Abstract
Introduction
The potential translation of mesenchymal stem cell (MSC) therapy into a multimodal protocol for traumatic brain injury requires evaluation of viability and cytokine production in a hyperosmolar environment. Optimization of MSC therapy requires delivery to the target area without significant loss of cellular function or viability. No model evaluating the potential efficacy of MSC therapy at varying osmolarities currently exists.
Methods
Rat MSCs were characterized with flow cytometric immunophenotyping. MSCs (passage 3) were placed in culture with multipotent adult progenitor cell media at varying osmolarities (250, 270, 290, 310, 330, 350 and 370 mOsm) potentially found with hypertonic saline infusion. After culture for 24 h, cellular viability was measured using flow cytometry (n = 6). Next, brain tissue supernatant was harvested from both normal rat brains and injured brains 6 h after cortical injury. Subsequently, MSCs were placed in culture with multipotent adult progenitor cell media ± 20% normal brain or injured brain supernatant (at the aforementioned osmolarities) and allowed to remain in culture for 24 h (n = 11). At this point, media supernatant cytokine levels were measured using a multiplex cytokine assay system.
Results
MSCs showed no clinically significant difference in viability at 24 h. MSCs cultured with 20% injured brain supernatant showed an decrease in proinflammatory cytokine production (IL-1α and IL-1β) with increasing osmolarity. No difference in anti-inflammatory cytokine production (IL-4 and IL-10) was observed.
Conclusion
Progenitor cell therapy for traumatic brain injury may require survival and activity in a hyperosmolar environment. Culture of MSCs in such conditions shows no clinically significant effect on cell viability. In addition, MSC efficacy could potentially be enhanced via a decrease in proinflammatory cytokine production. Overall, a multimodal traumatic brain injury treatment protocol based upon MSC infusion and hypertonic saline therapy would not negatively affect progenitor cell efficacy and could be considered for multicenter clinical trials.
Keywords: cytokines, inflammation, mesenchymal stem cells, osmolarity
In the USA, 1.5 million patients suffer a traumatic brain injury (TBI) each year, resulting in 50,000 deaths with an additional 230,000 patients requiring hospitalization [1]. The overall prevalence of TBI is estimated to be 6.5 million people [2]. Of those patients affected, up to 48% are impaired by physical, cognitive and psychosocial deficits [3]. Beginning aggressive rehabilitation early in the patient’s hospital course has been shown to lead to improvement in functional status [4,5]; however, neurons show little ability to repair and no treatment modality is currently available to reverse acute brain injury. A large body of work has failed to show significant efficacy from single-agent pharmacologic neuroprotective therapies. Therefore, the NIH recently convened a meeting to discuss the complex pathophysiology of neuronal injury and the failure of all trials based on current monotherapies (controlled hypothermia, hyperosmotic infusion) to date. Recommendations included the development of in vitro models to research multimodality treatments that could target several mechanisms of TBI’s complex pathophysiology [6,101]. Two therapeutic modalities currently under investigation for the treatment of TBI are the infusion of hypertonic saline (HTS) and progenitor cell therapeutics.
Preliminary research has shown that HTS infusion after TBI could offer potential neuroprotection. One possible pathway could be via a decrease in intracranial pressure (ICP). Both preclinical research using a dog model [7] and prospective randomized trials [8] have shown decreased ICP after HTS infusion without significant effect on cerebral blood flow (CBF). Additional investigation completed by Khanna et al. demonstrated that HTS infusion in a pediatric population was associated with improved CBF in accordance with a decrease in ICP [9]. HTS resuscitation also increases mean arterial pressure, thereby attenuating a significant increase in poor neurological outcomes seen with hypotension within 6 h of TBI [10]. Furthermore, Vialet et al. have shown HTS infusion to be more effective than mannitol as a hyperosmolar agent after TBI [11]. Despite promising initial results, the effect of HTS infusion upon ICP remains controversial as alternate prospective trials have failed to show improvement in ICP control when compared with crystalloid (lactated ringer’s) infusion [12,13]. HTS infusion decreased the number of complications, the number of required interventions and length of intensive care unit stay after TBI in a pediatric population [14]; however, additional studies completed with an adult population failed to show a favorable effect on the required interventions [13]. Despite the promising results noted in the acute and subacute setting, some trials have failed to show sustained improved neurological outcomes 6 months after TBI [6,15]. As a result of the controversial results derived from the initial preclinical and clinical trials, the Joint Section on Neurotrauma and Critical Care did not make any recommendations for the use HTS infusion for TBI [16]; however, the potential neuroprotection observed specifically in the pediatric population requires additional investigation.
A large amount of research has been completed to investigate the potential role of progenitor (stem) cell therapeutics for the treatment of TBI. While initial preclinical research has shown a potential benefit from progenitor cell therapeutics [17–20], the precise mechanism remains intensely controversial. Furthermore, the secondary injury observed with TBI is associated with an induction of the systemic inflammatory response. Analysis of rat brain supernatant from a TBI model has shown significant increase in the proinflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α in both the direct injury and penumbral areas [21]. In addition, previous preclinical work has shown progenitor cells to migrate towards the site of injury and potentially modulate cytokine production. Co-culture of mesenchymal stem cells (MSCs) with purified immune cells has shown a decrease in proinflammatory cytokine production (TNF-α and IFN-γ) with an increase in production of the anti-inflammatory cytokines IL-4 and IL-10 [22]. Therefore, MSCs could offer neuroprotection via modulation of locoregional proinflammatory cytokine production in accordance with the production of anti-inflammatory cytokines.
Previous research has shown HTS infusion and progenitor cell therapeutics to be ideal potential candidates for multimodal TBI treatment regimens. The potential translation of MSC therapy into such a multimodal protocol requires evaluation of cell viability and cytokine production in a hyperosmolar environment. Previous research has shown osmotic stress to induce apoptotic cell death in multiple cell lines including mononuclear cells [23], cardiomyocytes [24] and fibroblasts [25]. Furthermore, hyperosmotic stress has been shown to increase proinflammatory cytokine production from human peripheral blood mononuclear cells [26]. No model evaluating the potential efficacy of MSC therapy at varying osmolarities currently exists. We designed a series of in vitro experiments to investigate MSC viability and cytokine production while cultured at the osmolarities observed with HTS infusion for the treatment of TBI.
Experimental design
Mesenchymal stem cells (passage 3) were placed in culture with multipotent adult progenitor cell (MAPC) media with the varying osmolarities (250, 270, 290, 310, 330, 350 and 370 mOsm) potentially found with HTS infusion. After 24 h in culture, cellular viability was measured using flow cytometry (n = 6 samples per media osmolarity). Next, brain tissue supernatant was harvested from both normal rat brains and injured brains 6 h after cortical injury. Subsequently, MSCs were placed in culture with MAPC media ± 20% normal brain or injured brain supernatant (at the aforementioned osmolarities) and allowed to remain in culture for 24 h (n = 11 samples per media osmolarity). At this point, media supernatant cytokine levels were measured using a Bio-Plex cytokine assay system (Bio-Rad Laboratories, Hercules, CA, USA).
Ethical approval
All protocols involving the use of animals were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Institutional Animal Care and Use Committee (protocol HSC-AWC-07–055).
Data analysis
Unless otherwise indicated, all values are represented as mean ± standard error of the mean. Values were compared using analysis of variance (ANOVA) with a post-hoc Dunnett analysis using an osmolarity of 290 as the control. A p-value of ≤ 0.05 was used to denote statistical significance.
Isolation, characterization & labeling of rat MSCs
Mesenchymal stem cells were isolated from the bone marrow of Sprague-Dawley rats and expanded in MAPC media as previously described [27]. Flow cytometric immunophenotyping was used to ensure that the MSCs were CD11b−, CD45−, CD29+, CD49e+, CD73+, CD90+, CD105+ and Stro-1+. Passage 3 cells were used for all experiments.
Measurement of media osmolarity
The osmolarity of MAPC media was measured using the 5500 vapor pressure osmometer (Wescor Inc., Logan, UT, USA). Adjustments in media osmolarity were completed by addition of sterile distilled water or sodium chloride.
MSC viability
Mesenchymal stem cells were removed from the culture plates and incubated with propridium iodide for 10 min. Using flow cytometry, the fraction of dead cells was calculated.
Controlled cortical impact injury
A controlled cortical impact (CCI) device (eCCI Model 6.3; VCU, Richmond, VA, USA) was used to administer a unilateral brain injury as described previously [28]. Male Sprague Dawley rats weighing 225–250 g were anesthetized with 4% isoflurane and a 1:1 mixture of N2O/O2 and the head was mounted in a stereotactic frame. With the head held in a horizontal plane, a mid-line incision and subsequent 7- to 8-mm craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at the midpoint between bregma and lambda, 3 mm lateral to the midline and overlying the tempo-parietal cortex. Animals received a single impact of 3.1-mm depth of deformation with an impact velocity of 5.8 m/s and a dwell time of 150 ms (moderate-to-severe injury) at an angle of 10° from the vertical plane using a 6-mm diameter impactor tip, making the impact orthogonal to the surface of the cortex. The impact was delivered onto the parietal association cortex. Sham injuries were performed by anesthetizing the animals, making the midline incision, and separating the skin, connective tissue and aponeurosis from the cranium. The incision was then closed. The body temperature was maintained at 37°C by the use of a heating pad. Previously obtained serial arterial PaO2 and PaCO2 measurements have shown that animals do not become hypoxic or hypercarbic during this procedure [21].
Brain homogenate supernatant fluid collection
Rats were sacrificed 6 h after CCI or sham injury. Their brains were extracted and four regions, relative to the injury, were isolated: the site of direct injury, penumbral region, ipsilateral frontal region and contralateral region, as we have previously described [21]. The sections were weighed to ensure each section was 120 mg, gently minced with a pellet pestle, diluted in 1 ml (low-glucose Dulbecco’s modified Eagle medium with 10% fetal bovine serum; Gibco, Carlsbad, CA, USA), vortexed for 30 s and centrifuged for 6 min at 1000 g. The supernatant, containing intracerebral, interstitial fluid, was collected [21]. Of note, only the supernatant harvested from the direct injury region was used for stimulation of MSC cultures.
Cytokine analysis
Cytokines were detected in the MSC media supernatant using the Bio-Plex cytokine assay system (Bio-Rad Laboratories, Hercules, CA, USA). Concentrations of IL-1α, IL-1β, IL-4, IL-6, IL-10 and TNF-α were simultaneously evaluated using a commercially available multiplex bead-based immunoassay (Rat 9-Plex; Bio-Rad Laboratories). The assay was performed per the manufacturer’s instructions and the details have been previously published by our group and others [29,30]. High standard curves (low RP1 target value) for each soluble cytokine were used, ranging from 2 to 32,000 pg/ml. A minimum of 100 beads per cytokine region were evaluated and recorded. Values with a coefficient of variation beyond 10% were not included in the final data analysis. All samples were run in duplicate.
Results
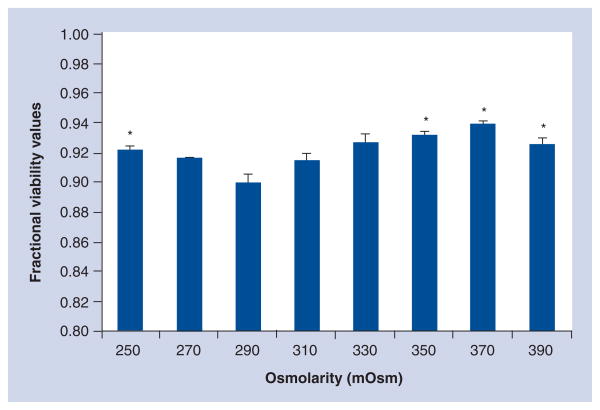
Mesenchymal stem cells placed in culture with MAPC media at the various potential osmolarities observed with HTS therapy (250–370 mOsm) showed a narrow range of viability (91.4–93.9%). Statistical analysis using an osmolarity of 290 to represent a physiologic control showed difference for osmolarities of 250, 330, 350 and 370. Figure 1 outlines MSC viability at the varying osmolarities.
Figure 1. Mesenchymal stem cell viability measured after 24 h in multipotent adult progenitor cell media of varying osmolarities.
Fractional viability values observed from 0.914 to 0.939 indicate that while there are statistically significant differences, adequate mesenchymal stem cell viability is observed under all culture conditions.
*Statistical significance (one-way ANOVA with Dunnett’s post-hoc) compared with control osmolarity (290 mOsm) (p < 0.05).
Table 1 shows the proinflammatory cytokine production (IL-1α, IL-1β, IL-6 and TNF-α) measured in the media (with and without 20% normal/injured brain supernatants) of MSC cultures after 24 h. Table 1 shows a significant (p = 0.004) decrease in IL-1α production at an osmolarity of 370 for MSCs cultured with injured brain supernatant (compared with the control value of 290). In addition, for MSCs cultured with injured brain supernatant there was a decrease in IL-1β production at serum osmolarities of 270 and 370 (p = 0.02) (Table 1). Further analysis failed to show a difference in the production of TNF-α or IL-6 when cultured in injured brain supernatant. In addition, no difference was observed for MSCs cultured in media alone or with 20% normal brain supernatant.
Table 1.
Proinflammatory cytokine concentration (pg/ml) measured in the media (with and without 20% normal/injured brain supernatant) of mesenchymal stem cell cultures at varying osmolarities.
| 250 mOsm | 270 mOsm | 290 mOsm* | 310 mOsm | 330 mOsm | 350 mOsm | 370 mOsm | |
|---|---|---|---|---|---|---|---|
| IL-1α | |||||||
| Media | 18.5 ± 0.4 | 17.6 ± 1.0 | 18.0 ± 0.5 | 21.0 ± 1.6 | 19.3 ± 1.9 | 20.6 ± 1.6 | 19.1 ± 1.2 |
| Normal brain supernatant | 26.0 ± 0.4 | 30.1 ± 1.2 | 25.7 ± 0.8 | 33.2 ± 3.8 | 35.1 ± 3.2 | 38.1 ± 4.1 | 38.3 ± 6.2 |
| Injured brain supernatant | 399 ± 13.3 | 414 ± 10.6 | 448 ± 6.7 | 463 ± 31.3 | 452 ± 18.2 | 422 ± 10.3 | 375 ± 7.2* |
| IL-1β | |||||||
| Media | 18.5 ± 0.4 | 17.6 ± 1.0 | 18.0 ± 0.5 | 21.0 ± 1.6 | 19.3 ± 1.9 | 20.6 ± 1.6 | 19.1 ± 1.2 |
| Normal brain supernatant | 33.3 ± 2.4 | 34.9 ± 2.6* | 32.4 ± 1.3 | 38.9 ± 2.8 | 39.0 ± 2.2 | 39.8 ± 2.9 | 43.4 ± 4.1* |
| Injured brain supernatant | 100 ± 3.7 | 92 ± 3.1 | 110 ± 6.5 | 108 ± 2.3 | 102 ± 3.7 | 100 ± 5.2 | 91 ± 4.8 |
| IL-6 | |||||||
| Media | 6.8 ± 0.4 | 6.8 ± 0.5 | 6.5 ± 0.3 | 6.4 ± 0.2 | 5.8 ± 0.4 | 6.4 ± 0.2 | 6.4 ± 0.3 |
| Normal brain supernatant | 123 ± 33 | 131 ± 37 | 108 ± 28 | 110 ± 28 | 112 ± 29 | 123 ± 25 | 140 ± 31 |
| Injured brain supernatant | 178 ± 45 | 180 ± 49 | 185 ± 51 | 161 ± 42 | 160 ± 42 | 157 ± 46 | 162 ± 49 |
| TNF-α | |||||||
| Media | 7.0 ± 0.3 | 6.6 ± 0.2 | 7.0 ± 0.5 | 7.2 ± 0.5 | 6.8 ± 0.4 | 6.5 ± 0.2 | 6.9 ± 0.1 |
| Normal brain supernatant | 9.5 ± 0.3 | 9.8 ± 0.4 | 10.2 ± 0.3 | 9.9 ± 0.7 | 15.0 ± 2.4 | 16.2 ± 2.6 | 15.8 ± 2.9 |
| Injured brain supernatant | 10.1 ± 0.8 | 10.6 ± 0.6 | 10.9 ± 0.9 | 10.8 ± 0.4 | 9.9 ± 0.6 | 9.6 ± 0.7 | 9.2 ± 0.5 |
Statistical significance (one-way ANOVA with Dunnett’s post-hoc) compared with control osmolarity (290 mOsm) (p < 0.05).
Table 2 shows anti-inflammatory cytokine production (IL-4 and IL-10) measured in the media (with and without 20% normal/injured brain supernatants) of MSC cultures after 24 h. No difference was observed for IL-4 or IL-10 at any osmolarity.
Table 2.
Anti-inflammatory cytokine concentration (pg/ml) measured in the media (with and without 20% normal/injured brain supernatant) of mesenchymal stem cell cultures at varying osmolarities.
| 250 mOsm | 270 mOsm | 290 mOsm* | 310 mOsm | 330 mOsm | 350 mOsm | 370 mOsm | |
|---|---|---|---|---|---|---|---|
| IL-4 | |||||||
| Media | 110 ± 3.6 | 103 ± 5.8 | 103 ± 2.2 | 107 ± 9.3 | 95 ± 7.8 | 119 ± 8.4 | 129 ± 9.1 |
| Normal brain supernatant | 213 ± 11.2 | 234 ± 10.4 | 207 ± 6.7 | 243 ± 16.6 | 222 ± 21.1 | 242 ± 17.6 | 252 ± 16.9 |
| Injured brain supernatant | 213 ± 34.7 | 220 ± 36.7 | 220 ± 39.3 | 204 ± 28.3 | 199 ± 23.5 | 184 ± 37.2 | 201 ± 35.6 |
| IL-10 | |||||||
| Media | 2.6 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.0 ± 0.0 | 2.6 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 |
| Normal brain supernatant | 3.5 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.3 | 3.1 ± 0.4 | 3.4 ± 0.2 | 3.3 ± 0.2 | 3.6 ± 0.4 |
| Injured brain supernatant | 3.7 ± 0.3 | 3.6 ± 0.3 | 4.4 ± 0.4 | 3.7 ± 0.3 | 4.0 ± 0.3 | 3.6 ± 0.4 | 3.5 ± 0.2 |
Statistical significance (one-way ANOVA with Dunnett’s post-hoc) compared with control osmolarity (290 mOsm) (p < 0.05).
Discussion
Multimodal protocols for the treatment of TBI could require the intravenous infusion of MSCs with HTS therapy. Optimal cell efficacy requires adequate viability and function at elevated serum osmolarities. Our data show that MSC viability (91.4–93.9%) is largely unaffected by the range of serum osmolarities (250–370 mOsm) that could be observed in a traumatically injured patient. While a significant increase in MSC viability is observed with increasing osmolarity, difference in viability fails to reach clinical significance.
The potential neuroprotection observed with HTS therapy after TBI could be derived from a decrease in proinflammatory cytokine production. Recent investigation has shown an increase in plasma matrix metalloproteinases (MMPs) as well as IL-6 after TBI in both animal models and injured patients [31,32]. MMPs are believed to contribute to further ischemic brain injury via breakdown of the blood–brain barrier. While initial work has shown controlled hypothermia to decrease the levels of MMP after TBI [32,33], little investigation into the effect of HTS has been completed. However, HTS therapy has been shown to mediate hepatic MMP production in a pancreatitis model and could offer neuroprotection via a similar pathway after TBI [34].
The observed benefit from MSC therapy for TBI could be explained by modulation of the locoregional or systemic inflammatory milieu [17]. While still controversial, the implanted MSCs could be responsible for the production in anti-inflammatory cytokines that lead to enhanced neuroprotection. Our data show a decrease in production of the proinflammatory cytokines IL-1α and IL-1β with increasing osmolarity. In addition, the production of the anti-inflammatory cytokines IL-4 and IL-10 remains unaffected with an increase in osmolarity. These data show that increasing osmolarity does not affect MSC function in the form of anti-inflammatory cytokine production. In addition, increasing osmolarity may be of some benefit as evidenced by a decrease in the pro-inflammatory cytokine concentration, which could lead to enhanced neuroprotection.
Conclusion
Traumatic brain injury is a major burden on the healthcare system worldwide. Current therapy in the acute setting is supportive with no pharmacologic treatment to attenuate neuronal cell death available today. The development of multimodal treatment protocols to attack TBI’s complex pathophysiology at multiple points could offer the most effective form of neuroprotection. The combination of HTS infusion and progenitor cell therapies could lead to improved CBF as well as modulation of the inflammatory response leading to reduced neuronal death. Our data have shown that the intravenous infusion of MSCs into the hyperosmolar environment seen with HTS therapy has no clinically significant effect on cell viability. In addition, MSC efficacy could potentially be enhanced via a decrease in pro-inflammatory cytokine production. Overall, a multimodal TBI treatment protocol based upon MSC infusion and HTS therapy would not negatively affect progenitor cell efficacy and could be considered for multicenter clinical trials.
Executive summary
Traumatic brain injury
Traumatic brain injury affects 1.5 million people per year in the USA. Of those affected, up to 48% have chronic physical, cognitive or psychosocial deficits. No single modality pharmacologic treatment has shown efficacy.
Hypertonic saline therapy
Preliminary trials have shown potential neuroprotection via control of intracranial pressure with improvement of cerebral blood flow; however, trials have failed to show cognitive improvement 6 months after injury.
Progenitor cell therapy
Initial in vivo models have shown potential neuroprotection based upon intravenous delivery; however, a review of the literature shows no evidence of mesenchymal stem cell (MSC) viability at hyperosmolar conditions. Owing to NIH recommendations to develop multimodality therapeutic regimens for traumatic brain injury, a model to look at MSC viability and function is needed.
MSC viability
MSCs cultured at various osmolarities (250–370) showed no clinically significant difference in viability (91.4–93.9%).
Inflammatory cytokine production
MSCs showed a decrease in IL-1α and -1β production with increasing osmolarity. No difference in anti-inflammatory cytokine production was observed.
Culture of MSCs at hyperosmolar conditions shows no clinically significant effect on cell viability. Additionally, MSC efficacy could potentially be enhanced via a decrease in pro-inflammatory cytokine production.
Acknowledgments
Financial & competing interests disclosure
The authors are supported by grants NIH T32 GM 08 79201, M01 RR 02558 R21, Texas Higher Education Coordinating Board, Children’s Memorial Hermann Hospital Foundation and The Brown Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
For reprint orders, please contact: reprints@futuremedicine.com
Bibliography
- 1.Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14(6):602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Consensus conference: Rehabilitation of persons with traumatic brain injury. NIH consensus development panel on rehabilitation of persons with traumatic brain injury. JAMA. 1999;282(10):974–983. [PubMed] [Google Scholar]
- 3.Hawley CA, Ward AB, Magnay AR, et al. Outcomes following childhood head injury: a population study. J Neurol Neurosurg Psychiatr. 2004;75(5):737–742. doi: 10.1136/jnnp.2003.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowen TD, Meythaler JM, DeVivo MJ, et al. Influence of early variables in traumatic brain injury on functional independence measure scores and rehabilitation length of stay and charges. Arch Phys Med Rehabil. 1995;76(9):797–803. doi: 10.1016/s0003-9993(95)80542-7. [DOI] [PubMed] [Google Scholar]
- 5.Gray DS, Burnham RS. Preliminary outcome analysis of a long-term rehabilitation program for severe acquired brain injury. Arch Phys Med Rehabil. 2008;81(11):1447–1456. doi: 10.1053/apmr.2000.16343. [DOI] [PubMed] [Google Scholar]
- 6.Walker P, Harting MT, Baumgartner JE, Fletcher S, Strobel N, Cox CS., Jr Modern approaches to pediatric brain injury therapy. J Trauma. 2009;67(Suppl 2):S120–S127. doi: 10.1097/TA.0b013e3181ad323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto FC, Capone-Neto A, Prist R, et al. Volume replacement with lactated Ringer’s or 3% hypertonic saline solution during combined experimental hemorrhagic shock and traumatic brain injury. J Trauma. 2006;60(4):758–763. doi: 10.1097/01.ta.0000214581.89316.73. discussion 763–754. [DOI] [PubMed] [Google Scholar]
- 8.Munar F, Ferrer AM, de Nadal M, et al. Cerebral hemodynamic effects of 7.2% hypertonic saline in patients with head injury and raised intracranial pressure. J Neurotrauma. 2000;17(1):41–51. doi: 10.1089/neu.2000.17.41. [DOI] [PubMed] [Google Scholar]
- 9.Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 10.Samant UB, 4th, Mack CD, Koepsell T, et al. Time of hypotension and discharge outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25(5):495–502. doi: 10.1089/neu.2007.0491. [DOI] [PubMed] [Google Scholar]
- 11.Vialet R, Albanese J, Thomachot L, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 ml/kg 7.5% saline is more effective than 2 ml/kg 20% mannitol. Crit Care Med. 2003;31(6):1683–1687. doi: 10.1097/01.CCM.0000063268.91710.DF. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Suarez JI, Castro A, et al. Use of hypertonic saline/acetate infusion in treatment of cerebral edema in patients with head trauma: experience at a single center. J Trauma. 1999;47(4):659–665. doi: 10.1097/00005373-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Shackford SR, Bourguignon PR, Wald SL, et al. Hypertonic saline resuscitation of patients with head injury: a prospective, randomized clinical trial. J Trauma. 1998;44(1):50–58. doi: 10.1097/00005373-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Simma B, Burger R, Falk M, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26(7):1265–1270. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291(11):1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- 16.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma. 2007;24:S14–S20. doi: 10.1089/neu.2007.9994. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Lee ST, Chu K, et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50. doi: 10.1016/j.brainres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Prough DS, McAdoo DJ, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol. 2006;201(2):281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Mahmood A, Lu D, Wang L, et al. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49(5):1196–1203. discussion 1203–1194. [PubMed] [Google Scholar]
- 20.Lu D, Sanberg PR, Mahmood A, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11(3):275–281. [PubMed] [Google Scholar]
- 21.Harting MT, Jimenez F, Adams SD, et al. Acute, regional inflammatory response after traumatic brain injury: implications for cellular therapy. Surgery. 2008;144(5):803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 23.Gastaldello K, Husson C, Dondeyne JP, et al. Cytotoxicity of mononuclear cells as induced by peritoneal dialysis fluids: insight into mechanisms that regulate osmotic stress-related apoptosis. Perit Dial Int. 2008;28(6):655–666. [PubMed] [Google Scholar]
- 24.Lee JW, Ko YE, Lee IH, et al. Osmotic stress induces loss of glutathione and increases the sensitivity to oxidative stress in H9c2 cardiac myocytes. Free Radic Res. 2009;43(3):262–271. doi: 10.1080/10715760802691471. [DOI] [PubMed] [Google Scholar]
- 25.Maeno E, Takahashi N, Okada Y. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett. 2006;580(27):6513–6517. doi: 10.1016/j.febslet.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 26.Otto NM, Schindler R, Lun A, et al. Hyperosmotic stress enhances cytokine production and decreases phagocytosis in vitro. Crit Care. 2008;12(4):R107. doi: 10.1186/cc6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harting M, Jimenez F, Pati S, et al. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10(3):243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- 28.Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1998;5(1):1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 29.Penolazzi L, Lambertini E, Tavanti E, et al. Evaluation of chemokine and cytokine profiles in osteoblast progenitors from umbilical cord blood stem cells by BIO-PLEX technology. Cell Biol Int. 2008;32(2):320–325. doi: 10.1016/j.cellbi.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Adams SD, Radhakrishnan RS, Helmer KS, et al. Effects of anesthesia on lipopolysaccharide-induced changes in serum cytokines. J Trauma. 2008;65(1):170–174. doi: 10.1097/TA.0b013e31805824ca. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi T, Kaneko Y, Yu S, et al. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009;1280:172–177. doi: 10.1016/j.brainres.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Suehiro E, Fujisawa H, Akimura T, et al. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004;21(12):1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 33.Truettner JS, Alonso OF, Dalton Dietrich W. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25(11):1505–1516. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- 34.Rios EC, Moretti AI, de Souza HP, et al. Hypertonic saline reduces metalloproteinase expression in liver during pancreatitis. Clin Exp Pharmacol Physiol. 2009 doi: 10.1111/j.1440-1681.2009.05220.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Website
- 101.NIH. Combination therapies for traumatic brain injury workshop. 2008 www.ninds.nih.gov/news_and_events/proceedings/Combination_Therapies_for_Traumatic_Brain_Injury_Workshop.htm.