Abstract
Background
The endothelium of normal aortic valves expresses different phenotypes on the aortic and ventricular sides. On the aortic side, which is susceptible to aortic valve sclerosis (AVS), there is a balanced co-expression of both pro-pathological and protective pathways. Side-specific global gene expression can address endothelial phenotype balance in early AVS.
Methods and Results
Adult male swine were fed a hypercholesterolemic (HC) or an isocaloric normal diet for 2-week and 6-month periods. HC induced localized lipid insudation confined to the aortic side of the leaflet. Transcript profiling of valve endothelial populations showed that the susceptible aortic side was more sensitive to 2-weeks hypercholesterolemia than the ventricular side (1325 vs 87 genes differentially expressed). Greater sensitivity, however, was not evidence of dysfunctional phenotype. Instead, pathway analyses identified differential expression of caspase3-, PPAR&[gamma], TNF&[alpha]-, and NF&[kappa]B-related pathways that were consistent with a protective endothelial phenotype; this was confirmed at the protein level at 2 weeks and persisted at 6 months.
Conclusions
In a large animal model at high spatial resolution, endothelium on the patho-susceptible side of the aortic valve leaflet is responsive to hypercholesterolemia. Transcript profiles indicative of a protective phenotype were induced and persisted on the side prone to AVS.
Keywords: Valves, Endothelium, Cholesterol, Genes, Molecular Biology
Introduction
Degenerative aortic valve disease is the most common cause of valvular heart disease and accounts for most of the 95,000 valve procedures in the US each year.1 Aortic valve sclerosis (AVS), which marks the first step in degenerative aortic valve disease, is present in more than 25% of people older than 65 and greater than half the population over 85.2 The pathology ranges from diffuse thickening to focal areas of calcific sclerosis which can progress to valve stenosis and compromised valvular function. Recent research has shown AVS to be a complex and active inflammatory process.3, 4 The pathogenesis is side-specific and occurs preferentially on the aortic side of the valve leaflets. The histology and composition of advanced lesions has been well characterized, but early AVS and the mechanisms that lead to lesion initiation and progression have received little attention. Better understanding of these early processes is crucial to finding either preventative options or therapeutic alternatives to valve replacement surgery.
AVS and arterial atherosclerosis share common systemic risk factors – hypercholesterolemia, smoking, hypertension, and male gender – and also an important local risk factor, flow disturbance, through which endothelial phenotype is linked to pathogenesis.5, 6 Endothelial dysfunction is a hallmark of arterial atherosclerosis and regulates many of the key processes integral to disease initiation and progression.7 The role of the endothelium in valvular disease, however, is not understood.4
There are few molecular studies of valve endothelium in situ. In vitro studies have shown that porcine aortic valve endothelial cells have distinct gene expression profiles compared to their arterial counterparts8 and, furthermore, may influence valvular interstitial cell phenotypes in co-culture.9 In vivo studies have mostly focused on endothelial signaling pathways during valve development.10 Early studies of valve development identified morphologic differences between the aortic (A) and ventricular (V) side endothelial cells and showed that hemodynamics played a role in patterning these differences.11, 12 More recently, the endothelial cells on the A and V sides of normal swine aortic valve leaflets were shown to have distinct phenotype profiles.6 In the A side endothelium of the leaflet, which is susceptible to calcific sclerosis, there was differential expression of genes that promote skeletal development and vascular calcification. Despite its pro-calcific phenotype, however, the A side endothelium of normal valves also expressed compensatory protective mechanisms, including an anti-inflammatory gene profile. Similar coexisting balances have been noted in endothelium located in atherosclerosis-susceptible regions of large arteries.5, 13
The coexistence of susceptible and protective endothelial phenotypes in the normal valve suggests a tenuous balance that preserves functional homeostasis and inhibits pathological processes. Here we report the use of a modest systemic insult (2 weeks hypercholesterolemia) in a swine model of early aortic valve disease to shift the existing equilibrium towards early pathology and to determine if there is side-specific differential sensitivity. A 2 week exposure was sufficient to affect both gene and protein phenotype changes, but brief enough to capture the transition from normal tissue to early pathology. A broad genomics-based approach, coupled with side-specific spatial resolution to probe endothelial populations, is necessary to begin to understand such a complex and multifactorial process. We found that the aortic side endothelium is markedly more sensitive to hypercholesterolemia than the ventricular side endothelium, specifically upregulating protective pathways including a PPAR&[gamma]-mediated program. Immunohistochemistry evidence suggests that these changes may be sustained at the protein level after 6-months of hypercholesterolemia.
Methods
Swine model and in vivo exposure to AVS risk factors
Please see www.ahajournals.org for Supplemental Materials. Briefly, aortic valves were harvested from mature castrated domestic male swine randomly assigned to a standard corn/soybean diet or an isocaloric diet high in fat (12%) and cholesterol (1.5%) for either 2 weeks or 6 months. Tissue from both the 2 week and 6 month cohorts were processed for histology and immunohistochemistry using standard techniques, and endothelium from the 2-week HC valves were processed for transcript profiling.
Aortic valve endothelial cell RNA microarray experiments
Endothelial cell RNA isolation procedures and bioinformatics are described in Supplemental Materials, and the genomic data are publicly available at ArrayExpress.
Results
Side-specific early pathological changes in swine model of early AVS
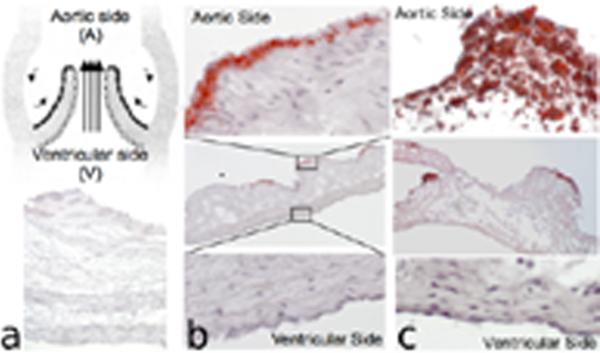
Initial signs of pathological change appeared on the aortic (A) side of valve leaflets following two weeks of hypercholesterolemia. Discrete regions of extracellular subendothelial lipid insudation and occasional early calcific nodules were restricted to the A side of the leaflet (Figure 1b, S1a). In contrast, no histological changes were observed on the ventricular side. CD11b/MAC1 and HAM 56 staining was negative (not shown), indicating the absence of macrophage and foam cell accumulation in areas of lipid deposition. These observations are consistent with evidence that lipoprotein particles associate with the extra-cellular matrix to form liposomes in the valve interstitium, often considered the first event in AVS,14 and indicate a shift from normal (but susceptible) tissue towards early pathology. Thus in a swine model, modest hypercholesterolemia for a limited period induced side-specific pathological changes consistent with that of early human AVS.
Figure 1.
Aortic valve schematic illustrating aortic (black) and ventricular (white) side endothelium (a,upper panel). Hematoxylin and Oil Red O staining shows no lipid insudation in normal valves (a,lower panel); aortic side extra-cellular lipid insudation at 2-weeks HC (b); and increasing extra- and intra-cellular lipid deposition on the aortic side at 6-months HC (c).
A longer exposure to hypercholesterolemia (6 months) produced similar, but more extensive, pathology including an increase in number and size of regions of lipid insudation (Figure 1c) and calcification (Figure S1b) restricted to the A side. In particular, there was greater extracellular lipid deposition and evidence of intracellular lipid deposition, but frank inflammation was not noted (See Supplemental Materials).
The side-specific endothelial responses that accompanied the initiation of early AVS (2 weeks) were investigated using broad gene expression analyses. Specifically, the susceptible A and protected V side endothelia of aortic valve leaflets of both normal (NC) and hypercholesterolemic (HC) male swine were profiled by microarray. Four transcript profile comparisons were conducted to analyze the effect of hypercholesterolemia in the context of valve sidedness. First, to confirm previous findings, a paired comparison of the A side endothelium to the V side endothelium was conducted in NC swine. Second, we separately examined the effects of hypercholesterolemia on each side of the valve. Finally, a paired comparison of the A side endothelium to the V side endothelium was conducted in HC swine.
Side-specific endothelial cell phenotypes in normocholesterolemia
In agreement with previous findings,6 many genes were differentially expressed between the A and V side endothelial cells of aortic valve leaflets from normal swine (see Supplemental Materials).
Heightened sensitivity of A-side endothelium to hypercholesterolemia
To investigate endothelial responses in early AVS, cells from each side of the valve leaflets were separately profiled in NC and HC swine. Each NC animal was randomly paired with an HC animal, and the A side endothelium of each NC animal was compared directly to the A side endothelium of a HC animal. Similarly, the V side endothelium of each NC animal was compared directly to the V side endothelium of a HC animal. The A side endothelium was markedly more responsive to hypercholesterolemia than was the V side. As shown in Table 1, hypercholesterolemia induced the differential expression of 1325 genes on the A side of the leaflet when compared with the normocholesterolemic state. In contrast, only 87 genes were differentially expressed by hypercholesterolemia on the V side.
Table 1.
Effects of hypercholesterolemia (HC) on endothelial cell gene expression on each side of the aortic valve. The number of genes differentially expressed on each side of the valve in HC relative to normocholesterolemic (NC) valves.
| Differential Endothelial Gene Expression: NC versus HC Swine | |||
|---|---|---|---|
| Up in HC | Number of Genes Down in HC | Total | |
| Aortic Side | 567 | 758 | 1325 |
| Ventricular Side | 82 | 5 | 87 |
Pathway analyses of hypercholesterolemia-induced differential gene expression in aortic (A) side endothelium indicate patho-protective responses
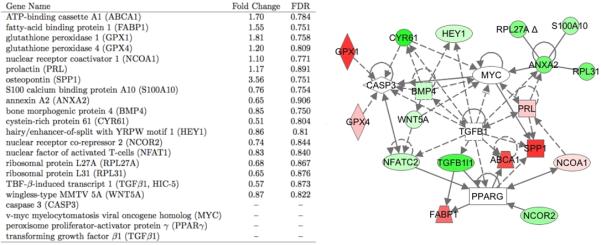
The response of the A side endothelium to hypercholesterolemia was examined using Ingenuity Pathway Analysis (IPA, as described in Supporting Materials). The 1325 genes differentially expressed by HC on the aortic side were interrogated for top-scoring networks, overrepresented canonical pathways, and functional categories. These analyses, in the context of the current AVS literature, identified a concise list of key differentially expressed genes (Figure 2). IPA then identified relationships between the genes based on either direct molecular or protein binding or indirect transcriptional or regulatory effects. Genes known to play a role in AVS pathology as well as genes involved in cellular proliferation, molecular transport, and lipid trafficking were differentially expressed and highly interconnected (Figure 2). A cluster of caspase 3 (CASP3) related genes was differentially expressed. These include downregulation of BMP4 in HC animals, an inflammatory mediator in endothelial cells capable of inducing ICAM expression.15, 16 BMP4 may play a role in aortic valve calcification17 and can also increase expression of cysteine-rich angiogenic inducer 61 (CYR61)18 which can in turn increase CASP3 activation.19 The downregulation of both genes is consistent with a decrease in CASP3 activation. Similarly, increases in glutathione peroxidase 1 and 4 (GPX1 and GPX4), potent anti-oxidants, both of which were upregulated in the HC animals, can decrease CASP3 activation.20, 21 A decrease in BMP4 expression and CASP3 activation indicates a protective, anti-apoptotic phenotype.22
Figure 2.
Pathway analysis of genes up- (red) and down- (green) regulated in HC relative to NC aminals on the aortic side revealed clusters of highly inter-connected CASP3, TGF&[beta], ANXA2, and PPAR&[gamma]-centric genes. Particularly prominent were hypercholerolemia-responsive upstream regulators and downstream targets of PPAR&[gamma].
Both upstream regulators and downstream targets of PPAR&[gamma] were differentially expressed (Figure 2). Nuclear receptor co-repressor 2 (NCOR2), a PPAR&[gamma] repressor, was downregulated, and PPAR&[gamma] activator nuclear receptor coactivator 1 (NCOA1) was upregulated. In the absence of ligand, PPAR&[gamma] recruits NCOR2, decreasing the transcription factor activity.23 Conversely, NCOA1 increases PPAR&[gamma] transcriptional activity.24 Several direct downstream targets of PPAR&[gamma] that are highly relevant to aortic valve pathology and cholesterol transport were the most highly upregulated genes in the HC animals. These included fatty-acid binding protein 1 (FABP1), which binds certain lipids and long-chain fatty acids and may be involved in their intracellular trafficking.25 PPAR&[gamma] has a complex relationship with FABP-1. It is known to bind FABP-1 promoter and increase transcription of the gene,26 but there is also evidence that FABP-1 may act as a PPAR&[gamma] ligand to regulate PPAR&[gamma] activation.27 PPAR&[gamma] is reported to increase expression of ATP-binding cassette A1 (ABCA1),28 which was also highly upregulated in the HC animals. Furthermore, PPAR&[gamma] has been shown to act on macrophages to decrease production of inflammatory mediator and increase oxidized LDL scavenger receptor in a lipid accumulation positive feedback loop.29 PPAR&[gamma] is an upstream regulator of SPP1 (osteopontin), which was the most highly upregulated gene and is known to have a protective, anti-calcific effect in the valve.30 The direction of differential expression of these genes is consistent with the induction of a protective PPAR&[gamma] phenotype in early AVS. Several other gene pathways, including ANXA2- and TGF&[beta]1-clusters, were differentially expressed in a similar manner consistent with a protective phenotype (See Supplemental Materials).
In contrast, gene expression in the V side endothelium was largely uninfluenced by hypercholesterolemia with only 87 non-specific genes differentially expressed (see Supplemental Materials)
Paired A side to V side comparisons of endothelial gene expression in hypercholesterolemia corroborates a protective A side response
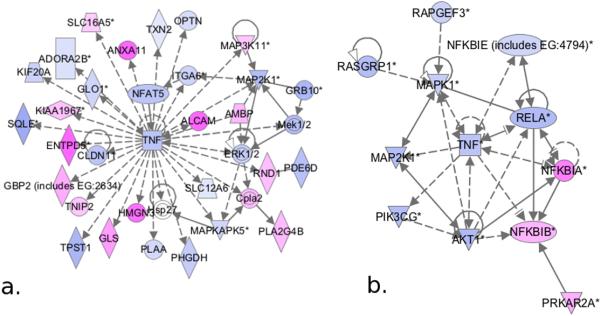
After examining the effect of HC on the A and V side endothelium separately as described above, paired comparisons of the A and V side phenotypes within each HC animal were conducted. Pathway analysis of 1638 genes differentially expressed (895 up- and 743 down-regulated) between the A and V sides of HC animals identified TNF&[alpha]-centric pathways as the top-scoring network. Hypercholesterolemia downregulated TNF&[alpha] on the A side and induced the differential expression of genes known to interact with TNF&[alpha], either directly at the protein level or through a transcriptional effect (Figure 3a). For example, the patterns of expression of squalene epoxidase, TNF&[alpha] inhibitor protein 3 interacting protein 2 (TNIP2), and cytosolic phospholipase A2(cPLA2) and its regulators was consistent with a protective phenotype on the A side (see Supplemental Materials).
Figure 3.
Pathway analysis of genes up- (pink) and down- (blue) regulated in A side endothelial cells relative to V side in aortic valves of HC swine. a. TNF&[alpha]-centric pathways were the highest scoring network of differentially expressed genes. b. TNF&[alpha] and NF&[kappa]B pathways were downregulated by hypercholesterolemia.
In addition to IPA network analysis, canonical pathway analysis of the genes differentially expressed between the A and V sides in HC animals was performed (Figure 3b). G-protein coupled receptor signaling, which includes NF&[kappa]B-related and mitogen-activated protein kinase- (MAPK) related genes, was identified as the most overrepresented canonical pathway. Inflammatory regulators Re1A and TNF&[alpha] were downregulated and NF&[kappa]B inhibitors IA and IB were upregulated on the A side, suggesting a shift towards an anti-inflammatory state on the A side endothelium. Re1A is the predominant and most active NF&[kappa]B species in endothelial cells and is inhibited by I&[kappa]Ba and I&[kappa]Bb.31
In summary, both the network and canonical pathway analyses suggest enhanced anti-inflammatory and anti-apoptosis profiles on the AVS-susceptible A side relative to the V side, consistent with the induction of a protective endothelium during hypercholesterolemia. Osteopontin (SPP1) also emerged from this analysis as the most highly differentially expressed gene, corroborating the protective phenotype on the A side relative to the V side in HC animals.
In situ protein expression of aortic valve endothelium
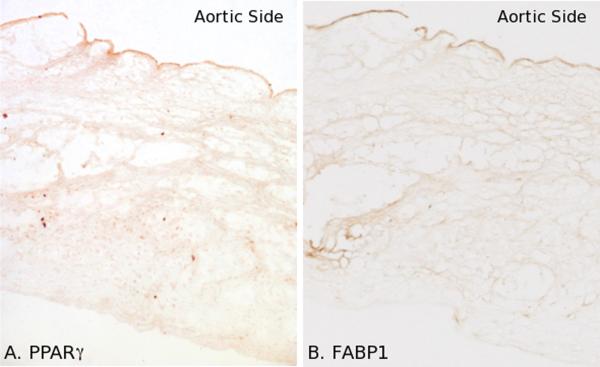
The spatial distribution of protein products was examined by immunohistochemistry (Figures 4 and 5). Following 2 weeks of HC, the endothelium expressed increased amounts of PPAR&[gamma]. Expression was confined to the aortic side and was often localized in endothelial nuclei, suggesting PPAR&[gamma] activation (Figure 4a). FABP1, a target of PPAR&[gamma], was similarly expressed (adjacent section, Figure 4b). Both PPAR&[gamma] and FABP1 expression was heterogeneous within the A side.
Figure 4.
Side-specific expression of endothelial proteins in aortic valves exposed to HC for 2 weeks. a. PPAR&[gamma] expression was heterogeneous and confined to the A side endothelium. b. FABP1 was expressed in A side endothelial cells in a heterogeneous distribution.
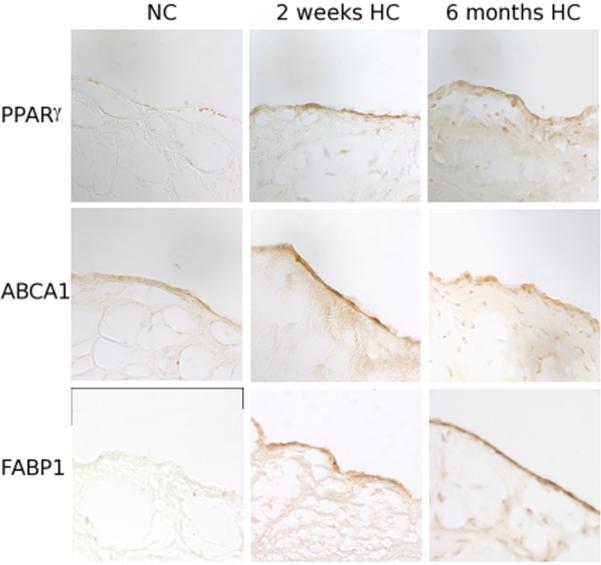
Figure 5.
Aortic side expression of PPAR&[gamma] pathway proteins in normal (NC), 2-week HC, and 6-month HC valves. There is minimal ABCA1, PPAR&[gamma], and FABP1 expression in normal valves. Upon exposure to HC, expression of these proteins is upregulated on the A side endothelium by 2-weeks and persisted at 6-months.
Normal swine expressed minimal amounts of PPAR&[gamma] in the endothelium (Figure 5). IHC of the A side following 2 weeks of HC, however, showed endothelial expression to be greatly enhanced and expression persisted at 6 months (Figure 5). Similarly, the direct downstream targets of PPAR&[gamma], including ABCA1 and FABP1 were minimally expressed in normal swine aortic valves. However, as with PPAR&[gamma], there was a clear increase in protein expression following 2 weeks of HC that persisted at 6 months. These findings are consistent with an HC-induced activation of a PPAR&[gamma]-mediated program that begins in the earliest stages of AVS.
Discussion
To examine the complex and multi-factorial processes in early AVS in vivo, we combined a broad genomics-based approach with precise spatial resolution in a large animal model. The present study is the first to examine the valve endothelium in the earliest stages of AVS and to identify a spatial shift of phenotype balance in response to a brief systemic insult. We found that the aortic side endothelium is much more responsive to hypercholesterolemia (HC) than the adjacent ventricular side endothelium, and unexpectedly expressed overall protective pathways on the side vulnerable to AVS.
Differential endothelial responses to HC were identified using four different genome-wide comparisons: two within-animal comparisons, in which the A and V sides were compared, and two between-animal comparisons, in which the effect of HC on a single side of the valve was considered. These single sides analyses between NC and HC swine provided insight about the within-animal comparisons. For example, the finding that TNF&[alpha] and NF&[kappa]B genes were down-regulated on the A side relative to the V side in HC animals could be interpreted as a pro-inflammatory profile on the V side rather than an anti-inflammatory profile on the A side. Furthermore, the between-animal genomic analyses were not sufficiently sensitive to identify TNF&[alpha] differences between NC and HC groups on either the A or the V side. However, since the effect of HC on the V side phenotype was minimal, the relative differences can be interpreted as a protective anti-inflammatory profile on the A side.
Furthermore, the four different genomic comparisons have inherently different sensitivities. Whereas the within-animal comparisons eliminated inter-animal variability, and were therefore more sensitive, the direct comparison of the A side endothelium in normal swine to the A side endothelium in HC swine probed the phenotypes associated with pathological changes. For example, the within-animal comparisons revealed the downregulation of TNF&[alpha] and NF&[kappa]B pathways on the A side, whereas the between-animal comparisons showed that HC induced several protective mechanisms including upregulating GPX1 and GPX4 expression and activating a PPAR&[gamma]-mediated pathway. Both analyses also identified osteopontin (SPP1) as the most responsive gene in early AVS. Osteopontin has been shown to inhibit and promote regression of calcification in aortic valves in vivo suggesting a further protective mechanism.30 These phenotype changes, identified by two independent analyses, are consistent with the upregulation of protective pathways by hypercholesterolemia on the AVS-susceptible A side and represent the earliest endothelial response in AVS.
Macromolecular transport studies in the aortic valve have shown that lipid can cross the endothelium focally, causing discrete lesions as seen in Figure 1, and passively, possibly at sites of apoptotic endothelial cells.32 Once in the sub-endothelial space, lipid can form liposomes and associate with the extra-cellular matrix to create a pro-pathological environment for underlying valvular interstitial cells. The endothelium may upregulate protective pathways, such as PPAR&[gamma], to protect the valve from the systemic insult. PPAR&[gamma] has been shown to have broad protective endothelial effects, including reduced endothelial activation and inflammation.33 The upregulation of downstream targets FABP1 and ABCA1 involved in lipid trafficking and cholesterol efflux pathway mark a second mechanism of protection. Furthermore, PPAR&[gamma] interacts with cytokines and NF&[kappa]B to suppress inflammatory processes in ECs.34 Despite recent interest and ongoing investigations into the role of the PPAR&[gamma] pathway in vascular disease and atherosclerosis, the role of PPAR&[gamma] agonists, such as rosiglitazone or pioglitazone, in valvular disease has not been explored and may prove highly relevant to AVS pathogenesis.
Although dietary and inter-animal variability in the within-animal comparisons was controlled by directly comparing A and V side endothelial cells, there was significant phenotype heterogeneity between sides of the aortic valve leaflets in both NC and HC swine. Both A and V sides of the valve endothelium derive from a common lineage,10 therefore differences in endothelial phenotype between the two populations of endothelial cells is an adaptive response to the local environment, which is characterized by prominent differences in surface hemodynamics and tissue biomechanics.35 The effect of hemodynamics on endothelial cell phenotype has been studied extensively in arterial endothelium. Unidirectional, laminar flow with high average shear stress (undisturbed flow) is athero-protective, whereas laminar and turbulent multi-directional flow containing low average shear stress (disturbed flow) is athero-permissive.36 The normal aortic valve functions in a complex flow environment where the V side is exposed to undisturbed flow throughout the cardiac cycle in contrast to the A side endothelium which is exposed to highly disturbed flow throughout most of the cycle.35 These differences may be critical in establishing not only endothelial phenotype heterogeneity but also differential endothelial responsiveness to a systemic insult, such as hypercholesterolemia.
The analyses presented here lead to a potentially important question that cannot be fully resolved in the present experiments: why does AVS appear and progress on the A side despite the expression of protective endothelial pathways? It is unclear if the endothelial phenotype switches from a predominantly protective phenotype to an athero-permissive phenotype or whether the endothelium continues to express protective pathways throughout AVS pathogenesis. The protective phenotype changes may be an adaptive (defensive) response to the altered lipid environment to retain normal functioning of the endothelium. There is no evidence of inflammatory mediation by the endothelium - as appears to occur in atherogenesis - at either 2 weeks (no A and V side differential transcript expression of adhesion molecules) or 6 months (no obvious differences in adhesion molecule expression by IHC).
Is it possible that the A side susceptibility is independent of the endothelium? This possibility would need to accommodate the distinct phenotype differences between the A side and V side in normocholesterolemia6 and the compelling data in this paper showing much greater sensitivity of the A side to HC when analyzed in 2 fundamentally different ways. However, it is conceivable that these are unrelated to the spatial localization of AVS. At 6 months, the practical difficulty of dealing with lesion heterogeneity within a leaflet side requires a more sensitive genomic analysis, including selection and isolation of endothelium from histological sections by laser capture microscopy to phenotype endothelial cells overlying discrete lesions. Such genomic analyses are feasible and necessary to further define the phenotype heterogeneity, possibly link it to hemodynamic biomechanics, and lead to a better spatio-temporal understanding of the molecular changes in valvular endothelial cells that underlie AVS.
Condensed Abstract.
A hypercholesterolemic swine model of early aortic valve sclerosis revealed early pathological changes after two-weeks of systemic insult. Side-specific transcript profiling of valve endothelium identified protective pathways upregulated on the patho-susceptible aortic side in response to hypercholesterolemia, including upregulation of PPAR&[gamma] pathway and downregulation of anti-inflammatory genes.
Supplementary Material
Acknowledgements
a) Supported by NIH grants HL62250 (PFD), NRSA HL079877 (MAG) and the University of Pennsylvania Medical Scientist Training Program.
b) We thank Dr. Daniel Rader for plasma lipid measurements, Dr. Elisabetta Manduchi for preliminary microarray validation studies, and Dr. Mete Civelek for thoughtful discussion.
Footnotes
The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the U.S. Food and Drug Administration, the Department of Health and Human Services or the Public Health Service.
c) The authors have no conflicts to disclose
References
- 1.Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–1213. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. A1396. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol. 2002;11:69–77. doi: 10.1016/s1054-8807(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 5.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr., Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 8.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 9.Butcher JT, Nerem RM. Valvular Endothelial Cells Regulate the Phenotype of Interstitial Cells in Co-culture: Effects of Steady Shear Stress. Tissue Eng. 2006;12:905–915. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurle JM, Colvee E. Changes in the endothelial morphology of the developing semilunar heart valves. A TEM and SEM study in the chick. Anat Embryol (Berl) 1983;167:67–83. doi: 10.1007/BF00304601. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Hutchins GM. The development of the semilunar valves in the human heart. Am J Pathol. 1974;74:331–344. [PMC free article] [PubMed] [Google Scholar]
- 13.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr., Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filip DA, Nistor A, Bulla A, Radu A, Lupu F, Simionescu M. Cellular events in the development of valvular atherosclerotic lesions induced by experimental hypercholesterolemia. Atherosclerosis. 1987;67:199–214. doi: 10.1016/0021-9150(87)90280-2. [DOI] [PubMed] [Google Scholar]
- 15.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 16.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 17.Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13:560–566. [PubMed] [Google Scholar]
- 18.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 19.Chien W, Kumagai T, Miller CW, Desmond JC, Frank JM, Said JW, Koeffler HP. Cyr61 suppresses growth of human endometrial cancer cells. J Biol Chem. 2004;279:53087–53096. doi: 10.1074/jbc.M410254200. [DOI] [PubMed] [Google Scholar]
- 20.Gouaze V, Andrieu-Abadie N, Cuvillier O, Malagarie-Cazenave S, Frisach MF, Mirault ME, Levade T. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–42874. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 21.Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ, 2nd, Herman B, Richardson A, Van Remmen H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 22.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J Biol Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 24.DiRenzo J, Soderstrom M, Kurokawa R, Ogliastro MH, Ricote M, Ingrey S, Horlein A, Rosenfeld MG, Glass CK. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol. 1997;17:2166–2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974;54:326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drori S, Girnun GD, Tou L, Szwaya JD, Mueller E, Xia K, Shivdasani RA, Spiegelman BM. Hic-5 regulates an epithelial program mediated by PPARgamma. Genes Dev. 2005;19:362–375. doi: 10.1101/gad.1240705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci U S A. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 29.Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000;129:823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Z, Yin Y, Huang AL, Jan KM, Rumschitzki DS. Macromolecular transport in heart valves I: Studies of rat valves with horseradish peroxidase. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01419.2006. [DOI] [PubMed] [Google Scholar]
- 33.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res. 2008;102:283–294. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 34.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 35.Sacks MS, Yoganathan AP. Heart valve function: a biomechanical perspective. Philos Trans R Soc Lond B Biol Sci. 2007;362:1369–1391. doi: 10.1098/rstb.2007.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER, Feldman CL, Stone PH. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.