Abstract
The interposed nuclei (IN) of the intermediate cerebellum are critical components of the circuits that control associative learning of eyeblinks and other defensive reflexes in mammals. The IN, which represent the sole output of the intermediate cerebellum, receive massive GABA-ergic input from Purkinje cells of the cerebellar cortex and are thought to contribute to the acquisition and performance of classically conditioned eyeblinks. The specific role of deep cerebellar nuclei and the cerebellar cortex in eyeblink conditioning are not well understood. One group of studies reported that blocking GABAA neurotransmission in the IN altered the time profile of conditioned responses (CRs), suggesting that the main function of the cerebellar cortex is to shape the timing of CRs. Other studies reported that blocking GABAA neurotransmission in the IN abolished CRs, indicating a more fundamental involvement of the cerebellar cortex in CR generation.
When examining this controversy, we hypothesized that the behavioral effect of GABAA blockers could be dose-dependent. The IN of classically conditioned rabbits were injected with high and low doses of picrotoxin and gabazine. Both GABAA blockers produced tonic eyelid closure. A high dose of both drugs abolished CRs, whereas a less complete block of GABAA-mediated inputs with substantially smaller drug doses shortened CR latencies. In addition, low doses of picrotoxin facilitated the expression of unconditioned eyeblinks evoked by trigeminal stimulation. These results suggest that the intermediate cerebellum regulates both associative and non-associative components of the eyeblink reflex, and that behavioral effects of blocking Purkinje cell action on IN neurons are related to collective changes in cerebellar signals and in the excitability of extra-cerebellar eyeblink circuits.
Keywords: Purkinje cells, interposed nucleus, classical conditioning, rabbit
1. Introduction
The intermediate cerebellar cortex and interposed nuclei (IN) are important parts of circuits controlling the learning and expression of anticipatory withdrawal responses, such as classically conditioned eyeblinks (CRs). The IN are the output of the intermediate cerebellum, and GABAergic Purkinje cells of the cerebellar cortex are the main source of their innervation (Ito, 1984). As a consequence of this functional arrangement, studies manipulating GABAA receptor-mediated neurotransmission in deep cerebellar nuclei offer important insights into cerebellar control of eyeblink motoneurons. It is known that activating GABAA receptors with muscimol suppresses the modulation and spontaneous activity of IN neurons (Aksenov et al., 2004), and this treatment blocks expression of CRs (Krupa et al., 1993; Bracha et al., 1994). Effects of suppressing IN neuronal activity in rabbits are not restricted to CRs. Cerebellar nuclear microinjections of muscimol also down-regulate the amplitude of airpuff-evoked unconditioned trigeminal eyeblinks (Bracha et al., 1994; Jimenez-Diaz et al., 2004) and block instrumental eyelid closure (Bracha et al., 2001). The inactivation data together with the fact that neuronal activity in intact IN correlates with stimuli and movements during conditioned and unconditioned blinks (Berthier and Moore, 1990; Aksenov et al., 2004; Jimenez-Diaz et al., 2004; Zbarska et al., 2008) suggest that the modulation of neuronal activity in the intermediate cerebellum, coupled with the out-going tonic excitatory drive, control a range of learned and reflexive eyeblink behaviors.
Additional understanding of the intermediate cerebellar role in eyeblink control can be gained from studies that block GABAergic neurotransmission in cerebellar output nuclei. In contrast to muscimol, infusing the IN with the chloride channel blocker picrotoxin (PTX) dramatically elevates the spontaneous firing of IN neurons and decreases modulation of their activity during CR expression (Aksenov et al., 2004). Thus far, behavioral effects of blocking cerebellar nuclear GABA neurotransmission were not studied systematically and several studies of CR performance yielded conflicting results. Mauk and Garcia (1998) reported that IN injections of picrotoxin or gabazine (GZ) invariably decreased CR latency, leading to so called short-latency conditioned responses (SLRs). In contrast, others reported that IN infusions of PTX abolish CRs (Mamounas et al., 1987; Attwell et al., 2002; Aksenov et al., 2004). The cause of these variable PTX effects is not clear. In addition to reports on the effects of cerebellar inactivations/lesions in rabbits, studies have also been conducted on human subjects, indicating similar involvement of the intermediate cerebellum in the control of the onset timing and amplitudes of eyeblink responses in humans (Gerwig et al., 2005).
Based on our previous report of a dose-dependent effect of PTX on IN neuronal activity (Aksenov et al., 2004), we propose that behavioral outcomes of injecting the IN with GABAA receptor antagonists are related to the extent of the block. We hypothesized that a partial disruption of GABAergic neurotransmission in eyeblink-related neurons should produce SLRs and that a complete block of inhibitory inputs will abolish CRs. To address this hypothesis, we infused the IN of classically conditioned rabbits with low and high doses of GABA antagonists. In the second part of this study we investigated the parallel effects of PTX infusions on CRs and visual and trigeminal unconditioned responses (URs). Here we report that a more complete block of GABAA receptor-mediated neurotransmission with high doses of PTX and GZ abolished CRs. In contrast, lower doses of PTX and GZ produced SLRs, increased tonic eyelid closure, and facilitated unconditioned trigeminal eyeblinks.
2. Results
2.1. General observations
When injected in the IN at sites where previous small injections of muscimol abolished CR expression, both GZ and PTX had a dose-dependent effect on eyeblink expression. At small doses, both drugs shortened CR latency and increased tonic eyelid closure. In addition, low-dose PTX increased the amplitude of URs to a weak airpuff US and altered the velocity and duration of URs evoked by the normal airpuff intensity. At higher doses both drugs suppressed CRs. Besides their effect on eyelid movements, both drugs exaggerated responses to the airpuff US causing a more generalized withdrawal response encompassing neck and forelimb movements that drew the animal’s head away from the air stimulation. Notably, tonic eyelid closure and withdrawal-related postural asymmetry disappeared immediately after the animal was removed from the restraint box. All of these effects were observed at injection sites located directly at or in the near vicinity of the left anterior IN (Fig. 1).
Fig. 1.
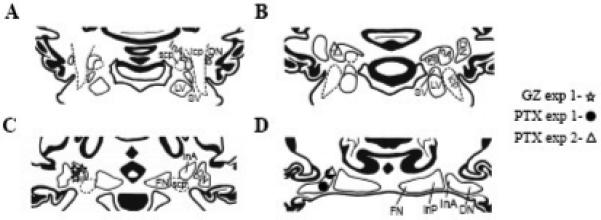
Reconstruction of injection sites in the IN for GZ (stars, n = 3) and PTX (circles, n = 3) for Experiment #1 and PTX (triangles, n = 4) for Experiment #2. The identified sites were transferred to a set of standardized coronal sections of the rabbit cerebellum. A-D: four adjacent, 0.5-mm sections through the cerebellum, arranged in rostral-caudal order. All injection sites were located directly on or in close proximity to the anterior interposed/dentate nuclear border. InA, anterior interposed nucleus; DN, dentate nucleus; LV, lateral vestibular nucleus; SV, superior vestibular nucleus; InP, posterior interposed nucleus; FN, fastigial nucleus; scp, superior cerebellar peduncle; icp, inferior cerebellar peduncle.
2.2. Effects of Gabazine and Picrotoxin on CR expression (Experiment #1)
The effects of both drugs on CR expression were dose-dependent, yielding either SLRs or CR abolition. Drug doses that led to these effects varied between rabbits. For example, a dose that shortened latencies of CRs in one rabbit could abolish CRs in another. Consequently, both the low and high doses were individually titrated for each animal. Our criterion for the high-dose was suppression of CR incidence to 30 % or less in at least one post-injection block of 10 trials. The low-dose was identified based on the appearance of SLRs lasting at least 10 minutes (about 30 trials).
2.2.1. Low-Dose Effects
When compared to control aCSF injections (Fig. 2C), low-dose GZ injections (0.13 – 0.51 nmol) significantly shortened CR latencies (Figs. 2A, 3A, F1,2 = 29.91, p = 0.032). The mean pre-injection eyeblink latency was 155.72 ± 15.03 ms and it declined immediately to 96.89 ± 15.80 ms in the first post-injection block of trials, peaking in the fifth at 78.41 ± 20.57 ms (Fig. 4C). Typically, SLRs were super-imposed on the background of GZ-induced tonic eyelid closure (Fig. 3A). Mean pre-injection eyelid aperture was 8.42 ± 1.28 % of maximum eyelid closure. Following GZ, rabbits had a tendency to ‘squint,’ significantly reducing their mean pre-eyeblink eyelid aperture nearly 6-fold to 56.69 ± 3.87 (Fig. 5A, F 9,18 = 6.97, p = 0.00025). In parallel to changes in eyeblink latency, GZ significantly reduced CR amplitude measured relative to eyelid aperture before the eyeblink (Figs. 2A, 3A and 5C, F9,18 = 2.96, p = 0.024). However, since these small amplitude CRs were executed on the background of a partially closed eye, the CR peak (measured relative to maximally opened eyelids) actually increased following the injection. Control injections of aCSF had no effect on CR latencies, amplitudes, or on baseline eyelid aperture (Figs. 4C, 5A, and 5C).
Fig. 2.
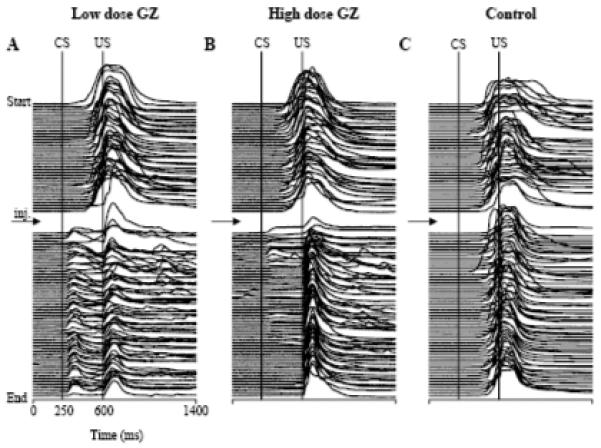
Examples of stack plots of eyeblink mechanograms showing the effects of GZ on conditioned eyeblink performance when injected in the IN. Each experiment begins at the top with each mechanogram representing 1 trial. All mechanograms were filtered by subtracting the mean pre-stimulation eyelid position in the corresponding trial. A: an experiment with an injection of low-dose GZ. Following the injection (indicated with arrow), the latency of conditioned responses (upward deflections between CS and US onset markers) was shortened for the remaining 60 trials. B: identical to plot A but in this experiment, high-dose GZ was injected. Following the injection, CRs were gradually abolished. C: control for both A and B in which aCSF was injected following 40 pre-injection trials. There was no vehicle effect on the expression of CRs in this experiment.
Fig. 3.
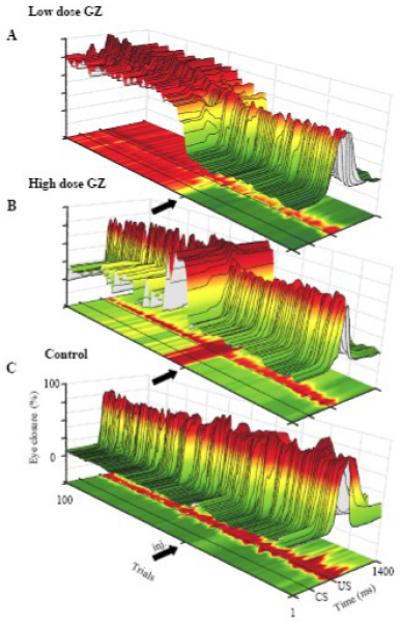
Three dimensional surface plots of the individual examples of eyeblink mechanograms shown in Fig. 2. In this display, eyeblink mechanograms were not filtered to preserve information about the tonic eyelid position. Each cut through the surface plot corresponds to one trial and the colors, together with the z axis, code the eyelid aperture (dark green = open, yellow = intermediate, dark red = mostly closed). A: one hundred trials illustrating the effect of low-dose GZ. The GZ injection (thick arrow) induced a long lasting tonic eyelid closure, coupled with decreased eyeblink amplitude and SLRs. B: Effect of high-dose GZ showing SLRs super-imposed on a tonically closed eye. Shortly thereafter, the tonic eyelid closure subsided and CRs were gradually abolished. C: control experiment for both A and B where aCSF, injected after 40 pre-injection trials, had no effect on tonic eyelid closure.
Fig. 4.
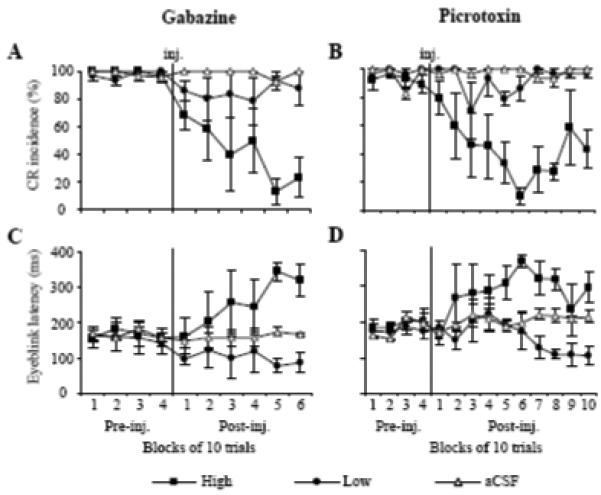
Effects of low (circle) and high doses (squares) of GZ and PTX on means (± SE, n = 3) for CR incidence and eyeblink latency. A: effect of GZ on CR incidence. While high-dose GZ suppressed CRs, low-dose GZ and control injections of vehicle (triangles) had minor or no effect on CR incidence. B: effect of PTX on CR incidence. Similar to GZ, high-dose PTX suppressed CRs, but CRs had a greater tendency to recover toward the end of the experiment. C: effect of GZ on eyeblink latency. High-dose GZ increased CR latency whereas low-dose decreased the CR onset time. D: effect of PTX on eyeblink latency. Effects of PTX are similar to GZ, except that low-dose PTX took 60 trials to shorten CR latency. In A-D, injections of aCSF (triangles) had no effect on CR expression.
Fig. 5.
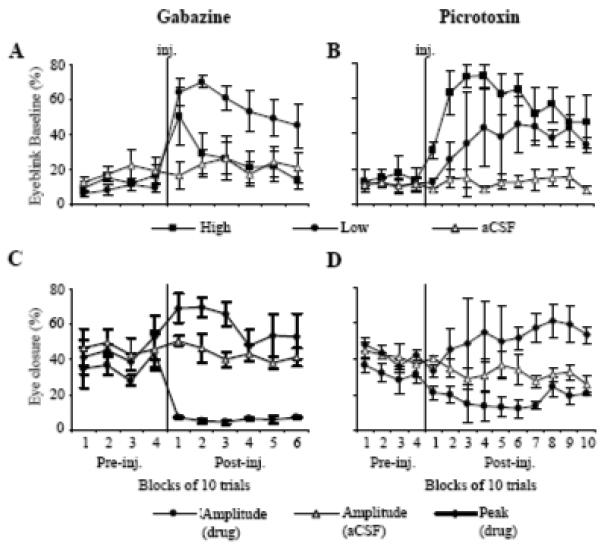
Effects of low (circle) and high-doses (square) of GZ and PTX on the means (± SE, n = 3) for eyeblink baseline, peak, and amplitude. A: effect of GZ on eyeblink baseline expressed in percentage of maximum eyelid closure. Low-dose GZ produced an immediate and long lasting eyelid closure. The eyelid closure resulting from high-dose GZ was short-lasting and it recovered within 20 trials. B: effect of PTX on eyeblink baseline. Both low and high-dose PTX induced long lasting eyelid closure. Control injections of aCSF (triangles) in A and B did not affect eyeblink baseline. C: effect of a low, SLR-inducing dose of GZ on eyeblink peak (diamonds) and amplitude (circles). Injected (inj.) after 40 pre-injection trials, GZ immediately increased eyeblink peak and decreased amplitude. D: effect of SLR-inducing, low-dose PTX on eyeblink peak and amplitude. Similar to GZ, PTX increased CR peaks but decreased eyeblink amplitudes.
Similar to GZ, injections of low-dose PTX (0.31 – 4.98 nmol) shortened eyeblink latency. However, this effect was notably delayed when compared to GZ and it developed only in the last four blocks of the 10-block post-injection period (Fig. 4D, F13,26 = 3.48, p = 0.0032). This difference in the effect onset was most likely related to differences in effectiveness of drug diffusion. Mean eyeblink latency decreased from 177.95 ± 9.53 ms pre-injection to 104.41 ± 25.79 ms by post-injection block 10. Also similar to GZ, PTX increased tonic eyelid closure (Fig. 5B, F13,26 = 2.13, p = 0.049) and it reduced CR amplitude (Fig. 5D F13,52 = 4.09, p = 0.00013) while increasing the absolute eyeblink peak in the CS - US period. Low doses of GZ and PTX had only a moderate effect on CR incidence, being slightly reduced and tending to recover toward the end of experiments (Figs. 4A-B).
2.2.2. High-Dose Effects
Effects of high doses of GZ (0.51 – 1.02 nmol) and PTX (0.62 – 7.47 nmol) were remarkably different from low-dose injections of these drugs. Most notably, both GZ and PTX at high doses suppressed CRs. An individual example in Figs. 2B and 3B shows that shortly after the injection of GZ, CRs were abolished, contrasting with the CR latency-shortening effects of low-dose GZ in the same animal and injection site (Figs. 2A, 3A). At the group level, high doses of GZ and PTX gradually, but significantly suppressed CR incidence (Figs. 4A-B) when compared to the pre-injection performance and to the control experiment (GZ: F9,18 = 9.21, p = 0.00004, PTX: F13,26 = 5.25, p = 0.0002). This gradual suppression of CRs was paralleled by a gradual increase of eyeblink latency in the CS - US period (Figs. 4C-D, GZ: F9,18 = 4.29, p = 0.0041, PTX: F13,26 = 5.23, p = 0.00017). While the effect of high-dose GZ on tonic eyelid closure was transient (Fig. 5A), high-dose PTX produced sustained eyelid closure, nearly doubling from 21.25 ± 3.40 % pre-injection to 41.42 ± 4.78 % of the full eyelid closure post-injection (Fig. 5B, F13,26 = 0.64, p = 0.000031).
2.3. Effects of Low-Dose Picrotoxin on CR and UR expression (Experiment #2)
In Experiment #1, we demonstrated how low doses of PTX affected CR expression. In considering whether disinhibiting the IN affects URs, four rabbits were injected with an SLR-eliciting dose of PTX so parallel effects of this treatment on CR and UR expression could be examined. In these animals, paired CS + US trials were intermixed with three different types of US trials: light, weak airpuff, and regular airpuff. As expected, injections of low-dose PTX (2.49 to 6.22 nmol) shortened CR latencies (Fig. 6A). The repeated-measures ANOVA revealed a significant drug and block-of-trials interaction (Fig. 7A, F3,9 = 8.061, p = 0.0064). Low-dose PTX shortened baseline CR latency from 197.20 ± 20.85 ms pre-injection to 99.43 ± 13.53 ms in the third block of post-injection trials. The tonic eyelid closure increased from 5.36 ± 0.76 % pre-injection to 20.02 ± 3.42 % in the second post-injection block and to 27.53 ± 8.26 % in the third (F3,9 = 4.23, p = 0.04). In parallel with tonic eyelid closure, the CR peak increased from 32.70 ± 7.41 % in pre-injection trials to 59.05 ± 12.04 % in the third post-injection block (Fig. 8B). This CR peak finding was revealed as main effects for the within-subject factor, blocks (F3,9 = 4.70, p = 0.031), and for the between-subject factor, PTX vs aCSF (F3,9 = 11.81, p = 0.041). Injections of vehicle had no effect on CR latency or tonic eyelid position. Since CR amplitudes were not significantly affected by PTX (Fig. 8A), it is likely that changes in the CR peak amplitude were due to increased tonic eyelid closure.
Fig. 6.
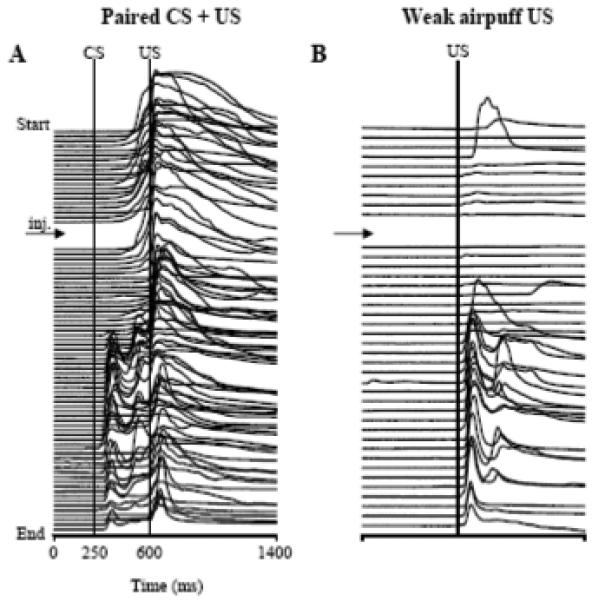
Example of effects of PTX injections in the IN on the performance of conditioned eyeblinks and on unconditioned responses evoked by weak airpuffs. Both stack plots of eyeblink mechanograms are a complete printout from the same experiment. The experiment begins at the top, with each trace representing one trial, and the time of injection is indicated by an arrow. A: in paired CS + US trials, PTX shortened the latency of CRs (positive trace deflections between the CS and US markers). B: In weak airpuff-alone trials, PTX increased UR amplitude approximately at the same time when SLRs were observed in A.
Fig. 7.
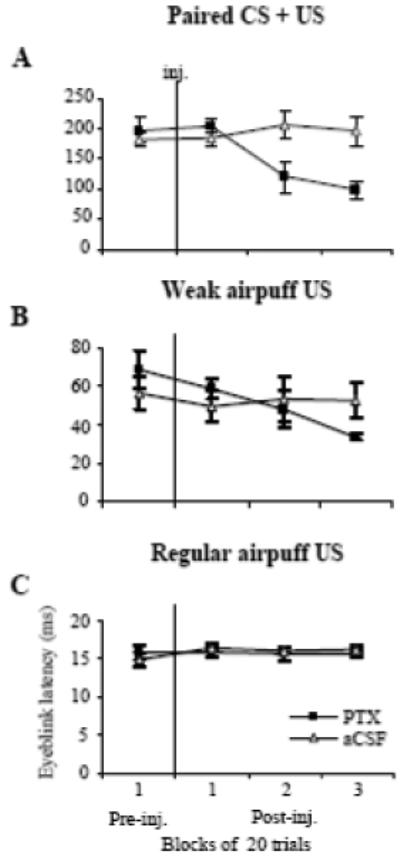
Effects of PTX (squares) injections (inj.) on the means (± SE, n = 4) for CR and UR latency. A: effect of PTX on eyeblink latency in paired CS + US trials. PTX reduced CR latency. B: effect of PTX injections to the IN on UR latency during weak airpuff trials. The UR latency gradually decreased when compared to the pre-injection level. C: PTX had no effect on UR latency during regular intensity airpuff trials. Injections of aCSF (triangles) had no effect on eyeblink latency during either type of trial.
Fig. 8.
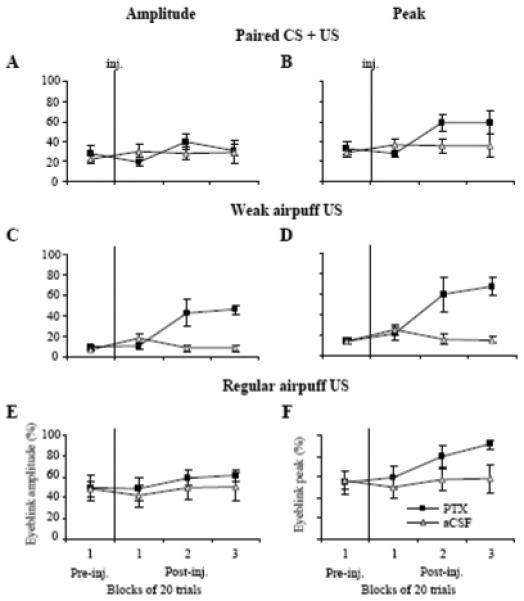
Effects of PTX (squares) on the means (± SE, n = 4) of amplitudes (left column) and peaks (right column) of CRs and airpuff-evoked URs. A: PTX injections (inj.) did not significantly affect CR amplitude in CS + US trials. B: PTX injections increased the CR peak in CS + US trials. C: The amplitude of responses to the weak airpuff US increased following PTX injections. D: The peak of responses to the weak airpuff US also increased following PTX injections. E: The amplitude of regular airpuff-evoked URs was not affected by PTX. F: The peak of regular airpuff URs slightly increased following PTX. Neither peaks nor amplitudes of responses in all three conditions were affected by control injections of aCSF (A-F).
2.3.1. URs to the weak airpuff
Among the three types of URs tested, eyeblinks to the weak airpuff were the most affected. Most notably, PTX increased UR amplitude during the same time period in which the drug affected CR expression (Figs. 6A-B). The mean amplitude of URs to the weak airpuff increased from 9.01 ± 2.65 % pre-injection to 45.88 ± 4.48 % during the third block of post-injection trials (Fig. 8C, F3,9 = 10.75, p = 0.00025). Mean UR peaks to the weak airpuff likewise increased from 13.66 ± 2.42 % during the pre-injection block of 10 trials to 67.64 ± 8.45 % during the third block of post-injection trials (Fig. 8D, F3,9 = 11.18, p = 0.0022). Similar to the PTX effect on CR latencies, mean UR latencies to the weak airpuff were shortened 50 % post-injection (Fig. 7B, F3,9 = 5.095, p = 0.025), steadily decreasing from 68.43 ± 9.80 ms pre-injection to 33.80 ± 1.91 ms in the third block of post-injection trials. Control injections of vehicle had no effect on latency or amplitude of URs to the weak airpuff (Fig. 7B, 8C).
2.3.2. URs to the strong airpuff and to the light
PTX did not significantly affect UR amplitude to the strong airpuff (Fig. 8E, 49.58 ± 11.83 % in the pre-injection and 61.87 ± 5.55 % in the third block of post-injection trials). Even though the size of eyeblinks did not change much, the resulting eyelid closure significantly increased due to the presence of the tonic squinting before blinks (Fig. 9A). The mean peak of URs to the strong airpuff (the response peak represents a sum of the pre-eyeblink tonic eyelid position and the eyeblink amplitude), increased from 54.33 ± 11.74 % pre-injection to 91.34 ± 3.84 % during the third block of post-injection trials. (Fig. 8F, F3,9 = 5.61, p = 0.019). The maximum instantaneous velocity of eyelid closure during eyeblinks to the strong airpuff nearly doubled from 0.99 ± 0.26 % per ms pre-injection to 1.93 ± 0.23 % per ms during the third block of post-injection trials (Fig. 9C, F3,9 = 4.49, p = 0.035). Besides its effect on eyelid closure velocity, PTX also delayed eye re-opening which was manifested as greater eyelid closure still present at the end of the 1400-ms recording period. Delayed eye re-opening was apparent in eyeblink averages (Fig. 9A). At the end of the 1400 ms recording period, eyelid closure had increased from 9.44 ± 1.30 % in pre-injection to 53.28 ± 12.76 % during the third block of post-injection trials (Fig. 9A, F3,9 = 9.65, p = 0.00036). Injections of vehicle had no effect on the peak, profile, or velocity of URs to the strong airpuff (Figs. 8E-F, 9B).
Fig. 9.
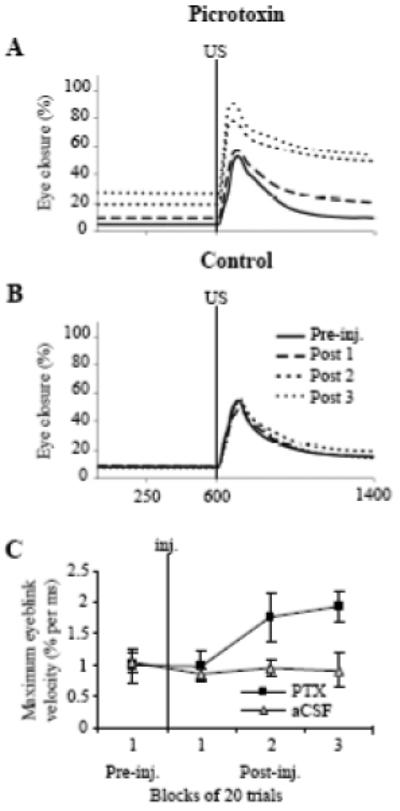
Effects of PTX on the time profile and instantaneous velocity of URs evoked by strong airpuff. A: average eyeblinks (n = 4) to the strong airpuff in one block of trials before (Pre-inj.) and three blocks of trials following (Post 1-3) PTX injections. PTX increased tonic eyelid closure seen as increased signal levels before the US onset. Also, eyelid re-opening was delayed in post-injection trials as indicated by the higher signal at the end of the recording period. B: average eyeblinks to the strong airpuff before and after control injections of aCSF. Injections of vehicle had no effect on the shape of strong airpuff-evoked eyeblinks. C: peak velocity of URs to the strong airpuff (mean ± SE, n = 4), which was calculated in a sliding window of 20 msec as the first derivative of rise-to-peak velocities of individual trials of URs to the strong airpuff, and then it was averaged by blocks. PTX significantly increased the maximum instantaneous velocity of strong airpuff URs.
PTX did not have a significant effect on URs to the light. At the group level, the amplitude of URs to the light was 22.86 ± 8.54 % in the pre-injection period, 29.13 ± 9.91 % (F1,9 = 0.28, p = 0.63) during the second and 20.69 ± 5.96 % (F1,9 = 0.090, p = 0.78) during the third block of post-injection trials. The latency of URs to the light tended to decrease from 132.27 ± 15.85 ms pre-injection to 114.69 ± 11.38 ms (F1,9=3.77, p = 0.15) during the third block of post-injection trials.
3. Discussion
The present study demonstrated that blocking GABAA neurotransmission in the IN with PTX or GZ affects CRs in a dose-dependent manner. While high doses of GABAA blockers suppressed CR expression, lower drug doses shortened CR latency. Besides their effects on CRs, the SLR-inducing doses of PTX also affected non-associative components of eyelid movements; they increased tonic eyelid closure and facilitated URs evoked by trigeminal stimulation.
3.1. Effects on CR expression
We hypothesized that prior variance in results of blocking GABAA receptor-mediated neurotransmission in the IN on CR expression could be related to the extent of the GABAA block. Our data presented here confirm this notion. High amounts of PTX and GZ suppressed CRs. This finding confirmed previous reports of CR abolition (Mamounas et al., 1987; Attwell et al., 2002; Aksenov et al., 2004). On the other hand, lower amounts of both drugs, when administered at sites where high doses abolished CRs, had a minor effect on CR incidence, but significantly shortened their latency. This supports previous reports of SLRs induced by cerebellar nuclear injections of similar amounts of PTX (0.2-0.4 nmol in Garcia and Mauk, 1998; 2 nmol in Medina et al., 2001) or GZ (0.04 nmol in Ohyama et al., 2006). Although all injection sites in this study were located in the IN region (Fig. 1), and their proximity to eyeblink-related parts of cerebellar nuclei was functionally confirmed with muscimol injections abolishing CRs, effective doses of GABAA blockers had to be titrated to optimize effects. In general, smaller amounts of drugs were required for SLRs and CR abolition at sites with the best muscimol effects, suggesting a dependency on the amount of drug diffusing around the eyeblink representation in deep cerebellar nuclei.
Differences between the effects of high and low drug doses could result from drug spreading to the overlying cerebellar cortex. Blocking GABAA neurotransmission in the cerebellar cortex increases the tonic firing rate of GABAergic Purkinje cells (Thomsen et al., 2004). Assuming the high drug dose did diffuse into the non-targeted cerebellar cortex, increased Purkinje cell activity would inhibit the IN and abolish CRs. However, this effect would be prevented by the simultaneous suppression of GABAA neurotransmission in the targeted deep cerebellar nuclei. Thus, conjectured drug diffusion to the cerebellar cortex does not explain CR abolition by high doses of GABAA blockers.
In agreement with previous reports (Medina et al., 2001), SLR-inducing doses of both blockers reduced CR latency and changed the temporal profile of CRs, which frequently peaked before the US onset (Figs. 2A, 3A). GZ reduced CR amplitude (Figs. 2 and 5) when measured relative to the eyelid position before application of the CS. Effects of PTX on CR amplitude were less pronounced, ranging from a small decrease in Experiment #1 (Fig. 5) to no change in Experiment #2 (Fig. 8). On the other hand, CR peaks measured relative to the maximally open eye, increased following both drugs and in both experiments (Figs. 5, 8). These seemingly contradictory effects were due to a drug-induced tonic eyelid closure. Following drug injections, CRs were evoked on a background of tonic eyelid closure and the same or smaller blinks resulted in absolute eyelid closure larger than baseline blinks recorded before injections or those recorded after control.
3.2. Effects on non-associative components of blinking
High and low doses of PTX and low-dose GZ elicited sustained tonic eyelid closure during which animals maintained partially closed eyelids both during and between trials. This finding confirms and extends our previous reports of PTX effects on tonic eyelid position in instrumental and classical conditioning tasks in the rabbit (Bracha et al., 2001; Aksenov et al., 2004). In our study of instrumental eyelid behavior we found that inactivating the IN with the GABAA agonist, muscimol, disrupts instrumentally conditioned tonic eyelid closure (Bracha et al., 2001). Since down-regulating the neuronal firing rate in the IN with muscimol produces tonic eyelid opening, whereas increasing the IN neuronal activity with GABAA antagonists increases tonic eyelid closure, it appears that tonic IN activity controls tonic eyelid aperture. In this regard, it was surprising that high doses of GZ produced only transient tonic eyelid closure, followed by eyelid opening at later stages of the experiment. Pertinent to this finding, in earlier work we observed that injecting the IN with the GABAA antagonist bicuculline at low concentrations increased the tonic activity of IN neurons and at high concentrations evoked bursting followed by long periods of inactivity (Bayev and Bracha, unpublished observations). If GZ has similar properties, then low doses would increase the tonic activity of IN neurons (Chen and Evinger, 2006) and enhance tonic eyelid closure. On the other hand, high doses would reduce the IN spontaneous firing rate, leading to eyelid opening and CR abolition. However, based on previously reported data, the different effects on tonic eye closure of PTX compared to GZ can be explained by the distinct mechanisms by which they affect GABAA channels (Bouairi et al., 2006).
To determine whether SLR-inducing doses of PTX affect URs, rabbits were presented with trigeminal and visual stimuli. Analyses of latencies, amplitudes, and velocity profiles showed no effect on photic URs. In contrast, PTX facilitated URs evoked by airpuffs. This was most pronounced in weak airpuff trials, where PTX shortened UR latencies and increased UR amplitudes. In strong airpuff trials, PTX increased the maximum instantaneous velocity of eyelid closure and delayed eyelid re-opening following the blink. These findings corroborate observations of GZ effects on URs in anesthetized rats (Chen and Evinger, 2006) and complement reports of opposite effects of IN lesions and inactivations in the rabbit (Welsh and Harvey, 1989; Welsh, 1992; Bracha et al., 1994). Here we have shown that SLR-inducing levels of GABAA neurotransmission affect non-associative eyelid movements. These data collectively demonstrate IN involvement in the control of tonic eyelid closure and trigeminal stimulation-evoked URs.
3.3. Implications for cerebellar control of eyeblinks
The present findings illuminate the controversy about PTX’s effect on CR expression (Garcia and Mauk, 1998; Attwell et al., 2002). We have shown that blocking GABAA neurotransmission affects CRs in a dose-dependent manner. Blocking either GABAA receptors or chloride channels with low drug doses induces SLRs. On the other hand, administering higher drug doses at the same injection sites abolishes CRs. In their original reports, Mauk and colleagues suggested that SLRs are evoked when GABAergic Purkinje cells are functionally disconnected from eyeblink representation in the deep cerebellar nuclei (Garcia and Mauk, 1998; Medina et al., 2001). Our results do not support this notion. We propose that during SLRs, cerebellar cortical projections are not disconnected completely because increasing the drug dose further aggravates behavioral effects, resulting in CR abolition.
An important contribution of the present study is showing that SLR-inducing injections of PTX affect non-associative components of blinking. This confirms previous suggestions that the intermediate cerebellum controls both classically conditioned and unconditioned eyeblink reflexes (Welsh and Harvey, 1989; Bloedel and Bracha, 1995; Delgado-Garcia and Gruart, 2006; Chen and Evinger, 2006). It is known that neurons in the interposed nuclei respond to both the tone CS and trigeminal US (Berthier and Moore, 1990; Aksenov et al., 2004; Jimenez-Diaz et al., 2004; Chen and Evinger, 2006). Consequently, it is possible that effects of PTX and GZ could be related to changes of IN task-related signals. However, the effects of IN pharmacological manipulations are also very likely related to changes in tonic IN activity. It is paramount to note that PTX and GZ dramatically enhance the spontaneous firing rate of IN neurons (Aksenov et al., 2004; Chen and Evinger, 2006). These IN neurons then send excitatory projections to the red nucleus and other mesencephalic eyeblink-related targets. In addition, neurons in the red nucleus receive CS and US information (Desmond and Moore, 1991) and project to sensory trigeminal (Davis and Dostrovsky, 1986; Godefroy et al., 1998) and facial nuclei (Holstege and Tan, 1988). Thus, it is possible that the elevated spontaneous IN activity increases excitability of extra-cerebellar eyeblink pre-motoneurons and this could affect CR and UR performance in a manner unrelated to learning. Since PTX and GZ affect both neuronal modulation and spontaneous activity simultaneously, dissociating contributions of these two processes to CR and UR performance is difficult and the present study cannot resolve this question (for review, see Bracha et al., 2008).
The most plausible explanation of the effects of low doses of PTX and GZ is that they partially block the inhibitory drive of Purkinje cells and IN GABAergic interneurons. This enhances the spontaneous firing rate of IN neurons, and reduces their depth of modulation (Aksenov et al., 2004; Chen and Evinger, 2006). The elevated IN firing in turn increases the activity of eyeblink pre-motoneurons and motoneurons, and modulates transmission of sensory information in the sensory trigeminal system. The high spontaneous firing within eyeblink circuits enhances tonic eyelid closure. Importantly, this tonic effect on eyelid position is context-dependent, because removing the animal from the restraining box restores normal eyelid aperture. This suggests a so far unknown and context-dependent gating mechanism that can over-ride the effect of the drug-induced high IN firing rate on pre-motoneurons. The reduced modulation of IN neurons is transmitted to mesencephalic pre-motoneurons, which themselves are now more excitable and respond more vigorously to IN signals as well as to direct CS and US inputs. The collective changes both inside and outside of the cerebellum are then responsible for facilitating responses to the CS and trigeminal US. It is not clear whether this mechanism of eyeblink facilitation extends to circuits controlling optic URs. Even though post-PTX amplitudes of optic URs tended to increase and their latencies slightly decreased, these changes were not significant. This seems to indicate that optic URs are not regulated by the cerebellum to the same extent as trigeminal eyeblinks. We cannot exclude, however, that the tonic eyelid closure following PTX injections reduced the perceived intensity of the light stimulus during some trials. This process could have obscured detecting facilitation of optic URs. Further clarification of this issue will require visual stimulus delivery that is independent of the tonic eyelid position. In our previous study we have shown that large doses of PTX dramatically increase IN firing rates and suppress neuronal responses to the CS and US (Aksenov et al., 2004). It is likely that this over-excitation of IN neurons, together with the associated high excitability in their efferent targets, saturate the circuit’s capacity to respond to the CS and this suppresses CRs on the background of pronounced eyelid closure. As addressed above, the mechanism of high GZ doses is different – it appears to suppress IN activity. The resulting suppression of cerebellar task-related signals and the decreased excitatory drive to eyeblink pre-motoneurons counter-balance tonic eyelid closure and suppresses CRs.
4. Material and Methods
4.1. Subjects
The experiments were performed on 10 male New Zealand White Rabbits (Harlan; Indianapolis, IN) weighing 2.5-3.0 kg (3-4 months old at time of surgery). Rabbits were housed individually on a 12-hour light/dark cycle and provided food and water ad libitum. All experiments were performed in accordance with the National Institutes of Health’s “Principles of Laboratory Animal Care” (publication No. 86-23, revised 1985), the American Physiological Society’s “Guiding Principles in the Care and Use of Animals,” and the protocol approved by Iowa State University’s Committee on Animal Care.
4.2. Surgery
Using aseptic techniques, surgery was performed on naive rabbits anesthetized with a mixture of ketamine (50 mg/kg), xylazine (6 mg/kg) and acepromazine (1.5 mg/kg). The head was secured in a stereotaxic apparatus with lambda positioned 1.5 mm ventral to bregma. A stainless steel injection guide tube (28-gauge thin-wall tubing) was stereotaxically implanted 0.5 mm dorsal to the expected location of the left anterior IN ((0.69x + 4.8) - x mm rostral from lambda, x being the horizontal distance between bregma and lambda in mm: 5.3 mm lateral and 13.5 mm ventral to lambda). A 33-gauge stainless steel stylet was inserted into the guide tube in-between experiments to protect its patency. The guide tube, anchor screws, and a small Delrin block designed to accommodate an airpuff delivery nozzle and eyeblink sensor were secured in place with dental acrylic. All animals were treated with antibiotics for 5 days during recovery from surgery.
4.3. Training procedures
Following recovery from surgery, rabbits were adapted to a restraint box in three daily 30-minute sessions. Adapted rabbits were trained in the standard classical conditioning paradigm until they reached at least 90 % CRs for 3 consecutive days. The conditioned stimulus (CS) was an 85-db, 450-ms, 1-kHz tone, super-imposed on a continuous 70-db white noise background. The CS co-terminated with a 40-psi, 100-ms airpuff unconditioned stimulus (US) directed to the left eye. The inter-stimulus interval was 350 ms and each training session consisted of 100 trials presented in pseudorandom, 15-25 sec inter-trial intervals. All experiments were conducted in a sound-attenuated chamber.
Animals tested in the UR performance experiments (Experiment #2) were adapted to a mixed paradigm following training. The paired presentation of the CS + US was alternated with three different types of US in Experiment #2: a normal airpuff US, a weak airpuff US (100 ms, 4-5 psi at the source), and a photic US (30 ms flash of four white LEDs positioned in front of the left eye; light intensity was dimmed to only elicit near-threshold URs). This mixed-stimulation paradigm consisted of repeated blocks of 10 trials: 4 paired CS + US, 2 normal US, 2 weak airpuff US, and 2 light US trials were pseudorandomly intermixed.
4.4. Injection procedures
Injections were delivered via a 33-gauge stainless steel injection needle which was connected via transparent Tygon tubing to a 10-μL Hamilton syringe. The injection tubing was first filled with nanopure water, and then a small bubble was drawn into the end of the injection needle before drawing in drug. The bubble was used for monitoring the injected volume relative to gradation marks on the tubing. The injection needle was inserted in the guide tube prior to beginning the experiment. A pre-injection period of 40 trials (or 50 trials in Experiment #2) was presented to rule out needle insertion effects and to assess baseline eyeblink performance. Following the pre-injection period, drug micro-injections were manually administered at a rate of 0.5 μL/min. To assess the drug effect, training continued for 60-150 additional trials.
The present study had two objectives. In the first group of rabbits (n = 6), CR performance was examined following injection of two GABA antagonists, picrotoxin (PTX, chloride channel blocker; Sigma-Aldrich, USA) and gabazine (GZ, GABAA receptor antagonist; Ascent Scientific, Weston-super-Mare, UK). Of the 6 animals, 3 were used in the PTX group and 3 were used in the GZ group. In preliminary experiments we found that effects of both drugs were dose-dependent besides being animal and injection site-dependent. For this reason, effective injection sites and drug doses in each animal were determined. The starting doses for PTX and GZ were 0.62 nmol and 0.51 nmol, respectively. If CRs were abolished after the injection, this drug concentration was considered the ‘high-dose,’ and the drug dose was progressively decreased on consecutive days until SLRs were observed (the ‘low-dose’ for the drug) or until no drug effect was detected. If no effect on CR performance was found following the initial drug injection, the drug dose was progressively increased until SLRs (the ‘low-dose’) and CR abolition (the ‘high-dose’) were detected. Only one drug was injected on any given experimentation day. Both GZ and PTX were dissolved in artificial cerebrospinal fluid and their pH was adjusted to 7.4 ± 0.1. All injections of PTX and GZ were performed at CR expression-related deep cerebellar nuclear sites where 0.5 μL of muscimol (1.75 nmol) completely suppressed conditioned eyeblinks (Bracha et al., 1994).
In the second group of animals (n = 4), the parallel effects of PTX on CR and UR expression were examined (Experiment #2). In this group of rabbits, PTX was injected in 0.5-μL (0.3 nmol) increments beginning immediately following 50 pre-injection trials. These injections were administered every 20 trials until SLRs were observed or until 2.5 μL of PTX had been cumulatively administered. In control experiments for both Experiment #1 and Experiment #2, an equal volume of drug vehicle (aCSF) was injected using the same injection protocol.
4.5. Data recording and analysis
Rabbit behavior was monitored using an infrared video system installed in the experiment chamber. Eyelid movements were recorded by a frequency-modulated infrared sensor that measures infrared light reflected from the eye and peri-orbital region (Ryan et al., 2006). The sensor, attached to an aluminum stage, was secured to the Delrin block on the rabbit’s head before every experiment. The output of the sensor was amplified, digitized (25 kHz, 12-bit A/D converter), and stored in a PC-based data acquisition system. During each trial, 1400 ms of the signal was recorded, beginning with 250 ms of baseline before the CS onset and extending for 800 ms beyond the US onset.
Eyeblink responses from each trial were examined off-line for the presence of CRs within the time window between CS and US onsets and for the presence of URs in US-alone trials. The threshold for eyeblink detection was set to 5 standard deviations of the baseline signal noise, which in the present setup corresponded to an approximately 0.15 mm decrease in eyelid aperture. The following response parameters were measured in each trial: baseline eyelid aperture, response latency, response amplitude and response peak. Response amplitude was defined as the difference between the baseline eyelid aperture and the maximum eyelid closure in the corresponding response time window for each trial. In normal conditions, the response amplitude reflects the degree of eyelid closure during the blink. However, in present experiments drugs infused in the IN induced squinting and blinks occurred on the background of partially closed eyelids. To assess the extent of eyelid closure in these conditions, we measured the response peak, which was derived not from the eyelid position before the blink, but rather from the eyelid position in an animal with completely open eyelids. To determine the signal corresponding to completely open eyelids, the software searched for the lowest signal value in each experiment, and this value was assigned to the completely open eyelid position. Thus, the response peak was calculated as the difference between the maximum eyelid aperture (openness) in the given session and the maximum eyelid closure in the corresponding response time window in each trial. All amplitudes were first measured in A/D units of the recording system. Typical eyeblinks in rabbits consist of eyelid closure and subsequent folding of external eyelids. Both of these response components were detected by our IR sensor (Ryan et al., 2006). The native amplitude measurements were normalized by converting them to a percentage of maximum eyeblink, assuming that the difference between minimum and maximum sensor signals in a particular daily session captures the eye both maximally open and closed. Means of eyeblink measures were calculated for consecutive blocks of 10 trials in Experiment #1. In Experiment #2, means of eyeblink measures were calculated for blocks of trials as follows: 20 paired CS + US, 10 light, 10 weak airpuff, and 10 strong airpuff trials, which were all randomly presented during blocks of 50 trials. In addition, instantaneous velocities were calculated in a sliding window of 20 msec as the first derivative of rise-to-peak velocities of URs to light and to the strong airpuff in Experiment #2. To compare time profiles of URs to the light and strong airpuff, response averages were normalized by expressing them as a percentage of their amplitude. We tested unique hypotheses about dose dependence by conducting separate repeated measures ANOVAs for PTX and GZ at each dose (high concentration: abolition-inducing, and low concentration: SLR-inducing). Response variables, divided into blocks of 20 trials as the within-subject repeated measures, were modeled against a two-factor treatment (drug vs vehicle) together with subject as a blocking factor. Reported F-ratios and their p-values refer to main effects only when there was no significant interaction between treatment and blocks-of-trials. All group data were reported as mean ± standard error of mean, and significance was declared by an alpha level = 0.05. All statistical analyses were performed using Statsoft Statistica software.
4.6. Histology
Upon the conclusion of experimentation, rabbits were deeply anesthetized with a cocktail of ketamine (100 mg/kg), xylazine (12 mg/kg), and acepromazine (3 mg/kg). Injection sites were marked by injecting 1 μL of tissue-marking dye. Animals were perfused transcardially with 1 L of a phosphate-buffered saline followed by 1 L of a tissue fixative (10 % buffered formalin). Carefully excised brains were stored in a solution of 30 % sucrose and 10 % formalin and subsequently sectioned coronally at 50 μm on a freezing microtome. The sections were mounted onto gelatin-coated slides, and once dry, stained with luxol blue and neutral red. Using bright light microscopy, injection locations were determined and plotted on standard sections of the rabbit cerebellum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aksenov D, Serdyukova N, Irwin K, Bracha V. GABA neurotransmission in the cerebellar interposed nuclei: involvement in classically conditioned eyeblinks and neuronal activity. J. Neurophysiol. 2004;91:719–727. doi: 10.1152/jn.00859.2003. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Ann. N. Y. Acad. Sci. 2002;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Exp. Brain Res. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav. Brain Res. 1995;68:1–44. doi: 10.1016/0166-4328(94)00171-b. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABA(A) receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J. Neurophysiol. 2006;96:3266–3272. doi: 10.1152/jn.00590.2006. [DOI] [PubMed] [Google Scholar]
- Bracha V, Webster ML, Winters NK, Irwin KB, Bloedel JR. Effects of muscimol inactivation of the cerebellar interposed-dentate nuclear complex on the performance of the nictitating membrane response. Exp. Brain Res. 1994;100:453–468. doi: 10.1007/BF02738405. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zbarska S, Parker K, Carrel A, Zenitsky G, Bloedel JR. The cerebellum and eye-blink conditioning: Learning versus network performance hypotheses. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.12.042. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Irwin K, Bloedel JR. Intermediate cerebellum and conditioned eyeblinks. Parallel involvement in eyeblinks and tonic eyelid closure. Exp. Brain. Res. 2001;136:41–49. doi: 10.1007/s002210000563. [DOI] [PubMed] [Google Scholar]
- Chen FP, Evinger C. Cerebellar modulation of trigeminal reflex blinks: Interpositus neurons. J. Neurosci. 2006;26:10569–10576. doi: 10.1523/JNEUROSCI.0079-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Dostrovsky JO. Modulatory influences of red nucleus stimulation on the somatosensory responses of cat trigeminal subnucleus oralis neurons. Exp. Neurol. 1986;91:80–101. doi: 10.1016/0014-4886(86)90028-2. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Gruart A. Building new motor responses: eyelid conditioning revisited. Trends Neurosci. 2006;29:330–338. doi: 10.1016/j.tins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Moore JW. Single-unit activity in red nucleus during the classically conditioned rabbit nictitating membrane response. Neurosci. Res. 1991;10:260–279. doi: 10.1016/0168-0102(91)90083-b. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37:471–480. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J. Neurosci. 2005;25:3919–3931. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy JN, Thiesson D, Pollin B, Rokyta R, Azerad J. Reciprocal connections between the red nucleus and the trigeminal nuclei: a retrograde and anterograde tracing study. Physiol. Res. 1998;47:489–500. [PubMed] [Google Scholar]
- Holstege G, Tan J, An HRP and autoradiographical tracing study Projections from the red nucleus and surrounding areas to the brainstem and spinal cord in the cat. Behav. Brain Res. 1988;28:33–57. doi: 10.1016/0166-4328(88)90075-7. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. Raven Press; New York: 1984. [Google Scholar]
- Jimenez-Diaz L, Navarro-Lopez JD, Gruart A, Delgado-Garcia JM. Role of cerebellar interpositus nucleus in the genesis and control of reflex and conditioned eyelid responses. J. Neurosci. 2004;24:9138–9145. doi: 10.1523/JNEUROSCI.2025-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Thompson RF, Madden J. Cerebellar GABAergic processes: evidence for critical involvement in a form of simple associative learning in the rabbit. Proc. Natl. Acad. Sci. USA. 1987;84:2101–2105. doi: 10.1073/pnas.84.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J. Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J. Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SB, Detweiler KL, Holland KH, Hord MA, Bracha V. A long-range, wide field-of-view infrared eyeblink detector. J. Neurosci. Methods. 2006;152:74–82. doi: 10.1016/j.jneumeth.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J. Physiol. 2004;560:181–189. doi: 10.1113/jphysiol.2004.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP. Changes in the motor pattern of learned and unlearned responses following cerebellar lesions: a kinematic analysis of the nictitating membrane reflex. Neuroscience. 1992;47:1–19. doi: 10.1016/0306-4522(92)90116-j. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA. Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J. Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbarska S, Bloedel JR, Bracha V. Cerebellar dysfunction explains the extinction-like abolition of conditioned eyeblinks after NBQX injections in the inferior olive. J. Neurosci. 2008;28:10–20. doi: 10.1523/JNEUROSCI.3403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


