Abstract
Study Objectives:
This study tested the ecological validity of actigraphy (ACT) for estimating objective sleep parameters in participants' homes. We also examined how well ACT and polysomnography (PSG) measures discriminated (1) individuals with and without insomnia; and (2) nights participants rated worse, the same as, or better than average.
Methods:
Thirty-one primary insomnia sufferers and 31 normal sleepers completed up to 3 consecutive monitoring nights with wrist ACT and PSG in their homes. They also rated how each night compared to their “average night's” sleep. ACT and PSG measures of sleep onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), and sleep efficiency (SE) were then compared using Bland and Altman correlational procedures and repeated measures ANOVAs. Differences between groups and among nights assigned distinctive ratings were tested via mixed-model ANOVAs.
Results:
Medium to large between- and within-subject correlations were observed for all measures in the insomnia sufferers sample and for most measures in the normal sleepers sample. Two (ACT vs. PSG) × 3 (nights) repeated measures ANOVAs showed that, in both samples, SOL derived from ACT was consistently lower than SOL derived from PSG across the 3 nights of recording. By contrast, ACT and PSG produced estimates of WASO, TST, and SE that did not differ from each other across nights. Subsequent 2 (insomnia vs. normal sleeper) × 3 (worse, same, better than average) mixed-model ANOVAs showed only ACT SOL discriminated those with and without insomnia and nights assigned distinctive ratings. Among the PSG-derived measures, only SE showed such a pattern.
Conclusions:
ACT provides informative data for insomnia sufferers and normal sleepers in their usual sleep environments. The ACT estimate of SOL seems sensitive to night-to-night differences in subjective sleep ratings. A possible strength of ACT lies in its assessment of nocturnal movement, a parameter different from PSG-based sleep measures.
Citation:
Sánchez-Ortuño MM; Edinger JD; Means MK; Almirall D. Home is where sleep is: an ecological approach to test the validity of actigraphy for the assessment of insomnia. J Clin Sleep Med 2010;6(1):21-29.
Keywords: Actigraphy, home polysomnography, insomnia, normal sleepers
Over the past 30 years actigraphy (ACT) has emerged as a less expensive and less invasive alternative to polysomnography (PSG) for the study of sleep/wake patterns in those with and without sleep disorders.1,2 This is due in part to the fact that PSG can be too cumbersome for applications in which the main focus of interest is an estimation of the time an individual spends sleeping and/or awake, as is the case for many patients with sleep pathology. In addition, studies with sleep disordered individuals, such as those with primary insomnia, have shown there can be great variability in sleep patterns from night to night.3 This observation, in turn, suggests that multiple nights of PSG recording would be needed to capture objectively how an insomnia sufferer truly sleeps. In situations when the use of PSG seems impractical, ACT offers an appealing approach to assess sleep/wake patterns.
A recent review from the American Academy of Sleep Medicine on the role of ACT in the study of sleep and circadian rhythms2 as well as the last update of the practice parameters for the use of ACT4 suggest that, for insomnia, ACT may be most valuable in assessing treatment effects or night-to-night variability in individuals' sleep. However, these reports also point out that the accuracy of ACT to detect sleep and wakefulness may decline as sleep efficiency decreases, a problem particularly relevant to insomnia. Since the publication of this report, only a few studies have assessed the validity of ACT for estimating sleep, as measured by PSG, within insomnia samples.5–7 None of these studies have included a sample of normal sleepers to confirm the hypothesis that the correspondence between ACT and PSG is diminished when assessing the disturbed sleep of insomnia sufferers.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The ultimate advantage of ACT is the relative ease to which it can be used to assess sleep at home across many nights. However, most actigraphic validation studies typically have been conducted in sleep laboratories.
Study Impact: When used in the home setting, ACT can not only provide a fairly accurate estimation of PSG-derived sleep/wake parameters (sleep onset, wake time during the night and sleep duration) but also seems sensitive to night-to-night differences in sleep quality. ACT provides informative data about the sleep of young insomnia sufferers and normal sleepers in their usual sleep environments.
On the other hand, when assessing the validity of ACT, a complete agreement between ACT and PSG-derived measures has been expected or required in many previous studies. In such investigations, both measures have then been treated as if they were alternative and equally valid measures of a neurobehavioral state, and researchers have thereby expected ACT to completely duplicate PSG results. Whenever a high level of agreement was not reached, the use of ACT was discouraged.8–10 Nevertheless, this conclusion seems arguable. When assessing the level of agreement between two methods of measurement, it is assumed that both methods evaluate the same “construct.”11 However, although PSG and ACT are meant to provide an estimation of the time an individual spends sleeping or awake, electrographic sleep-wake states and motor activity/inactivity are not equivalent. ACT measures movement of a limb, and, although there are sophisticated algorithms that claim to estimate the time an individual spends sleeping and awake based on movement, we cannot forget that ACT just provides an indirect approximation of sleep/wake as it is commonly defined.12 Nonetheless, the switch from wakefulness to sleep is not a discrete event, but a gradual process that entails a series of events occurring in a predictable order.13 For instance, and as pointed out by Tryon,14 if we see sleep onset latency as a gradual process that entails a series of changes, we can consider that ACT (evaluating the absence of movement) and PSG (evaluating an electrographic state) key on different phases of this process. Therefore, if we consider absolute values (i.e., number of minutes) provided by ACT and PSG, agreement between measures of sleep onset latency (SOL) may be low. This does not necessarily mean that ACT provides an inaccurate measure of SOL, but rather that there may be a systematic difference between measures, even if they are highly correlated.
The utility of ACT could also be considered in light of other evidence, such as demonstration that ACT tracks changes in sleep-wake parameters detected by PSG or that sleep/wake variables provided by ACT reflect differences between insomniacs and normal sleepers as well as PSG-derived measures do.
Yet another shortcoming concerning the application of ACT to the study of insomnia is the paucity of studies addressing the validity of this method in the home setting.10 One of the most attractive features of ACT is that it permits the evaluation of sleep in the individual's usual sleep environment, allowing for a more ecologically valid approach for sleep assessment. Interestingly, the studies assessing the comparability of ACT and PSG-derived sleep measures have been conducted almost invariably in controlled laboratory settings.15,16 Nonetheless, in-home ACT and PSG recordings might be differentially reactive to behavioral and environmental factors that are absent in the controlled laboratory setting. Thus, lab ACT/PSG comparisons do not necessarily provide impressions that generalize to in-home sleep settings.
The current study was designed to overcome some of the limitations of the previous literature about ACT and insomnia. We compared sleep/wake variables obtained from ACT and PSG in the individual's usual home sleep environment. As ACT and PSG provide two different ways of assessing sleep and wake, we first assessed the relationship of these measures across multiple nights of recording in a sample of insomnia sufferers and in a sample of normal sleepers. We then assessed whether the differences in sleep/wake variables derived by ACT and PSG, if existent, are held constant across different nights of recording. Secondly, we examined if the sleep/wake variables obtained from both ACT and PSG can be informationally equivalent for distinguishing clinically identified groups of insomnia sufferers and normal sleepers and for detecting subjective sleep quality differences across nights.
MATERIAL AND METHODS
Design
This study used a mixed factorial design. Independent groups of age- and gender-matched primary insomnia sufferers and non-complaining normal sleepers comprised the study sample. The participants for the current study were all young adults between the ages of 20 and 39 years, drawn from a larger study conducted to compare the home and laboratory sleep patterns of adult insomnia sufferers and normal sleepers. All study procedures were reviewed and approved by the Institutional Review Boards of the VA Medical Center and Duke University Medical Center in Durham, NC. All participants provided written informed consent to undergoing study-related procedures at their times of enrollment. Upon completion of their study participation, they received financial compensation ($250.00) for their study involvement, as well as reimbursement for the parking expenses they incurred.
Participants
Study participants were recruited between October 1999 and October 2001 via posted announcements at a VA and affiliated university medical center, flyers posted in public libraries, and face-to-face solicitations of patients presenting to our university sleep disorders center. Prior to their acceptance into the study, all participants underwent a thorough screening that included structured psychiatric17 and sleep interviews,18 a medical exam, thyroid (TSH level) screening, and 2 nights of screening PSG to rule out occult primary sleep disorders. The insomnia sufferers reported sleep complaints consistent with Diagnostic and Statistical Manual for Mental Disorders (DSM) criteria for primary insomnia (e.g., ≥ 6 months of difficulty initiating or maintaining sleep or nonrestorative sleep with accompanying daytime deficits).19,20 The normal sleepers enrolled were adults who reported no sleep complaints and did not meet structured interview criteria18 for any sleep disorder.
Exclusion criteria were: (a) a sleep-disruptive medical condition (e.g., rheumatoid arthritis); (b) a current major psychiatric (Axis I) condition on the basis of a Structured Clinical Interview for Psychiatric Disorders (SCID)17; (c) sedative hypnotic dependence and unwillingness/inability to abstain from these medications while in the study; (d) use of anxiolytics, antidepressants, or any other psychotropic medication; or (e) apnea/hypopnea index ≥ 15 or a periodic limb movement-related arousal index ≥ 15 during on screening PSG. In addition, we excluded insomnia sufferers who met structured interview criteria18 for another sleep disorder in addition to primary insomnia.
A total of 67 young adults were enrolled; 5 of these were dropped from the current study analyses because they either failed to complete any nights of home sleep monitoring or because technical problems resulted in data loss for the nights of home recording they completed (see section below). As a result, the final sample consisted of 62 participants. Thirty-one participants met criteria for primary insomnia, and the remaining 31 met selection criteria for normal sleepers. A total of 64.4% of participants within each group completed the 3 nights of recording. Eight insomnia sufferers and 8 normal sleepers had 2 nights available, and 3 participants in each group had just one night of recording.
Polysomnography
Participants were asked to complete 3 consecutive nights of PSG in their homes and another 3 consecutive nights in the sleep laboratory. The location of PSGs (lab vs. home) was randomly determined so that roughly one-half of the men and women in each study sample first underwent lab recording and the other half completed home monitoring first. All PSGs were conducted using 8-channel Oxford Medilog 9000 or 9200 model ambulatory cassette recorders. The monitoring montage included 2 electroencephalogram (EEG) channels (C3-A2, Oz-Cz), bilateral electrooculogram (EOG), submental electromyogram (EMG), 2 channels of anterior tibialis EMG (right and left leg), and a nasal-oral thermistor. Although PSG typically includes additional respiratory measures (respiratory effort, oximetry) to detect breathing abnormalities, it was thought that monitoring of nasal/oral airflow, along with our thorough interview screening for apnea, would be sufficient to identify individual in this young adult cohort with an apnea-hypopnea index above the exclusionary cut-off. Polysomnograms with 30-s epochs were recorded and scored using traditional scoring criteria for assignment of sleep stages, identification of respiratory events (e.g., apneas, hypopneas), and identification of periodic limb movements and periodic limb movement-related arousals.12,21–23
To address this study's objectives, values of time in bed (TIB), total sleep time (TST), sleep onset latency (SOL), wake time after sleep onset (WASO), and sleep efficiency (SE) were derived from the home PSGs obtained from each participant. SOL was defined as time (min) from lights-out to the first epoch of any sleep stage, WASO was defined as total time of wake after sleep onset and until final awakening, TST was the total time of sleep (all stages combined) recognized by PSG, and TIB was ascertained by an event marker that the participant activated upon retiring to and arising from bed each night. The total time between the indicated time of retiring and subsequent time of arising signaled by the event marker entries constituted TIB. SE was calculated with the formula [TST ÷ TIB] × 100%.
Actigraphy
On all nights participants completed PSG studies, they also were asked to wear an actigraph on their non-dominant wrists to derive movement-based estimates of sleep/wake parameters. Mini-Mitter Actiwatch devices (Mini-Mitter Co., Sun River, OR) were used to acquire the measures. The Actiwatch contains a calibrated accelerometer, an event marker, and 32 K memory storage apparatus, housed in a casing that, in size and shape, resembles a wristwatch. It is designed to interface with a PC computer via a specially designed reader/interface unit. PC Windows-style software accompanies the Actiwatch and is used to program the recording unit, download data into storage, and employ a scoring algorithm that provides estimates of various sleep parameters. The default threshold, i.e., medium sensitivity, was used for inferring wake. If the summed activity score was above the defined threshold, the epoch was scored as wake; otherwise, it was scored as sleep. Actigraphic data during 1-min epochs were then scored as sleep or wake. With its default parameters, the software estimated sleep onset automatically by searching for the fist 10-min immobility interval in which there was some measured activity in no more than one epoch. The software then established that the first minute of this 10-min immobility period was the time of sleep onset. In the same way, sleep offset was automatically inferred by detecting the last 10-min immobility period containing no more than one epoch with any motion count. The last minute of that period was determined as the end of sleep.
For the purposes of this study, actigraphic estimates of TIB, TST, SOL, WASO, and SE were obtained for each night of home monitoring. The definitions of these measures were the same as those used in PSG. When the ACT and PSG devices were initialized and programmed for each recording night, their internal clocks were synchronized to assure that data derived were obtained from comparable blocks of time. This procedure was accomplished using a computer containing scoring and programming software for each device.
Sleep Diaries
Participants completed paper and pencil sleep diaries each morning subsequent to each night of PSG/ACT monitoring. Sleep diary items included questions about the previous night's bedtime, rising time, sleep onset latency, wake time during the night, time of final awakening, final rising time, and sleep quality. In addition, the diary asked the respondent to evaluate each night as compared to the respondent's average night of sleep using a 5-point Likert scale (1 = much worse; 5 = much better). Only the data acquired in response to this last question about the subjective evaluation of sleep were extracted for use in this current study. For data analyses, the responses to this question were collapsed into 3 categories: “same”, “worse” (comprising the answers “much worse” and “worse”), and “better” (comprising the answers “much better” and “better”).
Procedure
All home PSG studies were scheduled for nights when participants planned to have no overnight houseguests. Participants who reported recent use of sleep medications were required to abstain from these medications ≥ 2 weeks prior to their first series of sleep monitoring nights and to not resume these medications until they completed all nights of monitoring. Finally, they were instructed to abstain from alcoholic beverages and to not consume caffeinated substances after 18:00 on study nights.
Prior to scheduling the monitoring nights, participants were interviewed to determine their customary bedtimes and rising times. Each participant was then instructed to adhere to his/her customary bed- and rising times on all monitoring nights. On dates home PSG studies were scheduled, participants reported to the sleep laboratory between 14:00 and 17:30 for electrode attachment and receipt of an actigraph before returning home where they were encouraged to follow their usual evening routines. Each individual was also instructed to sleep in her/his usual bedroom with her/his usual bed partner if such an individual was typically present. In the morning, they returned to the sleep laboratory for removal of electrodes and to return the actigraph.
Data Analyses
Differences between the 2 study samples in sociodemographic characteristics and health-related variables were examined using t-tests for continuous data and χ2 tests for categorical data.
Since the distributions for SOL, WASO, and SE were skewed, scores were transformed to normalize these distributions. SOL scores were normalized using the formula 1/(SOL+10), WASO scores were normalized by using log(WASO), and SE data were normalized by SE5. Hence, all statistical analyses were performed with normally distributed or normalized data.
Relations between the sleep/wake variables derived from PSG and ACT were assessed by correlation coefficients. As we had more than one night of recording for most participants, and the number of nights available for each participant varied (most of the participants had 3 nights of recordings, but others had just 2 or one), we calculated a weighted correlation coefficient using the procedure suggested by Bland and Altman.24 This analysis takes into account the number of nights each participant had available (i.e., 1, 2, or 3 nights), using the number of nights as weights. The coefficient obtained is interpreted as a standard correlation coefficient (Pearson correlation coefficient).
In addition, as we had repeated nights of recordings on most of the participants, we complemented the weighted correlation coefficient with another measure of association, the correlation within subjects. This approach tells us whether a change in PSG-derived variables across nights within the individual is associated with a change in ACT-derived variables. That is, this correlation coefficient is a measure of how changes in one of the measures are paralleled by changes in the other measure within participants across nights. The correlation coefficients within subjects were calculated via multiple regression according to the method described by Bland and Altman.25 In the regression model, the PSG-derived variable was designated as the outcome variable and the ACT-derived variable was used as a predictor variable (identical results are obtained if the model is specified the other way around). Participant was treated as a categorical factor; that is, indicator variables for each participant were also entered as predictors in the regression. The within-subject correlation coefficient was calculated from the sum of squares for the ACT-derived variable and the residual sum of squares, as described by Bland and Altman.25 The hypothesis test that there is no within-subject correlation is equivalent to the test that the regression slope corresponding to the ACT-derived variable is zero. These correlation coefficients were calculated separately in the group of normal sleepers and in the group of insomnia sufferers. We interpreted the magnitude of the correlation coefficients using the guidelines provided by Cohen26; correlation coefficients < 0.30 are considered small, those ranging from 0.30 to < 0.50 are considered medium, and those ≥ 50 are considered large. Correlation coefficients obtained in the sample of normal sleepers and in the sample of insomnia sufferers were compared to ascertain if they were significantly different. To aid in this determination, we used the reference tables provided by Millsap et al.27
We also wished to determine whether the ACT-derived and PSG-derived values of each of the sleep measures examined differed within and across recording nights. To address this objective we conducted a series of repeated measures analyses of variance (ANOVAs) using each of the 4 sleep measures, i.e., SOL, WASO, TST, and SE, as dependent variables. Our statistical approach included a first set of mixed-model ANOVAs with one fixed factor, i.e., group (insomnia sufferers vs. normal sleepers), and 2 repeated factors, method (ACT vs. PSG) and night. These analyses were conducted to determine if any differences noted between ACT and PSG measures were consistent across the 2 participant samples. To make this determination, we examined the group × method and group × method × night interaction terms for each ANOVA conducted. Inasmuch as none of these interaction terms was statistically significant (see Results section), we subsequently conducted a series of 2 (ACT vs. PSG) × 3 (nights) repeated-measures ANOVAs in each sample separately to examine the comparability of sleep measures derived from the 2 recording methods across nights.
Finally, to test the performance of ACT and PSG to detect differences in sleep/wake parameters (1) between insomnia sufferers and normal sleepers and (2) among nights assigned distinctive subjective evaluations (i.e., my sleep last night was worse, same or better than average), linear mixed models were conducted. This procedure was chosen for analysis as it provides omnibus tests for between-group (normal vs. insomnia participants) and within-group (subjective evaluation of sleep for each of the 3 nights of recording) effects. These models were run separately for ACT and PSG outcomes. Data were analyzed using Proc GLM for the repeated-measures ANOVAs and Proc MIXED for the linear mixed models with SAS statistical software, version 9.1.28 For all statistical hypothesis tests, a 2-tailed p value ≤ 0.05 was regarded as statistically significant.
RESULTS
Comparison of Sociodemographic and Health-Related Variables
A total of 19 women (61.3%) were included in the insomnia sufferers group. The mean age of this group was 28.2 y (SD = 6.0 y), and they averaged 16.6 y (SD = 2.2 y) of formal education. Of these individuals, 15 were Caucasians, 10 were African Americans, 3 were Asians, and the remaining 3 had other diverse ethnic backgrounds. Eighteen women were included in the group of normal sleepers (58.1%). The average age in this group was 28.2 y (SD = 5.0 y) and they had an average of 16.8 years (SD = 2.5 y) of formal education. Twenty-two of the normal sleepers were Caucasians, 5 were African Americans, 3 were Asian Americans, and the remaining individual had a biracial background. The 2 samples did not differ significantly in regard to these sociodemographic characteristics (all p values > 0.28). In terms of health-related characteristics, both samples had a mean body mass index within the normal range (< 25). The average number (SD) of caffeinated beverages consumed per day was 1.5 (2.3) in the group of normal sleepers and 1.2 (1.2) in the group of insomnia sufferers. Three individuals (6.6%) in the group of insomnia sufferers were smokers, whereas all normal sleepers reported being non-smokers. None of these 3 variables differed significantly across groups (all p values > 0.15). Descriptive data of demographic and health-related characteristics are summarized in Table 1.
Table 1.
Sociodemographic and health-related characteristics of participants
| Normal sleepers (n = 31) | Insomnia sufferers (n = 31) | |
|---|---|---|
| Age, mean (SD), y | 28.3 (4.9) | 28.4 (6.0) |
| Education duration, mean (SD), y | 16.7 (2.5) | 16.6 (2.2) |
| Sex, No. (%), Female | 18 (58.1) | 19 (61.3) |
| Ethnic group, No. (%) | ||
| Caucasian | 22 (71.0) | 15 (48.4) |
| African American | 5 (16.1) | 10 (32.3) |
| Asian | 3 (9.7) | 3 (9.7) |
| Other | 1 (3.2) | 3 (9.7) |
| Body mass index, mean (SD) | 25.1 (5.5) | 24.9 (5.1) |
| Non-smokers, No. (%) | 31 (100) | 29 (93.4) |
| Caffeine consumption, No. drinks/day, mean (SD) | 1.5 (2.3) | 1.2 (1.2) |
Correlations Between ACT and PSG-Derived Sleep/Wake Variables
The correlation coefficients between subjects and within subjects for each one of the sleep/wake variables recorded by ACT and PSG are presented in Table 2. Between-subject correlation coefficients were all positive and significant in the group of insomnia sufferers as well as in the group of normal sleepers. Comparisons of the correlation coefficients between subjects obtained in both samples showed that they did not differ significantly. Correlations within subjects in the group of insomnia sufferers were all positive and significant, ranging in magnitude from 0.41 for SE to 0.73 for TST. According to Cohen's guidelines for interpreting the magnitude of correlation coefficients, the correlations for WASO and TST were large, whereas correlations for SOL and SE were medium in size. By contrast, not all the within-subjects correlation coefficients were significant in the group of normal sleepers. The correlation value for SE in this group did not reach statistical significance. Correlation for TST was large, whereas correlations for SOL and WASO were just medium in magnitude. Again, the comparisons of the correlation coefficients within subjects obtained in both samples showed that they did not differ significantly.
Table 2.
Two types of correlation coefficients (between subjects and within subjects) of actigraphy- and polysomnography-derived sleep/wake variables
| Insomnia sufferers (n = 31) |
Normal sleepers (n = 31) |
|||
|---|---|---|---|---|
| Correlations between subjects | Correlations within subjects | Correlations between subjects | Correlations within subjects | |
| SOL | 0.57*** | 0.43** | 0.80*** | 0.41** |
| WASO | 0.85*** | 0.52*** | 0.78*** | 0.32* |
| TST | 0.92*** | 0.73*** | 0.93*** | 0.74*** |
| SE | 0.77*** | 0.41** | 0.81*** | 0.23 |
Tests of significance of correlation coefficients:
p < 0.05,
p < 0.01,
p < 0.001.
SOL, sleep-onset latency; WASO, wake after sleep onset; TST, total sleep time; SE, sleep efficiency
Discrepancies Between ACT and PSG-Derived Sleep/Wake Variables
The raw mean values and standard deviations of sleep/wake variables for ACT and PSG across the 3 nights of recordings are shown in Table 3. The group × recording method and group × recording method x night interaction terms tested for each sleep/wake variable (SOL, WASO, TST, and SE) were all not significant (p values = 0.15 to 0.84). These findings suggested that the differences between ACT- and PSG-derived variables did not vary across the 2 participant samples. Results of the subsequent 2 (PSG vs. ACT) × 3 (nights) repeated-measures ANOVA with SOL as the outcome showed a significant main effect for method of measurement within both samples (in the insomnia sufferers group, F1,152 = 10.10, p = 0.002, and in the normal sleepers group, F1,152 = 9.71, p = 0.002). These findings suggested that mean PSG-derived SOL was significantly higher than mean ACT-derived SOL in each of the 2 samples. By contrast, no significant main effect for night or interaction effect was found in either sample, indicating that ACT SOL consistently underestimated PSG-derived SOL across the 3 nights of recording. As for the other sleep/wake variables (WASO, TST, and SE), results of the 2 (PSG vs. ACT) × 3 (nights) repeated-measures ANOVA showed no significant main or interaction effects within either the insomnia or normal sleepers groups (all p values > 0.14). Hence, these sleep/wake measures obtained from ACT and PSG did not differ significantly from each other in either sample across the nights of sleep recordings conducted.
Table 3.
Raw means and standard deviations of sleep/wake variables derived by actigraphy (ACT) and polysomnography (PSG) across 3 nights of recording
| Insomnia sufferers (n1) |
Normal sleepers (n2) |
|||
|---|---|---|---|---|
| SOL | PSG | ACT | PSG | ACT |
| Night 1 | 28.92 (28.20) | 17.80 (15.60) | 10.75 (7.14) | 10.43 (10.10) |
| Night 2 | 32.21 (48.88) | 25.28 (44.45) | 15.28 (13.98) | 12.28 (19.28) |
| Night | 26.21 (28.81) | 16.58 (19.33) | 13.48 (11.45) | 10.92 (15.14) |
| WASO | ||||
| Night | 41.56 (26.66) | 39.84 (26.06) | 49.75 (41.61) | 36.04 (28.88) |
| Night 2 | 40.79 (21.83) | 45.75 (20.12) | 45.26 (63.89) | 40.40 (36.77) |
| Night 3 | 33.75 (20.56) | 40.85 (24.77) | 29.31 (14.06) | 36.42 (22.17) |
| TST | ||||
| Night 1 | 376.48 (62.96) | 387.80 (55.41) | 391.02 (74.32) | 407.11 (62.44) |
| Night 2 | 387.68 (79.19) | 398.11 (71.83) | 389.00 (81.15) | 395.72 (61.58) |
| Night 3 | 383.28 (71.62) | 386.46 (61.50) | 390.46 (49.43) | 382.58 (51.83) |
| SE | ||||
| Night 1 | 84.68 (6.53) | 85.85 (4.50) | 86.44 (10.32) | 87.70 (6.91) |
| Night 2 | 84.16 (10.09) | 83.80 (8.04) | 86.61 (15.17) | 87.08 (10.55) |
| Night 3 | 86.80 (6.99) | 85.14 (6.85) | 90.25 (3.83) | 86.80 (8.25) |
SOL, sleep-onset latency (in minutes); WASO, wake after sleep onset (in minutes); TST, total sleep time (in minutes); SE, sleep efficiency (in percentage). Sample size: Night 1: n1 = 25, n2 = 28; Night 2: n1 = 28, n2 = 25; Night 3: n1 = 26, n2 = 26
Performance of ACT and PSG for Discriminating Insomnia Sufferers from Normal Sleepers and for Detecting Differences in Subjective Sleep Assessments
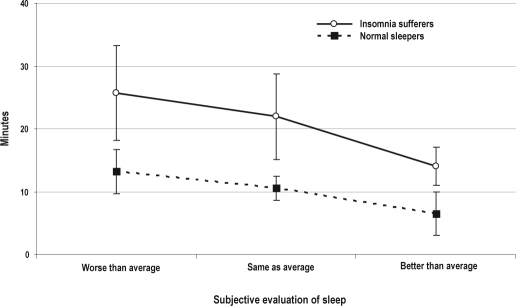
Results of the 2 (Group: insomnia vs. normal) × 3 (Subjective evaluation of sleep: worse vs. same vs. better) ANOVAs conducted with the ACT-derived SOL as outcome showed a significant group main effect (F1,60 = 7.8, p = 0.007). As shown in Figure 1, mean ACT-derived SOL was significantly higher in the group of insomnia sufferers than in the group of normal sleepers. ANOVA results also showed a significant main effect for inter-night subjective evaluation of sleep (F2,60 = 5,8, p = 0.005). Subsequent pairwise post hoc comparisons revealed that ACT-derived SOL was significantly lower when nights were rated as “better than average” than when they were rated as “same as average” or “worse than average” (both p values < 0.05). No other pairwise comparisons were significant. In addition, no group × subjective evaluation of sleep interaction effect was found. Figure 1 shows plots of the raw mean data of both groups across different subjective evaluations of sleep.
Figure 1.
Raw mean sleep-onset latency values (in minutes) and standard errors derived by actigraphy
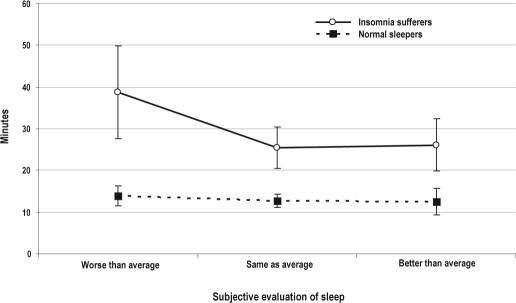
By contrast, for PSG-derived SOL we just found a significant group effect (F1,60 = 11.32, p = 0.0013). Subjective evaluation of sleep and the interaction effects were not significant, suggesting the lack of sensitivity of PSG-derived SOL for detecting differences in subjective evaluations of sleep across groups. Figure 2 shows plots of the raw mean data of the 2 groups (insomnia sufferers and normal sleepers) across different subjective evaluations of sleep.
Figure 2.
Raw mean sleep-onset latency values (in minutes) and standard errors derived by polysomnography
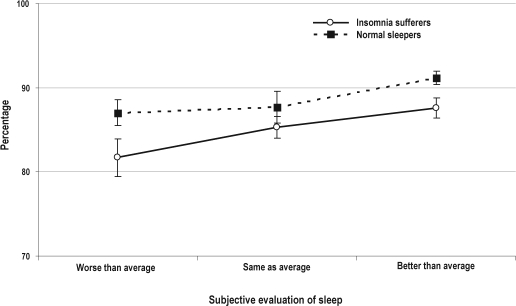
Surprisingly, results from the similar ANOVAs showed the groups of normal sleepers and insomnia sufferers did not differ significantly on measures of TST and WASO derived from both PSG and ACT. In addition, TST and WASO values did not show any significant variation across nights rated worse, the same as, or better than normal. When considering SE, PSG-derived values were significantly lower in the group of insomnia sufferers than in the group of normal sleepers (F1,60 = 5.4, p = 0.02). Furthermore, PSG-derived SE values differed significantly across nights assigned distinctive subjective evaluations (F2,60 = 3.9, p = 0.02), whereas no significant group × subjective evaluation of sleep interaction effect was noted for this measure. Pairwise post hoc comparisons revealed that PSG-derived SE was significantly lower when nights were rated worse than average than when they were rated as same as average or better than average (both p values < 0.04). Mean SE PSG-derived values for both groups and for the 3 distinctive subjective evaluations of sleep are shown in Figure 3. By contrast, the ANOVA conducted with ACT-derived SE values did not show any significant main or interaction effects.
Figure 3.
Raw mean sleep efficiency values (in percentage) and standard errors derived by polysomnography
DISCUSSION
Previous studies have led to the impression that ACT provides reasonable estimates of nocturnal sleep/wake measures in insomnia samples, although this technique may provide the most accurate sleep estimates among individuals without significant sleep disturbances.29 However, preceding studies concerning the validity of ACT failed to evaluate this technique across multiple nights of home recording in both insomnia and normal sleeper samples. In order to overcome these limitations and enhance the ecological validity of its results, the current study enrolled samples of normal sleepers and insomnia sufferers who then underwent multiple nights of simultaneous ACT and PSG monitoring while sleeping in their usual home sleep setting. The results obtained contrast somewhat with the impressions provided by previous studies30 and suggest that in-home ACT produces reasonably valid estimates of sleep/wake measures in both insomnia and normal sleeper samples.
Support for this contention comes from our within- and between-subjects correlational analyses as well as from comparisons of mean sleep/wake measures derived from ACT and PSG within each of our samples. The weighted between-subjects ACT/PSG correlations were, for the most part, moderately high, suggesting that ACT varied in a manner similar to PSG across participants within both study samples. Furthermore, our ANOVAs showed that the values of WASO, TST, and SE derived from ACT and PSG did not differ significantly in our study samples. Hence, if we use PSG as the “gold standard,” these sleep/wake parameters seemingly can be accurately inferred from ACT in young normal sleepers and insomnia sufferers. By contrast, ACT-derived SOL was significantly lower than PSG-derived SOL. Nevertheless, the differences between ACT-derived SOL and PSG-derived SOL were consistent across the 3 nights of recording, i.e., the interaction effect method × night was not significant. This finding is in agreement with Tryon's14 hypothesis stating that the fact that ACT-derived SOL systematically precedes PSG-derived SOL demonstrates that ACT validly keys on an earlier phase of the sleep onset spectrum; hence, differences between ACT and PSG are not random measurement error.
Of particular interest is the fact that ACT performed relatively well for estimating sleep among the insomnia sample. Indeed, the ACT/PSG correlations found in our group of insomnia sufferers did not differ significantly from those found in our group of normal sleepers. Moreover, the differences between ACT-derived and PSG-derived values were the same in both groups, e.g., the interaction term group (normal vs. insomnia participants) × method (ACT vs. PSG) × nights, tested for each sleep/wake variable, were all nonsignificant. These results are in stark contrast with the commonly held notion that the correspondence between ACT and PSG may be poorer among groups with marked sleep disruption,2,8,30 as is the case in insomnia. This view has been suggested repeatedly in many previous studies with insomnia samples,6,7 yet none of these studies have included a sample of normal sleepers, assessed with the same ACT device and the same scoring algorithm, to directly test this assumption. Of course, our correlational findings could be explained by the fact that, for our group of healthy young normal sleepers, the range of variability of sleep/wake variables was much more restricted than we observed for our insomnia sufferers. This restricted range, thus, could have produced small r values in the group of normal sleepers, and could have explained the absence of differences between the correlation coefficients computed in both groups. Nonetheless, our subsequent findings comparing the differences between ACT and PSG-derived measures across both groups tend to reduce concerns about the diminished validity of ACT for estimating sleep/wake measures among young insomnia samples.
In both samples, the within-subjects correlation coefficients obtained were, for the most part, significant and fell in the medium to large range of magnitude. These results offer a complementary view of the relationship between ACT and PSG. Such a finding points out that ACT seems rather sensitive to PSG-detected sleep/wake variability within participants across nights. This further suggests that ACT has the potential for tracking the natural night-to-night variability of a given individual's sleep, rather than indicating that ACT may be useful in the measurement of treatment effects, as has been suggested elsewhere.2,31 Although it has been concluded from previous studies that ACT is a useful device for measuring treatment response,5,32 it should be kept in mind that certain therapies could differentially affect the parameters assessed by ACT and PSG. For example, because research has shown that hypnotic use decreases motility,33 it could be hypothesized that ACT may be more sensitive than PSG to detect any treatment effect in this situation. Furthermore, after treatment, the correspondence between PSG and ACT may not be the same as before treatment.
Another question addressed in this paper was whether estimates of sleep/wake variables derived from in-home ACT were sufficiently sensitive to distinguish insomnia sufferers from normal sleepers. In addition, and as a subsidiary issue, this same question was asked of our in-home PSG monitoring. As expected, mean sleep-onset latencies derived from both ACT and PSG were significantly higher in the group of insomnia sufferers. This indicates that ACT may be as sensitive as PSG to detect a clinical group of insomnia sufferers based on their mean sleep-onset latencies.
Perhaps one of the most striking results of this study was the sensitivity of actigraphically derived SOL to discriminate among nights assigned ratings of “worse,” “the same as,” or “better than” average in both study groups. By contrast, sleep onset latency derived by PSG did not show such a pattern. A possible strength of the ACT lies in its assessment of small movements during sleep, a parameter that is very different from the ones assessed by PSG. Furthermore, since anxiety may be accompanied by greater movement, ACT assessment of SOL may be reflecting differences in the level of anxiety in our samples. In fact, it has been reported that high anxiety and worry in otherwise healthy individuals is related to greater percentage of light sleep relative to those with low anxiety and worry-proneness.34 Nonetheless, the hypotheses that our insomnia sample had higher anxiety levels than did our normal sleepers sample, and that higher levels of anxiety in both samples could have accounted for greater movement and lower sleep quality cannot be answered with our data. What we can surmise from our results is that perceived sleep quality may be related to the amount of movement that takes place while the individual is in bed attempting to fall asleep. This idea may open up a new research avenue about objective correlates of sleep quality that could not be pursued by PSG alone.
Of course, we should also note that our PSG measures of SE did perform like ACT SOL in discriminating our participant groups and nights they assigned distinctive subjective ratings. As is commonly recognized, SE is a composite measure that considers the balance between sleep time and wake time during the designated sleep period. In other words, this PSG-derived measure serves as an overall index of sleep consolidation and, thus, likely reflects group and night-to-night differences in qualitative aspects of sleep. It would appear from our data that PSG-derived SE functions much better in this regard than SE values derived from ACT. This would imply that the individual components that constitute ACT SE do not function in concert to reflect the same sleep dimensions as does PSG SE. Additional studies seem needed to help determine how ACT SE can be best employed in characterizing the sleep of groups such as those included herein.
The ultimate advantage of ACT is the relative ease to which it can be used to assess sleep at home across many nights, away from the distorting influences of the laboratory.1 However, most actigraphic validation studies have been conducted in sleep laboratories, where the individual is under close supervision and control. These circumstances eliminate many potential artifacts and measurement errors that exist in natural settings. In keeping with this, Sadeh et al.1 suggested that the accuracy of ACT might be compromised in settings where individuals are free to sleep in their natural environment. Nevertheless, as shown by the present study, when ACT is used in the home setting, one can get a fairly accurate determination of sleep onset, wake time during the night, and sleep duration.
In reviewing our results, it is important to consider this study's limitations. We used data drawn from a convenience sample that participated in a larger study designed and powered to address markedly different objectives. Admittedly, our sample for this study was, at best, moderate in size. Hence, it could be argued that the observed nonsignificant differences comparing means from ACT and PSG could be the result of insufficient power to detect differences between these devices. Yet, another study with a more sizeable sample of insomnia sufferers (n = 57) reported findings that are broadly in keeping with ours.6 Additionally, our sample consisted of only young normal sleepers and non-clinical insomnia sufferers who presented to us as research volunteers. Whether our findings apply to normal sleepers in general, other age groups, and clinical samples of insomnia patients, remains to be determined. Although we screened all enrollees with PSG to rule out sleep apnea, our recording montage did not include the array of respiratory indices usually employed in diagnostic PSG. Consequently, it is possible that some of our participants suffered from occult sleep disordered breathing rather than the primary insomnia diagnosis they were assigned. Another factor that precludes the generalization of these results is the algorithm used to score the data. The present study used the default scoring algorithm of a particular ACT device. However, it is possible that other scoring algorithms could yield different estimates of sleep/wake variables. Sadeh et al.,35 for example, have reported such results. In addition, it may be that an algorithm appropriate for young insomniacs may not be appropriate for elderly ones. Thus, replications of this study with clinical insomnia sufferers, other age groups, and other ACTs utilizing alternative algorithms may be useful. Despite such limitations, our findings suggest that ACT may provide informative data about the sleep of insomnia sufferers and normal sleepers in their usual sleep environments.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Edinger has received research support from Philips Respironics and Helicor Inc. and has consulted for Philips Respironics and Kingsdown Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by the Department of Veterans Affairs Merit Review Program, Grant # 0009 (Edinger: PI). M. Montserrat Sánchez-Ortuño was supported by a research fellowship award from Fundación Séneca, Murcia, Spain.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veteran Affairs.
REFERENCES
- 1.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 3.Coates TJ, Killen JD, George J, et al. Discriminating good sleepers from insomniacs using all-night polysomnograms conducted at home. J Nerv Ment Dis. 1982;170:224–30. doi: 10.1097/00005053-198204000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 5.Vallieres A, Morin CM. Actigraphy in the assessment of insomnia. Sleep. 2003;26:902–6. doi: 10.1093/sleep/26.7.902. [DOI] [PubMed] [Google Scholar]
- 6.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 7.Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–8. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 8.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 9.Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001;24:957–65. doi: 10.1093/sleep/24.8.957. [DOI] [PubMed] [Google Scholar]
- 10.Blood ML, Sack RL, Percy DC, Pen JC. A comparison of sleep detection by wrist actigraphy, behavioral response, and polysomnography. Sleep. 1997;20:388–95. [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 12.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems of sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 13.Tryon WW. Nocturnal activity and sleep assessment. Clini Psychol Rev . 1996;16:197–213. [Google Scholar]
- 14.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 15.Hauri PJ. Evaluation of a sleep switch device. Sleep. 1999;22:1110–7. doi: 10.1093/sleep/22.8.1110. [DOI] [PubMed] [Google Scholar]
- 16.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JBW, Gibbons M, First MB. Instruction manual for the structured clinical interview for DSM-IV (SCID-IV) New York: Biometrics Research Department, New York Psychiatric Institute; 1996. SCID 1996 Revision. [Google Scholar]
- 18.Schramm E, Hohagen F, Grasshoff U, et al. Test-retest reliability and validity of the structured interview for sleep disorders according to DSM-III-R. Am J Psychiatry. 1993;150:867–72. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Phillipson EA, Remmers JE. American Thoracic Society Consensus Conference on Indications and Standards for Cardiopulmonary Sleep Studies. Am Rev Respir Dis. 1989;139:559–68. doi: 10.1164/ajrccm/139.2.559. [DOI] [PubMed] [Google Scholar]
- 22.Coleman R. Periodic movements in sleep (nocturnal mioclonus) and restless leg syndrome. In: Guillerminault C, editor. Sleeping and waking disorders: Indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 265–95. [Google Scholar]
- 23.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 27.Millsap RE, Zalkind SS, Xenos T. Quick-reference tables to determine the significance of the difference between two correlation coefficients from two independent samples. Educ Psychol Meas. 1990;50:297–307. [Google Scholar]
- 28.Statistical Software System [computer program]. Version 9.1. Cary, NC: SAS Institute; 2006. [Google Scholar]
- 29.Jean-Louis G, von Gizycki H, Zizi F, et al. Determination of sleep and wakefulness with the actigraph data analysis software (ADAS) Sleep. 1996;19:739–43. [PubMed] [Google Scholar]
- 30.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Chambers MJ. Actigraphy and insomnia: a closer look. Part 1. Sleep. 1994;17:405–8. discussion 8-10. [PubMed] [Google Scholar]
- 32.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol . 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 33.van Hilten JJ, Middelkoop HA, Braat EA, et al. Nocturnal activity and immobility across aging (50-98 years) in healthy persons. J Am Geriatr Soc. 1993;41:837–41. doi: 10.1111/j.1532-5415.1993.tb06180.x. [DOI] [PubMed] [Google Scholar]
- 34.Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety and sleep architecture: a polysomnographic investigation. Sleep. 1997;20:370–6. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- 35.Sadeh A, Alster J, Urbach D, Lavie P. Actigraphically based autonomic bedtime sleep-wake scoring validity and clinical applications. J Amb Monitor. 1989;2:209–16. [Google Scholar]