Abstract
Objective:
To clarify the association of reported nightmare recall with polysomnographically defined obstructive sleep apnea (OSA) in a sleep laboratory population.
Methods:
This study included 393 individuals undergoing clinical polysomnography including a general intake questionnaire with questions on dream and nightmare recall frequency. Mean age was 50.5 and a range of 13 to 82 years, with 33% of the sample female and 67% male. Reported dream and nightmare recall were classified as infrequent when reported at less than once a month, or frequent when reported at a frequency greater than once per week.
Results:
Mean Apnea-hypopnea Index AHI was 34.9 (std. 32.0) indicating a high frequency of severe (AHI > 30) OSA in this clinical study population. Both AHI and Apnea Index (AI) were significantly higher (p = 0.000) for the grouping reporting infrequent nightmare recall. As the AHI score increased, the percent of participants with frequent nightmare recall decreased linearly.
Conclusion:
Patients with higher AHI report a lower nightmare frequency, indicating that significant OSA suppresses the cognitive experience of nightmare recall. Depressed nightmare recall may occur secondary to the REMS suppression know to occur in patients with significant OSA.
Citation:
Pagel JF; Kwiatkowski C. The nightmares of sleep apnea: nightmare frequency declines with increasing apnea hypopnea index. J Clin Sleep Med 2010;6(1):69-73.
Keywords: Nightmare, obstructive sleep apnea, dream, recall, REMS
The nightmare is defined as a disturbing mental experience that generally occurs during REM sleep and often results in awakening.1 The typical nightmare is a coherent dream sequence that seems real and becomes increasingly more disturbing as it unfolds. The negative emotions characterizing nightmares usually involve anxiety, fear or terror but also include anger, rage, embarrassment, and disgust. Nightmares have also been differentiated from other intense dreams by the characteristic of dream imagery expressed as an unmitigated perception of external reality.2,3 Nightmare content most often focuses on imminent physical danger to the individual (e.g., threat of attack, falling, injury, death) but may also involve aggression toward others, potential personal failures, and other distressing themes such as suffocation. Dream and nightmare recall frequency can be assessed by retrospective and longitudinal questionnaires, nocturnal REM sleep, awakenings, and by diaries. Studies designed to compare different assessments of dream and nightmare recall have generally found a remarkably high correlation between assessment methods for dream recall frequency with questionnaire reports of dream and nightmare recall having been shown to have longitudinal consistency and retest reliability.4,5
Five to eight percent of the general population reports a current problem with nightmares.6,7 In some clinical populations including insomniacs, nightmares are reported at much higher frequencies.8–10 Among individuals presenting at sleep laboratory facilities, nightmares are reported as a salient complaint by 16% of patients and positively correlated with reported lower sleep quality, and worse sleep and medical outcomes.11
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study was designed in an attempt to clarify the association of reported nightmare recall with polysomnographically defined OSA in the sleep laboratory population. This is among the first large studies to address both reported dream and nightmare recall frequency in a polysomnography evaluated study population including a high percentage of patients with severe OSA.
Study Impact: Sleep laboratory patients with more severe OSA, as based on higher AHI, report significantly lower nightmare frequency, indicating that OSA suppresses the cognitive experience of nightmare recall, an effect occurring independently of OSA effects on reported dream recall frequency. This effect is likely secondary to OSA induced REMS suppression emphasizing differences between nightmares that are REMS associated parasomnias, and dreaming that can be reported from all stages of sleep.
Nightmare Association with Sleep Apnea
Several lines of evidence suggest that obstructive sleep apnea is a diagnosis potentially associated with higher nightmare frequency. Somatic experiences are often incorporated into dream reports. As early as 1958, Dement and Wolpert reported the incorporation of exogenous stimuli (auditory tones, flashing lights, and cold water spray) into dream content.12 This finding of the incorporation of somatosensory stimulation into dream content has been replicated in several studies.13–15 Patient groupings with sleep paralysis and those with excessive perspiration are more likely to have dream content reflecting these symptoms.16 It would be surprising if patients experiencing the most physiologically disruptive of sleep disorders (OSA) did not include those experiences in their dreaming.
Patients with sleep apnea can report intense nightmares on waking from apneic events. Anecdotal examples from the author's sleep medicine practice include:
“I am buried under the sand and fighting my way to a surface that I can't seem to reach. I wake gasping for air.”
“I'm deep under water that is getting darker and darker. I'm holding my breath, but I can't any longer. As I breathe in water, I try to scream, but there's no sound. I wake up in a sweat.”
“I've been put in the refrigerator into a stoppered flask, formed to the shape of the glass. I'm turning darker by the minute, and then I wake. I'm terribly afraid when I awake.”
Based on clinical experience, such apnea related nightmares may be uncommon.17 However, studies have suggested that severe sleep apnea can present with dream enacting behaviors and unpleasant dreams, symptoms that can be eliminated with treatment with continuous positive airway pressure (CPAP).18 One small study (N = 20) reported that reported violent/highly anxious dreams in patients with severe OSA disappeared when assessed during REMS waking in the sleep laboratory in study patients treated for 2 years with CPAP.19 Wood et al. described a higher frequency of nightmares reported by patients with diagnoses of asthma and chronic obstructive pulmonary disease.20 Gross and Lavie found that dreams are more likely to be reported after apneas and these dreams were more likely to have negative content than the dreams of a control population.21 McFarland and Wilson reported that patients with waking respiratory problems were more likely to dream of suffocating and choking than other sleep laboratory patients; however, this finding was not present in patients with sleep apnea.16
Frequent nightmares are the most common symptom of posttraumatic stress disorder (PTSD).22–24 Sleep apnea has been reported to affect up to 56% of PTSD patients.25 Krakow et al .reported that 90% of a population of sexual assault survivors with PTSD have presumptive sleep disordered breathing based on symptomatology.26 A smaller study by the same authors utilizing standard polysomnography found significant OSA in 40 of 44 crime victims suffering from PTSD.27 Individuals reporting recurrent nightmares diagnosed with mild OSA based on apnea screening have been reported to have a significant improvement in both sleep and daytime well being with treatment of their OSA with CPAP.28 Nightmares have been proposed as symptomatic of a disordered emotional processing system in patients with PTSD.29
Studies Suggesting OSA Is Associated with Decreased Dream and Nightmare Recall
Several studies have suggested that OSA patients may report lower dream recall frequency. Pagel and Vann reported both a decline in dream recall frequency and use in waking behaviors by patients with OSA.30 Authors from the same sleep laboratory have reported that individuals reporting an absolute lack of dream recall are more likely to have OSA.31 This finding was postulated to be due to the effects of OSA induced sleepiness on dream recall, based on studies demonstrating that the level of arousability has a positive correlation with reported nightmare frequency, and the finding that individuals with shorter sleep latencies on multiple sleep latency testing report lower frequency of dream recall.32
Hicks and Bautisa found no correlation between reported snoring and nightmare frequency.33 Schredl et al., in a study of patients with severe apnea (mean respiratory disturbance index [RDI] 34.9) found a significant negative correlation between RDI and reported dream and nightmare recall frequency.34 A later study by the same author35 indicted that questionnaire reported pauses in breathing were associated with increased nightmare frequency. MacFarlane and Wilson found no increased incidence of dreams of choking and suffocation among patients with sleep apnea.16 In a previous study utilizing full-night polysomnography in the sleep laboratory and excluding spilt night studies, both dream and nightmare recall were nonsignificantly reduced in individuals with AHI > 15.10
This study was designed in the attempt to clarify the association of reported nightmare recall with polysomnographically defined OSA in the sleep laboratory population. The literature is supportive of 2 mutually exclusive hypotheses. Either:
patients with higher AHI and thus more significant OSA will report higher levels of nightmare frequency, suggesting that the physiological experience of sleep apnea induces the experience of nightmares, or
patients with higher AHI will report lower nightmare frequency, indicating that OSA suppresses the cognitive experience of nightmare recall for most patients.
This study includes a sample of sufficient size and variable severity of OSA so as to exclude at least one of these hypotheses as to the association of nightmares with OSA.
METHODOLOGY
This study included a consecutive series of 393 patients undergoing polysomnography (PSG) at an American Academy of Sleep Medicine (AASM) accredited sleep laboratory over a 2-year period. More than 90% of patients referred to this laboratory are being evaluated for potential OSA, with less than 10% of patients referred for potential narcolepsy, parasomnias, or nocturnal seizure disorders. In an effort to include patients with more severe apnea, this study included patients undergoing split-night studies (204/393 [51.9%]) in which OSA was diagnosed during a diagnostic portion of ≥ 120 min of untreated sleep and then treated with CPAP during the night of polysomnography. The PSG protocol utilized in this study included: EEG (4 leads, 2 channels), EOG (2 channels), chin and leg EMG, EEG, chest and abdominal strain gauges, snore microphone, positional marking, video taping, transcutaneous pulse oximetry, and oral/nasal airflow recorded by pressure cannula. Apnea hypopnea index (AHI) was based on number of apneas and hypopneas/ hour. Apneas are episodes > 10 sec characterized by total cessation of oral-nasal airflow. Apneas per hour are reported as an apnea index (AI). Hypopneas are respiratory events > 10 sec associated with 4% oxygen desaturation (O2 sat) and a reduction in airflow signal ≥ 30%. Periodic limb movements were scored based on AASM defined criteria and reported for analysis as number of events per hour in a periodic limb movement index (PLMI).
This retrospective noninvasive study utilizing a de-identified data base was approved by an institutional review board. Patients completed a general intake questionnaire on arrival in the sleep laboratory. Questions on dream and nightmare recall frequency were incorporated into a general sleep questionnaire in an effort to avoid the increase in dream recall frequency seen when questionnaires are specifically oriented towards dreaming.5 Dream and nightmare recall frequency were rated utilizing a previously validated Likert scale varying from 1 = never, 2 = monthly, 3 = weekly, 4 = twice weekly, to 5 = nightly.10,30,36 Reported dream and nightmare recall frequency was divided into the following categorical descriptions for statistical analysis: infrequent (including responses 1 = never and 2 = monthly), and frequent (including responses 3 = weekly, 4 = twice weekly, and 5 = nightly). Statistical analyses were conducted using SPSS version 16.0. Standard descriptive statistics were run on all variables. A Fisher exact χ2 test was performed to compare dream and nightmare recall. Normal log transformations were run on AHI, AI, and PLMSI to help normalize the data. For the dream recall and nightmare recall variables, independent sample t-tests were performed to compare those with frequent recall to those with infrequent recall. Pearson χ>2 was run to compare recall by 4 groups within AHI. Significance of all statistical tests was set at p < 0.05.
RESULTS
Age was normally distributed, with a mean of 50.5 years and a range of 13 to 82 years. With regard to gender, 33% of the sample was female and 67% was male. The mean AHI was 34.9 (SD = 32.0) indicating a high frequency of severe (AHI > 30) OSA in this clinical study population. Low oxygen saturation was normally distributed, with a mean of 71.8 (SD = 10.4), which was potentially affected by the laboratory location at altitude (4500 ft.). The mean AI was 17.1 (SD = 25.3) and the mean PLMSI was 11.3 (SD = 20.3). With regard to dream and nightmare recall, 205 patients (52%) reported frequent dream recall (at least weekly) and 134 patients (34%) reported frequent nightmare recall. A χ2 test confirmed that dream recall and nightmare recall are not associated (χ2 = 0.31, n = 350, p = 0.33).
Table 1 shows the results of independent sample t-tests to compare frequency of recall by AHI, Low O2 Sat, AI, and PLMSI. Both AHI and AI were significantly higher (p < 0.001) for the grouping reporting infrequent nightmare recall. PLMI was also significantly higher (p = 0.034) among those reporting infrequent nightmare recall. Low O2 Sat means were not significantly different according to nightmare recall. None of these variables were noted to significantly affect the reported frequency of dream recall in this study.
Table 1.
Recall frequency by sleep variables
| Variable | |||
|---|---|---|---|
| Nightmare Recall (n = 394) | Mean (SD) | Test statistic1 | p-value |
| AHI | 6.27 | <0.001 | |
| Infrequent recall | 40.3 (32.1) | ||
| Frequent recall | 24.6 (29.3) | ||
| AI | 6.64 | <0.001 | |
| Infrequent recall | 21.0 (27.4) | ||
| Frequent recall | 9.4 (18.3) | ||
| PLMI | 2.13 | 0.034 | |
| Infrequent recall | 12.5 (20.7) | ||
| Frequent recall | 9.1 (19.5) | ||
| Low O2 Sat | 1.42 | 0.16 | |
| Infrequent recall | 71.3 (10.3) | ||
| Frequent recall | 72.8 (10.6) | ||
| Dream Recall (n = 350) | |||
| AHI | 0.03 | 0.978 | |
| Infrequent recall | 32.8 (29.1) | ||
| Frequent recall | 35.6 (34.2) | ||
| AI | 1.09 | 0.276 | |
| Infrequent recall | 18.0 (27.3) | ||
| Frequent recall | 15.5 (24.0) | ||
| PLMI | 1.66 | 0.098 | |
| Infrequent recall | 12.8 (20.8) | ||
| Frequent recall | 9.8 (18.7) | ||
| Low O2 Sat | 0.31 | 0.76 | |
| Infrequent recall | 72.1 (9.8) | ||
| Frequent recall | 71.8 (10.6) |
Independent sample t-test. Test statistics and p-values are reported for tests using log transformed values for AHI, AI, and PLMSI, although actual means and standard deviations are reported.
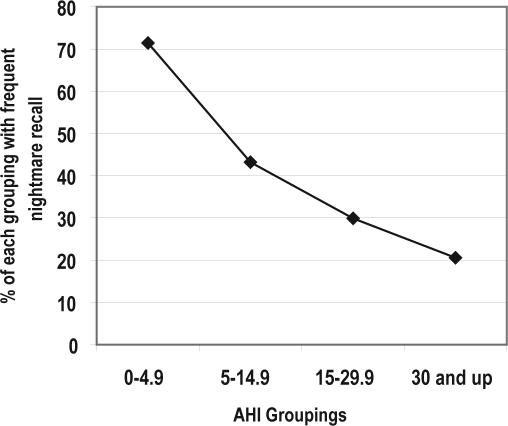
Chi-square analysis was run to compare recall of dreams and nightmares across 4 AHI groups (< 5.0, 5.0–14.9, 15–29.9, and > 30). There was no difference in the likelihood of recalling dreams across the 4 groups (p = 0.834). The results of comparing nightmare recall by the 4 AHI groups are presented in Table 2 and in Figure 1. Of individuals with an AHI < 5, 71.4% reported nightmares occurring more than once/week. As the AHI score increased, the percent of participants with frequent nightmare recall decreased linearly. Reported nightmare frequency was compared between high and low recall groupings for both the CPAP treated group and the no-CPAP group. Among those receiving CPAP, mean AHI in patients with frequent nightmare recall was 49.11 and mean AHI was 53.96 among those with infrequent nightmare recall (a nonsignificant difference). However, among patients who did not receive CPAP, AHI was significantly greater (p = 0.001) among those with infrequent recall (mean AHI = 19.81) compared to those with frequent recall (mean AHI 10.90).
Table 2.
Nightmare recall by four AHI groupings
| Number | Percent with frequent recall | Chi-square value | p-value | |
|---|---|---|---|---|
| AHI | 48.17 | < 0.001 | ||
| 0 through 4.9 | 49 | 71.4 | ||
| 5 through 14.9 | 88 | 43.2 | ||
| 15 through 29.9 | 87 | 29.9 | ||
| 30 through high | 170 | 20.6 |
Figure 1.
Nightmare recall by AHI groupings
DISCUSSION
This study clearly supports the hypothesis that sleep laboratory patients with higher AHI and AI report a significantly lower frequency of nightmares. This finding indicates that OSA suppresses the cognitive experience of nightmare recall. This effect on nightmare recall occurs independently of OSA effects on reported dream recall frequency. This is an important and somewhat surprising finding, given studies supporting the alternative contrary hypothesis, and may in part be due to this being one of the first large studies to address both reported dream and nightmare recall frequency in a polysomnography evaluated study population including a high percentage of patients with severe OSA. It should also be noted that the one other large polysomnography-based study including patients with severe OSA also showed a significant decline in nightmare recall in the high RDI grouping.34
Dream recall is known to increase with dream saliency—the greater novelty, bizarreness, affectiveness, or intensity of an experience—and with waking from a dream experience.3,37 Salience and waking from the dream are characteristic of nightmares. Yet this study clearly demonstrates that increasingly severe OSA has a much greater negative effect on reported nightmare recall frequency than it does on reported dream recall.
This finding, that worsening OSA results in a significant decline in reported nightmare recall frequency could potentially be secondary to the effects of OSA in inducing daytime sleepiness. Patients who are more difficult to arouse report lower nightmare frequency.33 The possibility that OSA associated sleepiness is contributing to lower nightmare recall frequency is also supported by the finding that individuals with shorter sleep latencies on multiple sleep latency testing report lower frequency of dream recall.32 However, daytime sleepiness is not present in all patients with OSA, with studies reporting excessive sleepiness in 15.5% to 22.5% of middle-aged OSA patients.38
In addition to daytime sleepiness, OSA is known to result in cognitive deficits that include declines in working memory and deficits in frontal cortex executive functions.39,40 The cognitive process of dream incorporation into waking behavior has been proposed to be a frontal cortex-based executive function.41 However, cognitive deficits are not present in all OSA patients and have been difficult to describe consistently for the disease process.42 Again, the potential effects of OSA induced cognitive impairment do not explain why worsening OSA should significantly affect nightmare as opposed to dream recall.
While dreaming occurs throughout sleep, nightmares are generally described as a REMS associated parasomnia.1,43 REMS is the sleep stage most susceptible to abnormal breathing events, with OSA selectively suppressing REMS.44 It is the authors' suspicion that diminished REMS in OSA patients is the basis for our finding of decreased frequency of reported nightmares when compared to dreaming. This suggestion could be clarified by further studies addressing the association of REMS percentages and times with dream and nightmare recall in OSA patients. An increase in nightmare recall would be expected as well in OSA patients experiencing increased REMS and restoration of normal sleep after therapy with CPAP. The effects of OSA on reported nightmare recall suggests that studies demonstrating an association of PTSD nightmares with OSA may reflect at least in part dysfunctions of an emotional processing system involving both nightmares and REMS.25–29 The potential finding that diminished REMS in OSA patients leading to fewer reported nightmares supports potential functional roles for both REMS and nightmares in emotional neuroregulation.29
Insomniacs are known to have higher nightmare frequency than the general population.8,9–10 The low AHI grouping in this study (the grouping least likely to be treated with CPAP) could include a higher proportion of insomniacs referred to the sleep center for polysomnographic evaluation, potentially introducing a selection variable that could account for the lower proportion of nightmares reported by higher AHI patients. Interestingly a significant difference in nightmare frequency based on AHI was even more apparent in the lower AHI – non-CPAP grouping likely to include a higher percentage of insomniacs. This finding indicates that even in this lower AHI grouping not treated in laboratory with CPAP, worsening OSA as characterized by higher AHI had significant negative effects on reported nightmare recall frequency. Other possible confounding variables potentially affecting nightmare recall frequency including PTSD, mood disorder/depression, and medication use are not addressed in this study.
CONCLUSION
Sleep laboratory patients with more severe OSA, as based on higher AHI, report a significantly lower frequency of nightmares. This finding indicates that OSA suppresses the cognitive experience of nightmare recall, an effect that occurs independently of OSA effects on reported dream recall frequency. It is postulated that this effect on nightmare recall occurs secondary to the REMS suppression know to occur in patients with significant OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Pagel JF, Nielsen T. The International Classification of Sleep Disorders – Diagnostic and Coding Manual (ICD-2) Westchester, IL: American Academy of Sleep Medicine; 2005. Parasomnias: recurrent nightmares. [Google Scholar]
- 2.Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep. 2002;25:205–12. [PubMed] [Google Scholar]
- 3.Kuiken D, Sikora S. The impact of dreams on waking thoughts and feelings. In: Moffitt A, Kramer M, Hoffman R, editors. The functions of dreaming. Albany, NY: State University of New York Press; 1993. pp. 419–76. [Google Scholar]
- 4.Schredl M. Questionnaires and diaries as research instruments in dream research: methodological issues. Dreaming. 2002;12:17–26. [Google Scholar]
- 5.Schredl M. Reliability in dream research: a methodological note. Consciousn Cogn. 2001;10:496–502. doi: 10.1006/ccog.2001.0522. [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–62. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 7.Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airway diseases. Chest. 1987;91:540–6. doi: 10.1378/chest.91.4.540. [DOI] [PubMed] [Google Scholar]
- 8.Wood JM, Bootzin RR. The prevalence of nightmares and their independence from anxiety. J Abnorm Psychol. 1990;99:64–8. doi: 10.1037//0021-843x.99.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Schredl M, Schafer G, Weber B, Heuser I. Dreaming and insomnia: Dream recall and dream content of patients with insomnia. J Sleep Res. 1998;7:191–8. doi: 10.1046/j.1365-2869.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 10.Pagel JF, Shocknasse S. Dreaming and insomnia: Polysomnographic correlates of reported dream recall frequency. Dreaming. 2007;17:140–51. [Google Scholar]
- 11.Krakow B. Nightmare complaints in treatment-seeking patients in clinical sleep medicine settings: diagnostic and treatment implications. Sleep. 2006;29:1313–9. doi: 10.1093/sleep/29.10.1313. [DOI] [PubMed] [Google Scholar]
- 12.Dement W, Wolpert E. The relation of eye movements, body motility, and external stimuli to dream content. J Exp Psychol. 1958;55:543–53. doi: 10.1037/h0040031. [DOI] [PubMed] [Google Scholar]
- 13.Berger R. Experimental modification of dream content by meaningful verbal stimuli. Br J Psychiatry. 1963;109:722–40. doi: 10.1192/bjp.109.463.722. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Foulkes D. Effects of visual stimuli on dream content. Percept Mot Skills. 1964;20:1149–60. doi: 10.2466/pms.1965.20.3c.1149. [DOI] [PubMed] [Google Scholar]
- 15.Koulack D. Effects of somatosensory stimulation on dream content. Arch Gen Psychiatry. 1969;20:718025. doi: 10.1001/archpsyc.1969.01740180102010. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane J, Wilson T. A relationship between nightmare content and somatic stimuli in a sleep-disordered population: a preliminary study. Dreaming. 2006;16:53–59. [Google Scholar]
- 17.Hartmann E. The Nightmare. New York: Basic Books; 1884. [Google Scholar]
- 18.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco E, Santamaria J, Iranzo A, et al. Changes in dreaming by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res. 2006;15:430–6. doi: 10.1111/j.1365-2869.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 20.Wood J, Bootzin R, Quan S, Klink M. Prevalence of nightmares among patients with asthma and chronic obstructive airways disease. Dreaming. 1993;3:231–41. [Google Scholar]
- 21.Gross M, Lavie P. Dreams in sleep apnea patients. Dreaming. 1994;4:195–204. [Google Scholar]
- 22.Fawzi MC, Pham T, Lin L, et al. The validity of posttraumatic stress disorder among Vietnamese refugees. J Trauma Stress. 1997;10:101–8. doi: 10.1023/a:1024812514796. [DOI] [PubMed] [Google Scholar]
- 23.Grillon C. Baseline startle amplitude and pre-pulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64:169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- 24.Yehuda R, McFarlane AC. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. Am J Psychiatry. 1995;152:1705–13. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]
- 25.De Groen J, Op den Velde W, Hovens J, Falger P, Schouten E, von Duijn H. Snoring and anxiety dreams. Sleep. 1993;16:35–6. [PubMed] [Google Scholar]
- 26.Krakow B, Melendrez D, Johnston L, et al. Sleep disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190:442–52. doi: 10.1097/00005053-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49:948–53. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 28.Krakow B, Lowry C, Germaine A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49:291–8. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 29.Levin R, Nielsen T. Disturbed dreaming, posttraumatic stress disorder and affect distress: a review and neurocognitive model. Psychol Bull. 2007;133:482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 30.Pagel J, Vann B. Polysomnographic correlates of reported dreaming: negative correlation of RDI with dreaming effects on waking activity. APSS Abstracts. 1995 [Google Scholar]
- 31.Pagel JF. Non-dreamers. Sleep Med. 2003;4:235–41. doi: 10.1016/s1389-9457(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 32.Myers P, Pagel JF. The effects of daytime sleepiness and sleep onset REMS period (SORP) on reported dream recall. Sleep. 2001;24:A183. [Google Scholar]
- 33.Hicks R, Fortin E, Brassington G. Arousability and dreaming. Dreaming. 2002;12:135–40. [Google Scholar]
- 34.Schredl M, Schmitt J, Hein G, Schmoll T, Eller S, Haaf J. Nightmares and oxygen desaturations: is sleep apnea related to heightened nightmare frequency? Sleep Breath. 2006;10:203–9. doi: 10.1007/s11325-006-0076-8. [DOI] [PubMed] [Google Scholar]
- 35.Schredl M. Snoring, breathing pauses, and nightmares. Percept Mot Skills. 2008;106:690–2. doi: 10.2466/pms.106.3.690-692. [DOI] [PubMed] [Google Scholar]
- 36.Pagel JF, Vann B. The effects of dreaming on awake behavior. Dreaming. 1992;2:229–37. [Google Scholar]
- 37.Zadra A, Donderi DC. Prevalence of nightmares and bad dreams and their relation to psychological well-being. J Abnorm Psychol. 2000;109:210–9. [PubMed] [Google Scholar]
- 38.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–35. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 39.Lis S, Krieger S, Hennig D, et al. Executive functions and cognitive subprocesses in patients with obstructive sleep apnoea. J Sleep Res. 2008;17:271–80. doi: 10.1111/j.1365-2869.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 40.Felver-Gant J, Bruce A, Zimmerman M, et al. Working memory in obstructive sleep apnea: construct validity and treatment effects. J Clin Sleep Med. 2007;3:589–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Pagel J, Vann B. Cognitive organization of dream mentation - evidence for correlation with memory processing systems. ASDA Abstracts. 1997 [Google Scholar]
- 42.Morrell M, Twigg G. Neural consequences of sleep disordered breathing: the role of intermittent hypoxia. Adv Exp Med Biol. 2006;588:75–88. doi: 10.1007/978-0-387-34817-9_8. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen TA, Zadra A. Dreaming disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia, PA: WB Saunders; 2000. pp. 753–72. [Google Scholar]
- 44.Sanders M, Givelber R. Overview of obstructive sleep apnea in adults. In: Lee-Chiong T, editor. Sleep: a comprehensive textbook. Hoboken, NJ: John Wiley & Sons; 2006. p. 235. [Google Scholar]