Abstract
Despite an armamentarium that is wide in range, scope of action and target; chemotherapy has limited success in colorectal cancer. Novel approaches are needed to overcome tumor barriers to chemotherapy that includes an abnormal tumor vasculature constituting a poor drug delivery system. We have previously shown that 5-methylselenocysteine (MSC) enhances therapeutic efficacy of irinotecan in various human tumor xenografts. We have recently demonstrated that MSC through vascular normalization leads to better tumor vascular function in vivo. In this study, we examined the role of MSC on tumor vasculature, interstitial fluid pressure (IFP) and drug delivery in two histologically distinct colorectal cancer (CRC) xenografts, HCT-8 (uniformly poorly differentiated) and HT-29 (moderately differentiated tumor with avascular glandular regions). The presence of specific histological structures as a barrier to therapy in these xenografts and their clinical relevance was studied using tissue microarray of human surgical samples of CRC. MSC led to a significant tumor growth inhibition, a reduced microvessel density, a more normalized vasculature in both colorectal xenografts. While IFP was found to be significantly improved in HCT-8, an improved intratumoral doxorubicin delivery seen in both xenografts could explain the observed increase in therapeutic efficacy. Differentiated, glandular, avascular and hypoxic region that contribute to tumor heterogeneity in HT-29 were also evident in majority of surgical samples of colorectal cancer. Such regions constitute a physical barrier to chemotherapy and can confer drug resistance. Our results indicate that MSC could enhance chemotherapeutic efficacy in human CRC, especially in CRC with few or no hypoxic regions.
Keywords: Vascular maturation, drug delivery, antiangiogenesis, selenium, tumor differentiation, tumor hypoxia
INTRODUCTION
Chemotherapy remains the mainstay of therapy in stage III patients with colorectal cancer (CRC) who have a 50%-60% chance of developing recurrent disease post surgical resection1. Systemic therapy has improved prognosis and median duration of survival among patients with metastatic CRC from 8 months to 21 months2. However results remain mainly palliative, with minimal evidence of increased cure rates. Additionally, costs associated with chemotherapy for CRC patients have increased dramatically2. Despite the availability of a chemotherapy armamentarium that is quite wide in scope of action and targets, therapeutic outcome is constrained by an abnormal tumor vascular network that constitutes a poor tumor drug delivery system, lack of selectivity and, a dose limiting toxicity. Unlike normal vasculature, tumor vessels are often dilated, lack pericyte coverage and exhibit abnormal branching patterns (tortuous) resulting in functional irregularities such as vascular shunting, pre-stasis, stasis and reversal of flow3. The widened endothelial gaps in tumor vessels contribute to increased vascular permeability and a high intratumoral interstitial fluid pressure (IFP) that retards intratumoral drug delivery and distribution, critical for optimal therapeutic response4. Morphologic heterogeneity within tumors such as the presence of avascular, hypoxic well-differentiated ‘islands’ in squamous cell carcinoma of the head and neck prevent delivery of therapeutically effective concentrations contributing to drug resistance5. Increasing drug doses in such a flawed delivery system is likely to increase dose limiting toxicities on healthy tissues deteriorating patient quality of life with minimal or no increase in therapeutic efficacy6. Several preclinical and clinical studies have shown that antiangiogenic agents can potentially overcome these barriers by ‘normalizing’ existing tumor vessels and improving their functionality4. Recently, Tong et al have shown that treatment with DC-101, an antibody against the vascular endothelial growth factor receptor (VEGF-R2) improves tumor vascular function, decreases IFP and subsequently improves drug penetration into tumors7. Similar results were shown with Bevacizumab in combination therapy which improved patient survival in colorectal cancer8. Results from our laboratory have revealed similar observations following treatment with the organoselenium compound, 5-methylselenocysteine (MSC) in human head and neck cancer xenografts 9.
Selenium (Se), an essential dietary trace element and a normal component of the mammalian physiology, has been in use as an antioxidant and in cancer prevention such as in the recently concluded SELCT trial10 where a daily dose of 200μg/patient or ~2.5μg/kg body weight (~ 80 kg body weight) is commonly used. The use of Se as a therapeutic agent at high but non-toxic doses has not been reported earlier other than reports from our laboratory11. We have previously shown that organo-Se compounds methylselenocysteine (MSC) and selenomethionine (SLM) acts as selective modulators of chemotherapeutic efficacy of a broad range of anti-cancer drugs including irinotecan (CPT-11), taxanes, platinum complexes, doxorubicin and cyclophosphamide11. The dose used for this chemomodulation effect is ~ 8-10 mg/kg (~ 20-25 mg mice) in the preclinical system or 180μg/patient that has been achieved in clinical trials12. This chemomodulation activity is MSC dose and schedule dependent and is not seen at the doses employed for chemoprevention studies but only at a much higher dose13. Treatment with MSC (0.2 mg/mouse/day per oral starting 7 days before CPT-11) in combination with CPT-11 (100 mg/kg i.v. weekly × 4) was found to significantly enhance therapeutic response to 100% complete remission (CR) compared to 20% CR with the drug alone in HCT-8, a poorly differentiated ileocecal carcinoma. However, the enhancement of therapeutic response was modest from 0% with the drug alone to 20% with the combination of MSC + CPT-11 in HT-29, a moderately differentiated colon adenocarcinoma xenografts11.
In this study, we examined the effect of MSC on tumor vascular phenotype, permeability, IFP and intratumoral drug gradient in the relatively resistant HT-29 and relatively sensitive HCT-8 tumor models that have different tumor vascular distribution and histomrophological pattern. Understanding the chemo-modulating effects of MSC in these histologically different clinically relevant CRC xenografts could improve our understanding of its potential clinical application as a chemomodulator in the treatment of CRC.
MATERIALS AND METHODS
Tumor model
The human CRC cell lines, HCT-8 and HT-29, were originally obtained from American Type Culture Collection (Manassas, VA) and xenografts established in six-to-eight week old female athymic nude mice (Foxn1nu, Harlan Sprague Dawley, Inc. Indianapolis, IN) as described previously(3). Tumor growth (N = 10 mice per group) was assessed using digital vernier calipers for measuring tumor burden (mm3) using the formulae: ½ (L × W2), where L and W are the longest and shortest axis in millimeters11. All animal studies were performed in accordance with Institute Animal Care and Use Committee approved protocols.
Patient Samples of CRC
Formalin/paraffin sections of human surgical tissue microarray (TMA) containing 0.6 mm cores from 90 CRC cases of which 24 had matching normal cores and 20% of the array consisting of normal tissues were studied for the presence or absence of glandular differentiated structure, microvessel distribution (MVD) and tumor hypoxia.
Drugs
MSC (Sigma, St. Louis, MO) was dissolved in sterile saline at a concentration of 1mg/ml) and administered orally at the maximum tolerated dose of 0.2 mg/mouse/day (3) for up to 16 days, beginning three days after tumor implantation. Doxorubicin (Bedford Laboratories, Bedford, OH) was administered intravenously (30 mg/kg) alone or 24 hours following administration of the MSC dose (day 14) for determining the effect of MSC on intratumoral drug gradient. Doxorubicin was selected for its auto-fluorescence properties in order to determine the effect of MSC induced tumor vascular maturation on intratumoral drug delivery and gradient using fluorescence microscopy 9, 14.
Immunohistochemistry
Immunohistochemical double staining was performed using CD31 and alpha-smooth muscle actin (α-SMA) for detection of endothelial cells and pericytes respectively as per previously described9. Briefly, 5-8μm cryosections were fixed in cold acetone (−20°C) for 15 minutes followed by endogenous peroxidase quenching, and, incubation with rabbit polyclonal SMA antibody (1μg/ml or 1/500) (Abcam, Cambridge, MA) and biotinylated goat anti-rabbit secondary antibody (1/250) (Vector Labs) for 30 min. CD31 antibody (B.D. Biosciences Pharmingen, Franklin Lakes, NJ) was used at 10μg/ml for 60 min. An isotype-matched rat IgG was used as a negative control. Endothelial cells were immunostained brown and pericytes were stained pink. There were a minimum of 4 tumors per group with 2 sections from each tumor at least 10 μm apart and multiple fields of view were used for quantification purposes. All immunohistochemical analysis and interpretation were carried out under the supervision of a board-certified and experienced pathologist (Károly Tóth MD PhD)
For TMA, the tumor vessels were detected using CD34 marker for endothelial cells while hypoxia was determined using carbonic anhydrase IX (CAIX) staining as per methods described earlier5. Briefly, the primary antibody M75 (gift from Dr Pastorek, Institute of Virology, Slovak Republic) and CD34 (Dako, Carpinteria, CA) were used at 20 μg/ml and 1/50 dilution for 90 minutes and 60 minutes respectively at room temperature. For CD34, pretreatment was done with antigen retrieval solution (Vector Laboratories, Burlingame, CA) for 10 minutes and secondary antibody used was mouse EnVision + kit (Dako). For CAIX, the primary antibody was followed by 30 minutes incubation with Streptavadin complex (Zymed Lab, Inc., San Francisco, CA).
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)
To determine MSC-induced changes in vascular volume and permeability, DCE-MRI using the intravascular contrast agent, albumin-GdDTPA was performed in a 4.7T/33-cm horizontal bore MR scanner (GE Instruments, Fremont, CA) on a separate cohort of mice 9. We have previously described the scan parameters of fast spin-echo (FSE) imaging sequence used in the study. Briefly, linear regression analysis of change in T1-relaxation rates (ΔR1) following contrast agent injection was performed to calculate the relative vascular volume (y-intercept) and permeability (slope) in tumors9.
In vivo tumor IFP measurements
Tumor IFP was measured using a micro catheter pressure transducer (Millar Instruments Inc., Houston, TX) in a manner similar to that described by an earlier report15. Briefly, the probe (SPR-1000 1F pressure transducer) was coupled to an infusion 23½ gauge needle catheter filled with de-gassed sterile saline. The output from the transducer was recorded at 1kHz data sampling rate using an USB analog to digital converter (DT-9816, Data Translation Inc., Marlboro, MA) connected to a laboratory computer. The data was recorded and analyzed using data analysis and display software (DT Measure Foundry, Data Translation Inc.). The probe was calibrated each time before and after an experiment using a manometer and the height of a column of water (cm water) was plotted against the measured voltage across the transducer (mV) to obtain a calibration curve. The output from the probe changed linearly with the applied hydrostatic pressure and had correlation coefficient R2 typically close to 0.999. At least five measurements were made from each tumor including measurements at the periphery and at the center without removing the needle as per method described earlier15. Mice bearing the tumor (N = 6-8 per group) at time points equivalent to 24 hours post 14 days of MSC were used to assess the IFP measurements under anesthesia.
Determination of intratumoral doxorubicin distribution gradient
The effect of MSC on drug distribution gradient (N = 89 or more linear paths 90 μ long using multiple sections from 4 different tumors per group) was assessed using and fluorescence microscopy as per procedure described earlier9. Doxorubicin was given at a dose of 30 mg/kg in order to facilitate easy detection and quantification of autofluorescence9, 14. Two hours post doxorubicin administration, animals were euthanized and tumor harvested was frozen and ~5-10 μm thick frozen sections were used. An average of 4 maximum intensity projection images with a resolution of 0.23μm were acquired using identical acquisition parameters under 63X objective of Leica confocal microscope similar to procedures described previously9. The digitized images were used for image analysis.
Image analysis
MVD counts were determined by counting all CD31 positive endothelial cell clusters in multiple high power fields (400X) covering non-necrotic areas of the whole tumor sections. The vascular maturation index (VMI) was derived by calculating the total number of CD31+ α-SMA+ areas and areas positive for CD31 alone in double-stained (CD31/ α-SMA) tissue sections using Analyze®(AnalyzeDirect, OverlandPark, KS)9. For intratumoral drug distribution studies, the mean intensity of doxorubicin auto fluorescence at various distances away from the blood vessels was calculated using Analyze®. At least 4 tumors for each group were analyzed for MVD, VMI and doxorubicin studies.
Statistical analysis
All results are expressed as mean ± standard error of the mean and the differences between the mean of the groups were analyzed using unpaired two-tailed student t test. For DCE-MRI measured parameters, a linear regression analysis was carried out to determine statistical significance (GraphPad Version 4.00, GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
RESULTS
Patient Samples of CRC
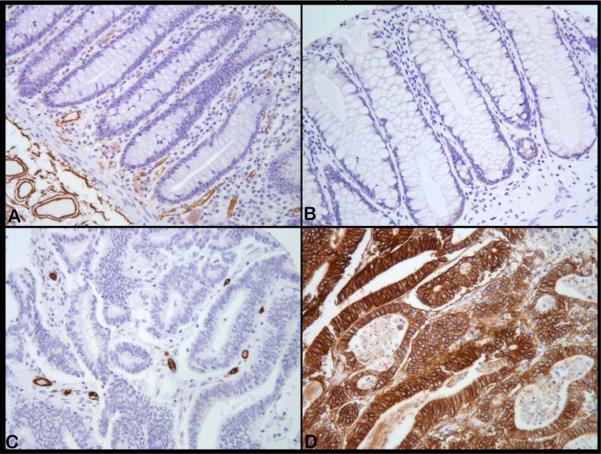
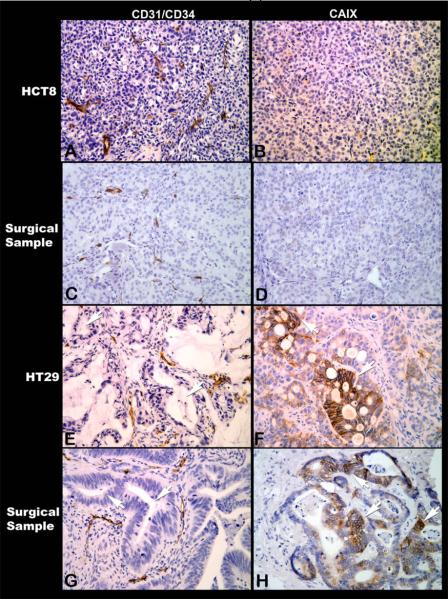
We first examined the histologic characteristcs of the two CRC xenograft models by comparing the tissue morphology and architecture to surgical specimens of CRC obtained from our TMA. Of the total 90 cores of surgical samples of human colorectal cancers, 78 were evaluable with 62 cores (79%) having regions of hypoxia as evidenced by presence of CAIX membrane staining. The remainder 16 cores (21%) did not show presence of tumor hypoxia. Histologically, ~80% of the clinical samples were moderately differentiated adenocarcinomas while the others were poorly differentiated tumors. The extent of hypoxic regions varied in tumors and ranged from 0% to 100% in the tumor cores. Large areas (75-100%) of the tumor fields were hypoxic in the majority (~70%) of the tumor cores. The hypoxia distribution with CAIX showed a unique characteristic and were evident in the most of the glandular lumen forming tumor cells (more differentiated parts of the tumor) and it was much less frequently observed when the cancer cells did not form glands (the poorly differentiated parts of the tumor). Photomicrographs show this pattern in representative tumor samples where the microvessel distribution was visualized by CD34 and compared the presence of hypoxic tumor cells detected using CAIX immunostaining. Figure 1 shows immunohistochemical staining for microvasculature (left panels) and hypoxia (right panels). As seen in Figure 1(i), normal colon mucosa (Panel A) contain microvessels (brown) in the peri-glandular area but not within the glandular structure and yet these glandular structures are not hypoxic (Panel B) while in the colon adenocarcinoma the glandular regions do not contain microvessels (Panel C) either but are strongly hypoxic (Panel D) despite the presence of vessels in the surrounding stroma (Panel C). Figure 1(ii) shows the microvessel distribution (left panels) and hypoxia (right panels) in the human colorectal xenografts HCT-8 and HT-29 along with the corresponding representative formalin/paraffin sections from the TMA of patient surgical specimens of CRC. Panel A shows the uniform distribution of tumor vasculature (brown) in the poorly differentiated xenograft HCT-8 and as a consequence there are no hypoxic regions (Panel B). Poorly differentiated surgical samples of CRC with similar uniform distribution of tumor vasculature (Panel C) are non-hypoxic (Panel D). The moderately differentiated human colon xenograft HT-29 has glandular avascular regions (Panel E, arrows) that are hypoxic (Panel F, brown membrane staining for CAIX) and have a histological structure similar to differentiated human CRC as shown in Panel G(arrows show glandular avascular regions) and Panel H (arrows shows numerous hypoxic glandular lumen forming cancer cells). These observations demonstrate the histological differences between HCT-8 and HT-29 CRC xenografts and highlight the similarities between the two xenografts and human CRC specimens. The findings also demonstrate presence of morphologic heterogeneity with glandular avascular tumor regions in human surgical samples similar to that seen in HT-29 xenograft model used in the current study.
Figure 1.
Microphotographs of CD31/CD34 immunostaining to visualize microvessels (left panels) and CAIX immunostaining to demonstrate tumor hypoxic regions (right panels) (All magnifications, X200). [1(i)] Normal colon mucosa (A) with microvessels (brown) uniformly distributed except for the glandular structures that do not contain vessels and is yet not hypoxic (B). In contrast, adenocarcinoma do not contain microvessels in the glandular regions, but only in the surrounding stroma (C) and the cancer cells are strongly positive for hypoxia marker CAIX (brown) in many such glandular regions (D). [1(ii)] Photomicrographs of HCT-8 and HT-29 human colon cancer xenografts along with human surgical samples of colorectal cancers with similar histomorphological characteristics. Poorly differentiated HCT-8 is uniformly well vascularised (A) and has no regions of hypoxia (B). Similar vascular arrangement (C) and absence of hypoxia (D) are seen in this surgical sample of poorly differentiated colorectal cancer. In contrast, in HT-29, the cancer cells form glandular structures (E, arrows) that being avascular are hypoxic (F, arrows). Most surgical samples of colorectal cancers have such glandular structures (G, arrows) containing hypoxic cancer cells within the glandular regions (H, arrows).
Treatment with MSC Results in Significant Tumor Growth Inhibition
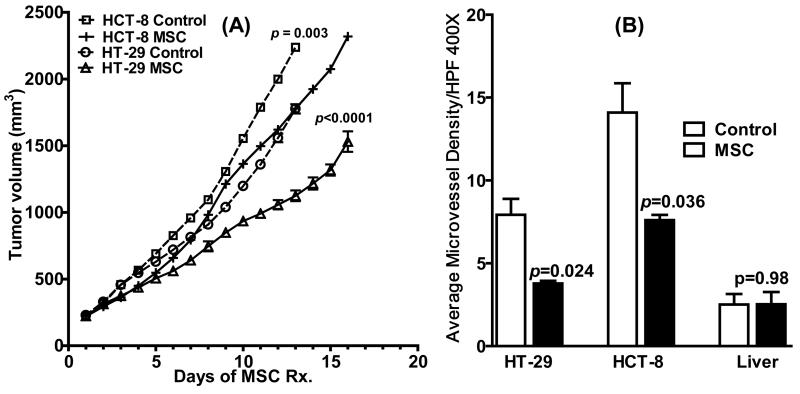
To investigate the antitumor activity of MSC against the two CRC xenografts, tumor-bearing mice were treated daily with MSC (0.2 mg/mouse/day for 16 days) and tumor growth monitored till the end of the experiment. As shown in Figure 2A, a significant reduction in tumor growth was observed following MSC treatment compared to untreated controls in both HCT-8 (P<0.0001) and HT-29 (P=0.003). Thus, antiangiogenic effects of MSC contributed to tumor growth inhibition in both CRC xenografts.
Figure 2.
(A) MSC had a tumor inhibitory effect in both the HCT-8 and HT-29 xenografts (n=10 per treatment group) as depicted by the reduction in tumor volume between MSC-treated tumors and untreated controls over a 16-day period following treatment with MSC (0.2 mg/mouse/day). (B) Bar graphs show MVD counts per high power field (HPF) (original magnification, 400X) in the untreated vs. MSC-treated HCT-8 and HT-29 xenografts (n=4 per treatment group)following daily MSC treatment (0.2 mg/mouse/day × 14). Significant reduction in MVD was seen in both the xenografts after 14 days of MSC treatment with no changes seen in liver.
Antiangiogenic MSC Lowers Tumor Microvessel Density in both colorectal xenografts
Microvessel density (MVD) estimates of untreated HT-29 xenografts were ~50% lower (P=0.04) compared to untreated HCT-8 xenografts. As shown in Figure 2B, treatment with MSC (0.2 mg/mouse/day x 14) resulted in a ~ 46% and 52% reduction in MVD compared to untreated control tumors in HCT-8 (7.6 ± 0.32 vs. 14.1 ± 1.77, P = 0.04) and HT-29 (3.8 ± 0.16 vs. 7.93 ± 0.96, P = 0.02) respectively. This effect was tumor specific with no significant change in MVD observed in normal tissue such as liver.
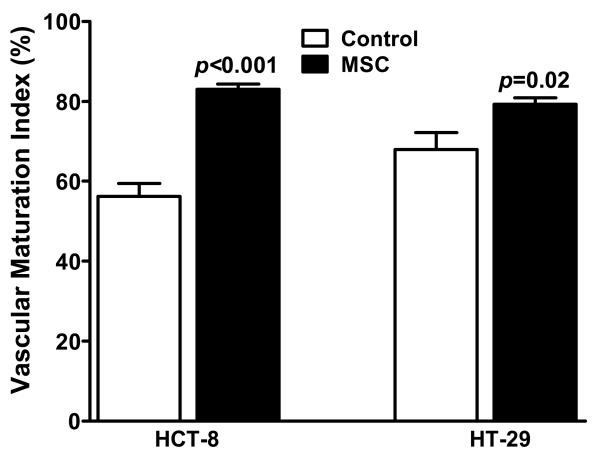
MSC Induces Tumor Vascular Maturation in CRC
We have previously shown that MSC treatment results in an increase in the vascular maturation index (VMI) in a xenograft model of human head and neck cancer9. Using the same method, we examined if treatment with MSC resulted in increased α-SMA coverage of tumor vasculature compared to untreated controls in both HCT-8 and HT-29 xenografts. As shown in Figure 3, quantitative estimates of VMI, i.e. the percentage of endothelial cells associated with pericytes showed a ~48 and ~17 % increase in VMI in MSC-treated HCT-8 and HT-29 tumors respectively, compared to untreated control tumors. MSC-induced increase in VMI was higher in well vascularised HCT-8 tumors compared to the relatively resistant HT-29 xenografts.
Figure 3.
Quantitative estimates of VMI in untreated controls vs. MSC-treated HCT-8 and HT-29 xenografts (n=4 per treatment group) indicating tumor vascular normalization in both the xenografts by MSC.
Altered vascular function in CRC xenografts following MSC treatment
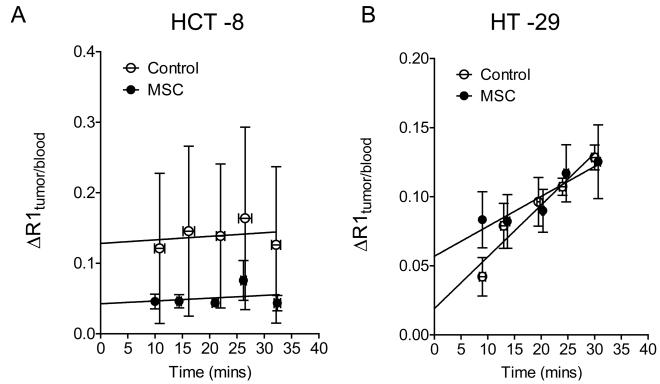
To investigate the consequence of MSC-induced alterations in MVD and VMI on tumor vascular function, we utilized contrast-enhanced DCE-MRI to examine changes in tumor vascular volume and permeability following MSC treatment. Consistent with differences in MVD between the two xenografts, estimates of tumor vascular volume were higher in control HCT-8 xenografts compared to HT-29 tumors. Treatment with MSC induced a significant (P<0.001) decrease in tumor vascular volume in HCT-8 xenografts compared to volume-matched controls (Figure 4A). However, no significant decrease in permeability was observed following treatment. In comparison, no significant reduction in relative vascular volume or permeability was observed in the relatively resistant HT-29 xenografts.
Figure 4.
Vascular response of HCT-8 (A) and HT-29 (B) CRC xenografts to MSC assessed using contrast-enhanced MRI. Relative vascular permeability and vascular volume in control and MSC-treated tumors were calculated from the normalized change in the longitudinal relaxation rate (ΔR1) as described in the Materials and Methods. The sample sizes for the individual groups are as follows: HCT-8: Control (n=6), MSC (n=5) with a P < 0.0001 between intercepts and P > 0.05 between slopes; HT-29: Control (n=4); MSC (n=6) with a P > 0.05 between intercepts and slopes.
MSC Reduces and Improves Tumor Interstitial Fluid Pressure
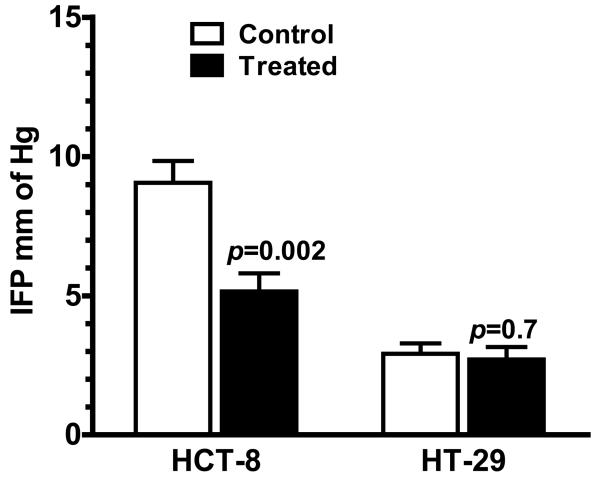
Recent studies have shown that antiangiogenic agents decrease IFP and facilitate enhanced drug delivery to tumors. To determine if the antiangiogenic activity of MSC contributed to a similar effect, we measured IFP levels in control and MSC treated tumors using the microcatheter transducer technique. As shown in Figure 5, treatment with MSC led to a significant reduction in tumor IFP in HCT-8 (9.06 ± 0.78 vs. 5.15 ± 0.65, P = 0.002) with no significant change in HT-29 xenografts (2.92 ± 0.37 vs. 2.71 ± 0.44, P = 0.7). IFP in relatively resistant HT-29 were significantly lower (~68%) lower than that of HCT-8 (P<0.0001).
Figure 5.
Treatment with MSC led to a significant reduction in IFP in HCT-8 [Control (n=8); MSC (n=8)] but not in HT-29 [Control (n=8); MSC (n=6)] xenografts.
MSC Improves Tumor Drug Delivery
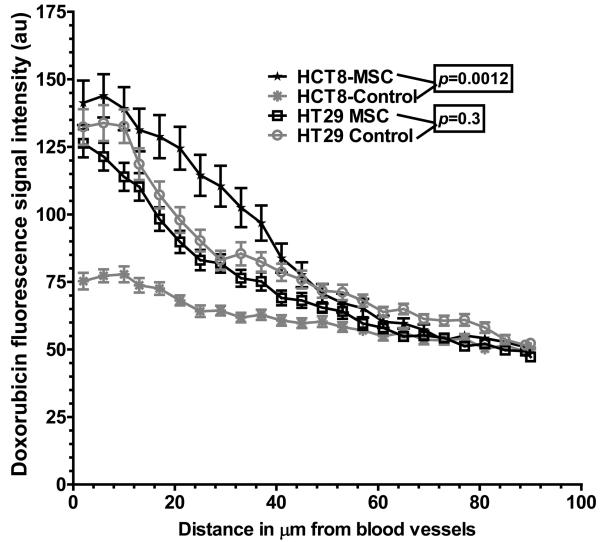
We then examined the effect of MSC on drug delivery by quantitating the levels of doxorubicin in tumors using autofluorescence. Treatment with MSC led to a significant increase in doxorubicin intensity at regions close to the vessel wall and away from the blood vessel (Figure 6) in HCT-8 compared to the untreated HCT-8 tumors, highlighting the improvement in intratumoral drug delivery and penetration in the relatively sensitive and well vascularised HCT-8. In case of the relatively resistant and morphologically heterogeneous HT-29 xenograft, there was no significant difference in intratumoral doxorubicin intensity gradient between the control and the MSC treated groups at regions adjacent to the blood vessel (131.6 ± 6.8 vs. 115.4 ± 5.2, P = 0.6) but there was a significant increase at a distance of 90 μm (micrometers) away from the vessels in the MSC treated HT-29 xenografts (47.22 ± 1.34 vs. 52.25 ± 1.56, P = 0.02).
Figure 6.
Quantitation of doxorubicin fluorescence intensity (au) in HCT-8 and HT-29 colorectal cancer xenografts (n=4 per treatment group) treated with doxorubicin ± MSC. Pretreatment with MSC showed an improved tumor delivery and penetration of doxorubicin in HCT-8 but not in the HT-29 xenografts, in the combination treatment group.
DISCUSSION
Normal colon mucosa does not show presence of microvessels in the glandular structures and yet these glandular structures are not hypoxic because the well vascularised periglandular tissue microenvironment allows diffusion of nutrients and oxygen to the cells within the glandular structure. In contrast, colerectal adenocarcinoma are characterized by the presence of such irregular, atypical glandular structures with lumens formed by the multi layered tumor cells as seen in HT-29 human tumor xenografts [Figure 1(ii), panels E, arrows] and in majority of surgical samples [Figure 1(ii), panels G, H arrows]. This characteristic glandular structure is maintained in the HT-29 xenografts even after many passages. While the histological characteristics of uniformly poorly differentiated HCT-8 without any glandular formationby the tumor cells were observed in some clinical specimens, majority of clinically seen recalcitrant human colorectal adenocarcinomas are similar in histological characteristics to the HT-29 xenograft model. Intratumoral microvessels detected by CD31 (mouse xenografts) /CD34 (human tumor) are found present in the tumor stroma, but the glandular regions with the lumen do not contain any vessels [Figure 1(ii), panel E & G], and as a consequence these areas are hypoxic, as demonstrated by the positive CAIX immunostaining [Figure 1(ii), panel F & H]. Further, HT-29 contains hypoxic regions (panels F) similar to majority (79%) of human surgical samples of CRC. Such glandular regions in adenocarcinomas are avascular, hypoxic and therefore have limited access to therapeutic agents. These regions are likely to provide sanctuaries to some proliferating tumor cells that can escape chemotherapy and contribute to tumor re-growth and drug resistance. We have previously reported a similar observation in the well differentiated avascular hypoxic regions in head and neck squamous cell carcinoma where we demonstrated the limited drug delivery using radio labeled drug and autoradiography16. This barrier caused by a specific histological structure (glandular formation) provides additional new rationale for the limited clinical success of chemotherapy in CRCs where a majority of such tumors show a histomorphology similar to HT-29.
Despite the presence of higher (~4x) tumor blood flow compared to normal tissues such as muscle17, tumor vasculature is predominantly abnormal in function and constitute a faulty intratumoral drug delivery system. Antiangiogenic agents have been shown to cause vascular pruning, arborization, maturation and retard formation of new vessel while ‘normalizing’ the existing vessels4. Tumor vascular normalization leads to further consequent changes in tumor microenvironment including a reduction in tumor IFP, blood flow, hypoxia and acidosis resulting in significant potentiation of chemo- and radiation- therapy7, 18, 19. Clinically, a high IFP is associated with poor survival after radio- and chemo-therapy20. In general a lower IFP has been found to be conducive for higher tumor specific drug delivery21 though changes in IFP may not influence all drugs in a similar fashion. In our study, MSC was found to significantly reduce tumor MVD, inhibit tumor growth and improve VMI in both the tumor models but this was translated into a significantly reduced favorable tumor IFP by MSC only in the relatively sensitive untreated HCT-8 with a higher IFP. MSC as a monotherapy had very limited tumor inhibitory effects but in combination chemotherapy, the response in terms of complete remissions was extremely significant as reported earlier 11. While organoselenium compound as a suicide prodrug substrate has been previously reported to result in significant tumor inhibition22, its' use as an antiangiogenic agent and a chemomodulator in combination chemotherapy is novel and holds great translational promise in the clinic. While the intratumoral drug gradient was significantly improved in HCT-8, this improvement was noticed in HT-29only at a distance of 90 μm away from the vessels with no significant difference in the regions immediately adjoining the vessels. Thus, treatment with MSC led to an improved tumor drug delivery and penetration in both the xenografts. This increase was significantly higher in the uniformly well vascularized HCT-8 xenografts compared to HT-29 xenografts. The relatively resistant CRC xenograft HT-29 had a low IFP (~68% lower than HCT-8). A lower IFP should have facilitated a better intratumoral drug delivery and, thus, an improved chemotherapeutic efficacy. However, due to limited tumor vascularization as reflected by a lower MVD (14.1 ± 1.77 in HCT-8 vs. 7.93 ± 0.96 in HT-29) and the presence of histomorphological heterogeneity conferred by presence of avascular hypoxic glandular regions that impede free drug diffusion and delivery into the tumor, HT-29 xenografts are relatively resistant to chemotherapy. Treatment with MSC led to a significant reduction in MVD and an improvement in VMI and consequently leading to a higher tumor drug delivery gradient that could at least partially explain the dramatic increase in cures from 20% and 0% with CPT-11 alone vs. 100% and 20% with the MSC+CPT-11 combination in HCT-8 and HT-29 respectively as reported earlier11. Despite not a significant improvement in IFP and DCE-MRI measured vascular volume and permeability in HT-29, MSC in combination with CPT-11 still lead to a marginally higher cure rate of 20% compared to 0% with CPT-11 alone11. This effect of MSC was not limited to ectopic models but was found to similarly impact orthotopically implanted GEO colon xenografts in nude mice with a significant reduction in MVD (24 ± 2.42 in control vs. 9.73 ± 1.01 in MSC treated group, P <0.0001) and an improved tumor vascular maturation (60.59% in control vs. 71.14 in MSC treated group, P = 0.02) on day 15. As a consequent, a significant reduction in tumor growth was seen as measured for volume using non-invasive MRI imaging on day 27 (176.98 ± 36 mm3 vs. 38.29 ± 16.9 mm3, P = 0.01) (A. Bhattacharya, personal communication).
To the best of our knowledge, this issue of glandular, avascular histological barrier to therapy has been largely overlooked in the quest for discovering new and novel anti-cancer agents. However effective such an individual agent may be, it is unlikely to achieve the anticipated therapeutic results in the clinic if it is unable to reach all individual cancer cells in the different histological regions within the tumor. Our study suggests an important role of avascular and hypoxic glandular differentiated regions in tumor drug delivery and therapeutic outcome in xenografts. Since most human cancers grow relatively slowly compared to xenografts that grow in an immune compromised preclinical animal, such unique histological structures are likely to play a more important role in therapy resistance in CRC in the clinic.
Clinical trials with Organo-Se compounds have shown encouraging results including durable response and disease stabilization in patients who previously failed combination chemotherapy in lung, gastric, and pancreatic adenocarcinomas12. In order to achieve the pharmacokinetic plasma profile of 20-30 μM Se found optimal in the in vivo preclinical models, a modified phase I trial was initiated with Se dose calculated to achieve the desired plasma concentrations. This desired plasma Se concentration has been clinically achieved with 7200 μg bid/day × 7 and concurrently at 7200 μg/day of SLM in combination with CPT-11, without any significant adverse toxicity12. Phase II clinical trials with SLM are currently ongoing at Roswell Park Cancer Institute in lung and rectal cancers.
CONCLUSION
Most antiangiogenic agents available today were designed to target a single specific proangiogenic molecule that most tumors can easily overcome through bypassing on to other angiogenic markers/pathways. Besides, currently available antiangiogenic agents are limited by dose limiting toxicity including to normal vasculature and are cost prohibitive23. In contrast, selenium being part of the mammalian physiology is relatively well tolerated affecting multiple targets (HIF-1α, VEGF, Cox-2 etc)24 important for cancer survival and progression25. The data presented in this study along with the feasibility of attaining the plasma Se levels comparable to that seen in the preclinical model for therapeutic synergy makes MSC a clinically viable antiangiogenic agent that can possibly inhibit tumor growth and enhance therapeutic efficacy of neo-adjuvant, adjuvant or primary palliative chemotherapy in patients with CRC. This chemo-modulating effect is likely to be more pronounced and significant in tumors that share histomorphological features of HCT-8 i.e. uniformly well vascularised with little or no hypoxic regions. Furthermore, since MSC have been shown to have a significant protective effect on normal tissues from the adverse effects of cytotoxic drugs 11, drug dose escalation is an additional attractive possibility with use of MSC in the clinic since in the preclinical system, the MTD of the some anticancer drugs was found to double with MSC11. The results obtained here demonstrate that non-toxic dose of MSC is a marked inhibitor of angiogenesis and tumor growth, while concomitantly improving vascular maturation, function and chemotherapeutic efficacy in CRCs. Further, CRCs that have little or no hypoxic regions will be more responsive to antiangiogenic agents used in combination chemotherapy while those with many hypoxic glandular structures will not be easily amenable to therapy with most anti-cancer agents.
Acknowledgments
Supported by National Cancer Institute Grant 1 R21 CA133682-01A2 (A Bhattacharya) and a Comprehensive Cancer Center Support Grant CA016056 from the National Cancer Institute, Bethesda, MD.
REFERENCE
- 1.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J of Med. 2004;351:317–9. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya A, Toth K, Durrani FA, et al. Hypoxia specific drug tirapazamine does not abrogate hypoxic cells in combination therapy with irinotecan and methylselenocysteine in well-differentiated human head and neck squamous cell carcinoma A253 xenografts. Neoplasia. 2008;10:857–65. doi: 10.1593/neo.08424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Understanding barriers to drug delivery: high resolution in vivo imaging is key. Clin Cancer Res. 1999;5:1605–6. [PubMed] [Google Scholar]
- 7.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Eng J of Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya A, Seshadri M, Oven SD, Toth K, Vaughan MM, Rustum YM. Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clin Cancer Res. 2008;14:3926–32. doi: 10.1158/1078-0432.CCR-08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippman SM, Klein EA, Goodman PJ, et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Am Med Assoc. 2009;301 in-press. [Google Scholar]
- 11.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–9. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 12.Fakih MG, Pendyala L, Brady W, et al. A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemotherapy and Pharmacol. 2008;62:499–508. doi: 10.1007/s00280-007-0631-4. [DOI] [PubMed] [Google Scholar]
- 13.Azrak RG, Cao S, Pendyala L, et al. Efficacy of increasing the therapeutic index of irinotecan, plasma and tissue selenium concentrations is methylselenocysteine dose dependent. Biochem Pharmacol. 2007;73:1280–7. doi: 10.1016/j.bcp.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 15.Ozerdem U, Hargens AR. A simple method for measuring interstitial fluid pressure in cancer tissues. Microvascular Res. 2005;70:116–20. doi: 10.1016/j.mvr.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharya A, Toth K, Mazurchuk R, et al. Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clin Cancer Res. 2004;10:8005–17. doi: 10.1158/1078-0432.CCR-04-1306. [DOI] [PubMed] [Google Scholar]
- 17.Brix G, Bahner ML, Hoffmann U, Horvath A, Schreiber W. Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT-initial experience. Radiology. 1999;210:269–76. doi: 10.1148/radiology.210.1.r99ja46269. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorn ME, Strieth S, Dellian M. Anti-vascular tumor therapy: recent advances, pitfalls and clinical perspectives. Drug Resist Updat. 2004;7:125–38. doi: 10.1016/j.drup.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–35. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milosevic M, Fyles A, Hill R. Interstitial fluid pressure in cervical cancer: guide to targeted therapy. Am J of Clin Oncol. 2001;24:516–21. doi: 10.1097/00000421-200110000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure - an obstacle in cancer therapy. Nature reviews. 2004;4:806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 22.Miki K, Xu M, Gupta A, et al. Methioninase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer research. 2001;61:6805–10. [PubMed] [Google Scholar]
- 23.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nature reviews. 2007;7:475–85. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 24.Yin MB, Li ZR, Toth K, et al. Potentiation of irinotecan sensitivity by Se-methylselenocysteine in an in vivo tumor model is associated with downregulation of cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia-inducible factor 1alpha expression, resulting in reduced angiogenesis. Oncogene. 2006;25:2509–19. doi: 10.1038/sj.onc.1209073. [DOI] [PubMed] [Google Scholar]
- 25.Whanger PD. Selenium and its relationship to cancer: an update. The British journal of nutrition. 2004;91:11–28. doi: 10.1079/bjn20031015. [DOI] [PubMed] [Google Scholar]