Abstract
Rationale
The concurrent use of cocaine and nicotine is associated with increases in their relative rates of intake. While this increase could be due to a high reinforcing effect of the drug combination, higher rates of intake could also be explained by a decrease in the drugs’ relative reinforcing effects.
Objectives
To determine if nicotine could modulate cocaine’s reinforcing effects, the current study compared the reinforcing potency and strength of cocaine to cocaine mixed with various concentrations of nicotine.
Method
Five rhesus monkeys were allowed to self-administer cocaine (25–400 μg/kg/inj), nicotine (12–50 μg/kg/inj), or combinations of the two under a progressive-ratio schedule of reinforcement.
Results
Nicotine alone did not function as a reinforcer. Cocaine injections increased in a dose-dependent manner when taken alone and when taken as a mixture with nicotine. Furthermore, adding nicotine to cocaine shifted the cocaine dose-response function to the left in four of the five monkeys. Analysis of the ED50 values for cocaine and the mixtures indicated that some mixtures of cocaine and nicotine were more potent than cocaine alone. There were no differences in maximum injections between cocaine or any of the mixtures of cocaine and nicotine.
Conclusion
These results suggest that nicotine, under certain conditions, can increase cocaine’s potency as a reinforcer without affecting its maximum reinforcing strength.
Keywords: cocaine, nicotine, polydrug, rhesus monkey, self-administration, progressive-ratio
Introduction
Cocaine and nicotine are commonly co-abused, and concurrent use of these compounds is associated with increases in their relative rates of intake. Cocaine users who also smoke cigarettes generally take more cocaine than their non-smoking counterparts (Budney et al 1993; Roll et al 1996), and during bouts of cocaine use, cocaine users increase their rates of cigarette smoking relative to periods of non-use (Roll et al 1997). This reciprocal increase in drug intake may increase the likelihood of users developing dependence on one or both drugs. Furthermore, nicotine and cocaine are each associated with increased risk of heart disease (Giovino 2007; Vasica and Tennant 2002), and their concurrent use can exacerbate this risk by compounding cardiac stress beyond what is observed with either drug alone (Moliterno et al 1994). These risk factors emphasize the need to understand the physiological and behavioral bases of cocaine and nicotine co-administration.
The concurrent use of cocaine and nicotine may be motivated by an increase in the reinforcing strength of one or both compounds. Supporting this, cocaine abusers who also smoke cigarettes report that cigarettes enhance cocaine’s reinforcing effects (Wiseman and McMillan 1998). Furthermore, smokers pretreated with the indirect dopamine agonist, methylphenidate, but not the indirect noradrenergic agonist, atomoxetine, increased their rates of smoking relative to a placebo condition, an indication that increased nicotine intake associated with cocaine use may be mediated by dopaminergic processes related to cocaine’s reinforcing effects (Vansickel et al 2007). The animal literature provides further support for an increased reinforcing effect of cocaine and nicotine combinations. Rats pretreated with nicotine show higher breakpoints for cocaine self-administration under a progressive-ratio (PR) schedule of reinforcement than rats pretreated with saline (Bechtholt and Mark 2002). Furthermore, nicotine pretreatment enhances cocaine- and amphetamine-induced locomotor activation in rats (Jutkiewicz et al 2008), a behavior mediated by dopaminergic processes that also mediate drug reinforcement (Kelly and Iversen 1976; Koob et al 1987). At the neurochemical level, the co-administration of cocaine and nicotine has been reported to increase nucleus accumbens dopamine in an additive manner in rats (Zernig et al 1997), and acute nicotine pretreatment increases dopamine overflow in rat striatal slices treated with amphetamine, a psychostimulant with a similar pharmacological profile to cocaine (Jutkiewicz et al 2008). Taken together, these results suggest that nicotine can enhance cocaine’s reinforcing effects, and may do so by facilitating dopaminergic activity.
However, contrary to the rat data showing increased cocaine reinforcement with nicotine pretreatment, Kouri et al (2001) reported that, in humans, nicotine pretreatment via a transdermal patch lowered subjective reports of being “high” or “stimulated” by an acute cocaine challenge. These results were not due to changes in drug availability because there were no pharmacokinetic alterations in cocaine metabolism following nicotine pretreatment. As such, it could be the case that cocaine users who also smoke increase their cocaine intake to compensate for a diminution of cocaine’s reinforcing effects.
The goal of the current study was to determine if nicotine could modulate the reinforcing effects of cocaine in non-humans, and if so, to determine the direction of the modulation, i.e., diminution or enhancement. Rhesus monkeys were allowed to self-administer cocaine and nicotine, both alone and in combination, under a PR schedule of reinforcement. A PR schedule was used because it allows for the measurement of both the potency and the strength (maximum reinforcing effect) of a reinforcer by including both a dose-response function and an extinction criterion (breakpoint), the latter of which is thought to index an animal’s motivation to take a drug (Richardson and Roberts 1996; Rowlett et al 1996). The doses selected for cocaine spanned an ascending limb of a dose-response function previously characterized in this preparation (25–400 μg/kg/injection; Woolverton et al 2008a,b). The doses for nicotine (12–50 μg/kg/injection) were chosen based on previous self-administration reports using monkeys (Le Foll et al 2007; Wakasa et al 1995). It was predicted that if nicotine enhanced cocaine’s reinforcement potency or strength, leftward shifts in the dose-response function or higher breakpoints, respectively, would be observed relative to cocaine alone when the two drugs were self-administered as mixtures. Alternatively, if nicotine diminished cocaine reinforcement, rightward shifts or lower breakpoints would be observed. Lastly, if nicotine had no effect on cocaine’s reinforcing effects at the doses tested, the expected outcome would be no systematic shifts in the dose-response functions and no change in the maximum number of injections.
Materials and Methods
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Animals and Apparatus
The subjects were five male rhesus monkeys (Macaca mulatta) weighing between 9.75 and 12 kg at the beginning of the study. Four monkeys (R0697, M1338, R02050 and CK6R) had a history of cocaine and remifentanil self-administration under progressive ratio (PR) schedules similar to the ones used in the current study (Woolverton et al 2008b). The fifth monkey (R01025) had no history of drug self-administration. All monkeys were provided with sufficient food to maintain stable body weights (140–200g/day, Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI, USA) and had unlimited access to water. Fresh fruit was provided daily, and a vitamin supplement was given three times a week. Lighting was cycled to maintain 16 h of light and 8 h of dark, with lights on at 0600 hours.
Each monkey was fitted with a stainless-steel harness (EandH Engineering, Chicago, IL, USA) or a jacket (Lomir Biomedical, Malone, NY, USA) that was attached by a tether to the rear wall of the experimental cubicle (1.0 m3, Plaslabs, Lansing, MI, USA). The front door of the cubicle was made of transparent plastic, and the remaining walls were opaque. Two response levers (PRL-001, BRS/LVE, Beltsville, MD, USA) were mounted on the inside of the door. Four jeweled stimulus lights, two red and two white, were mounted above each lever. Drug injections were delivered by a peristaltic infusion pump (Cole-Parmer, Chicago, IL, USA). A Macintosh computer with custom interface and software controlled all events in an experimental session and recorded data.
Procedure
Monkeys were surgically implanted with intravenous catheters and connected to drug infusion pumps in a manner identical to what has been previously described (Woolverton et al 2008a, b). Experimental sessions began at 1100 hours each day and were conducted 7 d per week. Thirty minutes before the beginning of each session, catheters were filled with drugs for the sessions at volumes calculated not to result in infusions into the monkey. At the start of a session, the white lights were illuminated above both levers, and pressing the right lever resulted in the delivery of a 10 s drug injection. During the injection, the white lights were extinguished, and the red lights were illuminated. Responding was maintained under a PR schedule of reinforcement comparable to that described by Wilcox et al (2000). Briefly, a session consisted of 20 trials, with one injection available per trial. The response requirement started at 50 responses per injection in monkeys R02050 and M1338, 100 responses in monkey R0697, and 200 responses in monkey CK6R. For all monkeys, the response requirement per injection doubled after every fourth trial. Because of response-variability between monkeys, the set point of the initial response requirement was adjusted in each monkey to make the total number of injections during baseline drug sessions as comparable between monkeys as possible (range approximately 12–15 inj/session). There was an inter-trial interval of 30 min duration after each injection during which lights were extinguished and levers were inactive. A subject had 30 min to complete a trial (limited hold 30 min: LH 30’). A trial ended with a drug injection or the expiration of the LH. If the response requirement was not completed for two consecutive trials (i.e., the LH expired) or the animal self-administered all 20 injections, the session ended. Depending on the number of injections taken, and with two LH 30’ periods in effect, session lengths ranged from approximately 1.5 to 13 h. After the session, catheters were filled with 0.9 % saline containing heparin (40 U/ml).
In baseline sessions, injections of cocaine or saline were available. The baseline dose of cocaine was the lowest dose that maintained the maximum injections in individual monkeys (either 0.2 or 0.4 mg/kg/injection). For initial training, the baseline dose of cocaine or saline was available under a double-alternation schedule, i.e., two consecutive daily cocaine sessions were followed by two consecutive daily saline sessions. This sequence was sometimes modified to allow extra sessions for responding to be maintained by cocaine or to extinguish with saline injection, according to the behavior of the individual monkey. Responding was considered stable in baseline sessions when injections per session varied by no more than two for both cocaine and saline for at least two consecutive double-alternation sequences. At this point, test sessions were inserted to the daily sequence between two saline or two cocaine sessions. To prevent monkeys from learning this session sequence, a randomly determined saline or cocaine baseline session was inserted after every other test session. Thus, the final daily sequence of sessions was C, S, T, S, C, T, R, where “C”, “S”, “R” and “T”, respectively, represent sessions for cocaine baseline, saline, randomly determined cocaine or saline, and tests.
Five doses of cocaine (25, 50, 100, 200 or 400 μg/kg/injection) and three doses of nicotine (12, 25 or 50 μg/kg/injection) were made available in test sessions that were otherwise identical to baseline sessions. Following this, monkeys were tested with mixtures of cocaine and nicotine. The lowest dose of nicotine (12 μg/kg) was tested in only the first four monkeys to enter the study (R0697, R02050, CK6R, M1338) because the effects of nicotine were more apparent at higher doses. Therefore, the final monkey to enter the study (R01025) was only tested with 25 and 50 μg/kg nicotine in the mixtures. Once the drug mixtures were tested, the cocaine and nicotine dose-response functions were re-determined in each monkey. For nicotine, the initial and re-determined functions were combined into a single dose-response function by averaging the number of injections received at a single dose. In monkey R01025, only the lowest and highest doses of nicotine (12 and 50 μg/kg) were re-determined. ED50 values were calculated for the initial and re-determined cocaine dose-response functions. For monkeys in which the initial and re-determined functions were within 25% of their ED50 mean (R0697, R02050, and M1338), the functions were combined into a single dose-response function by averaging the number of injections received at each dose. However, the re-determined function for cocaine shifted to the right in one monkey (CK6R) and to the left in another (R01025) such that the ED50s for these functions were outside the 25% mean range. In these cases, the cocaine dose-response functions with ED50s closest to the range of values for the cocaine/nicotine mixtures were used to make the most conservative comparisons between cocaine alone and the mixtures.
Testing was conducted in irregular order across all monkeys. After a test session, a monkey was returned to baseline conditions until responding again met stability criteria or a new stable baseline was established. All doses were tested at least twice in each monkey, once with a saline session the day before and once with a cocaine session the day before. When the two test sessions of a dose showed high variability (each of the two determinations were different from the mean by ± three injections), the dose was re-tested twice, once after a saline and once after a cocaine baseline session and the data from the second determinations were used in the analysis.
Data Analysis
The mean number of injections per session were calculated individually from the two test sessions at a dose. A single drug or combination was considered to be a positive reinforcer in a monkey if the mean value for these two sessions exceeded the mean value for saline test sessions and the ranges did not overlap. For each monkey, the mean dose-effect data for cocaine alone and for the cocaine-nicotine mixtures were fitted by non-linear regression (GraphPad Prism 4.0) and ED50 values were calculated for each function (i.e., cocaine alone and cocaine mixed with each concentration of nicotine). Mean ED50 values and maximum injections for cocaine alone and for mixtures of cocaine and nicotine were calculated and compared using one-way repeated measures ANOVA tests with the single factor of nicotine dose (0, 12, 25 and 50 μg/kg). Because ANOVA testing requires balanced groups, the monkeys receiving four doses of nicotine in the mixture (0, 12, 25, and 50 μg/kg/inj) and three doses (0, 25 and 50 μg/kg/inj) were analyzed separately (note that the “zero” dose condition is included as a level in the factor of dose). Paired t-tests were used to compare group means when there was a main effect of nicotine. Significance was set at p ≤0.05.
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA), and Nicotine bitartrate was purchased commercially from Sigma-Aldrich (St. Louis, MO). Final solutions were prepared using 0.9% saline, and mixtures of cocaine and nicotine were prepared by adding both drugs to a single bag. All solutions containing nicotine were buffered to meet the stated pH range of the drug-free saline bags (pH range 4.5–7.0). All test bags containing nicotine were shielded from light by aluminum foil wrap. Doses for cocaine are expressed as the salt in this report. Doses for nicotine are expressed as the base.
Results
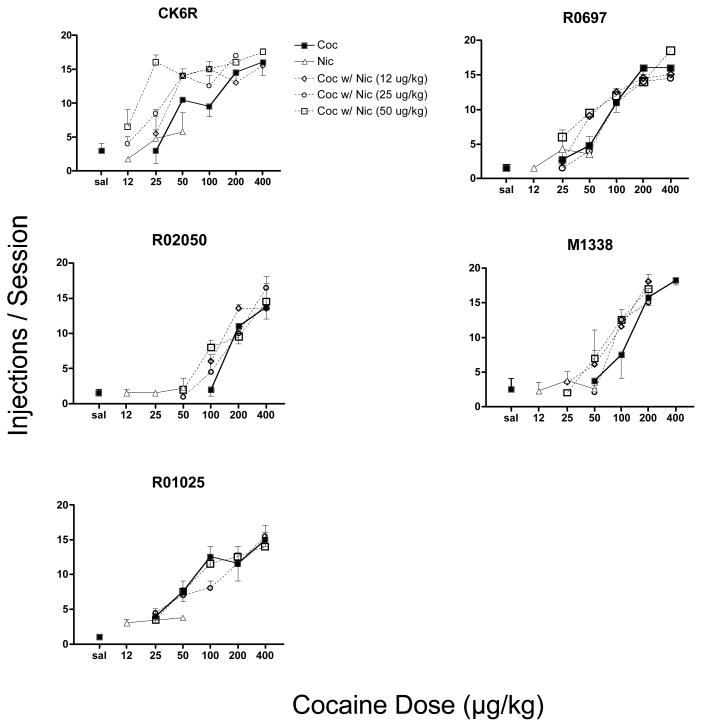
Figure 1 illustrates the number of injections each monkey received of saline, cocaine, nicotine, and cocaine mixed with various doses of nicotine. Monkeys took an average of 1.9 saline injections per session, with a range of 1 to 4 injections. Consistent with previous reports, the number of cocaine injections increased in a dose-dependent manner in each monkey. The range of nicotine injections overlapped the range of saline injections in all cases with the exception of one monkey. For monkey R01025, mean nicotine injections were higher than saline and did not overlap with the saline range, but did not vary with dose. Injections of all cocaine/nicotine mixtures increased directly with cocaine dose, indicating that the mixture was a positive reinforcer in all cases. In four of the five monkeys (CK6R, R0697, R02050, and M1338), adding nicotine shifted the cocaine dose-response function to the left in a dose-dependent manner, with the most pronounced effect occurring in monkey CK6R. In monkey R01025, adding nicotine to cocaine had a negligible effect on the cocaine dose-response function.
Figure 1.
Dose-response functions for self-administration of cocaine (Coc, bold solid line, squares), nicotine (Nic, thin solid line, triangles), and cocaine mixed with various concentrations of nicotine (hatched lines) under a progressive-ratio schedule of reinforcement. Nicotine concentrations were mixed according to dose per injection. Each point is the mean value for two test sessions at each dose or combination, vertical lines are the range (S.E.M.). Numbers a the top of each graph are the monkey numbers.
Inactive lever responses were counted for each monkey during periods of drug availability. For all test conditions, all monkeys with the exception of M1338 made relatively few inactive lever responses per session, and did so within ranges that were lower than the lowest response requirement for the initial injection (i.e., <50 inactive lever responses per session). Monkey M1338, however, showed higher activity on the inactive lever during periods of drug availability (range 0–781), and session response totals greater than fifty occurred only under test conditions that included cocaine (either alone or as part of a mixture with nicotine).
ED50 values were calculated for cocaine and the cocaine/nicotine mixtures and means were calculated for analysis (see Table 1). There were statistically significant main effects of nicotine dose in both repeated-measures ANOVAs (i.e., the analyses for four and three nicotine doses; all F’s ≥ 4.9; all p’s ≤.04). ED50s for cocaine mixed with 12 and 50 μg/kg nicotine in the four-dose analysis and 50 μg/kg nicotine in the three-dose analysis were significantly lower than cocaine alone (all p’s ≤.033), indicating that these mixtures of cocaine and nicotine were more potent reinforcers than cocaine alone.
Table 1.
ED50 values for cocaine alone (Coc) and cocaine mixed with various doses of nicotine (Nic 12, 25 and 50 μg/kg/inj) in individual monkeys (upper section) and as means for all monkeys tested for the noted conditions (lower section). Numbers in parentheses represent S.E.M.
| ED50 (μg/kg) | ||||
|---|---|---|---|---|
| Subject | Coc | Coc/Nic 12 | Coc Nic 25 | Coc Nic 50 |
| CK6R | 64 | 33 | 32 | 34 |
| R0697 | 77 | 50 | 75 | 61 |
| R02050 | 165 | 104 | 174 | 125 |
| M1338 | 124 | 83 | 95 | 72 |
| R01025 | 53 | 77 | 51 | |
| N = 4 | 108 (23) | 68 (16)* | 94 (30) | 73 (19)* |
| N = 5 | 97 (21) | 91 (23) | 69 (15)* | |
= significant difference from cocaine ED50.
Mean maximum injections/session of cocaine and cocaine mixed with each dose of nicotine are presented for each subject in Table 2. Nicotine did not affect the maximum number of injections for cocaine at any dose (all F’s ≤ 3.562, all p’s ≥.078), indicating that it did not change the reinforcing strength of cocaine.
Table 2.
Maximum injections per session for cocaine alone (Coc) and cocaine mixed with various doses of nicotine (Nic 12, 25 and 50 μg/kg/inj) in individual monkeys (upper section) and as means for all monkeys tested under the noted conditions (lower section). Numbers in parentheses represent S.E.M.
Discussion
As has been found previously using variants of the present procedure (Rowlett et al 1996; Woolverton et al 2008a,b), cocaine functioned as a positive reinforcer in a dose-dependent manner. On the other hand, nicotine did not function as a positive reinforcer under these conditions in four of the five monkeys. In one monkey (R01025; the drug-naïve monkey) that took nicotine above saline levels, the conclusion that nicotine was a positive reinforcer is weakened somewhat by the fact that responding did not vary with dose. Previous studies examining nicotine self-administration in monkeys have had mixed results with some reporting it to be a reinforcer and others not (see Le Foll and Goldberg 2006; also see Le Foll et al 2007). One factor that may have contributed to nicotine’s lack of reinforcing effects in the current study is the relatively slow infusion speed. Previous reports demonstrating positive reinforcing effects of nicotine have used relatively high infusion rates, and rate of infusion has been reported to be directly related to the reinforcing effectiveness of nicotine (Le Foll et al 2007; Wakasa et al 1995). Another factor that appears to be important in the acquisition and maintenance of nicotine self-administration in both rodents and non-human primates is the pairing of environmental cues such as lights or tones with drug injections (Caggiula et al 2001, 2002; Donny et al 2003; Le Foll and Goldberg 2006). Recently, Le Foll et al (2007) reported that nicotine was self-administered in squirrel monkeys under both fixed-ratio and PR schedules of reinforcement when a colored light stimulus was paired with nicotine injections. In the PR sessions, monkeys took approximately 20 injections per session and reached breakpoints of approximately 600 responses per injection at the most effective dose (30 μg/kg/inj). However, in the current study, monkeys earned an average of 3.4 injections of nicotine per session at a comparable dose (25 μg/kg/inj) with lower response demands required for this injection range (between 50 to 200 responses/inj). These different outcomes may be due to differences in the use of paired injection cues. Unlike the Le Foll et al methodology, the current study did not use a stimulus cue associated exclusively with nicotine injections. Rather, the lights that were illuminated during injections were the same for all injection conditions (i.e., for saline, nicotine, cocaine, and the drug combinations). It is well known that the strength of associative learning depends on the contiguity of stimulus presentation, and that non-contiguous presentations of one of the paired stimuli will preclude the learning of a stimulus association (Domjan 2005). As such, it is possible that the light cues paired with injections in the current study, having been repeatedly paired with both cocaine and saline, did not acquire the secondary reinforcement properties necessary to produce and encourage nicotine self-administration (Caggiula et al 2001, 2002).
Adding nicotine to cocaine resulted in leftward shifts in the cocaine dose-response function in four of the five monkeys. The ED50 values for cocaine mixed with 12 and 50 μg/kg were significantly lower than for cocaine alone, indicating that nicotine increased the reinforcing potency of cocaine. This suggests that the co-administration of these compounds is, at least in part, motivated by an enhanced reinforcing effect of the drug combination relative to either drug alone. Interestingly, there was no significant effect of the intermediate dose of nicotine, 25 μg/kg, on cocaine self-administration. This may be, in part, due to the relatively high ED50 valueof monkey R02050 at this mixture concentration and the associated variability at this data point (see Table 1). It is noteworthy, however, that in three of the four monkeys tested with all three doses of nicotine in the mixture, the ED50s for cocaine with 12 and 50 μg/kg nicotine were lower than cocaine with 25 μg/kg nicotine. While these data are too preliminary to make assumptions about mechanism, they do indicate that, qualitatively, the underlying mechanisms mediating nicotine’s modulatory effects on cocaine reinforcement may vary by dose.
As previously mentioned, asymptotes in dose-response functions under PR schedules of reinforcement are thought to reflect a drug’s maximum reinforcing strength (Rowlett et al 1996). In the current study, nicotine administration did not change the maximum number of cocaine injections relative to cocaine alone. As such, doses of nicotine that increased cocaine’s potency as a reinforcer did not affect its reinforcing strength. Similar results have been reported with mixtures of cocaine and heroin (Rowlett and Woolverton 1997) and cocaine and remifentanil (Woolverton et al 2008b), with the enhancing effects of these compounds occurring at moderate doses of cocaine only (i.e., doses along the ascending limb of the dose-response function). Therefore, at least in the current preparation, cocaine’s maximally reinforcing effects appear to be insurmountable by mixtures with mu opioid and nicotinic agonists, two classes of compounds that are commonly co-abused with cocaine (Kreek 1987; Roll et al 1996, 1997; Rowlett and Woolverton 1997). It should be noted, however, that the highest possible dose of cocaine and nicotine were limited to 400 and 50 μg/kg, respectively, to avoid toxicity. The possibility remains that higher doses of these drugs could have resulted in more injections.
It is noteworthy that nicotine, which has well-characterized punishing effects (Goldberg and Spealman 1983; Spealman 1983; Stolerman 1988), did not function as a punisher of cocaine self-administration as has previously been reported for other punishing compounds (e.g., histamine; Negus 2005; Woolverton 2003). As previously noted, Kouri et al (2001) reported that humans given nicotine pretreatment via a transdermal patch reported feeling less “high” or “stimulated” by a cocaine challenge than those receiving a placebo patch. The current results are not consistent with these findings. While the differences may be related to the species used, it is also possible that a constant infusion of nicotine (as would be the case with a transdermal patch) may affect the reinforcing effects of cocaine differently than a bolus method of administration. Another possibility is that non-contingent nicotine delivery may affect cocaine’s reinforcing effects differently than contingent nicotine. Recently, Woolverton and Wang (2009) reported that contingently administered pentobarbital modulated cocaine’s reinforcing potency but that non-contingent pentobarbital (given as a pretreatment) did not. As such, behavioral control of drug intake may be an important factor in the modulation of cocaine’s reinforcing effects by other drugs. At the very least, the current results demonstrate that, in monkeys, nicotine does not necessarily limit or diminish cocaine intake.
Depending on the site of intracranial infusion, nicotinic receptor antagonists have been reported to both augment and diminish cocaine-induced increases in extracellular dopamine in mice (Zanetti et al 2006). However, previous research in rats suggests that the neurochemical basis of nicotine’s enhancing effects on cocaine self-administration may be an additive increase in extracellular dopamine (Zernig et al 1997). Indeed, drugs that act on dopamine, both as direct and indirect agonists, have been reported to increase cocaine’s potency as a reinforcer (i.e., result in leftward shifts in the cocaine dose-response function; Rowlett et al 2007; Woolverton et al 2008a). One intriguing possibility is that users of cocaine increase their rates of smoking to compensate for declining dopamine levels between instances of cocaine intake. Future investigations may focus on the temporal distribution of cocaine and nicotine intake when the two are concurrently available, and correlate behavior with changes in neurochemistry.
In summary, the current results indicate that nicotine can enhance the reinforcing potency of cocaine. However, there was no evidence that nicotine affects cocaine’s reinforcing strength. Available evidence indicates that the co-abuse of cocaine and nicotine may be mediated by converging effects on the dopaminergic system. According to the current results, these neurochemical effects may be manifest through an increase in the reinforcement potency of cocaine, a result that could encourage the co-abuse of these drugs.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant R01 DA-019471 to W.L.W. and by the Robert M. Hearin Foundation. We gratefully acknowledge Lindsey Halley and Jeremy Wilson for their technical assistance.
References
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharm. 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse Treat. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaundhri N, Perkins KA, Evans-Martin FF, et al. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaundhri N, Booth S, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: A functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaundhri N, Caggiula AR, Evans0Martin FF, Booth S, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharm. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Giovino GA. The tobacco epidemic in the United States. Am J Prev Med. 2007;33:S318–S326. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224:334–340. [PubMed] [Google Scholar]
- Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharm. 2008;200:93–103. doi: 10.1007/s00213-008-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6-OHDA-induced destruction of mesolimbic dopamine neurons: Abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Vaccarino F, Amalric M, Bloom FE. Positive reinforcement properties of drugs: Search for neural substrates. In: Engel J, Oreland L, Ingvar DH, Pernow B, Rossner S, Pellborn LA, editors. Brain Reward Systems and Abuse. Raven Press; New York: 1987. pp. 35–50. [Google Scholar]
- Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine’s subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacol Biochem Behav. 2001;69:209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Multiple drug abuse patterns and medical consequences. In: Meltzer H, editor. Psychopharmacology, the third generation of progress. Raven Press; New York: 1987. pp. 1597–1604. [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharm. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230, 1–9. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliterno DJ, Willard JE, Lange RA, Negus BH, Boehrer JD, Glamann DB, Landau C, Rossen JD, Winniford MD, Hills LD. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med. 1994;330:454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharm. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules of drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Dep. 1996;40:195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: Evidence from laboratory and naturalistic settings. Exp Clin Psychopharm. 1997;5:263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Massey BW, Kleven MS, Woolverton WL. Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharm. 1996;125:361–370. doi: 10.1007/BF02246019. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharm. 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Maintenance of behavior by postponement of scheduled injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1983;227:154–159. [PubMed] [Google Scholar]
- Stolerman IP. Characterization of central nicotinic receptors by studies on the nicotine cue and conditioned taste aversion in rats. Pharmacol Biochem Behav. 1988;30:235–242. doi: 10.1016/0091-3057(88)90451-0. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PEA, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharm. 2007;193:305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- Vasica G, Tennant CC. Cocaine use and cardiovascular complications. Med J Aust. 2002;177:260–262. doi: 10.5694/j.1326-5377.2002.tb04761.x. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Takada K, Yanagita T. Reinforcing effect as a function of infusion speed in intravenous self-administration of nicotine in rhesus monkeys. Nihon Shinkei Sieshin Yadurigaku Zasshi. 1995;15:53–59. [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: Practical and theoretical concerns. Psychopharm. 2000;153:139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Wiseman EJ, McMillan DE. Rationale for cigarette smoking and for mentholation preference in cocaine- and nicotine-dependent outpatients. Compr Psychiatry. 1998;39:358–363. doi: 10.1016/s0010-440x(98)90048-7. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. A novel choice for studying drugs as punishers. Pharmacol Biochem Behav. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Self-administration of cocaine-pentobarbital mixtures by rhesus monkeys. Drug Alc Dep. 2009;100:272–276. doi: 10.1016/j.drugalcdep.2008.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Carroll FI, Tallarida R. Self-administration of drug mixtures by monkeys: Combining drugs with comparable mechanisms of action. Psychopharm. 2008a;196:575–582. doi: 10.1007/s00213-007-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Tallarida R. Self-administration of cocaine-remifentanil mixtures by monkeys: An isobolographic analysis. Psychopharm. 2008b;198:387–394. doi: 10.1007/s00213-008-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti L, Picciotto MR, Zoli M. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharm. 2007;190:189–199. doi: 10.1007/s00213-006-0598-6. [DOI] [PubMed] [Google Scholar]
- Zernig G, O’Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337:1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]