Abstract
Background
Cardiac cachexia is characterized by an exaggerated loss of skeletal muscle, weakness, and exercise intolerance, although the etiology of these effects remains unknown. Here we hypothesized that the heart functions as an endocrine organ in promoting systemic cachexia by secreting peptide factors such as myostatin. Myostatin is a cytokine of the transforming growth factor β(TGFβ) superfamily that is known to control muscle wasting.
Methods and Results
We used a Cre/loxP system to ablate myostatin (Mstn gene) expression in a celltype-specific manner. As expected, elimination of Mstn selectively in skeletal muscle with a myosin light chain 1f (MLC1f)-cre allele induced robust hypertrophy in all skeletal muscle. However, heart-specific deletion of Mstn with a Nkx2.5-cre allele did not alter baseline heart size or secondarily affect skeletal muscle size, but the characteristic wasting and atrophy of skeletal muscle that typifies heart failure was not observed in these heart-specific null mice, indicating that myocardial myostatin expression controls muscle atrophy in heart failure. Indeed, myostatin levels in the plasma were significantly increased in wildtype mice subjected to pressure overload-induced cardiac hypertrophy, but not in Mstn heart-specific deleted mice. Moreover, cardiac-specific overexpression of myostatin, which increased circulating levels of myostatin by 3–4-fold, caused a reduction in weight of the quadriceps, gastrocnemius, soleus, and even the heart itself. Lastly, to investigate myostatin as a potential therapeutic target for the treatment of muscle wasting in heart failure, we infused a myostatin blocking antibody (JA-16), which promoted greater maintenance of muscle mass in heart failure.
Conclusions
Myostatin released from cardiomyocytes induces skeletal muscle wasting in heart failure. Targeted inhibition of myostatin in cardiac cachexia might be a therapeutic option in the future.
Keywords: Heart failure, atrophy, gene-deleted mice, myostatin, hypertrophy
Clinical Perspective.
The prevalence of chronic heart failure is steadily increasing in our population. Individuals suffering from this disease face high rates of mortality (which were reported to be as high as 50% in 5 years) and dire symptoms like dyspnoea, edema, exercise intolerance and muscle loss. Skeletal muscle atrophy occurs in up to 68% of patients with heart failure and has been identified as a strong independent risk factor for mortality. Despite these facts, the etiology of muscle wasting in heart failure is unclear and a targeted treatment option is lacking. In this study we show that the mouse heart, when exposed to pathological hemodynamic burden consistent with heart failure, releases the protein Myostatin into the systemic circulation - a well-known inhibitor of skeletal muscle growth. Genetic ablation of Myostatin specifically in the heart prevented skeletal muscle atrophy when heart failure was induced. Antithetically, genetically modified mice with enhanced Myostatin expression in the myocardium showed skeletal muscle rarefaction, indicating that cardiac Myostatin is sufficient to induce skeletal muscle wasting. Therapeutically, injection of a Myostatin-blocking antibody in mice with preexisting heart failure preserved muscle mass. Thus, Myostatin inhibition might be a medically relevant avenue for the treatment of muscle wasting in heart failure.
INTRODUCTION
In the year 2005 more than 5 million people were estimated to suffer from heart failure in the US.1 Heart failure is associated with high rates of morbidity and mortality, which are comparable to many forms of cancer. Similar to cancer, body wasting occurs in heart failure that affects skeletal muscle, fat and bone tissues.2 Strikingly, the presence of body wasting in heart failure was recently identified as a strong independent risk-factor for mortality.3 Skeletal muscle atrophy occurs in up to 68% of patients with heart failure and it is thought to contribute to the muscle weakness and low exercise tolerance typically observed in this disease.4 Despite recent progress in the therapies for heart failure, an effective treatment strategy for wasting is currently lacking.
Plasma levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) or neurohormones such as epinephrine, norepinephrine and cortisol are upregulated in heart failure patients, and hence could contribute to the general catabolic state observed in this disease.5 However, a direct causal association between increased neurohormones and total body wasting has not been established nor does this association hold true in all heart failure patients. Therefore, we hypothesized that an atrophy-inducing factor might be directly released from the myocardium in heart failure, such as myostatin, a cytokine of the transforming growth factor β (TGFβ) superfamily that functions as a potent inhibitor of skeletal muscle growth.6,7 Indeed, myostatin is upregulated in the heart after infarction injury, volume overload injury, and in transgenic mice with diseased hearts due to Akt overexpression.8–12 In the absence of pathology, myostatin is predominantly expressed in skeletal muscle, although some weak expression is observed in the heart and adipose tissue.7,9,10,13
Myostatin uniquely functions to control skeletal muscle mass, as loss of this gene in mice, cattle, and dogs leads to profound increases in size that results from both hypertrophy and hyperplasia of muscle.6,7 Deletion of myostatin specifically in adult mice employing a tamoxifen inducible Cre/loxP strategy still resulted in a 25% increase in skeletal muscle weight mainly through the development of muscle fiber hypertrophy rather than hyperplasia.14 Consistent with these observations, systemic overexpression of myostatin (such as injection of myostatin producing Chinese hamster ovary cells) in adult mice resulted in cachexia with a significant reduction in individual skeletal muscle weights.15 These data demonstrate the effectiveness of myostatin in the modulation of skeletal muscle mass in adulthood.
We demonstrate here that myostatin from cardiomyocytes plays a crucial role for the development of muscle wasting in a mouse model of heart failure and that systemic myostatin inhibition with a blocking antibody might be a valuable approach for the treatment of heart failure associated skeletal muscle atrophy.
MATERIALS AND METHODS
Animals and Animal Procedures
The generation of Myostatin (Mstn) loxP site targeted (fl/fl) embryonic stem cells and mice was described previously.16 Mice carrying an Nkx2.5-cre knock-in allele and mice expressing cre recombinase under the control of the myosin light chain 1f (MLC1f) genomic locus (knock-in) were described previously.17,18 The Mstn fl/fl, the Nkx2.5-cre and the MLC-cre mice were all maintained in the C57Bl/6 genetic background. Myostatin transgenic mice (FVBN strain) were generated by fusing the full-length myostatin mouse cDNA to the cardiac-specific murine α-myosin heavy chain (MHC) promoter. Transverse aortic constriction (TAC) was performed in 8–10 week-old mice as previously described.19 For echocardiography, mice were anesthetized with 2% isoflurane and the heart was visualized with a Hewlett Packard Sonos 5500 instrument and a 15 MHz transducer. Left ventricular dimensions were taken on M mode in triplicate for each mouse.19 PCR to detect Mstn recombination or the un-recombined allele was described previously.14 All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center.
Antibody Administration
The monoclonal anti-myostatin blocking antibody (clone JA-16, Wyeth Research, Collegeville, PA) inhibits the binding of myostatin to its receptor ActRIIB. Mice were treated with weekly intraperitoneal injections of JA-16 at 60 mg/kg, or mouse control antibody for 6 weeks starting at 8 weeks after TAC or sham surgery.20
Semiquantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RNA was prepared from quadriceps muscles and hearts using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was generated through reverse transcription with the SuperScript III first-strand synthesis system (Invitrogen) following the protocol provided by the manufacturer. Myostatin cDNA was amplified by PCR (34 cycles) using the following primers: 5′-acgctaccacggaaacaatc-3′ and 5′-ctggtcctgggaaggttaca-3′. L7 was used as a control and was amplified using the following primers: 5′-gaagctcatctatgagaaggc-3′ and 5′-aagacgaaggagctgcagaac-3′.
Western blotting
Protein concentration from mouse plasma samples was determined using the DC protein assay (Biorad, Hercules, CA). Plasma and homogenized protein tissue samples were supplemented with Laemmli loading buffer were boiled, subjected to electrophoresis on 16% Tris-Tricine gels, and western blotted with rabbit anti-myostatin antibody (Millipore. Billerica, MA). Protein extracts from tissues were obtained by homgenization in RIPA buffer (10 mMTris/HCl at pH 7.5, 150 mM NaCl, 4% Glycerol, 1% Triton X-100, 0.1% NaDeoxycholate, 0.05% SDS, 1mM DTT, Halt protease inhibitorscocktail (Thermo Scientific), and phosphatases cocktail inhibitors I & II (Calbiochem)). Insoluble material was precipitated bycentrifugation at 14,000 rpm for 30 min at 4°C.
Statistics
All values are presented as mean ± SEM. The unpaired Student’s t test was used to analyze differences between 2 groups in figures 1 and 3. Statistical differences for multiple comparisons in figures 2 and 4 were determined using two-way ANOVA followed by Holm-Sidak’s post-hoc test. Non-Gaussian distributed data were analyzed by Kruskal-Wallis test followed by Dunn’s post-hoc test for the data shown in Figure 2G. A 2-tailed P value of less than 0.05 was considered significant.
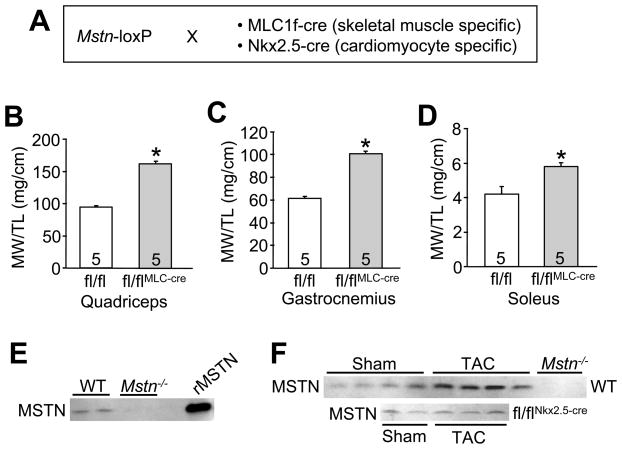
Figure 1.
Genetic deletion of Mstn in skeletal muscle produces hypertrophy. (A) Schematic of the crosses performed to delete the Mstn gene in skeletal muscle with the MLC1f-cre line or in heart with the Nkx2.5-cre line. Muscle weights (MW) normalized to tibia length (TL) of the (B) quadriceps (C) gastrocnemius and (D) soleus from the indicated groups of mice at 2 months of age. The number of mice per group is indicated within the bar *p<0.01 vs. control. (E) Western blot for myostatin protein from the plasma of wildtype (WT) or global Mstn−/− mice. Recombinant myostatin protein is shown as a control. (F) Western blots for myostatin protein in the plasma of the indicated mice after 2 weeks of TAC stimulation or a control sham procedure. Mstn−/− plasma is shown as a control.
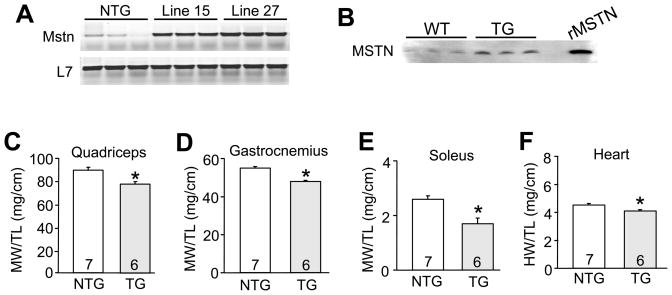
Figure 3.
Cardiomyocyte specific overexpression of myostatin reduces cardiac and skeletal muscle mass. (A) Representative RT-PCR analysis of myostatin and L7 (control) expression in cardiac muscle from non-transgenic (NTG) mice and lines 15 and 27 transgenic mice. (B) Western blot for myostatin protein in plasma of wildtype (WT) and myostatin transgenic mice. Recombinant myostatin protein is shown as a migration control. (C,D,E,F) Muscle weights (MW) or heart weight (HW) normalized to tibia length (TL) from 3 month-old non-transgenic (NTG) and transgenic (TG) mice (Line 27). The number of mice per group is indicated within the bar. *p<0.01 vs. NTG.
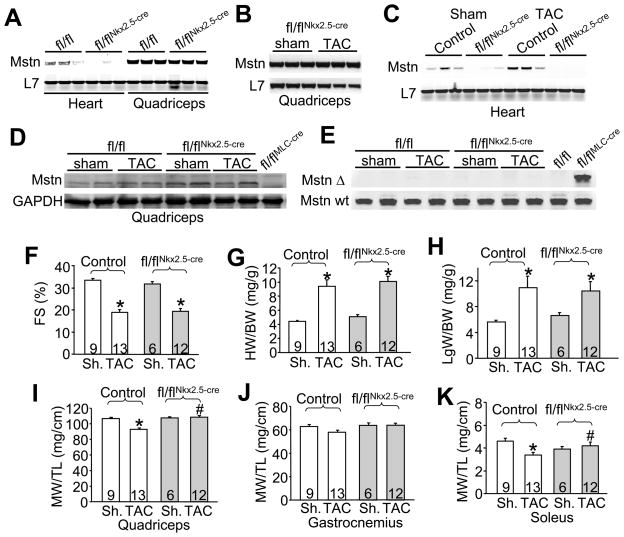
Figure 2.
Mstn deletion from heart prevents skeletal muscle atrophy in heart failure. (A) Representative RT-PCR analysis of myostatin and L7 (control) in heart and quadriceps from control and Mstn fl/flNkx2.5-cre mice. (B) RT-PCR analysis of myostatin and L7 (control) in the quadriceps from Mstn fl/flNkx2.5-cre mice 12 weeks after sham or TAC surgery. (C) RT-PCR analysis of myostatin and L7 (control) in the heart from control and Mstn fl/flNkx2.5-cre mice 12 weeks after sham or TAC surgery. (D) Western blot from quadriceps of Mstn fl/fl and Mstn fl/flNkx2.5-cre mice 12 weeks after sham or TAC surgery. As a control the last lane shows loss of pre-promyostatin from quadriceps in Mstn fl/flMLC-cre mice. (E) PCR (30 cycles) from quadriceps genomic DNA isolated from Mstn fl/fl or Mstn fl/flNkx2.5-cre mice 12 weeks after sham or TAC surgery to assay for the recombined allele (Δ, top) or the un-recombined allele (Wt, bottom). The last lane shows recombination of the Mstn locus in Mstn fl/flMLC-cre mice from quadriceps and negative control without Cre in the adjacent lane. (F) Fractional shortening (FS) determined by echocardiography in control and Mstn fl/flNkx2.5-cre mice 12 weeks after sham (Sh.) or TAC surgery. *p<0.05 vs. sham. (G) Heart weight normalized to body weight (HW/BW) in control and Mstn fl/flNkx2.5-cre mice 12 weeks after sham (Sh.) or TAC surgery. *p<0.05 vs. sham. (H) Lung (Lg) weight normalized to body weight in control and Mstn fl/flNkx2.5-cre mice 12 weeks after sham (Sh.) or TAC surgery. *p<0.05 vs. sham. (I,J,K) Muscle weights (MW) normalized to tibia length (TL) for quadriceps, gastrocnemius and soleus from control and Mstn fl/flNkx2.5-cre mice 12 weeks after sham or TAC surgery. *p<0.05 vs. sham control, #p<0.05 vs. TAC control. The number of mice per group in panels F-K is indicated within the bars of the graphs.
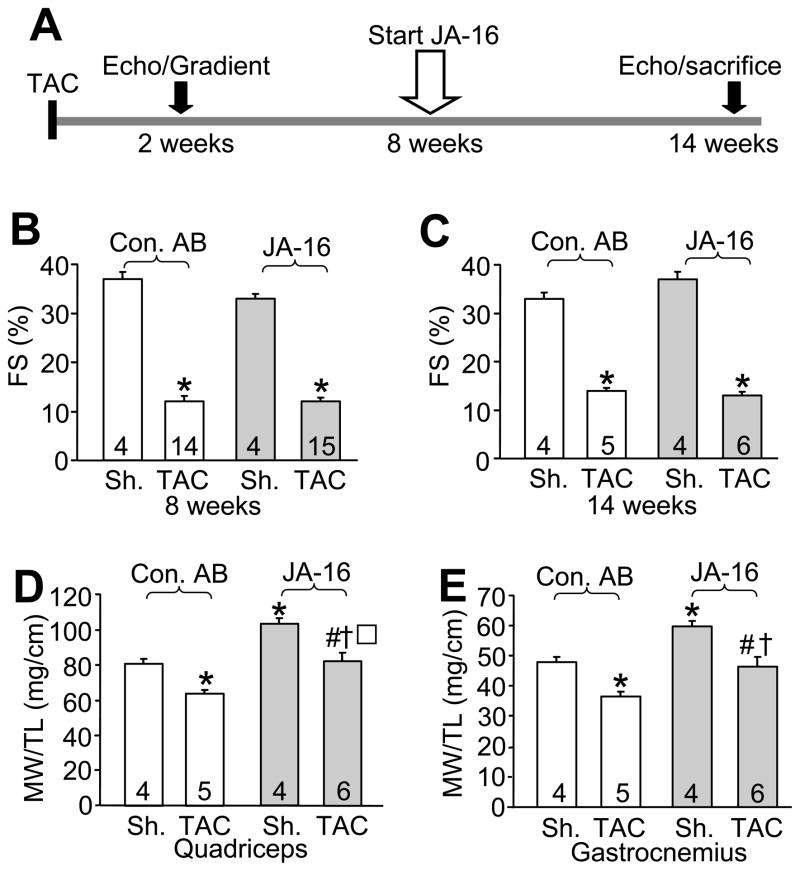
Figure 4.
Systemic antibody inhibition of myostatin enhances muscle mass in heart failure. (A) Schematic representation of the experimental design for TAC surgery and JA-16 (anti-myostatin) or control antibody (Con AB) infusion and echocardiographic assessment of heart function. (B,C) Cardiac fractional shortening (FS) before treatment at 8 weeks and at sacrifice at 14 weeks after surgery in sham or TAC operated mice treated with Con AB or JA-16. The number of mice per group is indicated within the bar. *p<0.001 vs. sham. (D,E) Muscle weights (MW) normalized to tibia length (TL) were analyzed in quadriceps and gastrocnemius muscles from sham or TAC-operated mice at sacrifice at 14 weeks after surgery. Mice were treated with either Con AB or JA-16. *p<0.05 vs. sham Con AB, # p<0.01 vs. TAC Con AB, † p<0.01 vs. sham JA-16.
RESULTS
Genetic deletion of Mstn in skeletal versus cardiac muscle cells
While myostatin is primarily expressed in skeletal muscle, some expression is observed in heart muscle although its function there remains unknown. Here we employed Mstn gene-targeted mice in which the third exon is flanked by loxP sites (Mstn fl/fl) to permit tissue-specific deletion with an appropriate cre-expressing line. Skeletal muscle- and cardiac muscle-specific deletion was separately achieved by crossing mice containing an MLC1f-cre or Nkx2.5-cre knock-in allele, respectively (Figure 1A). At 2 months of age Mstn fl/flMLC-cre mice showed a large increase in total skeletal muscle mass (normalized to tibia length) compared to Mstn fl/fl control mice, as specifically quantified from quadriceps, gastrocnemius and soleus (Figure 1B,C,D).
Previous reports have shown that myostatin mRNA and protein are up-regulated in the heart following injury or induction of hypertrophy and heart failure.8–12 Given the known role of myostatin as a muscle atrophy factor, it suggested the hypothesis that myostatin secretion from the injured or failing heart could alter skeletal muscle mass. First, we confirmed that myostatin protein could be detected in mouse plasma, employing the global Mstn−/− mouse as a control for the antibody, as well as recombinant myostatin protein. The data show that myostatin protein is easily detected in the plasma of wildtype mice, but is absent in plasma from Mstn−/− mice (Figure 1E). More importantly, plasma harvested from mice subjected to cardiac injury by TAC-induced pressure overload exhibited a 2–3-fold increase in circulating myostatin levels (Figure 1F). However, this increase in circulating myostatin after 2 weeks of TAC was blocked in Mstn fl/flNkx2.5-cre mice, suggesting that the heart was producing this increased level of myostatin in the plasma (Figure 1F).
Next we analyzed mice with cardiomyocyte specific Mstn deletion (Mstn fl/flNkx2.5-cre). Myostatin mRNA levels were quantified from quadriceps and hearts of Mstn fl/flNkx2.5-cre mice by RT-PCR, showing that myostatin was markedly deleted in the heart but not the quadriceps, indicating that the cre allele was effective and specific to the heart (Figure 2A). To evaluate our hypothesis that myostatin from the heart could affect skeletal muscle mass in cachexia we subjected Mstn fl/flNkx2.5-cre and control mice to 12 weeks of TAC stimulation to induce heart failure. Controls consisted of either Mstn fl/fl mice or Nkx2.5-cre mice, since each showed no difference in their response to TAC over 12 weeks. Importantly, myostatin mRNA levels in the quadriceps did not change after TAC in Mstn fl/flNkx2.5-cre mice (Figure 2B). In the heart, myostatin mRNA levels were still essentially absent in Mstn fl/flNkx2.5-cre mice after TAC compared to controls (Figure 2C). We also examined myostatin protein levels in the quadriceps of Mstn fl/flNkx2.5-cre mice to ensure that this Cre allele was not expressed outside the heart at baseline or after TAC. The data show no loss of pre-promyostatin protein from skeletal muscle of Mstn fl/flNkx2.5-cre mice (Figure 2D), nor was there detectable recombination of the Mstn genetic locus by PCR compared with abundant recombination in Mstn fl/flMLC-cre control mice (Figure 2E).
After 12 weeks of TAC, echocardiography revealed a significant reduction in left ventricular function (measured as fractional shortening, FS) in both control and Mstn fl/flNkx2.5-cre mice, suggesting equivalent dysfunction in both groups (Figure 2F). Measurements of heart weight normalized to body weight and lung weight normalized to body weight showed equivalent hypertrophy and edema, respectively, in each group (Figure 2G,H). Other parameters of decompensation, such as left ventricular dilation and wall thinning were also similarly affected in control mice and mice lacking myostatin in their hearts (data not shown). Analysis of skeletal muscle weights revealed a reduction of muscle mass in control mice after TAC in quadriceps, gastrocnemius and soleus muscles compared to sham operated mice (Figure 2I,J,K). However, this decrease in muscle mass after TAC was blocked in Mstn fl/flNkx2.5-cre mice (Figure 2I,J,K). Analysis of the gastrocnemius (Figure 2J) showed a trend towards significance in prevention of atrophy with a 2-way ANOVA, although a pair wise analysis between the control and deleted TAC groups did produce significance. Skeletal muscle weights did not differ in any group of mice subjected to sham operation. These results indicate that deletion of Mstn from the heart mitigates skeletal muscle atrophy during long-term pressure overload stimulation.
Cardiomyocyte specific overexpression of myostatin induces muscle wasting in mice
To complement our results observed in Mstn fl/flNkx2.5-cre mice with respect to muscle wasting in heart failure, we specifically overexpressed myostatin within cardiomyocytes to determine its ability to affect skeletal muscle through the blood. Here we generated heart-specific myostatin overexpressing transgenic mice using the mouse α-MHC cardiac-specific promoter. Two independent lines (Line 15 and Line 27) were obtained and shown to produce similar levels of myostatin overexpression in the heart at 3 months of age by RT-PCR (Figure 3A). Western blotting from heart protein extracts of these transgenic mice showed abundant expression of the pre-promyostatin peptide (data not shown). More importantly, line 27 transgenic mice showed about 3–4-fold more myostatin in the plasma compared to wildtype mice, mimicking the known enhancement in circulating myostatin levels in response to cardiac injury (Figure 3B).
Myostatin transgenic mice of both lines were viable and fertile and did not show any obvious signs of disease. However, skeletal muscle showed a significant reduction in mass that was apparent in both transgenic lines compared to non-transgenic control mice (data from line 27 are presented in Figure 3C,D,E). Interestingly, the heart weight normalized to tibia length was also significantly, albeit subtly, reduced in both transgenic lines (data from line 27 are presented in Figure 3F). These data indicate that myostatin produced in cardiomyocytes alone is sufficient to inhibit cardiac and skeletal muscle growth, respectively.
Myostatin blocking antibody (JA-16) maintains more muscle in preexisting heart failure
We next wanted to examine whether pharmacological inhibition of myostatin in preexisting heart failure could be therapeutically effective in mice. For this purpose, we performed TAC or sham procedures in 2 month-old mice (C57Bl/6 background) and started treatment with a myostatin blocking antibody (JA-16) or a control antibody (AB) 8 weeks after surgery. Cardiac performance was evaluated by echocardiography at multiple time points throughout the procedure and equivalent pressure gradients across the aortic constrictions were observed after TAC in the beginning of the protocol (Figure 4A). Echocardiography revealed markedly reduced left ventricular function after 8 weeks of TAC in mice of both treatment groups, even before AB was administered (Figure 4B). During the 6 weeks of treatment the mortality rate was 64.3 % in the control AB TAC group and 60% in the JA-16 TAC group. Echocardiography at the end of treatment demonstrated similar reductions in left ventricular performance in both TAC treatment groups, indicating that the inhibition of myostatin during heart failure did not improve survival or cardiac performance over this period of time (Figure 4C). However, inhibition of myostatin with JA-16 maintained muscle weights (quadriceps and gastrocnemius) during heart failure to values observed in sham mice without treatment (Figure 4D,E). While JA-16 treatment also increased muscle weights in sham-operated mice compared to control AB, anti-myostatin antibody nonetheless prevented atrophy as defined by a change from a baseline value of muscle mass observed in sham control mice (Figure 4D,E). Thus, systemic inhibition of myostatin maintains more musculature in a mouse model of heart failure due to pressure overload, preventing frank muscle cachexia.
DISCUSSION
In this study we identified cardiac myostatin as an important mediator of muscle atrophy in heart failure. Myostatin is highly and almost exclusively expressed in skeletal muscle where it strongly inhibits myoblast proliferation before birth, as well as muscle fiber hypertrophy before birth and in adulthood.6,7 While myostatin is generated in skeletal muscle where it acts in an autocrine manner, it is also present in the plasma, implying a systemic role of this TGFβ-family member protein, and the possibility that it is made and secreted by other organs.15 Indeed, myostatin is also expressed in heart and fat tissue, which could serve as another source of this factor and contribute to total plasma levels.7,9,10,13
Our analysis of gene-targeted mice suggests that it is the local production of myostatin within skeletal muscle itself that dominantly regulates developmental growth and hypertrophy in adulthood, which is a novel finding not previously reported. For example, deletion of Mstn only in skeletal muscle with the MLC1f-cre knock-in allele resulted in a robust 72% increase in the weight of the quadriceps at 2 months of age. Prior to this work, another group crossed Mstn-loxP targeted mice with transgenic mice expressing cre under the muscle creatine kinase (MCK) promoter, which similarly induced skeletal muscle hypertrophy.21 However, the MCK promoter is also expressed in the heart so this approach did not unequivocally prove that deletion of Mstn from skeletal muscle is the reason for hypertrophy induction.
Our data suggest that heart myostatin expression at baseline does not dominantly affect skeletal muscle mass. For example, cardiomyocyte-specific deletion of Mstn with the Nkx2.5-cre allele did not increase skeletal muscle mass in adult mice, suggesting that local production of myostatin within skeletal muscle is the primary regulator of myofiber growth, and that the heart does not secrete enough myostatin under normal physiologic conditions to impact skeletal muscle. However, myostatin production is induced in the heart by pathologic insults, which may then significantly contribute to plasma levels to secondarily impact skeletal muscle mass.8–12 Indeed, TAC stimulation in wildtype mice, but not in heart-specific Mstn-deleted mice, enhanced circulating myostatin levels. The simplest interpretation of this observation is that the heart secretes myostatin, causing/contributing to an increase in total plasma levels after injury or prolonged cardiac stress stimulation. Myostatin protein expression is also induced in cultured cardiomyocytes in response to cyclic stretching.22 Thus, cardiac stress likely induces physiologically meaningful myostatin expression or release, which can have an effect on skeletal muscle. Indeed, α-MHC-myostatin transgenic mice showed skeletal muscle wasting and atrophy, indicating that cardiac production is competent to regulate muscle mass through an endocrine-like mechanism. From a teleological perspective, it might be beneficial to rarify skeletal muscle during heart failure to secondarily reduce total circulatory burden on the heart, although too much rarefaction likely becomes maladaptive, leading to excessive morbidity.
Many patients with heart failure present with skeletal muscle atrophy, reductions in fiber strength normalized to cross-sectional area, and a disproportionate loss of exercise tolerance.3,23 Indeed, patients in advanced heart failure showed an approximate 10–30% reduction in limb muscle weight estimated by dual x-ray absorptiometry.23 Here we showed that induction of heart failure in mice, by applying long-term pressure overload, similarly led to muscle wasting. More specifically, we observed a 10%, 13% and 26% reduction in the weight of the gastrocnemius, quadriceps and soleus muscles, respectively, in wildtype mice 12 weeks after TAC. Thus, while this degree of muscle wasting is not highly robust, it is similar to clinically observed cachexia in heart failure. Remarkably, cardiomyocyte-specific deletion of Mstn with Nkx2.5-cre completely prevented skeletal muscle atrophy after 12 weeks of TAC, indicating that cardiac-generated myostatin influences skeletal muscle mass in heart failure. However, the function of myostatin proposed here does not preclude a role for inflammatory cytokines like TNF-α or neurohormones like epinephrine, norepinephrine and cortisol in also contributing to muscle wasting in heart failure.5 Indeed, we identified a significant increase in local TNF-α from the quadriceps after TAC-induced heart failure, although JA-16 antibody treatment did not reduce this expression (data not shown).
Beside its inhibitory effect on skeletal muscle growth, Mstn−/− mice were shown to develop significantly more cardiac hypertrophy in response to phenylephrine infusion, suggesting that it can also mildly affect heart growth as well.8 Consistent with these results, we observed that cardiac overexpression of myostatin results in a small, but significant reduction of heart weight. Similarly, transgenic mice with MCK promoter-driven myostatin overexpression also showed a small reduction in heart weight.24 Together these data indicate that while myostatin is primarily a regulator of skeletal muscle mass, it can negatively influence cardiac muscle hypertrophy as well.
Genetic deletion of Mstn from the heart appeared to be more effective in combating muscle wasting in heart failure than JA-16 antibody treatment (compare figures 2 vs 4). However, the genetic approach rendered the heart without Mstn from the onset of the TAC experiment, while JA-16 antibody treatment began after the induction of heart failure by TAC. This might suggest that early inhibition of myostatin is more effective, or simply that JA-16 antibody results in only partial inhibition of myostatin, especially given the increased plasma levels that are produced in heart failure. Regardless of this issue, we demonstrate that systemic inhibition of myostatin with a blocking antibody in preexisting heart failure in mice can maintain overall muscle weights to values of sham operated control mice. The effectiveness of JA-16 had been previously reported in the treatment of some forms of muscular dystrophy, where antibody mediated inhibition of myostatin increased muscle weights, strength, and also reduced fibrosis.20,25 Although drugs commonly used for the treatment of heart failure can reduce muscle atrophy (ACE inhibitors, for example, reduce the risk of cachexia in heart failure by 19%), a targeted and more efficient treatment for cardiac cachexia is needed.26,27 Interestingly, although muscle weights were increased in our study, this effect did not correlate with an improvement in mortality or cardiac function after long-term TAC. Thus, while combating muscle atrophy in heart failure may improve the quality of life in patients, it remains to be determined if it will ultimately extend lifespan.
Acknowledgments
None
Sources of funding
This work was supported by grants from the NIH (J.D.M. and S.W.) and by the Fondation Leducq (J.D.M), and the Howard Hughes Medical Institute (J.D.M.).
Footnotes
Disclosures
The JA-16 antibody was obtained from Wyeth Pharmaceuticals for a previous study.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988;18:187–195. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 3.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1034. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 7.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 8.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36:713–719. doi: 10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 11.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 12.Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, Schuler G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11:342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 13.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–E927. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 14.Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab. 2007;292:E985–E991. doi: 10.1152/ajpendo.00531.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 16.Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab. 2006;290:E409–E415. doi: 10.1152/ajpendo.00433.2005. [DOI] [PubMed] [Google Scholar]
- 17.Bothe GW, Haspel JA, Smith CL, Wiener HH, Burden SJ. Selective expression of Cre recombinase in skeletal muscle fibers. Genesis. 2000;26:165–166. [PubMed] [Google Scholar]
- 18.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 20.Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis. 2003;35:227–238. doi: 10.1002/gene.10188. [DOI] [PubMed] [Google Scholar]
- 22.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;68:405–414. doi: 10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Strassburg S, Springer J, Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol. 2005;37:1938–1947. doi: 10.1016/j.biocel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876–E888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 27.von Haehling S, Doehner W, Anker SD. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res. 2007;73:298–309. doi: 10.1016/j.cardiores.2006.08.018. [DOI] [PubMed] [Google Scholar]