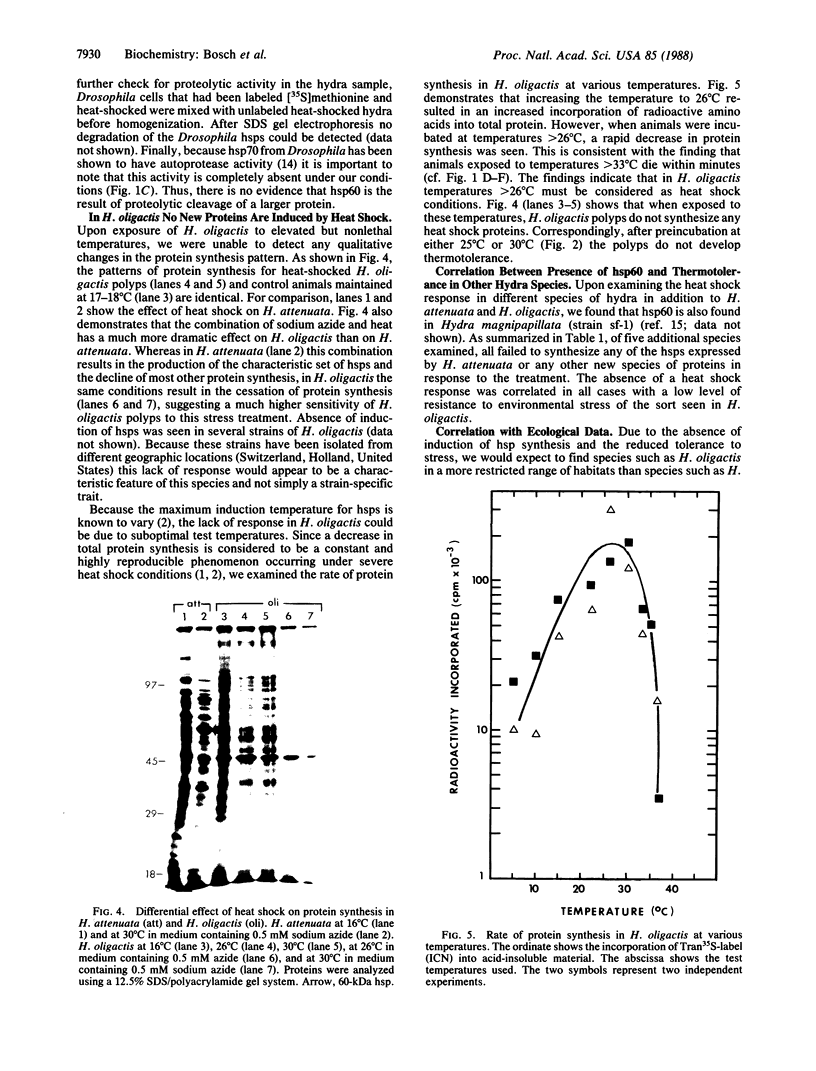
Abstract
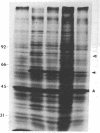
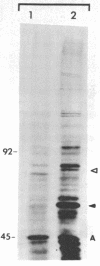
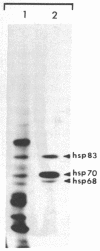
Organisms respond to environmental stress by synthesizing a small number of highly conserved heat shock proteins. In organisms as diverse as bacteria, plants, invertebrates, and vertebrates, synthesis of these proteins is directly correlated with the acquisition of thermotolerance. While studying the freshwater coelenterate hydra, we observed that Hydra oligactis was extremely sensitive to thermal stress. In contrast, the related species Hydra attenuata survives short-term exposure to high temperatures. Furthermore, after incubation at an elevated but nonlethal temperature, H. oligactis did not become thermotolerant. H. attenuata, however, acquired thermotolerance after such a preincubation. In H. attenuata the major heat shock protein was found to be 60 kDa in size. H. oligactis did not synthesize detectable levels of this protein or any new species of proteins in response to stress. Several other species of hydra were found to behave like H. oligactis in response to stress. Thus, these findings provide direct support for the hypothesis that heat shock proteins are required for stress tolerance and that the major heat shock protein in hydra does not have any effects on normal growth or physiology. The findings also indicate that the presence of a heat shock response might be related to the natural environment in which an organism lives.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- David C. N., Campbell R. D. Cell cycle kinetics and development of Hydra attenuata. I. Epithelial cells. J Cell Sci. 1972 Sep;11(2):557–568. doi: 10.1242/jcs.11.2.557. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhoff H. M., Brown R. D. Mass culture of hydra: an improved method and its application to other aquatic invertebrates. Lab Anim. 1970 Apr;4(1):139–154. doi: 10.1258/002367770781036463. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Fulford W. D., Moran L. A. Mouse and Drosophila genes encoding the major heat shock protein (hsp70) are highly conserved. Mol Cell Biol. 1983 Aug;3(8):1540–1543. doi: 10.1128/mcb.3.8.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S., Buzin C. H. Self-degradation of heat shock proteins. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4969–4973. doi: 10.1073/pnas.82.15.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Fujisawa T. Genetic analysis of developmental mechanisms in Hydra. II. Isolation and characterization of an interstitial cell-deficient strain. J Cell Sci. 1978 Feb;29:35–52. doi: 10.1242/jcs.29.1.35. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]