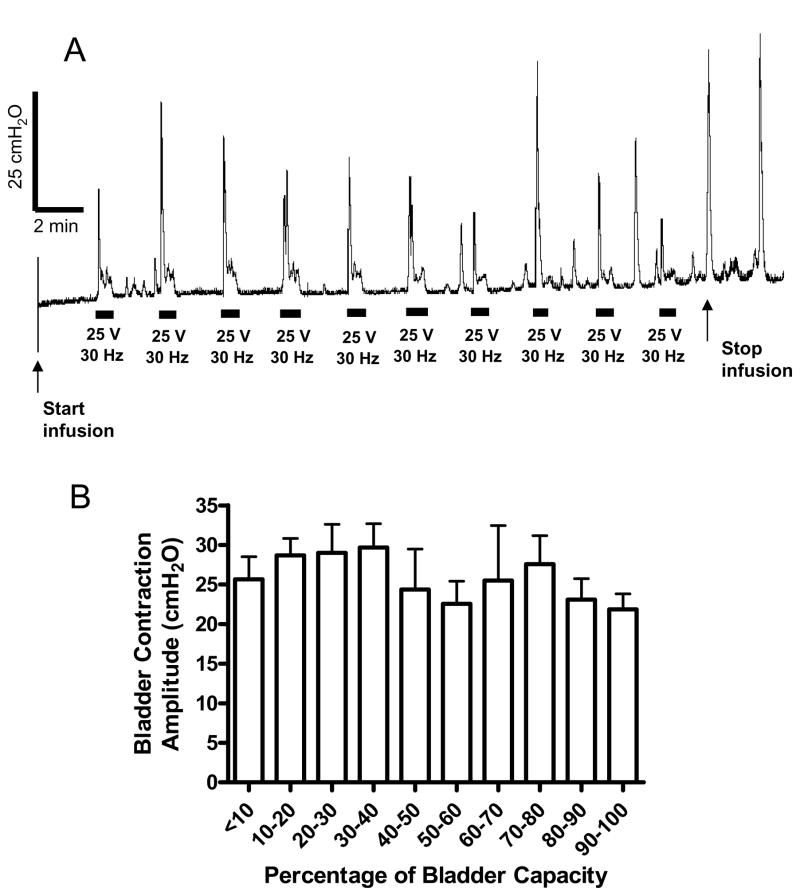
In addition to inhibiting the bladder-to-bladder micturition reflex, the perianal afferent input could also induce an excitatory perianal-to-bladder reflex in awake chronic SCI cats, which was not dependent on the bladder volume. Fig. 5A shows a CMG recording where a short burst (40 second duration) of electrical stimulation (25 V, 30 Hz) was repeatedly applied to the perianal area at regular intervals following approximately 4 ml increments in bladder volume. The stimulation induced large (greater than 25 cm H2O) amplitude bladder contractions at a variety of bladder volumes. Fig. 5B shows the average amplitude of bladder contractions induced by electrical (30 Hz) stimulation at different bladder volumes. Both ANOVA analysis and linear regression analysis showed that the amplitude of bladder contractions was not significantly (P>0.05) increased when the bladder volume was increased (Fig. 5B).
Fig. 5.
Bladder contractions induced by electrical perianal stimulation during cystometrogram (CMG). Electrical stimulation: 30 Hz; 25 V in A, but 11–30 V in B; 0.2 ms pulse width. The black bars under bladder pressure traces mark the stimulation duration. Saline infusion was started with the bladder empty. Infusion rate: 4 ml/min. N = 6 (2 tests on each cat).
DISCUSSION
This study revealed that in awake chronic SCI cats electrical stimulation of afferent nerves located in the perianal skin area could elicit either an inhibitory or an excitatory spinal reflex to the bladder depending on the frequency of stimulation. The inhibitory effect was significant at a stimulation frequency of 3–10 Hz (Fig. 1), but the excitatory effect was maximal at 30 Hz (Fig. 3C). The inhibitory stimulation at 7 Hz significantly increased bladder capacity and inhibited the pre-micturition contractions (Fig. 4). The excitatory electrical (30 Hz) perianal stimulation induced large bladder contractions that were not dependent on bladder volume (Fig. 5). The results obtained in this study are similar to those obtained from electrical stimulation of perigenital area in our previous study [25], indicating that the pudendal afferents from both anal and genital areas could have the same modulatory role on bladder activity in chronic SCI cats.
Mechanical anal stimulation could also induce either an inhibitory or an excitatory effect on bladder activity. Inhibition of bladder contractions by stretching the anal sphincter was observed in people with or without SCI [30–31]. Excitation of the bladder was also seen in SCI subjects by light perianal touch, pinprick, introduction of rectal catheter, or alternatively inflation and deflation of a rectal balloon [32]. It seems that mild stretch or light touch of the anus tends to be excitatory while vigorous stretch tends to cause inhibition. In this study, we further demonstrated that the perianal-to-bladder spinal reflex in awake chronic SCI cats could be either inhibitory or excitatory depending on the frequency of electrical stimulation. Previous studies [26–28] in human SCI subjects using electrical stimulation (20 Hz) of the anal area only demonstrated bladder inhibition. Our study indicated that using perianal stimulation at different frequencies might also induce bladder excitation in people with SCI. Inducing either inhibition or excitation using the same stimulation electrode will be very useful in clinical applications.
Since the skin and muscle around the anal area are innervated by pudendal nerve [33], the electrical stimulation used in this study must have activated a branch or branches of this nerve. The inhibitory pudendal-to-bladder reflex has been demonstrated in spinal intact and acute SCI cats by intra-vaginal electrical stimulation [15,17,34]. It was suggested that stimulation of pudendal afferents could inhibit bladder activity via activation of hypogastric nerve when bladder volume was low, and via spinal inhibition of parasympathetic activity in the pelvic nerve when bladder volume was high [17,34]. A previous study [24] in anesthetized chronic SCI cats also showed the involvement of both hypogastric and pelvic efferent nerves in the inhibitory pudendal-to-bladder spinal reflex. The inhibitory perianal-to-bladder reflex demonstrated in this study in awake chronic SCI cats might involve the same efferent pathways (i.e. hypogastric and pelvic) as revealed previously. Meanwhile, the electrical perianal stimulation can also activate an excitatory perianal-to-bladder spinal reflex to induce large amplitude bladder contractions at both low and high bladder volumes (see Fig. 5), indicating that the excitatory perianal-to-bladder reflex is independent on the afferent input from the bladder. This suggests that the excitatory afferent input from the perianal area may have projections to the parasympathetic preganglionic neurons via spinal circuitry separate from that activated by the pelvic afferent input from the bladder.
The electrical perianal stimulation is inhibitory to the bladder at 3–10 Hz (Fig. 1), but becomes excitatory at 20–50 Hz (Fig. 3). Since at the same stimulation intensity the same afferent nerve fibers are activated, the frequency selection of the perianal-to-bladder spinal reflex must occur in the spinal cord. One possible explanation is that the afferent firing at different frequencies may trigger the release of different neurotransmitters at the first spinal synapse between the primary afferent axons and spinal interneurons resulting in either an inhibitory or an excitatory effect on the bladder activity. Another possible explanation is that the spinal interneuronal networks are optimally tuned at different frequencies for bladder inhibition or excitation. Afferent firing between 20–50 Hz is optimally transmitted through the excitatory spinal neural network, but 3–10 Hz is optimal for the inhibitory network. Frequency tuning of spinal neural networks is also evident in previous studies in cats [15,34]. The maximal inhibition via the hypogastric nerve could be obtained when the pudendal afferent pathway was stimulated at 5 Hz, whereas the spinal inhibition via pelvic nerve could be optimally activated at frequencies between 5 and10 Hz [15,34].
Our current study in awake, chronic SCI animals tested the hypothesis that frequency dependent activation of the inhibitory or excitatory pudendal-to-bladder spinal reflex could be induced by applying electrical stimulation to the perianal skin area. A previous study [18] using restrained, awake, chronic SCI cat only investigated the inhibitory effect on bladder by inserting fine wire electrodes percutaneously to stimulate the pudendal nerve. Whether the excitatory bladder response induced in this study could produce efficient voiding depends on the relaxation of external urethral sphincter (EUS). Although the EUS activity was not investigated in this study, the perianal stimulation presumably activated the EUS due to the excitatory pudendal-to-pudendal spinal reflex. However, the voiding problem due to dyssynergic EUS contraction could be overcome by inducing post-stimulus voiding [3]. The approach employed in this study in awake SCI cats to characterize reflexes from the perianal skin to the urinary bladder might provide an alternative and practical clinical method to manage bladder function after SCI.
Acknowledgments
This study is supported by the NIH grants RO1-DK-068566 and RO1-DK-077783, and by the Christopher and Dana Reeve Foundation.
References
- 1.Yoshimura N, Smith CP, Chancellor MB, de Groat WC. Pharmacologic and potential biologic interventions to restore bladder function after spinal cord injury. Cur Opin Neurol. 2000;13:677–681. doi: 10.1097/00019052-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Jamil F. Toward a catheter free status in neurogenic bladder dysfunction: a review of bladder management options in spinal cord injury (SCI) Spinal Cord. 2001;39:355–361. doi: 10.1038/sj.sc.3101132. [DOI] [PubMed] [Google Scholar]
- 3.Brindley GS. The first 500 sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 4.Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958–963. doi: 10.1016/s0022-5347(17)54147-6. [DOI] [PubMed] [Google Scholar]
- 5.Fall M. Electrical pelvic floor stimulation for the control of detrusor instabililty. Neurourol Urodynam. 1985;4:329–335. [Google Scholar]
- 6.Groen J, Amiel C, Bosch JLHR. Chronic pudendal nerve neuromodulation in women with idiopathic refreactor detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodynam. 2005;24:226–230. doi: 10.1002/nau.20131. [DOI] [PubMed] [Google Scholar]
- 7.Vodusek DB, Plevnik S, Vrtacnik P, Janez J. Detrusor inhibition on selective pudendal nerve stimulation in the perineum. Neurourol Urodynam. 1988;6:389–393. [Google Scholar]
- 8.Andre R, Schmid DM, Curt A, Knapp PA, Schurch B. Afferent fibers of the pudendal nerve modulate sympathetic neurons controlling the bladder neck. Neurourol Urodynam. 2003;22:597–601. doi: 10.1002/nau.10134. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJM. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005;173:2035–2039. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 10.Kirkham APS, Shah NC, Knight SL, Shah PJR, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420–428. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 11.Previnaire JG, Soler JM, Perrigot M, Boileau G, Delahaye H, Schumacker P, Vanvelcenaher J, Vanhee JL. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia. 1996;34:95–99. doi: 10.1038/sc.1996.17. [DOI] [PubMed] [Google Scholar]
- 12.Previnaire JG, Soler JM, Perrigot M. Is there a place for pudendal nerve maximal electrical stimulation for the treatment of detrusor hyperreflexia in spinal cord injury patients. Spinal Cord. 1998;36:100–103. doi: 10.1038/sj.sc.3100440. [DOI] [PubMed] [Google Scholar]
- 13.Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodynam. 1986;5:381–389. [Google Scholar]
- 14.Wheeler JS, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100–103. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- 16.Sundin T, Carlsson CA, Kock NG. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents: An experimental study in cats. Investigative Urol. 1974;11:374–378. [PubMed] [Google Scholar]
- 17.Fall M, Erlandson BE, Carlsson CA, Lindstrom S. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder: neuronal mechanisms. Scand J Urol Nephrol suppl. 1978;44:19–30. [PubMed] [Google Scholar]
- 18.Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodynam. 1993;12:241–253. doi: 10.1002/nau.1930120306. [DOI] [PubMed] [Google Scholar]
- 19.Chartier-Kastler EJ, Ruud Bosch JLH, Perrigot M, Chancellor MB, Richard F, Denys P. Long-term results of sacral nerve stimulation (S3) for the treatment of neurogenic refractory urge incontinence related to detrusor hyperreflexia. J Urol. 2000;164:1476–1480. [PubMed] [Google Scholar]
- 20.Chartier-Kastler EJ, Denys P, Chancellor MB, Haertig A, Bussel B, Richard F. Urodynamic monitoring during percutaneous sacral nerve neurostimulation in patients with neurogenic detrusor hyperreflexia. Neurourol Urodynam. 2001;20:61–71. doi: 10.1002/1520-6777(2001)20:1<61::aid-nau8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Ishigooka M, Suzuki Y, Hashimoto T, Sasagawa I, Nakada T, Handa Y. A new technique for sacral nerve stimulation: a percutaneous method for urinary incontinence caused by spinal cord injury. Br J Urol. 1998;81:315–318. doi: 10.1046/j.1464-410x.1998.00569.x. [DOI] [PubMed] [Google Scholar]
- 22.Kirkham APS, Knight SL, Craggs MD, Casey ATM, Shah PJR. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40:272–281. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 23.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Tai C, Shen B, Wang J, Chancellor M, Roppolo JR, de Groat WC. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in cat. Am J Physiol Renal Physiol. 2007;294:F591–602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godec C, Cass AS, Ayala GF. Bladder inhibition with functional electrical stimulation. Urology. 1975;6:663–666. doi: 10.1016/0090-4295(75)90791-8. [DOI] [PubMed] [Google Scholar]
- 27.Godec C, Cass AS, Ayala GF. Electrical stimulation for incontinence; technique, selection and results. Urology. 1976;7:388–397. doi: 10.1016/0090-4295(76)90253-3. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Sakurai T, Tsujimoto Y, Tada Y. Bladder inhibition by electrical stimulation of the perianal skin. Urol Int. 1986;41:62–63. doi: 10.1159/000281160. [DOI] [PubMed] [Google Scholar]
- 29.Thor KB, Roppolo JR, de Groat WC. Naloxone induced micturition in unanesthetized paraplegic cats. J Urol. 1983;129:202–205. doi: 10.1016/s0022-5347(17)51984-9. [DOI] [PubMed] [Google Scholar]
- 30.Kock NG, Pompeius R. Inhibition of vesical motor activity induced by anal stimulation. Acta Chir Scand. 1963;126:244–250. [PubMed] [Google Scholar]
- 31.Rodriquez AA, Awad E. Detrusor muscle and sphincteric response to anorectal stimulation in spinal cord injury. Arch Phys Med Rehabil. 1979;60:269–272. [PubMed] [Google Scholar]
- 32.Rossier A, Bors E. Detrusor responses to perianal and rectal stimulation in patients with spinal cord injuries. Urol Int. 1964;18:181–190. doi: 10.1159/000279237. [DOI] [PubMed] [Google Scholar]
- 33.Martin WD, Fletcher RF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- 34.Fall M, Lindstrom S. Electrical stimulation: A physiologic approach to the treatment of urinary incontinence. Urol Clin North Amer. 1991;18:393–407. [PubMed] [Google Scholar]