Abstract
Hypoxia contributes to the resistance of tumors to conventional therapies. We hypothesized that their replication in hypoxic environments like brain or oral mucosa would make oncolytic herpes simplex viruses (HSVs) such as G207 (which has undergone clinical trials) replicate to a greater extent in hypoxic tumors like glioblastoma. Hypoxic cultured U87 cells yielded 4% more wild-type HSV (P = 0.04) and 3.6-fold more G207 (P = 0.001) after 48 hours of infection when compared with normoxic cells. Real-time RT-PCR confirmed a fivefold hypoxia-induced U87 upregulation of GADD34 mRNA, a factor complementing the γ34.5 gene deletion in G207. The viral yield under conditions of hypoxia, as against normoxia, in GADD34 siRNA-treated U87 cells was 65% of that in control siRNA-treated cells. Treating subcutaneous U87 tumors in athymic mice with erythropoietin lowered the tumoral hypoxic fraction from 57.5 to 24.5%. Tumoral hypoxia dropped to 2.5% during 4 hours/day of hyperbaric chamber treatment. Each tumor-oxygenating maneuver reduced the G207 yield fourfold (P = 0.0001). Oncolytic HSV G207 exhibited enhanced replication in hypoxic environments, partly on account of increased GADD34 expression in hypoxic cells. The unique tropism of oncolytic HSVs for hypoxic environments contrasts with the hypoxia-mediated impairment of standard (radiation, chemotherapy) and other experimental therapies, and enhances HSV's appeal and efficacy in treating tumors like glioblastoma.
Introduction
The theory that hypoxia is prevalent in human tumors was first postulated by Thomlinson and Gray 50 years ago,1 and was confirmed in several studies on tumors in the 1990s, after the introduction of the Eppendorf oxygen electrode.2 Since then, several investigators have demonstrated that hypoxia causes resistance of tumor cells to radiation therapy3 and chemotherapy.4
Ionizing radiation produces free radicals around DNA, and these can be stabilized into additional free radical species in the presence of oxygen or reduced into nontoxic compounds by free sulfhydryl groups in the absence of oxygen.3 Hypoxia mediates chemotherapy resistance through multiple mechanisms: (i) hypoxic cells are distant from blood vessels, leading to reduced exposure to systemically administered agents;5 (ii) hypoxia decreases cellular proliferation, a requirement for most chemotherapy agents;6 (iii) hypoxia selects for cells that have lost sensitivity to p53-mediated apoptosis, a common mechanism of chemotherapy-mediated cell death;7 (iv) some chemotherapies resemble radiation in that hypoxia decreases the cytotoxicity of the free radical-induced DNA lesions that they cause;8 and (v) hypoxia upregulates genes involved in drug resistance, such as P-glycoprotein.4
Oncolytic viruses with natural selectivity for tumor cells or viruses such as herpes simplex virus (HSV) or adenovirus engineered in the laboratory to replicate selectively in tumor cells have generated considerable interest based on laboratory data in experimental cancer models.9,10 Phase I and II clinical trials confirmed the safety of these agents but failed to show definitive efficacy.10,11,12,13 This could be because of failure to reach a maximum tolerated dose or the presence of deficiencies in the delivery methods.10,13
While it will be important to continue studying these agents in further clinical trials given the verification of their safety in initial trials, it will also be important to go further and understand how oncolytic viruses might be affected by the tumor microenvironment, in the context of differences between the tumor microenvironment and the natural milieu of the virus. For example, data from recent studies showing that hypoxia inhibits adenoviral replication by reducing translation of adenoviral E1A and fiber proteins14,15 are causing concern in the light of the profound hypoxia demonstrated in human tumors, such as glioblastomas (PO2 = 5 mm Hg), pancreatic cancers (PO2 = 2.7 mm Hg), and prostate cancers (PO2 = 2.4 mm Hg).16
We hypothesized that, unlike adenovirus, oncolytic HSVs, such as the virus G207 that has undergone clinical trials in glioblastoma patients,12 would exhibit increased replication in hypoxic tumor cells. This hypothesis was based on two features of HSV. First, wild-type HSV normally replicates in environments such as the brain or oral mucsoa, whose oxygen tensions of 34 mm Hg17 and 40.5 mm Hg18, respectively, better approximate the 2.4 to 18 mm Hg oxygen tension of human tumors16 rather than the 150 mm Hg oxygen tension found in the respiratory epithelium in which wild-type adenovirus normally replicates.14 Second, DNA damage, which can be induced by free radicals formed in a hypoxic environment, and the resulting cellular DNA repair response have been shown to stimulate HSV replication19,20 while inhibiting adenoviral replication.20
Results
Hypoxia enhances the replication of G207 in cultured cells
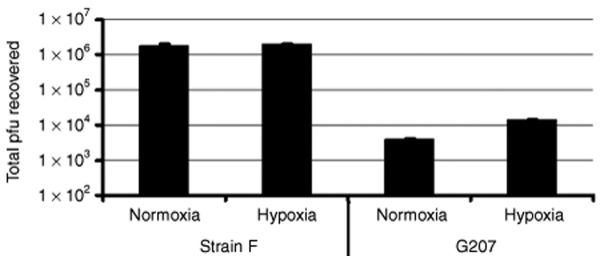
To determine the effect of hypoxia on oncolytic HSV replication, the rates of production of infectious wild-type HSV strain F and strain F-derived oncolytic HSV G207 were evaluated in cultured U87 human glioma cells infected under normoxic or hypoxic (1% oxygen) conditions. At a multiplicity of infection of 0.4, the yield of G207 after 24 hours was 8% higher in hypoxic conditions than in normoxic conditions (P = 0.01), while there was no difference in the yield of strain F in hypoxic conditions as compared to normoxic conditions. At 48 hours after a multiplicity of infection of 0.4, the viral yield of strain F in plaque forming units (pfu) was 4% higher in hypoxic conditions than it was in normoxic conditions (Figure 1), while the yield of G207 was 3.6-fold higher in hypoxic conditions than in normoxic conditions (Figure 1). These enhancements of replication under hypoxic conditions for the viruses, and the relative degrees of such enhancement, were much greater for G207 than strain F. The results were reproducible (experiment repeated three times) and statistically significant (P = 0.04 for strain F, P = 0.001 for G207 by Student's t-test). By 72 hours, U87 cells under hypoxic conditions began to exhibit signs of early cell death, consistent with data from earlier studies that had used this cell line,21 and therefore the viral yield could no longer be assessed. Lowering the multiplicity of infection to 0.1 and infecting for 48 hours led to a slightly more robust fivefold increase in the yield of G207 in hypoxic cells relative to normoxic cells, and a 7% increase in the yield of strain F in hypoxic cells relative to normoxic cells (P = 0.02 for strain F and P = 0.0007 for G207, by Student's t-test).
Figure 1.
Yield of oncolytic HSV is greater in hypoxic cultured U87 cells than in normoxic U87 cells. U87 cells (7 × 105) were infected with strain F and oncolytic HSV G207 at a multiplicity of infection (MOI) of 0.4 for 48 hours in normoxic and hypoxic conditions, after which the yield of infectious virus was measured in terms of plaque-forming units (pfus) by plaque assay on Vero cells. The results shown are the mean values, with the error bars representing standard deviations (triplicate plates used for each grouping of virus and oxygen concentration) from a representative experiment. The experiment was repeated three times, each time showing similar results.
Hypoxia induces GADD34 expression, not mammalian ribonucleotide reductase expression
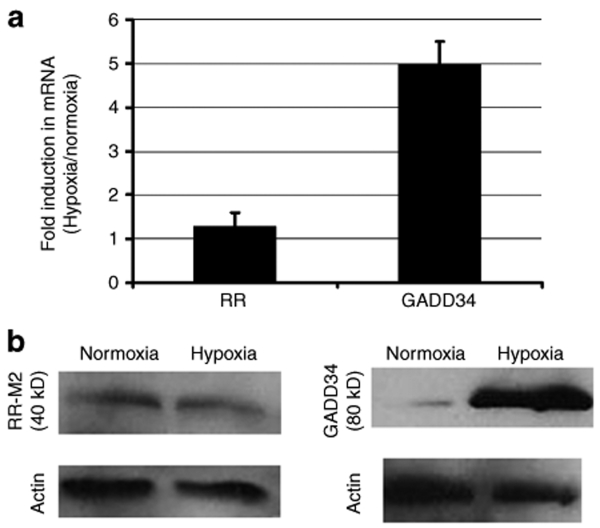
G207 carries mutations in viral genes γ34.5 and ribonucleotide reductase (RR), which enables its selective replication in tumor cells, because these express mammalian proteins GADD34 and RR which complement the respective viral mutations.22 We have previously shown that chemotherapy-induced upregulation of GADD34 and mammalian RR can enhance G207 replication.19 In this study, therefore, we investigated whether the hypoxia-mediated enhancement of G207 replication that we observed was caused by hypoxia-induced expression of GADD34 or RR. While real-time RT-PCR identified only a slight, statistically insignificant (P = 0.5) 30% increase in the mRNA of RR, it demonstrated a significant (P = 0.008) fivefold increase in GADD34 mRNA in hypoxic U87 cells relative to normoxic ones (Figure 2a), and western blot identified elevated levels of GADD34 protein, and not RR M2 subunit protein, in hypoxic U87 cells relative to normoxic ones (Figure 2b).
Figure 2.
Hypoxia upregulates GADD34, not the M2 subunit of ribonucleotide reductase. U87 cells were cultured in hypoxic or normoxic conditions for 48 hours. (a) mRNA levels were measured under hypoxic conditions as compared to normoxic conditions, by relative quantification real-time RT-PCR. The bar labeled “RR” represents the mRNA levels for the M2 subunit of ribonucleotide reductase, and a similar lack of induction was seen with the M1 subunit of RR. Error bars represent standard deviations. (b) GADD34 and the M2 subunit of ribonucleotide reductase were also assessed by western blot in normoxic and hypoxic U87 cells.
GADD34 induction contributes to hypoxia-enhanced G207 replication
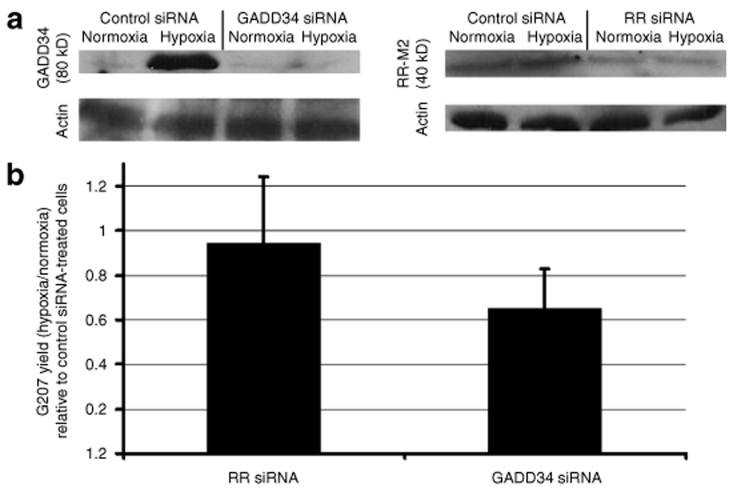
To determine whether hypoxia-induced GADD34 expression contributes to hypoxia-induced enhancement of G207 replication, U87 cells were treated with siRNAs targeting GADD34, mammalian RR, or control sequences not present in the mammalian genome prior to G207 infection. The siRNAs reduced the expression of the proteins corresponding to the transcripts they targeted (Figure 3a). Control siRNA-treated cells exhibited the same 3.6-fold more G207 yield in hypoxic cells as compared to normoxic cells. However, the viral yield under hypoxic conditions (relative to normoxic conditions) in GADD34 siRNA-treated cells was 65% that in control siRNA-treated cells, representing a significant reduction (P = 0.01). In contrast, in RR siRNA-treated cells the relative viral yield was 95% that in control siRNA-treated cells, an insignificant reduction (P = 0.8) (Figure 3b). Taken together, these data suggest that the elimination of GADD34 mRNA blocks more than one-third of the hypoxia-enhanced G207 replication in cultured cells.
Figure 3.
GADD34 siRNA blocks hypoxia-mediated enhancement of G207 replication. U87 cells were transfected with siRNA targeting GADD34, the M2 subunit of ribonucleotide reductase (RR), and control sequences. (a) western blot showing siRNA knockdown of GADD34 (left)and the M2 subunit of ribonucleotide reductase protein expression (right) under hypoxic and normoxic conditions. (b) The cells were then infected with G207 at an multiplicity of infection (MOI) of 0.4, and cultured under hypoxic and normoxic conditions for 48 hours. The viral yield was then measured. The data show the ratio of G207 yields in hypoxic cells to those in normoxic cells transfected with siRNA targeting GADD34 or RR, normalized to the hypoxic-to-normoxic G207 yield ratio of cells transfected with control siRNA. Error bars represent standard deviations.
Decreasing the hypoxia of tumors in vivo reduces G207 yield
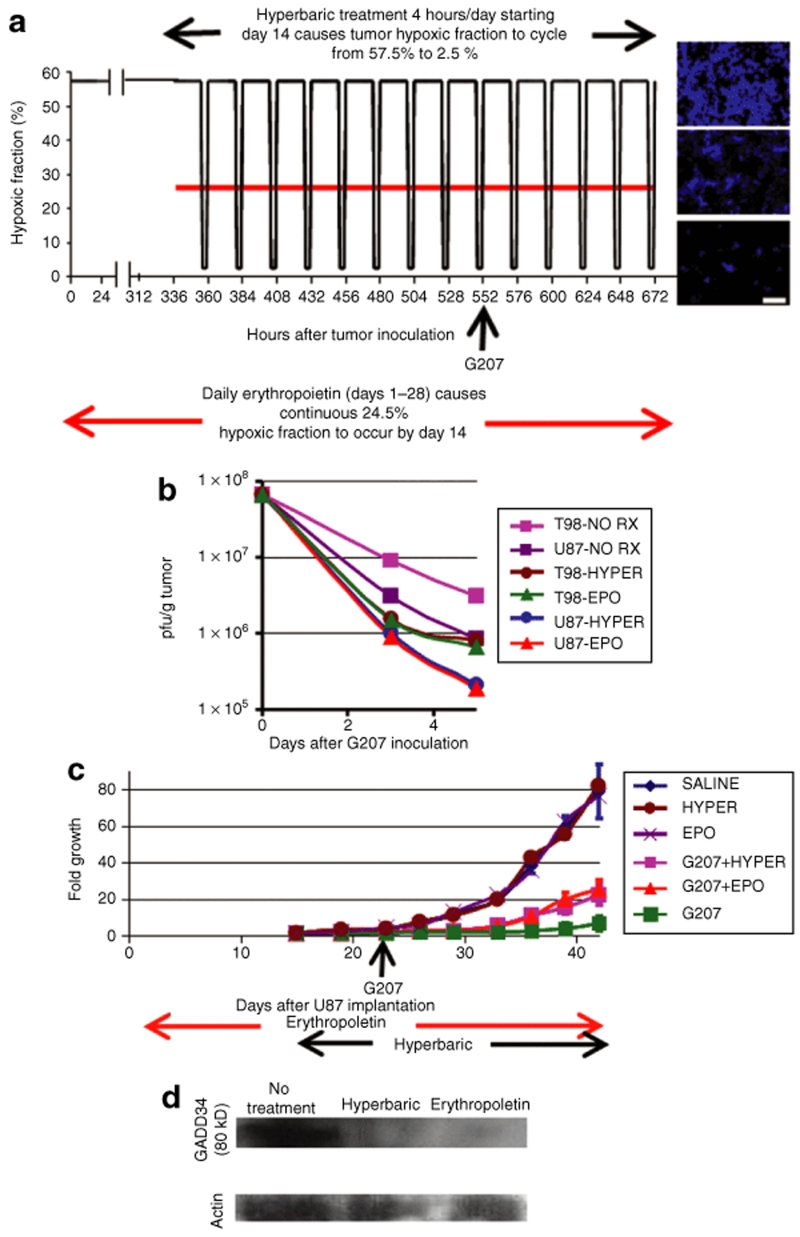
We determined the difference in G207 replication in athymic mice bearing subcutaneous tumors derived from the U87 human glioblastoma cell line. Although this is an ectopic anatomic location, it causes severe tumor hypoxia that better approximates the hypoxia of human glioblastomas than do orthotopic intracranial murine tumors derived from human glioma cell lines.16 Further, the hypoxia of these subcutaneous tumors can be experimentally reduced by one of two different methods: erythropoietin administration or hyperbaric oxygen treatment. The administration of erythropoietin daily, starting from tumor implantation and continuing throughout the period of tumor growth (28 days, until tumors became large enough to require that the animals be killed), reduced the mean hypoxic fraction in subcutaneous U87 tumors, as determined by pimonidazole staining, from 57.5 to 24.5% (Figure 4a). The administration of hyperbaric oxygen for 4 hours a day (the maximum tolerated duration), starting 14 days after tumor implantation and continuing throughout the remaining 14 days of tumor growth, reduced the mean hypoxic fraction to 2.5% immediately after treatment, with a gradual escalation to baseline values of 57.5% during the 20 hours of absence of treatment (Figure 4a). Neither erythropoietin nor hyperbaric oxygen treatment affected tumor growth (Figure 4c). Treatment of U87 or T98 human glioma cell line-derived subcutaneous tumors with either hyperbaric oxygen or erythropoietin lowered the yield of G207 to an extent of four- to fivefold 5 days after viral inoculation (the virus was inoculated 23 days after tumor implantation), which is a statistically significant difference (P = 0.00001–0.00004) (Figure 4b). At the conclusion of the experiment (42 days after tumor implantation), subcutaneous U87 tumors treated with 5 × 106 pfu G207 for 23 days after tumor implantation exhibited an average sevenfold growth, which is much less than the 79-fold growth exhibited when tumors were treated with saline alone (P = 0.0002) (Figure 4c). U87 tumors treated with hyperbaric oxygen or with erythropoietin-plus-G207 exhibited 22-fold and 25-fold growth, respectively, 42 days after tumor implantation, which is greater than the sevenfold growth seen with G207 treatment alone at the same time point (P = 0.0004–0.0007). These findings confirm that artificial correction of the tumor hypoxia lowers the inhibitory “oncolytic” effect of G207 on tumor growth (Figure 4c). U87 tumors in mice treated with either hyperbaric oxygen or erythropoietin exhibited reduced GADD34 protein levels (Figure 4d). This finding supports our hypothesis that hypoxia-induced GADD34 expression contributes to hypoxia increased G207 viral yield in vivo.
Figure 4.
Increasing the oxygenation of subcutaneous U87 or T98 tumors in athymic mice lowers oncolytic HSV yield in vivo. (a) Athymic mice with subcutaneous tumors derived from U87 or T98 glioma cells underwent daily erythropoietin treatment starting from tumor implantation and continuing until the tumor size required that the animals be killed (28 days after implantation) or hyperbaric oxygen treatment for 4 hours/day from day 14 through day 28 after tumor implantation. The graph shows the tumor hypoxic fraction for animals treated with erythropoietin (red) or hyperbaric oxygen (black). At the right are representative immunohistochemical micrographs in which hypoxic areas (detected using antibodies targeting the adducts formed by pimonidazole) are blue. The picture at the top represents hypoxic tumors that were not treated or prior to hyperbaric treatment; the picture in the middle represents tumors in erythropoietin-treated mice; and the picture at the bottom represents the nonhypoxic tumors seen after hyperbaric treatment. For the experiments in b, G207 was inoculated 23 days (552 hours) after T98 or U87 tumor implantation. Scale bars, 50 µm. (b) Athymic mice with subcutaneous T98 or U87 tumors either underwent no treatment (pink or purple squares, “NO RX”), or underwent one of two treatments that increased tumor oxygenation: hyperbaric chamber treatment for 4 hours per day (brown or blue circles, “HYPER”) or daily treatment with human dose erythropoietin (green or red triangles, “EPO”). Viral yields were measured 3 and 5 days after G207 inoculation. Both tumor-oxygenating treatments lowered viral yields 5 days after G207 inoculation, fourfold in U87 tumors (P = 0.00003–0.00004) and four- to fivefold in T98 tumors (P = 0.00001–0.00002). Error bars represent standard deviations but are smaller than the symbols. (c) The growth of subcutaneous U87 tumors expressed as a multiple, plotted against days after U87 implantation, for tumors treated with saline (blue), hyperbaric oxygen (HYPER, brown), erythropoietin (EPO, purple), G207 (green), G207 plus erythropoietin (G207+EPO, red), and G207 plus hyperbaric oxygen (G207+HYPER, pink). Error bars represent standard deviations. (d) Western blot showing GADD34 protein expression in single subcutaneous U87 tumors grown for 28 days in mice that were left untreated, or treated with erythropoietin, or treated in the hyperbaric chamber. Similar protein expression differences were found within five tumors in each treatment group, and the GADD34 protein expression did not vary during or between the final hyperbaric oxygen treatments (the blot shown is from a representative experiment performed between the hyperbaric oxygen treatments on days 27 and 28).
Discussion
Hypoxia is a well characterized feature of solid tumors, and the hypoxic environment has a detrimental effect on the response of tumors to radiation3 and chemotherapy.4 Oncolytic viruses that selectively replicate in tumor cells have made the transition from bench to bedside over the past decade. Data from recent studies, showing reduced adenoviral replication in hypoxic cells,14,15 raise concerns that, much like the effects of chemotherapy and radiation, hypoxic conditions in cells could also diminish the efficacy of oncolytic viruses.
We found that the oncolytic HSV G207 exhibited increased replication in hypoxic cells, while wild-type HSV strain F exhibited a smaller increase in replication in such cells. The reproducible half-log enhancements in G207 replication that we describe after 48 hours of viral replication under hypoxic conditions (as compared to normoxic culture conditions) in culture, and after 5 days under hypoxic conditions (as compared to normoxic conditions) in vivo may seem small, but are actually significant in the light of several studies showing that relatively small increases in viral yield in vivo, comparable to the increase we observed under hypoxic conditions, can have large impacts on inhibition of tumor growth. For example, ionizing radiation was shown to increase oncolytic HSV yield in vivo by approximately threefold, and yet it increased the tumor cure rate from 14 to 56%.23 We have shown that temozolomide increases G207 yield in vivo by two- to sixfold, and yet the combination increases long-term survival from 10 to 100%.13
In addition, our in vivo observations held true not only in “glucose sensitive” glycolytic-dependent U87 cells, which are less tolerant of low glucose concentrations because of cellular expression of the LDH-B isoform alone,24,25 but also in “glucose resistant” T98 cells, which can tolerate low glucose concentrations26 and which rely on oxidative phosphorylation (aerobic respiration) for survival, probably as a result of expression of both the LDH-A and the B isoforms. The in vivo data presented here, therefore, suggest that hypoxia can enhance oncolytic HSV replication in glioma cell lines, whether these are glycolytic-dependent or independent.
The enhanced replication in wild-type strain F in hypoxic cells relative to normoxic cells, which was much smaller than the hypoxia-related enhancement of oncolytic HSV G207 replication but still statistically significant, might reflect the natural tropism of HSV for cells with reduced oxygen tension and the stimulation of HSV replication by DNA damage induced by oxygen-derived free radicals. Indeed, if hypoxia naturally enhances HSV replication, this could reflect HIF-1α-mediated transcription of cellular factors that enhance HSV replication or direct HIF-1α effects on the HSV genome, given the recent demonstration that the genome of Kaposi's sarcoma-associated herpesvirus contains hypoxia-responsive elements that can be specifically activated by HIF-1α.27
However, the larger hypoxia-mediated enhancement of G207 replication shows that the viral gene deletions in G207 dramatically enhance the slight hypoxia-related enhancement of HSV replication that is intrinsic to strain F. A portion of the hypoxia-mediated enhancement of G207 replication may reflect our finding of hypoxia-mediated upregulation of GADD34 and our further finding that blocking of the GADD34 expression impairs one-third of the hypoxia-mediated upregulation of viral replication. GADD34 is a mammalian gene whose product complements the replication of HSVs such as G207 that are deficient in the viral gene γ34.5.22 This finding is consistent with previous demonstrations that GADD34 is expressed in response to cellular stressors such as hypoxia,28 and suggests that, in addition to HSV's natural tropism for hypoxic environments, the specific viral gene deletion found in G207 may cause an even greater augmentation of viral replication in hypoxic environments. In addition to the explanation relating to hypoxia-mediated GADD34 upregulation, other potential explanations for the finding that hypoxia upregulates G207 replication far more than strain F does could be: (i) hypoxia, which upregulates protein phosphatase 1 expression,29 causes protein phosphatase 1 levels to rise high enough that protein phosphatase 1 causes some dephosphorylation and activation of eIF-2α in a γ34.5-independent fashion, thereby promoting viral protein synthesis; and (ii) hypoxia upregulates the expression of other GADD proteins to sufficient levels as to make them achieve a degree of complexing with proliferating-cell nuclear antigen (PCNA), similar to the action of GADD34 in cells infected with γ34.5-deficient HSVs.
A review of the literature shows that oncolytic HSV, which was investigated in this study, is the fourth oncolytic virus to be studied by investigators to characterize how hypoxia affects viral replication. Also, this is the first study to report hypoxic upregulation of oncolytic viral replication. An earlier study found that hypoxia impairs replication of oncolytic adenovirus,14 a DNA virus. Another study found that hypoxia has no effect on adenoviral uptake or exogenous gene expression, and therefore does not affect gene delivery by replication-incompetent adenoviruses; however, hypoxia impairs adenoviral replication by reducing E1A levels and thereby affects the propagation of oncolytic adenoviruses.15 While one study showed that GADD34 expression (induced by hypoxia in our study) impairs replication of vesicular stomatitis virus,30 an RNA virus, another study argued that hypoxia does not affect vesicular stomatitis virus replication, using indirect evidence in the form of viral replication in hypoxic areas of a tumor.31 Hypoxia has also been shown to inhibit the replication of the oncolytic DNA virus, Minute virus of mice.32 Further, hypoxia has been demonstrated to inhibit the replication of other DNA viruses that have not yet been used as oncolytic viruses, such as simian virus 40 (ref. 33). These reports from earlier studies underscore the fact that the hypoxia-mediated upregulation of oncolytic HSV replication, as identified by us in this study, is a unique feature among viruses in general.
Importantly, our study is the first to find an oncolytic virus (oncolytic HSV) that demonstrates an increase in replication in hypoxic environments. Other researchers have attempted to use HIF-1α-driven promoters to regulate the expression of essential viral genes in oncolytic adenovirus34 or oncolytic HSV.35 However, before embarking on these viral engineering approaches that are designed to increase the safety of these viruses by limiting their replication to hypoxic areas, it is important to keep in mind the direct effects of hypoxia on the oncolytic virus itself. In other words, hypoxia-driven promoters in the virus might not overcome the ability of hypoxia to limit the translation of proteins from adenoviruses14 and might not improve upon the intrinsic hypoxia-mediated upregulation of viral replication that we found with the HSV G207.
Further, in this study we not only showed replication in hypoxic areas in vivo but also used manipulations of tumor oxygenation in vivo to demonstrate that there is greater viral replication in tumors that are more hypoxic. Such preclinical data, along with other considerations such as the ability to generate large titers and the rate of viral replication relative to the rate of tumor migration and growth, will likely need to be considered when choosing which oncolytic viruses warrant further clinical study. Given the high levels of hypoxia found in most human solid tumors,16 and the recent demonstration that oncolytic viruses such as vaccinia virus and vesicular stomatitis virus eventually cause reduced blood supply to the tumor36 thereby potentially worsening tumoral hypoxia, the ability of oncolytic HSVs to not only tolerate hypoxia but to benefit from it may provide these viruses with a key intrinsic therapeutic advantage.
Materials and Methods
Cell lines and culture. U87 and T98 human glioblastoma cells were obtained from American Type Culture Collection (Manassas, VA). The cells were cultured under either normoxic conditions (5% CO2, 21% O2, 74% N2) in a humidified incubator at 37 °C, or under hypoxic conditions (5% CO2, 1% O2, 94% N2) at 37 °C, in an ESPEC triple gas-sealed incubator (Tabai-Espec, Osaka, Japan) kindly provided by O. Iliopoulos (Massachusetts General Hospital). The incubator contained only the U87 cells under study and was never opened during the duration of incubation.
Viruses. Wild-type HSV-1 strain F (obtained from B. Roizman, University of Chicago, Chicago, IL) and strain F-derived γ34.5−ICP6−LacZ+ G2079 were grown, purified, and titered by plaque assay on Vero cells, as described.9
Quantitative real-time RT-PCR. U87 cells were grown in hypoxic or normoxic environments for 48 hours. RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA). A high-capacity cDNA archive kit (Applied Biosystems; Foster City, CA) was used for generating cDNA. Real-time RT-PCR was performed on an ABI Prism 7000 (Applied Biosystems) machine using human GADD34 (forward: 5′ GGAGGAAGAGAATCAAGCCA 3′; reverse: 5′ TGGGGTCGGAGCCTGAAGAT 3′) primers (Invitrogen) combined with SYBR Green Master Mix (Applied Biosystems) or primer-probe combinations for RR subunits M1 and M2 (Applied Biosystems Part Nos. Hs00357247_g1 and Hs00168784_m1) and 18S rRNA (Applied Biosystems Part No. 4308329) combined with TaqMan Master Mix (Applied Biosystems). Relative quantification was performed using 18S rRNA as an endogenous control. All reactions began with 10 minutes at 95 °C for AmpliTaq Gold activation, followed by 40 cycles at 95 °C for 15 seconds for denaturation, and then 60 °C for 1 minute for annealing/extension.
Western blot. Total protein from cultured cells or homogenized subcutaneous tumors suspended in an equal volume of phosphate buffered saline was extracted by RIPA buffer, and 30 µg of protein was separated on a 8% SDS-PAGE gel, transferred to PVDF membrane, and incubated with antibodies to GADD34 (Imgenex Corp.; San Diego, CA) or RR M2 subunit (GenWay Biotech; San Diego, CA) at 4 °C overnight. The membranes were incubated with peroxidase-conjugated secondary antibodies for 40 minutes the next day. Protein was visualized using the enhanced chemiluminescence kit (Amersham Biosciences; Piscataway, NJ).
siRNA. To silence gene expression, duplex RNA targeting human GADD34 (5′ GGACACUGCAAGGUUCUGA), the M2 subunit of human RR (5′ UGCUGUUCGGAUAGAACAG), and control siRNA with medium GC content (comparable to the other siRNAs used) targeting no known vertebrate sequences were synthesized with d(TT) at the 3′ terminus of each strand (Invitrogen). siRNA was transfected into U87 cells using Lipofectamine 2000 in accordance with the manufacturer's protocol (Invitrogen). The levels of GADD34 and RR M2 subunit mRNA relative to mock-transfected cells were assessed at 24, 48, and 72 hours after transfection using real-time RT-PCR, and were found to be reduced to 27–30%, 3–7%, and 25–28% of baseline values, respectively. Control siRNA maintained GADD34 and RR MR subunit mRNA levels at 95–100% of nontransfected cells 24–72 hours after transfection. Knockdown of protein levels by siRNA was confirmed by western blot analysis.
Animals. Athymic mice (20 g) were inoculated subcutaneously with 106 U87 or T98 cells. Some of the mice were treated with recombinant human erythropoietin (Amgen; Thousand Oaks, CA) at human-equivalent dose (150 IU/kg) thrice per week intraperitoneally (i.p.) from the day of tumor cell implantation until the conclusion of the experiment. Two weeks after the treatment, the mice with 12–36 mm3 tumors were assigned to three different groups (10 mice per group), each group having the same mean tumor volume. One group continued to receive erythropoietin for the remainder of the experiment; a second group was treated for 4 hours/day (maximum tolerated amount) in a hyperbaric chamber (Seachirst; Santa Monica, CA) with 100% O2 at two absolute atmospheres, translating to an arterial PO2 of 1,500 mm Hg for the remainder of the experiment; and a third group received no tumor oxygenation promoting treatments.
The tumors were measured biweekly using calipers to calculate length, width, and height, with the measurer blinded as to each animal's treatment group. Tumor volume was calculated as the product of these three dimensions, and growth was expressed as a multiple relative to the volume on treatment day one. Each mouse tumor continued to be measured up to the time point when the animal was killed because of excess tumor burden (2.1 cm maximal dimension).
For assessing viral replication in these subcutaneous tumors, 5 × 106 pfu G207 was inoculated intratumorally into each tumor at the time point of 23 days after subcutaneous U87 cell inoculation, when tumors had achieved volumes of 90–140 mm3. The animals were killed and the tumors were excised at 3 and 5 days after G207 inoculation (five mice per group per time point). To measure tumor oxygenation, some mice received 60 mg/kg pimonidazole (Chemicon) i.p. 1 hour before they were killed. The tumors were weighed, cut into small pieces, suspended in a volume of phosphate buffered saline twice the tumor volume, homogenized manually, sonicated, and centrifuged. The supernatant was isolated, freeze-thawed three times, and titered on Vero cells.
Immunohistochemistry. The tumors were removed and frozen in liquid nitrogen-cooled N-methylbutane, and the entire tumor was sectioned coronally by cryostat to 8 µm-thick slices. Every third tumor-containing slide was immunostained for hypoxyprobe-1 adducts (mouse; Chemicon). Secondary staining was carried out using Jackson ImmunoResearch antibodies. A Nikon Eclipse TE2000-U inverted microscope was used for imaging five fields from five animals per tumor type. The images were captured using a Retiga EXi CCD digital camera (Qimaging, Surrey, British Columbia), and Metavue imaging software (version 6.2r4; Molecular Devices; Downington, PA) was used to calculate tumoral hypoxia, defined as the percentage of tumor tissue that stained positive for the hypoxyprobe-1 adduct.
Acknowledgments
This work was supported in part by National Institutes of Health Grants NS32677 (to R.L.M.) and P30 NS045776 (to S.R.) for the real-time PCR core. Robert Martuza and Samuel Rabkin are consultants to MediGene AG, which has a license from Georgetown University for G207.
REFERENCES
- Thomlinson RH., and , Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Schlenger K, Knoop C., and , Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- Nordsmark M, Overgaard M., and , Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- Tannock IF.Conventional cancer therapy: promise broken or promise delayed Lancet 1998351SII9–SII16.Suppl 2 [DOI] [PubMed] [Google Scholar]
- Primeau AJ, Rendon A, Hedley D, Lilge L., and , Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22:258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermeus A, Cosse JP, Crespin M, Mainfroid V, de Longueville F, Ninane N, et al. Hypoxia induces protection against etoposide-induced apoptosis: molecular profiling of changes in gene expression and transcription factor activity. Mol Cancer. 2008;7:27. doi: 10.1186/1476-4598-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe E, Zhou X, Li D, Oku N, Yamamoto T., and , Osaki T. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD., and , Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA., and , Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Aghi M., and , Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Pipiya T, Sauthoff H, Huang YQ, Chang B, Cheng J, Heitner S, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12:911–917. doi: 10.1038/sj.gt.3302459. [DOI] [PubMed] [Google Scholar]
- Shen BH., and , Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12:902–910. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- Brown JM., and , Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- Zauner A, Daugherty WP, Bullock MR., and , Warner DS.Brain oxygenation and energy metabolism: part I-biological function and pathophysiology Neurosurgery 200251289–301.discussion [PubMed] [Google Scholar]
- Thorn JJ, Kallehave F, Westergaard P, Hansen EH., and , Gottrup F. The effect of hyperbaric oxygen on irradiated oral tissues: transmucosal oxygen tension measurements. J Oral Maxillofac Surg. 1997;55:1103–1107. doi: 10.1016/s0278-2391(97)90290-1. [DOI] [PubMed] [Google Scholar]
- Aghi M, Rabkin S., and , Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- Lilley CE, Carson CT, Muotri AR, Gage FH., and , Weitzman MD. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci USA. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chou J, Liebermann DA, Hoffman B., and , Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR., and , Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol. 2005;74:123–133. doi: 10.1007/s11060-004-6404-6. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Parajuli P., and , Sloan AE.Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study Neurosurgery 2004551410–1419.discussion [DOI] [PubMed] [Google Scholar]
- Nakayama T, Mikoshiba K, Yamamori T., and , Akagawa K. Activation of syntaxin 1C, an alternative splice variant of HPC-1/syntaxin 1A, by phorbol 12-myristate 13-acetate (PMA) suppresses glucose transport into astroglioma cells via the glucose transporter-1 (GLUT-1) J Biol Chem. 2004;279:23728–23739. doi: 10.1074/jbc.M314297200. [DOI] [PubMed] [Google Scholar]
- Haque M, Davis DA, Wang V, Widmer I., and , Yarchoan R. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J Virol. 2003;77:6761–6768. doi: 10.1128/JVI.77.12.6761-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann DA., and , Hoffman B. Myeloid differentiation (MyD)/growth arrest DNA damage (GADD) genes in tumor suppression, immunity and inflammation. Leukemia. 2002;16:527–541. doi: 10.1038/sj.leu.2402477. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lim CJ, Min JK, Lee JK, Kim YM, Lee JY, et al. Protein phosphatase 1 nuclear targeting subunit is a hypoxia inducible gene: its role in post-translational modification of p53 and MDM2. Cell Death Differ. 2007;14:1106–1116. doi: 10.1038/sj.cdd.4402111. [DOI] [PubMed] [Google Scholar]
- Minami K, Tambe Y, Watanabe R, Isono T, Haneda M, Isobe K, et al. Suppression of viral replication by stress-inducible GADD34 protein via the mammalian serine/threonine protein kinase mTOR pathway. J Virol. 2007;81:11106–11115. doi: 10.1128/JVI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JH, Naczki C, Koumenis C., and , Lyles DS. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J Virol. 2004;78:8960–8970. doi: 10.1128/JVI.78.17.8960-8970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais C, Caillet-Fauquet P, Draps ML, Velu T, de Launoit Y., and , Brandenburger A. Hypoxic-response elements in the oncolytic parvovirus Minute virus of mice do not allow for increased vector production at low oxygen concentration. J Gen Virol. 2006;87:1197–1201. doi: 10.1099/vir.0.81754-0. [DOI] [PubMed] [Google Scholar]
- Riedinger HJ, van Betteraey M., and , Probst H. Hypoxia blocks in vivo initiation of simian virus 40 replication at a stage preceding origin unwinding. J Virol. 1999;73:2243–2252. doi: 10.1128/jvi.73.3.2243-2252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DE, Sandberg EM, Kyle MM, Devi NS, Brat DJ, Xu Z, et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67:6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin RH, Reinblatt M., and , Fong Y. Employing tumor hypoxia to enhance oncolytic viral therapy in breast cancer. Surgery. 2004;136:199–204. doi: 10.1016/j.surg.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]