Abstract
Developmental differences in the neurocognitive networks for phonological and semantic processing in Chinese word reading were examined in 13 adults and 13 children using functional magnetic resonance imaging (fMRI). Rhyming and semantic association judgments were made to two‐character words that were presented sequentially in the visual modality. These lexical tasks were compared with a nonlinguistic control task involving judgment of line patterns. The first main finding was that adults showed greater activation than children in right middle occipital gyrus on both the meaning and rhyming task, suggesting adults more effectively engage right hemisphere brain regions involved in the visual‐spatial analysis of Chinese characters. The second main finding was that adults showed greater activation than children in left inferior parietal lobule for the rhyming as compared with the meaning task, suggesting greater specialization of phonological processing in adults. The third main finding was that children who had better performance in the rhyming task on characters with conflicting orthographic and phonological information relative to characters with nonconflicting information showed greater activation in left middle frontal gyrus, suggesting greater engagement of brain regions involved in the integration of orthography and phonology. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: Chinese, rhyming, meaning, development
INTRODUCTION
Writing systems vary in how their visual forms represent units of spoken language, which suggests that reading should involve specialized brain areas in different languages in addition to a shared network. Chinese characters map onto phonology at the monosyllable level, with no parts in a character corresponding to phonological segments such as phonemes. It is never the case in Chinese that a phonetic component maps onto a subsyllabic phonological representation in the way that a letter maps onto a part of a word's phonological form in an alphabetic system [Perfetti et al.,2005]. However, many Chinese characters encode meaning by including a semantic radical. Semantic radicals can provide useful cues such as category information of the character, and these radicals are important in character acquisition and recognition [Leck et al.,1995]. Although there is some controversy [Liu et al.,2002; Pollatsek et al.,2000; Spinks et al.,2000], researchers have speculated that phonological processing plays a less important role than semantics in visual identification of Chinese words or that phonology is activated only after semantics [Chen and Shu,2001; Feng et al.,2001; Leck et al.,1995; Zhou,1997]. In terms of visual form itself, Chinese is also very different from alphabetic writing systems. Written Chinese uses characters as a basic writing unit that possesses a number of intricate strokes packed into a square configuration, whereas written English is a linear layout of letters.
Neuroimaging research has revealed a set of cortical regions shared by mature readers of Chinese and English. The common areas include left fusiform gyrus, left inferior parietal lobule, left middle temporal gyrus, and left inferior frontal gyrus. Left fusiform gyrus is thought to be involved in orthographic processing and visual word form recognition in both English and Chinese reading [Bookheimer et al.,1995; Booth et al.,2002a; Chee et al.,1999; Cohen et al.,2000; Fiez and Petersen,1998; Kuo et al.,2001,2004; Petersen et al.,1989; Tan et al.,2000,2001]. Left inferior parietal lobule is thought to be involved in mapping between orthographic and phonological representations in both English and Chinese [Bitan et al.,2007a; Booth et al.,2006]. Left middle temporal gyrus is thought to be involved in representing verbal semantic information in both English and Chinese [Booth et al.,2002b,2006; Luke et al.,2002; Poldrack et al.,1999; Tan et al.,2001]. Left inferior frontal gyrus is thought to be involved in subvocal rehearsal, articulatory preparation [Pugh et al.,1996], phonological processing [Herbster et al.,1997; Rumsey et al.,1997], and in higher‐level modulation to the posterior language areas in lexical tasks [Bitan et al.,2005,2006]. Bitan et al. [2005] found that the converging influence of left inferior frontal gyrus on left lateral temporal cortex was significantly modulated by rhyming task, whereas the converging influence of left inferior frontal gyrus on left intraparietal sulcus was significantly modulated by spelling task. This study suggests that left inferior frontal gyrus may play a pivotal role in top–down modulation of task‐selective regions. Moreover, this top–down modulation of inferior frontal gyrus is weaker in children than in adults [Bitan et al.,2006].
There are two brain areas that seem to be specialized for Chinese reading. One is right middle occipital gyrus and fusiform gyrus, and the other is left middle frontal gyrus. There is greater involvement of right middle occipital gyrus in Chinese reading as compared with English reading [Bolger et al.,2005; Tan et al.,2005a], presumably because Chinese character recognition requires greater spatial analysis in visual association regions. Left middle frontal gyrus also seems to be a critical area in Chinese reading [Tan et al.,2005a]. It has been argued that this region is associated with addressed phonology in Chinese reading, and with the integration of visual orthographic information with phonology. Left middle frontal gyrus also appears to be associated with developmental dyslexia in Chinese. One study found that children with dyslexia show significantly less activation in left middle frontal gyrus than their age‐matched control children for both a homophone judgment and a lexical decision task [Siok et al.,2004]. On the other hand, there also appears to be regions specialized for English reading as compared with Chinese reading. The posterior portion of left superior temporal gyrus seems to be important in English reading, presumably because this region is involved in assembled phonology from letters to phonemes [Eden et al.,2004; Simos et al.,2000,2002; Temple et al.,2001]. This region is not significantly activated in many Chinese reading studies [Siok et al.,2003; Tan et al.,2003].
Although some studies have shown that phonological skills are related to reading skills in Chinese [Ho and Bryant,1997], most studies have shown that phonological skills are not related to reading skill or other cognitive processes that are more strongly related to reading skill. Studies have reported that visual skill but not phonemic awareness is correlated with reading skill [Siok and Fletcher,2001], orthographic skill is strongly correlated with pseudocharacter reading [Ho et al.,2007], writing skill is more strongly associated with reading skill than phonological awareness [Tan et al.,2005b], and syllable awareness is more strongly related with reading skill than phoneme awareness [McBride‐Chang et al.,2004]. Studies have also suggested that the importance of visual orthographic processing in character processing may increase with age, whereas the importance of phonology may decrease with age. The phonological (homophone) Stroop effect is larger in younger and less‐skilled readers [Guo et al.,2005], and younger and less‐skilled readers make more visual errors, whereas older and more‐skilled readers make more phonological errors [Chan and Siegel,2001]. Studies on dyslexic children have also supported the importance of visual orthographic skills in character processing [Ho et al.,2004].
Cross‐linguistic and bilingual studies have suggested that the cognitive and linguistic mechanisms for processing English and Chinese are different. In terms of cross‐linguistic studies, phonological awareness shows a stronger relationship with reading skills in English, but morphological awareness and visual skills show a stronger relationship with reading skills in Chinese [Huang and Hanley,1995; McBride‐Chang et al.,2005]. In terms of bilingual studies, Chinese orthographic processing skill in the native language is not correlated with English reading skill in the second language, and English orthographic processing skill in the second language is not correlated with Chinese reading skill in the native language [Wang et al.,2005]. In addition, studies have shown that phonological skill in Chinese dyslexics is not correlated with reading skill in their native language, but is correlated with reading skill in their second English language [Ho and Fong,2005]. Taken together, behavioral research suggests that visual orthographic skill is more important than phonological skill for Chinese character processing and that visual orthographic skill is relatively unique to reading in this writing system.
The primary motivation of this study was to examine how neural systems responsible for reading in Chinese change throughout development. We adopted a paradigm used in our previous study on adults [Booth et al.,2006] to examine phonological and semantic processing differences between children and adults. Subjects were asked to determine whether a target word rhymed with or was semantically related to one of the two preceding words. The formulation of specific predictions regarding developmental differences in brain activation patterns during lexical processing in Chinese is difficult, because no neuroimaging studies have examined developmental differences. Most neuroimaging research on reading development has been done in English. Studies have found that reading development is characterized by increasing involvement of left fusiform gyrus in visual word form recognition [Booth et al.,2003,2004; Brem et al.,2006], by more elaborated mapping between orthography and phonology representations in left inferior parietal lobule [Bitan et al.,2007b; Booth et al.,2003,2007], by more extensive semantic representations in left middle temporal gyrus [Booth et al.,2003; Chou et al.,2006; Schmithorst et al.,2006; Turkeltaub et al.,2003], and by greater involvement of left inferior frontal gyrus in lexical processing in general [Booth et al.,2001,2003,2004; Gaillard et al.,2003; Schlaggar et al.,2002; Szaflarski et al.,2006; Turkeltaub et al.,2003]. Based on the adult studies showing similarities between Chinese and English word processing, we expected similar developmental changes as in English. However, we also expected that Chinese adults would show greater activation in right middle occipital gyrus due to more in‐depth visual‐spatial analysis of characters, and greater activation in left middle frontal gyrus due to more advanced integration of orthography and phonology.
MATERIALS AND METHODS
Participants
Thirteen adults (M age = 22.3, range: 20–30; 7 males) and 13 children (M age = 11.2, range: 9.7–12.4; 7 males) participated in this study. All participants met the following inclusionary criteria: (1) native Chinese speaker, (2) right‐handed, (3) free of neurological disease or psychiatric disorders, (4) no attention deficit hyperactivity disorder (ADHD), and (5) no learning disability. Four children were eliminated due to low accuracy (less than 60%) in the lexical tasks, and one was eliminated due to excessive movement.
Rhyming and Meaning Tasks
The rhyming and meaning tasks required the participant to determine the relation of the final word with two previous words according to a predefined criterion. All words were presented visually. If there was a match, they pressed a button with their index finger of their right hand; if there was no match, they pressed a different button with their middle finger of their right hand. Applying different criteria for a match in these tasks (either rhyming or meaning) allowed us to measure the effects of conscious access to phonologic and semantic representations. Participants were encouraged to respond as quickly as possible without making errors.
Rhyming task
In the rhyming judgment task, participants determined whether the final word rhymed with either of the two preceding words. Stimuli were two‐character words with the final character (foot) consisting of two radicals. Most of the final characters had a left–right radical structure with less than 5 having an up–down radical structure. O+P+ pairs (30% of trials) had similar orthography and phonology in that they shared the same phonetic radical and same vowel phoneme. O−P+ pairs (30% of trials) had different orthography and similar phonology in that they had different phonetic radicals, but shared the same vowel phoneme. O−P− pairs (40% of trials) had different orthography and phonology in that they had different radicals and did not share the same vowel phoneme (they did not rhyme). All pairs had a different initial consonant phoneme. Refer to Figure 1 for examples of rhyming stimuli. One of the three conditions included O−P+ pairs to make it so that the subjects could not just base their judgment only on the spelling of the word. Including words that rhymed but were spelled differently encouraged subjects to access phonology.
Figure 1.

Examples of Chinese stimuli used for the rhyming tasks with their English interpretation and their pronunciation in pinyin. Numbers for the pinyin translations indicate tone. There are four different tones including the high‐level tone (first tone), the rising tone (second tone), the falling rising tone (third tone), and the falling tone (fourth tone).
Meaning task
In the meaning judgment task, participants determined whether the final word was associated with either of the two preceding words. Thirty percent of the trials contained pairs with a high association, 30% contained pairs with a low association, and 40% contained pairs of words that were unrelated. A 7‐point scale was used to assess the association between prime and target. Forty adult subjects in Beijing were asked to judge to what extent pairs of words were related. An average score across subjects below 4.2 was considered as low association (M = 3.7), whereas an average score over 5.0 was considered as high association (M = 5.5). Refer to Figure 2 for examples of meaning task stimuli. We included a manipulation of difficulty in the meaning task so that it would be comparable to the rhyming task that included O−P+ pairs that were likely to be more difficult [Kramer and Donchin,1987] and we wanted to equate difficulty across tasks.
Figure 2.

Examples of Chinese stimuli used for the meaning tasks with their English interpretation and their pronunciation in pinyin. See Figure 1 caption.
Control conditions
The control conditions were designed to equate the experimental and control blocks in terms of response characteristics. The experimental setup and timing (see below) for the control blocks were exactly the same as for the word blocks. For control blocks, the three stimuli were nonlinguistic symbols consisting of straight lines (e.g., / /, \ \, / \). Participants determined whether the third stimulus was the same as one of the first two stimuli.
Timing
Each task lasted 9 min, consisting of 10 blocks of 54s (including a 4‐second introduction screen to each block). The five experimental blocks alternated with the five control blocks. Because the design of the study was blocked with random presentation of trial types, we could not effectively contrast words of different orthographic similarity (O+P+, O−P+, O−P−) in the rhyming task or words of different association (high, low) in the meaning task. In each trial, three consecutive words were presented with each word presented for 800 ms followed by a 200‐ms blank interval. A yellow fixation cross (+) appeared on the screen after the third stimulus was removed, indicating the need to make a response during the subsequent 2,000 ms interval. Participants were told that they could respond before the yellow cross (+) appeared on the screen. Each trial lasted a total of 5,000 ms and there were 10 trials in each block. During the scanning procedure, brief written instructions were given before each block for 4 s: “Rhyme” for phonological, “Meaning” for semantic, and “Lines” for visual control. Each participant was scanned during each task and each task was in a separate run in the same session.
Stimulus characteristics
Several stimulus variables were controlled across tasks so that our effects of interest were not confounded by nuisance variables. First, all of the words contained two syllables. Second, the tasks consisted of words with similar written and spoken word frequency. Chinese written frequency was determined by a corpus (1.3 million words and 1.8 million characters) that covers almost all fields of human activity, such as politics, economy, philosophy, literature, biology, and medicine [Wang et al.,1985]. Chinese spoken word frequency was determined by a corpus of 1.7 million characters that came from 374 persons living in Beijing with different age, gender, education level, and occupation [Lu,1993]. Third, the number of strokes was the same across tasks. Stroke is the smallest component of Chinese characters and is a measure of visual spatial complexity, i.e., it is the number of steps required to write a character. Refer to Table I for information on word frequency and strokes for the Chinese stimuli for the fMRI session. We confirmed that there were no significant main effects or interactions on these nuisance variables by calculating the ANOVAs including the following independent variables: 2 sessions (practice, test) by 2 tasks (rhyming, meaning), 3 conditions (O+P+, O−P+, O−P− for the rhyming task and high association, low association, unrelated for the meaning task).
Table I.
Means (standard deviations) for written and spoken word frequency and stroke count for the rhyming and meaning tasks during the fMRI session
| Written | Spoken | Strokes | ||||
|---|---|---|---|---|---|---|
| Rhyming | Meaning | Rhyming | Meaning | Rhyming | Meaning | |
| Target | 38.5 (63.4) | 32.6 (63.7) | 10.9 (18.8) | 17.6 (26.3) | 17.3 (4.6) | 16.9 (4.8) |
| Prime | 37.1 (51.8) | 31.6 (27.3) | 12.2 (24.6) | 21.1 (26.5) | 17.2 (4.2) | 16.0 (3.9) |
| Filler | 38.7 (47.0) | 33.9 (28.9) | 16.9 (45.4) | 22.4 (32.8) | 17.2 (3.4) | 15.3 (4.5) |
MRI Procedure and Data Analysis
The participant practiced a full‐length version of each experimental task before the fMRI scanning session. Different stimuli were used in the practice and fMRI sessions.
MRI acquisition
All the images were acquired using a 2T GE/Elscint Prestige. For the functional imaging studies, a susceptibility‐weighted single‐shot echo planar imaging (EPI) method with blood oxygenation level‐dependent (BOLD) was used with the following scan parameters: TE = 45 ms, flip angle = 90°, matrix size = 128 × 72, field of view = 37 × 21 cm, slice thickness = 5 mm, number of slices = 28; TR = 3,000 ms. At the end of the functional imaging session, a high resolution, T1‐weighted 3D image was acquired. The following scan parameters were used: TR = 25 ms, TE = 6 ms, flip angle = 28°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 2 mm, number of slices = 62.
Data preprocessing
Data analysis was performed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). The functional images were realigned to the last functional volume in the scanning session. This step used affine transformations to estimate a set of six rigid‐body transformation parameters for each image by using an iterative procedure to minimize the mean‐squared difference between each individual image to the reference image. No individual runs had more than 2‐mm maximum movement for any subject in the x‐plane (M = 0.52, range = 0.1–2.0 for the adults; M = 0.57, range = 0.1–1.7 for the children), y‐plane (M = 0.45, range = 0.1–1.0 for the adults; M = 0.28, range = 0.1–0.8 for the children), or z‐plane (M = 0.12, range = 0.1–0.2 for the adults; M = 0.12, range = 0.1–0.2 for the children). Furthermore, no individual runs had more than 3° of maximum displacement in rotation for pitch (M = 0.27, range = 0.1–0.8 for the adults; M = 0.42, range = 0.1–0.8 for the children), yaw (M = 0.40, range = 0.1–0.8 for the adults; M = 0.41, range = 0.1–0.9 for the children), or roll (M = 0.54, range = 0.2–1.5 for the adults; M = 0.94, range = 0.4–3.0 for the children). The group differences between the adults and children for the movement were not significant. All statistical analyses were conducted on these movement‐corrected images. Images were then segmented and the gray‐white matter information was used to coregister the structural and functional images. The images for each individual were normalized to the Montreal Neurological Institute (MNI) average template (12 linear affine parameters for brain size and position, eight nonlinear iterations and 2 × 2 × 2 nonlinear basis functions for subtle morphological differences). The size of the voxel was 3 mm × 3 mm × 3 mm after normalized.
Statistical analyses
Statistical analyses were calculated on the smoothed data (7‐mm isotropic Gaussian kernel) using a delayed boxcar design with a 6‐s delay from onset of the block to account for the lag in hemodynamic response. Statistics were also calculated with a high‐pass filter equal to two cycles of the experimental and control conditions (216 s). We used global normalization to scale the mean of each scan to a common value to correct for whole brain differences over time. Parameter estimate images were calculated for the 26 subjects across the entire brain. Using random effect statistics, we calculated contrasts comparing the experimental to control conditions separately for the two‐word judgment tasks (rhyming and meaning) separately for the two groups and also directly between the two tasks using the control as a baseline for each contrast separately for each group. Direct comparisons between groups were also examined using two‐sample t‐tests for the rhyming minus control, meaning minus control, rhyming minus meaning, and meaning minus rhyming contrasts. Using multiple regressions, we also examined the correlation of behavioral performance with signal intensity separately for each task and separately for each group with age partialed out as a covariate. Positive and negative correlations were calculated for two kinds of analyses. First, overall accuracy was correlated with signal intensity. Second, the difference in accuracy between nonconflicting and conflicting trials for the rhyming task and the difference in accuracy between high‐association and low‐association trials for the meaning task were correlated with signal intensity. Larger difference scores indicate that subjects had relatively low accuracy on the more difficult conflicting trials in the rhyming task and low‐association trials in the meaning task. All the reported areas of activation were significant using P < 0.001 uncorrected at the cluster >20 level.
RESULTS
Behavioral Performance
Table II presents accuracy and reaction time for adults and children on the meaning, rhyming, and control trials in the scanner. We calculated task (meaning, rhyming, and control) by group (adults, children) ANOVAs separately for accuracy and reaction time on correct trials. There were significant main effects of group showing that children were less accurate, F(1,24) = 37.649, P = 0.000, and slower, F(1,24) = 17.503, P = 0.000, than adults. There were significant main effects of task for accuracy, F(2,48) = 21.006, P = 0.000, and reaction time, F(2,48) = 85.434, P = 0.000. Multiple comparisons found that the accuracy on the control task was significantly higher than that on the rhyming task (t(25) = 4.610, P = 0.000) and the meaning task (t(25) = 4.541, P = 0.000). The reaction time on the control task was significantly faster than that on the rhyming task (t(25) = −10.814, P = 0.000) and the meaning task (t(25) = −11.969, P = 0.000). However, there was no significant differences between the rhyming and meaning tasks for accuracy (t(25) = 0.941, P = 0.356) or reaction time (t(25) = 0.186, P = 0.854). There was a significant interaction between group and task for accuracy, F(2,48) = 11.507, P = 0.000, but not for reaction time F(2,48) = 1.740, P = 0.186. Simple effect analysis found that the difference between adults and children was larger for the two lexical tasks than for the control task, although children were less accurate than adults on the control task, t(24) = 2.365, P = 0.026, as well as the meaning task, t(24) = 4.490, P = 0.000, and the rhyming task, t(24) = 5.957, P = 0.000.
Table II.
Means (and standard deviations) for accuracy and reaction time for adults and children in the meaning task, the rhyming task, and the control trials
| Meaning | Rhyming | Control | |
|---|---|---|---|
| Accuracy (%) | |||
| Adults | 93.1 (4.1) | 94.6 (5.1) | 96.6 (4.0) |
| Children | 80.3 (9.1) | 77.9 (7.4) | 92.5 (4.6) |
| Reaction time (ms) | |||
| Adults | 1,171 (143) | 1,140 (123) | 831 (152) |
| Children | 1,438 (207) | 1,443 (254) | 1,199 (188) |
Table III presents accuracy and reaction time for adults and children on the three conditions of the meaning and rhyming tasks. We calculated condition (high association and low association for the meaning task; O+P+ and O−P+ for the rhyming task) by group (adults, children) ANOVAs separately for accuracy and reaction time. There were significant main effects for group showing that children were less accurate (F(1,24) = 5.957, P = 0.022 for the meaning task; F(1,24) = 23.783, P = 0.000 for the rhyming task), and slower (F(1,24) = 19.006, P = 0.000 for the meaning task; F(1,24) = 16.292, P = 0.000 for the rhyming task) than adults. There were significant main effects for conditions showing that subjects were more accurate (F(1,24) = 15.245, P = 0.001 for the meaning task; F(1,24) = 34.433, P = 0.000 for the rhyming task), and faster (F(1,24) = 28.454, P = 0.000 for the meaning task, F(1,24) = 25.958, P = 0.000 for the rhyming task) on the easier condition (high association for the meaning task and O+P+ for the rhyming task) than on the difficult condition (low association for the meaning task and O−P+ for the rhyming task). There was a significant interaction between group and condition for accuracy on the rhyming task (F(1,24) = 8.187, P = 0.009) but not for reaction time (F(1,24) = 0.370, P = 0.549). Simple effect analysis found that the difference between adults and children was clearly larger for the O−P+ trials than for the O+P+ trials, although children were less accurate than adults on O−P+ trials, t(24) = 4.325, P = 0.000, as well as O+P+ trials, t(24) = 3.170, P = 0.004. There were no significant interactions between group and condition for the meaning task on either accuracy (F(1,24) = 0.089, P = 0.768) or reaction time (F(1,24) = 0.711, P = 0.408).
Table III.
Means (and standard deviations) for accuracy and reaction time for adults and children on the three conditions of the meaning and rhyming tasks
| Meaning | Rhyming | |||||
|---|---|---|---|---|---|---|
| High | Low | Unrel | O+P+ | O−P+ | O−P− | |
| Accuracy (%) | ||||||
| Adults | 96.3 (4.4) | 85.6 (14.6) | 94.6 (5.6) | 96.8 (3.6) | 87.2 (17.4) | 93.9 (5.8) |
| Children | 86.7 (11.2) | 74.2 (18.3) | 83.1 (7.2) | 87.7 (9.7) | 60.0 (17.3) | 81.5 (14.8) |
| Reaction time (ms) | ||||||
| Adults | 1,063 (177) | 1,164 (136) | 1,485 (208) | 1,002 (123) | 1,163 (182) | 1,285 (166) |
| Children | 1,318 (165) | 1,457 (196) | 1,276 (136) | 1,346 (261) | 1,472 (272) | 1,464 (263) |
Brain Activation Patterns
Table IV shows activation for meaning minus control and rhyming minus control contrasts within each group, and Figure 3 shows the brain activation maps for these comparisons. For meaning minus control trials, both children and adults showed activation in bilateral inferior frontal gyrus, left middle frontal gyrus, left inferior occipital gyrus, left fusiform gyrus, left middle temporal gyrus, medial frontal gyrus, and right middle occipital gyrus. For rhyming minus control trials, both children and adults showed activation in left inferior frontal gyrus, left middle frontal gyrus, left inferior occipital gyrus, left fusiform gyrus, medial frontal gyrus, and right middle occipital gyrus; however, only adults showed activation in left inferior parietal lobule (BA 40, 7) if the threshold was lowered to cluster >10 voxels (x = −30, y = −60, z = 45, Z = 3.59).
Table IV.
Activations for adults and children in meaning minus control and rhyming minus control contrasts
| Contrast | Region | H | BA | z‐test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Adults (meaning) | Middle occipital gyrus | R | 18, 19 | 5.83 | 175 | 27 | −90 | 6 |
| Cuneus, hippocampus | L/R | 18 | 5.60 | 240 | −3 | −96 | 15 | |
| Inferior occipital gyrus, fusiform gyrus | L | 18, 37 | 5.48 | 327 | −24 | −90 | −9 | |
| Middle frontal gyrus, inferior frontal gyrus | L | 46, 47 | 5.38 | 819 | −45 | 27 | 21 | |
| Cerebellum (culmen, declive) | R/L | 5.20 | 177 | 6 | −63 | −30 | ||
| Middle temporal gyrus | L | 21 | 4.57 | 47 | −60 | −45 | 0 | |
| Medial frontal gyrus, anterior cingulate | L/R | 6, 8, 24 | 4.37 | 158 | −3 | 33 | 39 | |
| Inferior temporal gyrus | L | 20 | 4.32 | 48 | −36 | −3 | −33 | |
| Cerebellum (tuber, uvula) | R | 3.90 | 20 | 30 | −69 | −36 | ||
| Parahippocampal gyrus | R | 35/36 | 3.86 | 57 | 33 | −24 | −24 | |
| Inferior frontal gyrus | R | 47 | 3.76 | 113 | 39 | 30 | −3 | |
| Children (meaning) | Inferior frontal gyrus middle frontal gyrus | L | 45, 46, 47 | 5.25 | 1,033 | −39 | 24 | 24 |
| Fusiform gyrus, inferior occipital gyrus | L | 37, 18, 19 | 4.98 | 208 | −39 | −66 | −18 | |
| Inferior occipital gyrus | R | 18 | 4.72 | 51 | 33 | −84 | −12 | |
| Superior temporal gyrus, middle temporal gyrus | L | 22, 21 | 4.50 | 46 | −57 | −39 | 6 | |
| Middle occipital gyrus | R | 18 | 4.28 | 25 | 24 | −99 | 0 | |
| Superior frontal gyrus, medial frontal gyrus | L/R | 8, 6 | 4.11 | 160 | −6 | 21 | 54 | |
| Uncus, parahippocampal gyrus | L | 36 | 4.09 | 36 | −36 | −6 | −33 | |
| Posterior cingulate, cuneus | R/L | 31, 18 | 4.06 | 165 | 21 | −69 | 6 | |
| Inferior frontal gyrus, superior temporal gyrus | R | 47, 38 | 3.98 | 59 | 36 | 27 | −6 | |
| Adults (Rhyming) | Middle occipital gyrus, cuneus | R | 19, 18 | 5.25 | 532 | 33 | −87 | 0 |
| Inferior frontal gyrus | L | 47 | 4.55 | 47 | −39 | 27 | −12 | |
| Inferior frontal gyrus, middle frontal gyrus | L | 45, 44, 46 | 4.54 | 289 | −54 | 18 | 9 | |
| Inferior occipital gyrus, fusiform gyrus, middle occipital gyrus | L | 18, 19, 37 | 4.49 | 208 | −24 | −90 | −9 | |
| Medial frontal gyrus | L/R | 8 | 3.92 | 54 | −3 | 30 | 33 | |
| Children (Rhyming) | Inferior occipital gyrus, fusiform gyrus | L | 18, 19, 37 | 5.40 | 224 | −30 | −84 | −6 |
| Inferior occipital gyrus, middle occipital gyrus, cuneus | R | 18 | 5.09 | 131 | 30 | −90 | −12 | |
| Inferior frontal gyrus, middle frontal gyrus | L | 46, 10 | 4.73 | 273 | −42 | 36 | 3 | |
| Superior frontal gyrus, medial frontal gyrus | L/R | 6, 9 | 4.62 | 138 | −3 | 15 | 57 | |
| Middle frontal gyrus, inferior frontal gyrus | L | 9, 45 | 3.77 | 146 | −48 | 15 | 30 |
Note: Peaks of activation are listed in bold for areas spanning different regions; H, hemisphere; L, left; R, right; BA, Brodmann's Area.
Figure 3.
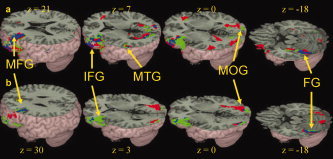
Main effects for children and adults. Brain activations for the meaning versus control trials (Panel a) and rhyming versus control trials (Panel b) in children (green) and in adults (red). Overlap between groups is represented in blue. For meaning versus control and rhyming versus control, both children and adults showed activation in left middle frontal gyrus (MFG), left inferior frontal gyrus (IFG), left fusiform gyrus (FG), and right middle occipital gyrus (MOG). For meaning versus control, both children and adults additionally showed activation in left middletemporal gyrus (MTG). See Table IV for a full listing of activations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table V shows greater activation for adults than for children on meaning minus control, rhyming minus control, and rhyming minus meaning (with control as a baseline) contrasts, and Figure 4 shows the brain activation maps for these comparisons. Adults showed greater activation than children in right middle occipital gyrus (BA 18, 19) on both meaning and rhyming contrast. Adults showed greater activation than children in left inferior parietal lobule (BA 40, 39) on rhyming minus meaning contrast, and in left inferior frontal gyrus (BA 44) if the threshold was lowered to cluster >10 voxels (x = −51, y = 12, z = 15, Z = 3.69). Table V also shows greater activation for children than adults in right postcentral gyrus (BA 43) on the rhyming contrast. The rhyming versus the meaning contrasts within the children revealed no significant differences. The rhyming versus the meaning contrasts within the same adults as used in this study has been reported previously [Booth et al.,2006]. We showed greater activation for the rhyming than for meaning task in a posterior dorsal portion of left inferior/middle frontal gyrus (BA 9, 44) and left inferior parietal lobule (BA 40). We also showed greater activation for the meaning than for the rhyming task in an anterior ventral portion of the left inferior frontal gyrus (BA 45, 47) and left superior/middle temporal gyrus (BA 22, 21).
Table V.
Direct comparisons between adults and children for meaning minus control, rhyming minus control, and rhyming minus meaning using control as a baseline
| Contrast | Region | H | BA | z‐test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Adults–Children | ||||||||
| Meaning | Middle occipital gyrus | R | 18/19 | 4.91 | 29 | 27 | −90 | 6 |
| Rhyming | Middle occipital gyrus | R | 18/19 | 4.40 | 82 | 27 | −90 | 9 |
| Rhyming–meaning | Inferior parietal lobule, postcentral gyrus, precuneus | L | 40,39 | 3.83 | 95 | −45 | −36 | 51 |
| Children–Adults | ||||||||
| Rhyming | Postcentral gyrus | R | 43 | 4.20 | 24 | 57 | −9 | 21 |
Note: See Table IV note.
Figure 4.
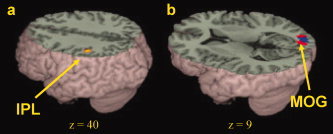
Developmental differences in brain activation. (a) Greater activation for adults than for children in rhyming versus meaning using control as a baseline in left inferior parietal lobule (IPL). (b) Greater activation for adults than for children in rhyming versus control (red) and meaning versus control (green) in right middle occipital gyrus (MOG). Overlap between these two contrasts is in blue. See Table V for a full listing of activations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table VI shows brain‐behavior correlations in children on rhyming minus control and meaning minus control contrasts, and Figure 5 shows the maps for these contrasts. Children who had a larger difference between nonconflicting and conflicting conditions in the rhyming task showed greater activation in anterior cingulate gyrus (BA 24), whereas children with a smaller difference showed greater activation in left middle frontal gyrus (BA 8, 9) and bilateral precuneus (BA 7). Children who had a larger difference between high‐association and low‐association conditions in the meaning task showed greater activation in left fusiform gyrus (BA 19, 37), whereas a smaller difference was not correlated with brain activation. No correlations were significant for adults using this difference measure. However, we reported previously that greater overall accuracy was correlated with less activation in right fusiform gyrus (BA 37) in the rhyming task, whereas there were no correlations for the meaning task using the same adults as in this study [Booth et al.,2006]. There were no brain‐behavior correlations for the children in this study using overall accuracy as the behavioral measure.
Table VI.
Correlations of the difference in accuracy between nonconflicting and conflicting trials in rhyming judgment with activation in the rhyming minus control contrast in children; correlation of the difference in accuracy between high‐association and low‐association trials in meaning judgment with activation in the meaning minus control contrast in children
| Contrast | Region | H | BA | z‐test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Rhyming+ | Corpus callosum | R | 3.88 | 28 | 9 | 18 | 24 | |
| Anterior cingulate, insula | R | 13, 24 | 3.48 | 28 | 24 | 18 | 6 | |
| Rhyming− | Precuneus | R | 31 | 4.60 | 68 | 15 | −63 | 33 |
| Precuneus | L | 31 | 3.91 | 42 | −12 | −57 | 42 | |
| Middle frontal gyrus | L | 9, 8 | 3.88 | 33 | −39 | 21 | 36 | |
| Meaning+ | Fusiform gyrus, lingual gyrus | L | 37,19 | 3.81 | 28 | −30 | −63 | −15 |
Note: See Table IV note; +, larger difference scores for accuracy are correlated with greater activation; −, smaller difference scores for accuracy are correlated with greater activation.
Figure 5.
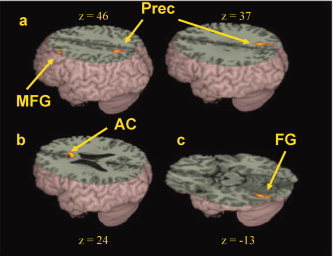
Brain‐behavior correlations for children. (a) Negative correlations of the difference in accuracy between nonconflicting and conflicting trials in rhyming judgment with activation in the rhyming minus control contrast in left middle frontal gyrus (MFG) and bilateral precuneus (Prec). (b) Positive correlations of the difference in accuracy between nonconflicting and conflicting trials in rhyming judgment with activation in the rhyming minus control contrast in bilateral anterior cingulate gyrus (AC). (c) Negative correlation of the difference in accuracy between high‐association and low‐association trials in meaning judgment with activation in the meaning minus control contrast in left fusiform gyrus (FG). Positive correlations indicate that larger difference scores for accuracy are correlated with greater activation and negative correlations indicate that smaller difference scores for accuracy are correlated with greater activation. See Table VI for a full listing of activations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To disentangle developmental differences from performance differences, we conducted two more analyses. The first analysis chose subgroups of adults and children matched for accuracy on each task. For the meaning task, we chose a subgroup of seven adults (M = 0.90, SD = 0.03) and nine children (M = 0.87, SD = 0.04; t(14) = 2.052, P = 0.052). For the rhyming task, we chose a subgroup of five adults (M = 0.90, SD = 0.05) and six children (M = 0.84, SD = 0.04; t(9) = 2.225, P = 0.07). For meaning minus control trials, adults showed greater activation than children (P < 0.001 uncorrected) in right middle occipital gyrus (x = 27, y = −84, z = 3, Z = 4.51, voxels = 29) and left lingual gyrus (x = −27, y = −72, z = −9, Z = 4.47, voxels = 29). For rhyming minus control trials, adults showed greater activation than children (P < 0.001 uncorrected) in right middle occipital gyrus (x = 30, y = −81, z = 3, Z = 3.94, voxels = 24) and left inferior parietal lobule (x = −36, y = −51, z = 57, Z = 4.44, voxels = 47). There were no group differences when directly comparing the rhyming to the meaning tasks. The second analysis conducted ANCOVAs examining group differences with accuracy as a covariate. For meaning minus control trials, adults showed greater activation than children (P < 0.001 uncorrected) in right middle occipital gyrus (x = 27, y = −87, z = 6, Z = 4.47, voxels = 14). For rhyming minus control trials, adults showed greater activation than children (P < 0.005 uncorrected) in right middle occipital gyrus (x = 27, y = −90, z = 6, Z = 3.11, voxels = 10). Adults also showed more activation than children (P < 0.005 uncorrected) in left superior parietal lobule (x = −18, y = −66, z = 60; Z = 3.30, voxels = 5) for the rhyming task minus the meaning task. Results from both analyses suggest that group differences revealed when including all subjects are not accounted for by behavioral performance differences.
DISCUSSION
Both children and adults showed activation during both the rhyming and meaning tasks in left middle/inferior frontal gyri, medial frontal gyrus, left fusiform gyrus, and bilateral middle occipital gyri. This network has been revealed in a number of previous Chinese reading studies [Chee et al.,1999; Chen et al.,2002; Kuo et al.,2001,2004; Peng et al.,2003; Tan et al.,2001,2003]. In addition, both children and adults showed activation during the meaning task in left middle temporal gyrus. This is consistent with previous studies that suggest that the left middle temporal gyrus includes verbal semantic representations [Blumenfeld et al.,2006; Booth et al.,2002b; Price et al.,1997].
Our first major developmental finding is that adults show greater activation than children in right middle occipital gyrus for both the rhyming and the meaning tasks. Two recent meta‐analyses show that right middle occipital gyrus is involved to a greater degree in Chinese reading than in English reading [Bolger et al.,2005; Tan et al.,2005a]. In Chinese, the correspondence between a character and a syllable is arbitrary, and therefore, in learning to read Chinese, character forms—the visual layout of strokes—play a critical role in reading acquisition [Ho et al.,2004,2007; Siok and Fletcher,2001; Tan et al.,2005b]. This role is not shared in alphabetic reading, which can rely to a greater degree on phonologically decoding alphabetically spelled words [Huang and Hanley,1995; McBride‐Chang et al.,2005]. Chinese characters are comprised of strokes packed into a square shape, usually including two spatially arranged radicals, and therefore visual recognition of Chinese characters requires substantial holistic and visual‐spatial processing [Xue et al.,2005]. An ERP study found that Chinese–English bilinguals reading Chinese words showed an early response in a left visual region and shift to a right visual region by 200 ms, whereas they only showed an early response in a left visual region to English words [Liu and Perfetti,2003]. This finding suggests that left visual cortex, which is specialized for processing local and high spatial frequency information, supports radical identification, whereas right visual cortex, which is specialized for global and low spatial frequency information, supports processing of the spatial layout of the character. Several studies suggest that right visual cortex is specialized for holistic and spatial processing as compared with its homologous structure in the left hemisphere [Ellis et al.,1988; Jonides et al.,1993; Kosslyn et al.,1993; Smith et al.,1995]. Neuroimaging studies examining visual processing have also demonstrated developmental differences in right visual cortex. One study found that children with better performance showed greater activation in right middle occipital gyrus than children with poor performance during a global processing task [Moses et al.,2002]. Another study found that adults showed greater activation than children in a right occipital region during face processing, which also requires holistic processing [Aylward et al.,2005]. Greater activation in right middle occipital gyrus in adults than in children during Chinese character reading presumably reflects that holistic processing in right visual cortex develops throughout childhood.
Our second major developmental finding is that adults showed greater activation than children in left inferior parietal lobule for the rhyming task as compared with meaning task. Left inferior parietal lobule is thought to be involved in mapping between orthography and phonology in English [Booth et al.,2002a]. Activation in this region has also been demonstrated during phonological processing in Chinese reading [Chee et al.,2004; Tan et al.,2001,2003]. Booth et al. [2003,2004] have demonstrated developmental differences for English speakers in left inferior parietal lobule, with adults showing more activation than children during tasks that require conversion between orthography and phonology such as rhyming judgments to visually presented words and spelling judgments to orally presented words. Studies have also shown that fractional anisotropy in inferior parietal cortex is related to reading ability [Beaulieu et al.,2005; Deutsch et al.,2005; Klingberg et al.,2000; Niogi and McCandliss,2006], so developmental changes in activation may be associated with changes in white matter integrity. Our finding is consistent with previous studies suggesting that both Chinese and English reading development is characterized by increasing specialization of left inferior parietal lobule for processes involved in converting between phonology and orthography.
This inferior parietal cortex has also been implicated in verbal working memory [Tan et al.,2005a; Xue et al.,2004]. Olesen et al. [2007] found that activity in bilateral intraparietal cortex was stronger in adults than in children during a delay, when information was maintained in working memory. Developmental changes in white matter may be associated with these activation changes as fractional anisotropy in fronto‐parietal cortex is correlated with activation in intra‐parietal cortex and behavioral performance during a working memory task [Nagy et al.,2004; Olesen et al.,2003]. In this study, the demands on working memory may have been enhanced in the rhyming task as compared with the semantic task, because in rhyming judgment, subjects must separate the second character from the word, decompose the syllable of the second character into onset and rhyme, and then compare the rhymes. In contrast, in semantic judgment, subjects do not need to separate one character from the word, or decompose the syllable. They only need to compare the meanings of three words. Greater activation in left inferior parietal lobule in adults on the rhyming task as compared with the meaning task may also indicate that adults are more effective than children in engaging their verbal working memory system.
In addition to the developmental differences in the intensity of brain activation, we also found several brain‐behavior correlations within the children. Children with higher accuracy on word pairs with conflicting orthographic and phonological information in rhyming task (see Fig. 1 for examples) showed greater activation in left middle frontal gyrus than those with lower accuracy. Left middle frontal gyrus has been argued to be a “Chinese reading area” [Perfetti et al.,2005], though it is also involved in English reading but with a less consistent and dominant role than in Chinese reading [Bolger et al.,2005; Tan et al.,2005a]. This area has been consistently activated in Chinese processing including homophone judgments [Chee et al.,2004; Kuo et al.,2004; Tan et al.,2001,2003], rhyming judgments [Booth et al.,2006], lexical decisions [Siok et al.,2004], and semantic judgments [Booth et al.,2006; Tan et al.,2001]. Some researchers have hypothesized that this area is responsible for integrating visual orthographic information with phonology, whereas others have argued that it serves as a long‐term storage center of phonological representations of Chinese words, specifically, for addressed phonology [Tan et al.,2005a]. Our finding that lower accuracy children showed less activation in left middle frontal gyrus is consistent with a previous study, which found that Chinese children with poor reading ability showed less activation in this area than age‐matched control readers during both a homophone judgment task and a lexical decision task [Siok et al.,2004].
We also found that children with higher accuracy on word pairs with conflicting orthographic and phonological information in the rhyming task (see Fig. 1 for examples) showed greater activation in bilateral medial precuneus than those with poorer performance. Studies have found that midline areas within posterior cingulate cortex and adjacent precuneus show deactivation during attention‐demanding tasks as compared with the rest [Raichle et al.,2001]. The posterior cingulate cortex and adjacent precuneus can be posited as a tonically active region of the brain that may continuously gather information about the world around, and possibly within, us. Only when successful performance demands focused attention, such broad information gathering activation should be curtailed. The positive correlation between activation in this area and accuracy in this study suggests that children with higher skill do not have to engage their attention resources as robustly as those with lower skill. In contrast to those with higher accuracy, children with lower accuracy showed greater activation in anterior cingulate gyrus. Anterior cingulate cortex has been argued to be involved in conflict detection, selective attention, and error monitoring [Kiehl et al.,2000; Weissman et al.,2003]. Both of these brain‐behavior correlations suggest that lower skilled children need to more robustly engage their attention resources as compared with higher skilled children when making rhyming judgments.
Finally, we found that children with lower accuracy on word pairs with low semantic association (see Fig. 2 for examples) in the meaning task showed greater activation in left fusiform gyrus as compared to children with higher accuracy. This may be a compensatory strategy of orthographic analysis used by children when they are not able to easily identify semantic relationships between words. Although left fusiform gyrus has been found to be involved in semantic processing of reading [Devlin et al.,2006; Kiehl et al.,2002; Wheatley et al.,2005], it seems to be related to a larger degree with retrieval of perceptual information of object form and color [Martin et al.,1995; Thompson‐Schill et al.,1999; Wiggs et al.,1999]. Therefore, it is also possible that lower skill children rely more on perceptual information than higher skill children when they make semantic judgments.
This is the first study to examine developmental differences in brain activation when processing Chinese characters. The results suggest that adults have greater specialization for processing phonological representations in left parietal cortex and have a more developed system for the visual‐spatial analysis of characters in right visual regions. In addition, children with higher skill show greater engagement of left middle frontal gyrus that has been consistently implicated in Chinese word reading and is assumed to be involved in the integration of visual orthographic information with phonology.
REFERENCES
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, Meltzoff AN (2005): Brain activation during face perception: Evidence of a developmental change. J Cogn Neurosci 17: 308–319. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L (2005): Imaging brain connectivity in children with diverse reading ability. Neuroimage 25: 1266–1271. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM (2005): Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci 25: 5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone N, Gitelman DR, Mesulam MM, Booth JR (2006): Weaker top‐down modulation from left inferior frontal gyrus area in children. Neuroimage 33: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Dong L, Cone NE, Cao F, Bigio JD, Booth JR (2007a): The interaction of orthographic and phonological information in children: An fMRI study. Hum Brain Mapp 28: 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, Booth JR. (2007b): Developmental changes in activation and connectivity of phonological processing. Neuroimage 38: 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD (2006): Differential prefrontal‐temporal neural correlates of semantic processing in children. Brain Lang 99: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W (2005): Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp 25: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W (1995): Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp 3: 93–106. [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TR, Mesulam MM (2001): The development of specialized brain systems in reading and oral‐language. Child Neuropsychol 7: 119–141. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM (2002a) Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16: 7–22. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM (2002b) Modality independence of word comprehension. Hum Brain Mapp 16: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Choy J, Gitelman DR, Parrish TR, Mesulam MM (2003): Modality‐specific and ‐independent developmental differences in the neural substrate for lexical processing. J Neurolinguistics 16: 383–405. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Gitelman DR, Parrish TR, Mesulam MM (2004): Development of brain mechanisms for processing orthographic and phonological representations. J Cogn Neurosci 16: 1234–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L (2006): Specialization of phonological and semantic processing in Chinese word reading. Brain Res 1071: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Cho S, Burman DD, Bitan T (2007): Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev Sci 10: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Bradeis D (2006): Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage 29: 822–837. [DOI] [PubMed] [Google Scholar]
- Chan CK, Siegel LS (2001): Phonological processing in reading Chinese among normally achieving and poor readers. J Exp Child Psychol 80: 23–43. [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan EW, Thiel T (1999): Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci 19: 3050–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Soon CS, Lee HL, Pallier C (2004): Left insula activation: A marker for language attainment in bilinguals. Proc Natl Acad Sci USA 101: 15265–15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H‐C, Shu H (2001): Lexical activation during the recognition of Chinese characters: Evidence against early phonological activation. Psychon Bull Rev 8: 511–518. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM (2002): Testing for dual brain processing routes in reading: A direct contrast of Chinese character and pinyin reading using FMRI. J Cogn Neurosci 14: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Dong L, Cone NE (2006): Developmental changes in the neural correlates of semantic processing. Neuroimage 29: 1141–1149. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene‐Lambertz G, Henaff M‐A, Michel F (2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123: 291–307. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B (2005): Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 41: 354–363. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM (2006): The role of the posterior fusiform gyrus in reading. J Cogn Neurosci 18: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NA, Agnew JA, Flowers DL (2004): Neural changes following remediation in adult developmental dyslexia. Neuron 44: 411–422. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Young AW, Anderson C (1988): Modes of word recognition in the left and right cerebral hemispheres. Brain Lang 35: 254–273. [DOI] [PubMed] [Google Scholar]
- Feng G, Miller K, Shu H, Zhang H, Miller K (2001): Rowed to recovery: The use of phonological and orthographic information in reading Chinese and English. J Exp Psychol Learn Mem Cogn 27: 1079–1100. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE (1998): Neuroimaging studies of word reading. Proc Natl Acad Sci USA 95: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Humter K, Xu B, Grandin CB (2003): Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp 18: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Peng D, Liu Y (2005): The role of phonological activation in the visual semantic retrieval of Chinese characters. Cognition 98: B21–B34. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT (1997): Regional cerebral blood flow during word and nonword reading. Human Brain Mapp 5: 84–92. [DOI] [PubMed] [Google Scholar]
- Ho CS, Bryant P (1997): Phonological skills are important in learning to read Chinese. Dev Psychol 33: 946–951. [DOI] [PubMed] [Google Scholar]
- Ho CS, Fong KM (2005): Do Chinese dyslexic children have difficulties learning English as a second language? J Psycholinguist Res 34: 603–618. [DOI] [PubMed] [Google Scholar]
- Ho CS, Chan DW, Chung KK, Lee SH, Tsang SM (2007): In search of subtypes of Chinese developmental dyslexia. J Exp Child Psychol 97: 61–83. [DOI] [PubMed] [Google Scholar]
- Ho CS, Chan DW, Lee SH, Tsang SM, Luan VH (2004): Cognitive profiling and preliminary subtyping in Chinese developmental dyslexia. Cognition 91: 43–75. [DOI] [PubMed] [Google Scholar]
- Huang HS, Hanley JR (1995): Phonological awareness and visual skills in learning to read Chinese and English. Cognition 54: 73–98. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awah E, Minoshima S, Mintun MA (1993): Spatial working memory in humans as revealed by PET. Nature 363: 623–625. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: An event‐related fMRI study. Psychophysiology 37: 216–223. [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF (2002): Reading anomalous sentences: An event‐related fMRI study of semantic processing. Neuroimage 17: 842–850. [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA (2000): Microstructure of temporo‐parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging [see comments]. Neuron 25: 493–500. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Malijkovic V, Weise SB, Chabris CF, Hamilton SE, Rauch SL, Buonanno FS (1993): Visual mental imagery activates topographically organized visual cortex: PET investigations. J Cogn Neurosci 5: 263–287. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Donchin E. (1987): Brain potentials as indices of orthographic and phonological interaction during word matching. J Exp Psychol Learn Mem Cogn 13: 76–86. [DOI] [PubMed] [Google Scholar]
- Kuo W‐J, Yeh T‐C, Cuann J‐R, Wu T‐T, Ho L‐T, Hung D, Tzeng OJL, Hsieh J‐C (2001): A left‐lateralized network for reading Chinese words: A 3 T fMRI study. Neuroreport 12: 3997–4001. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, Ho LT, Hung DL, Tzeng OJ, Hsieh JC (2004): Orthographic and phonological processing of Chinese characters: An fMRI study. Neuroimage 21: 1721–1731. [DOI] [PubMed] [Google Scholar]
- Leck KJ, Weekes BK, Chen MJ (1995): Visual and phonological pathways to the lexicon: Evidence from Chinese readers. Mem Cogn 23: 468–476. [DOI] [PubMed] [Google Scholar]
- Liu W, Inhoff AW, Ye Y, Wu C (2002): Use of parafoveally visible characters during the reading of Chinese sentences. J Exp Psychol Hum Percept Perform 28: 1213–1227. [DOI] [PubMed] [Google Scholar]
- Liu Y, Perfetti CA (2003): The time course of brain activity in reading English and Chinese: An ERP study of Chinese bilinguals. Hum Brain Mapp 18: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. 1993. Modern Beijing spoken Chinese corpus. Beijing: Beijing Language University Language Education Institute. [Google Scholar]
- Luke KK, Liu HL, Wai YY, Wan YL, Tan LH (2002): Functional anatomy of syntactic and semantic processing in language comprehension. Hum Brain Mapp 16: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105. [DOI] [PubMed] [Google Scholar]
- McBride‐Chang C, Bialystok E, Chong KK, Li Y (2004): Levels of phonological awareness in three cultures. J Exp Child Psychol 89: 93–111. [DOI] [PubMed] [Google Scholar]
- McBride‐Chang C, Cho JR, Liu H, Wagner RK, Shu H, Zhou A, Cheuk CS, Muse A (2005): Changing models across cultures: Associations of phonological awareness and morphological structure awareness with vocabulary and word recognition in second graders from Beijing, Hong Kong, Korea, and the United States. J Exp Child Psychol 92: 140–160. [DOI] [PubMed] [Google Scholar]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J (2002): Functional MRI of global and local processing in children. Neuroimage 16: 415–424. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T, Klingberg T (2004): Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16: 1227–1233. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD (2006): Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia 44: 2178–2188. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T (2003): Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto‐parietal network. Cogn Brain Res 18: 48–57. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T (2007): Brain activity related to working memory and distraction in children and adults. Cereb Cortex 17: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Peng DL, Xu D, Jin Z, Luo Q, Ding GS, Perry C, Zhang L, Liu Y (2003): Neural basis of the non‐attentional processing of briefly presented words. Hum Brain Mapp 18: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Tan LH (2005): The Lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112: 43–59. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1989): Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1: 153–170. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Tan LH, Rayner K (2000): The role of phonological codes in integrating information across saccadic eye movements in Chinese character identification. J Exp Psychol Hum Percept Perform 26: 607–633. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS (1997): Segregating semantic from phonological processes during reading. J Cogn Neurosci 9: 727–733. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC (1996): Cerebral organization of component processes in reading. Brain 119: 1221–1238. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Horowitz B, Donohue BC, Nace K, Maisog JM, Andreason P (1997): Phonological and orthographic components of word recognition: A PET‐rCBF study. Brain 120: 739–759. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002): Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science 296: 1476–1479. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E (2006): Cognitive modules utilized for narrative comprehension in children: A functional magnetic resonance imaging study. Neuroimage 29: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, Papanicolaou AC (2000): Brain mechanisms for reading: The role of the superior temporal gyrus in word and pseudoword naming. Neuroreport 11: 2443–2447. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC (2002): Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex 12: 297–305. [DOI] [PubMed] [Google Scholar]
- Siok WT, Fletcher P (2001): The role of phonological awareness and visual‐orthographic skills in Chinese reading acquisition. Dev Psychol 37: 886–899. [PubMed] [Google Scholar]
- Siok WT, Jin Z, Fletcher P, Tan LH (2003): Distinct brain regions associated with syllable and phoneme. Hum Brain Mapp 18: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH (2004): Biological abnormality of impaired reading is constrained by culture. Nature 431: 71–76. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S (1995): Spatial versus object working memory: PET investigations. J Cogn Neurosci 7: 337–356. [DOI] [PubMed] [Google Scholar]
- Spinks JA, Liu Y, Perfetti CA, Tan LH (2000): Reading Chinese characters for meaning: The role of phonological information. Cognition 76: B1–B11. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK (2006): A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol 59: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Gao J‐H, Liu H‐L, Perfetti CA, Xiong J, Stofer KA, Pu Y,Liu Y, Fox PT (2000): Brain activation in the processing of Chinese characters and words: A functional MRI study. Hum Brain Mapp 10: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH (2001): The neural system underlying Chinese logograph reading. Neuroimage 13: 836–846. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH, Kalogirou E (2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Karl L, Fox PT (2005a) Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Eden GF, Perfetti CA, Siok WT (2005b) Reading depends on writing, in Chinese. Proc Natl Acad Sci USA 102: 8781–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE (2001): Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport Int J Rapid Commun Res Neurosci 12: 299–307. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Aguirre GK, D'Esposito M, Farah MJ (1999): A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia 37: 671–676. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Garaeu L, Flowers DL, Zefirro TA, Eden G (2003): Development of the neural mechanisms for reading. Nat Neurosci 6: 767–773. [DOI] [PubMed] [Google Scholar]
- Wang H, Chang RB, Li YS (1985): Modern Chinese frequency dictionary. Beijing: Beijing Language University Press. [Google Scholar]
- Wang M, Perfetti CA, Liu Y (2005): Chinese‐English biliteracy acquisition: Cross‐language and writing system transfer. Cognition 97: 67–88. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG (2003): Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage 19: 1361–1368. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A (2005): Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci 17: 1871–1885. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A (1999): Neural correlates of semantic and episodic memory retreival. Neuropsychologia 37: 103–118. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Chen C (2004): Mapping of verbal working memory in nonfluent Chinese‐English bilinguals with functional MRI. Neuroimage 22: 1–10. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen K, Jin Z, Chen C, Zeng Y, Reiman EM (2005): Cerebral asymmetry in children when reading Chinese characters. Cogn Brain Res 24: 206–214. [DOI] [PubMed] [Google Scholar]
- Zhou XL (1997): Phonology plays a limited role in semantic activation In: Peng DL, Shu H, Chen XC, editors. Cognitive Research on the Chinese Language. Shandong: Shandong Educational Publishing; pp 159–194. [Google Scholar]


