Abstract
Several reactions mediated by lithium diisopropylamide (LDA) with added hex-amethylphosphoramide (HMPA) are described. The N-isopropylimine of cyclohex-anone lithiates via an ensemble of monomer-based pathways. Conjugate addition of LDA/HMPA to an unsaturated ester proceeds via di- and tetra-HMPA-solvated dimers. Deprotonation of norbornene epoxide by LDA/HMPA proceeds via an intermediate metalated epoxide as a mixed dimer with LDA. Ortholithiation of an aryl carbamate proceeds via a mono-HMPA-solvated monomer-based pathway. Dependencies on THF and other ethereal cosolvents suggest that secondary-shell solvation effects are important in some instances. The origins of the inordinate mechanistic complexity are discussed.
Introduction
Hexamethylphosphoramide (HMPA) is one of the most prominent additives used to influence the yields, rates, and selectivities of organolithium reactions.1 A preponderance of what is known about solvation of lithium ions by HMPA derives from the studies of Reich and coworkers.2 Their spectroscopic analyses of lithium salts in the limit of slow exchange of free and lithium-ion-coordinated HMPA offer intimate details of HMPA-mediated deaggregation and ionization. Despite the sound understanding of how HMPA influences the structures of lithium salts, the oft-cited influence of HMPA on reactivity is not well understood and leaves many questions unanswered.3 Does the marked tendency of HMPA to serially solvate organolithiums foreshadow a similar structural diversity in the rate-limiting transition structures? Do dramatic accelerations result from the capacity of HMPA to promote high solvation numbers and low aggregation numbers in the rate-limiting transition structures? Why does HMPA sometimes fail to elicit high reactivities?2
We have begun addressing some of these questions in the context of lithium diisopropylamide (LDA),4-9 a prevalent base in organic synthesis.10 We describe herein investigations of four reactions mediated by LDA/HMPA--lithiation of imine 1 (eq 1),7f,8b,11,12 1,4-addition to unsaturated ester 3 (eq 2),13,14 opening of epoxide 5 (eq 3),10,15-17 and ortholithiation-Fries rearrangement of carbamate 7 (eq 4).18 All have found niches in organic synthesis, and all but the 1,4-addition have proved useful in studies of LDA structure-reactivity relationships.19 The results support an emerging picture of unusual mechanistic diversity imparted by HMPA;4,5 they are summarized at the start of the discussion for the benefit of the non-specialist.
 |
(1) |
 |
(2) |
 |
(3) |
 |
(4) |
Background
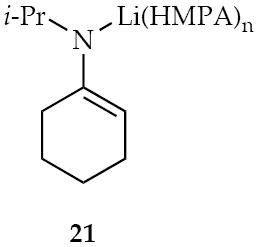
Any examination of structure-reactivity relationships must be prefaced by a clear understanding of structure.19,20 LDA offers an optimal template in that it forms exclusively disolvated dimer 9 over all THF concentrations and disolvated dimer 10 at ≥ 1.0 equiv of HMPA.6a,21 (The absence of deaggregation is notable given that HMPA usually deaggregates organolithiums.2)

A brief survey of previous rate studies of LDA/HMPA-mediated reactions is instructive. LDA/HMPA-mediated reactions afford considerable mechanistic variability.4,5 For example, enolizations of a hindered ester by LDA/THF and LDA/HMPA/THF proceed at similar rates by distinctly different mechanisms.4b The sole detectable pathway in neat THF involves disolvated monomers (11), whereas HMPA causes enolization to proceed via monosolvated monomers (12) and putative triple ions (13). (Three dimensional depictions of transition structures throughout this paper derive from computational studies,7,8 analogies with observable structural forms,4,6,7f and conjecture.)

Dehydrobrominations of alkyl bromides proceed by mono-, di- and trisolvated monomers (14-19) as well as triple ions (20).5 The highly variable solvation numbers are unusual when compared with LDA-mediated reactions in standard ethereal solvents.19

A seemingly minor point has baffled us. LDA/HMPA-mediated dehydrobrominations are insensitive to the proportions of THF in THF/hexane cosolvent. Conversely, enolization proceeding via putative triple ion 13 is inhibited by THF.4b A model based on the solvation of free (uncoordinated) HMPA by THF22 with an affiliated net stabilization of the ground state was postulated,4b but this explanation was subsequently dismissed as incorrect or at least inadequate.5 The dual role of ethers as both ligands and media continues to be vexing.23,24
Results
General Methods
Reactions carried out under standard conditions using 1.0-4.0 equiv of [6Li,15N]LDA6a confirm that a number of mixed aggregates are formed, as described in the context of each case study.25 6Li and 15N NMR spectroscopic data are summarized in Table 1. To avoid autoinhibition often caused by mixed aggregation4c,26 during the rate studies, pseudo-first-order conditions were established by using substrates at low concentrations (0.004 M). LDA, HMPA, and THF were maintained at high, yet adjustable, concentrations using inert cosolvents.27 The loss of 5 was monitored using gas chromatography relative to an internal dodecane standard.9 The loss of 1, 3, and 7 were monitored using in situ IR spectroscopy.4,28 All follow first-order decays, affording pseudo-first-order rate constants (kobsd) that are independent of the initial concentrations of substrate (±10%).29 Isotope effects (kH/kD) determined using deuterated analogues 1-d4 and 7-d5 (Table 2) are consistent with rate-limiting proton transfers for 1 and 7. The isotope effect obtained with epoxide 5-d2 is unusually small, emblematic of a proton transfer that is not rate limiting.
Table 1.
6Li and 15NMR spectroscopic data.a
| compd | δ 6Li(mult, JLiN) | δ15N (mult, JLiN) |
|---|---|---|
| 21 | 0.72 (s) | --- |
| 27a | 0.88 (d, 5.2) | 74.8 (q, 5.4) |
| 27b | 1.03 (d, 5.2) | 76.7 (q, 5.2) |
| 38 | 0.88 (d, 5.0) | 76.0 (q, 5.0) |
| 39 | 0.91 (d, 5.1) | 75.0 (q, 5.0) |
| 43 | 0.78 (d, 5.2) | 76.4 (q, 5.2) |
Spectra were recorded in THF solutions of 0.10 M [6Li,15N]LDA total lithium titer, 0.40 M total HMPA (bound and unbound), and 0.025 M substrate. Coupling constants are reported in hertz. Multiplicities are denoted as follows: s = singlet, d = doublet, t = triplet, q = quintet. The chemical shifts are reported relative to 0.30 M 6LiCl/MeOH at -90 °C (0.0 ppm) and neat Me2NEt at -90 °C (25.7 ppm). Spectra also contained [6Li,15N]LDA dimer 10: 6Li NMR δ 1.64 (t, 4.9); 15N NMR δ 73.3 (q, 4.8).
Table 2.
| Substrate | T (°C) | solvent | THF order | HMPA order | LDA order | kH/kD |
|---|---|---|---|---|---|---|
| 1 | 20 | THF | 0 | --- | 0.54±0.02a | 10±1b |
| 1 | -55 | THF/HMPA | 2.4±0.2 | 0 | 0.60±0.03c | ---- |
| 0 | 2.0±0.3d | 0.54±0.07e | 7.8±0.1e | |||
| 3 | -78 | THF/HMPA | 2.0±0.3 | 0 | 1.0±0.1f | --- |
| --g | 1.87±0.06h | 1.10±0.06i | --- | |||
| 5 | 20 | THF | 0 | --- | 0.73±0.04j | 3.6±0.4k |
| 5 | 0 | THF/HMPA | 0 | 0 | 1.0±0.1a | 1.3±0.1f |
| -0.6±0.2 | ||||||
| 7 | -40 | THF | 0 | --- | 0.49±0.01 | 16k |
| 7 | -40 | THF/HMPA | 0 | 0 | 0.51+0.04 | 14.2±0.2l |
[LDA] = 0.10 M in 10.0 M THF/hexane.
See ref 8b.
[HMPA] = 0.10 M in 8.0 M THF/hexane.
[LDA] = 0.10 M; [THF] = 8.0 M in hexane cosolvent.
[HMPA] = 0.40 M in 8.0 M THF/hexane.
[HMPA] = 0.40 M in 10.0 M THF/hexane.
The order in THF could not be measured at high HMPA concentration due to insolubility.
[LDA] = 0.10 M; [THF] = 10.0 M in hexane cosolvent.
[HMPA] = 0.10 M in 10.0 M THF/hexane.
[LDA] = 0.10 M; [THF] = 10.0 M in hexane cosolvent.
See ref 43.
[LDA] = 0.10 M; [HMPA] = 0.50 M in 10.0 M THF/hexane.

Selected rate data are depicted in Figures 1-6, and the reaction orders constituting the rate laws are summarized in Table 2. Rate data for the LDA/THF-mediated lithiations of 1 and 7 in the absence of HMPA reported previously are also included in Table 2 for comparison.8b,26 Rate studies of LDA/THF-based lithiation of 59a in THF with no added HMPA have not been reported previously and are described below.
Figure 1.

Plot of kobsd vs [HMPA] for the lithiation of imine 1 (0.005 M) by 0.10 M LDA in THF/hexane at -55 °C: (A) 2.0 M THF; (B) 8.0 M THF. Curve A derives from a linear least squares fit. Curve B depicts the result of an unweighted least-squares fit to kobsd = k[HMPA]n + k′ (k = (1.3 ± 0.1) × 10-3; k′ = (1.0 ± 0.2) × 10-4; n = 2.0 ± 0.3).
Figure 6.
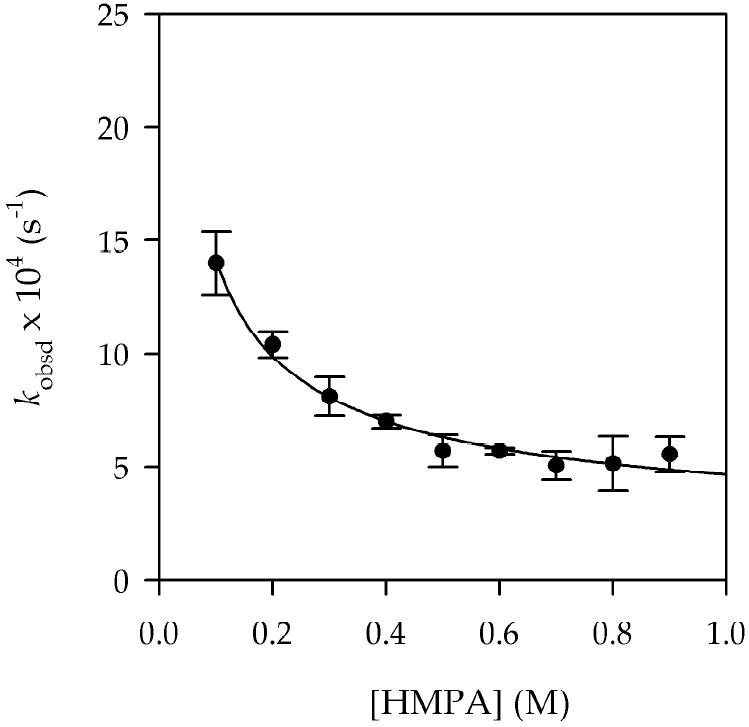
Plot of kobsd vs [HMPA] for the deprotonation of epoxide 5 (0.004 M) by 0.10 M LDA in THF (10.0 M)/hexane at 0 °C. The curve depicts the result of an unweighted least-squares fit to kobsd = k [HMPA]n + k′ (k = (3.3 ± 1.9) × 10-4; k′ = (1.3 ± 2) × 10-2; n = -0.6 ± 0.2).
Imine Lithiations
Previous 6Li and 15N NMR spectroscopic studies indicate that lithiated imine 2 is a monomer in THF.30 Analogous studies in which imine 1 is metalated by a modest excess of [6Li,15N]LDA (2.0-4.0 equiv) in the presence of 0.40 M HMPA reveal LDA dimer 10 along with a species displaying a singlet consistent with (but not rigorously assigned to) monomer 21. Mixed aggregates are not formed.
LDA/THF-mediated metalations of imine 1 proceed via mono-solvated monomer (22).8b,11 Conversely, the LDA/HMPA/THF-mediated metalation of imine 1 displays a stifling complexity. A plot of kobsd versus HMPA concentration shows a first-order HMPA dependence in 2.0 M THF and a second-order HMPA dependence in 8.0 M THF (curves A and B, respectively, in Figure 1). The HMPA concentration-independent pathway--the non-zero intercepts in curves A and B--is also independent of the THF concentration. The THF concentration dependence can be illustrated from a different perspective in which the HMPA concentration is held constant and the THF concentration is varied (Figure 2). At low HMPA concentration the rates are independent of the THF concentration (curve A), whereas at high HMPA concentration an exponential (2.4 ± 0.4 order) dependence on the THF concentration is evident (curve B).
Figure 2.
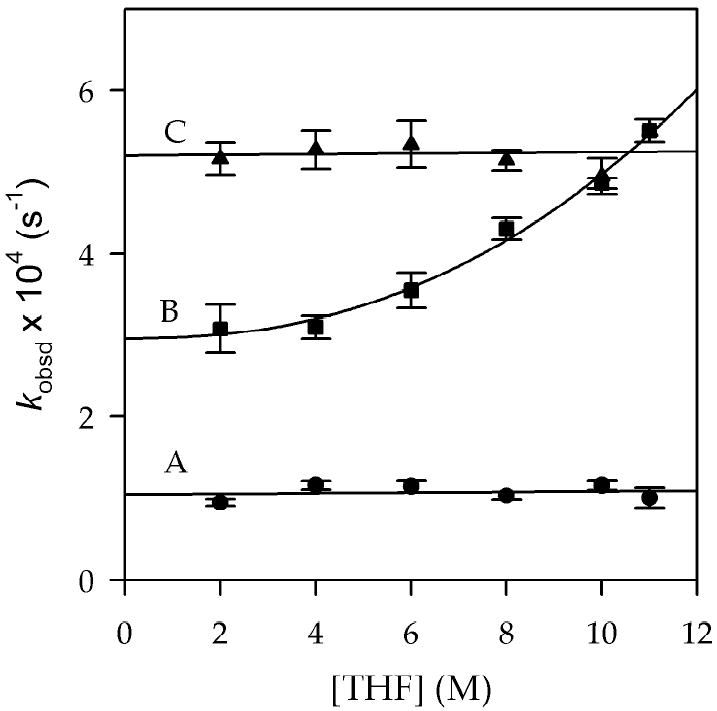
Plot of kobsd vs [THF] for the lithiation of imine 1 (0.005 M) by 0.10 M LDA in hexane with HMPA at -55 °C: (A) 0.10 M free HMPA in cyclopentane; (B) 0.50 M free HMPA in cyclopentane; (C) 0.50 M free HMPA in 2,5-Me2THF. Curves A and C derive from linear least squares fits. Curve B depicts the result of an unweighted least-squares fit to kobsd = k [THF]n + k′ (k = (7.6 ± 0.8) × 10-7; k′ = (3.0 ± 0.1) × 10-4; n = 2.4 ± 0.4).
The solvent dependencies are consistent with three independent terms in the idealized31 partial rate law described by eq 5. We wondered whether the THF dependence derives from a sterically sensitive, primary-shell solvation by THF or a sterically insensitive secondary-shell effect.4b,23,24 Compelling evidence of secondary-shell solvation was uncovered in a plot of kobsd versus THF concentration using the weakly coordinating 2,5-dimethyltetrahydrofuran (2,5-Me2THF) as cosolvent (Figure 2, curve C). When the polarity of the medium is held constant using a poorly coordinating cosolvent,32 the apparent second-order THF dependence disappears.33 Thus, although the rate law is formally described by eq 5, we choose to cull out the apparent secondary-shell solvation effects and provide the simplified version described by eq 6.
| (5) |
| (6) |
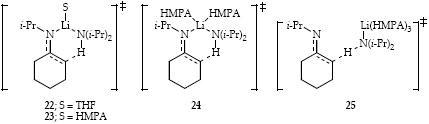
Plots of kobsd versus LDA concentration reveal half-order LDA dependencies at both low and high HMPA concentrations consistent with monomer-based metalations (Figure 3). The fractional LDA orders in conjunction with HMPA and ethereal cosolvent dependencies are consistent with the idealized rate law31 in eq 7. A literal interpretation of eq 7 implicates transition structures [(i-Pr2NLi)(HMPA)(1)]‡, [(i-Pr2NLi)(HMPA)2(1)]‡, and [(i-Pr2NLi)(HMPA)3(1)]‡ for which we offer depictions 23, 24, and 25, respectively; however, we advise caution against over interpreting such a three-term rate law.
| (7) |
Figure 3.

Plot of kobsd vs [LDA] for the lithiation of imine 1 (0.005 M) in 0.40 M free HMPA/8.0 M THF/cyclopentane at -55 °C. The curves depicts the result of an unweighted least-squares fit to kobsd = k[LDA]n (k = (1.4 ± 0.2) × 10-3; n = 0.54 ± 0.07).
Conjugate Additions
LDA in THF solution undergoes conjugate addition to 3 in lieu of either α- or γ-deprotonation.13a However, the results are highly unusual and part of an emerging mechanistic story to be discussed in another context.34 LDA/HMPA-mediated conjugate additions proceed smoothly and provide tractable rate data as follows.
Conjugate addition of LDA to unsaturated ester 3 in HMPA/THF (eq 2) affords β-amino ester 26 in 76% yield. Analogous addition with 2.0-4.0 equiv of [6Li,15N]LDA forms two mixed dimers that we believe are the two geometric isomers (27a,b). Enolization of amino ester 26 with 2.0-4.0 equiv of [6Li,15N]LDA affords only one isomer. The stereochemical assignments of the E and Z isomers has not been made. Enolization with 1.25 equiv of LDA affords enolate 4 in an unknown aggregation state.35

Rate studies carried out under pseudo-first-order conditions afforded evidence of several mechanisms. A plot of kobsd versus HMPA concentration reveals a second-order HMPA dependence in 10.0 M THF (Figure 4). An HMPA concentration-independent pathway is evidenced by a significant non-zero y-intercept. Plots of kobsd versus LDA concentration at both low and high HMPA concentrations reveal first-order dependencies, indicating that both the HMPA-independent and HMPA-dependent pathways are dimer based. In principle, transition structures [(R2NLi)2(HMPA)4]‡ (30) and [(R2NLi)2(HMPA)2]‡ (possibly 28 or 29) are consistent with the rate data. However, second-order dependencies on the THF concentration with distinct non-zero intercepts at both low and high HMPA concentrations (Figure 5, curve A) suggest two additional transition structures corresponding to [(R2NLi)2(HMPA)2(THF)2]‡ and [(R2NLi)2(HMPA)4(THF)2]‡.36 One could imagine describing the former transition structure as mixed-solvated triple ion 31. The high solvation number of [(R2NLi)2(HMPA)4(THF)2]‡--formally a hexasolvated dimer--stretches our imaginations. A plot of kobsd versus THF concentration at low HMPA concentration (0.10 M) using 2,5-Me2THF as the cosolvent to hold the polarity of the medium relatively constant (Figure 5, curve B) simply adds further confusion by revealing a first-order THF dependence rather than the second-order dependence noted in hexane.
Figure 4.

Plot of kobsd vs [HMPA] for the Michael addition to ester 3 (0.004 M) by 0.10 M LDA in THF (10.0 M)/hexane at -78 °C. The curve depicts the result of an unweighted least-squares fit to kobsd = k[THF]n + k′ (k = (7.1 ± 0.3) × 10-3; k′ = (8.2 ± 0.2) × 10-2; n = 1.87 ± 0.06).
Figure 5.

Plot of kobsd vs [THF] for the Michael addition to ester 3 (0.004 M) by 0.10 M LDA with 0.10 M free HMPA at -78 °C in (A) 2,5-Me2THF as cosolvent; (B) hexane as cosolvent. Curve A derives from a linear least squares fit. Curve B depicts an unweighted least-squares fit to kobsd= k[THF]n + k′ (k = (3.8 ± 0.5) × 10-6; k′ = (3.8 ± 0.3) × 10-4; n = 2.0 ± 0.4).

Epoxide Metalation
We examined the LDA/HMPA-mediated metalation of several epoxides and confirmed16a,b that epoxides 32 and 33 are unreactive even at ambient temperatures, above which the integrity of LDA/HMPA/THF becomes suspect.37,38 Metalation of epoxide 5 in HMPA/THF (eq 3) affords alcohol 34 deriving from a transannular C-H insertion by a carbenoid intermediate in 80% isolated yield.

LDA/THF-mediated metalations of epoxide 5 display an odd (0.73 ± 0.04) fractional order in LDA and a clean zeroth-order dependence on the THF concentration. The idealized rate law (eq 8)31 is consistent with reaction via mono-solvated monomers (eq 9) and disolvated dimers (eq 10). (Previous studies of the LDA-mediated metalation of epoxide 5 using chelating ligands showed no evidence of a monomer-based pathway.) Transition structures 35-37 seem reasonable,7,8,39 although the deviation from an optimal 180° C-H-N angle appears significant in 35.7,9a,40
| (8) |
| (9) |
| (10) |
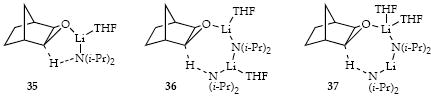
Metalation of epoxide 5 (0.25 M) using [6Li,15N]LDA (0.10 M) with HMPA (0.40 M) reveals a mixed dimer that is replaced by another mixed dimer. The latter was confirmed to be 38 by mixing [6Li,15N]LDA with alcohol 34. The first-formed mixed dimer is believed to be 39 derived from the observable lithiation before carbenoid formation/C-H insertion.41 Quenching with D2O failed to afford significant levels of deuterated 34, which is not particularly surprising in light of Seebach’s studies of D2O quenches when i-Pr2NH is present. 42 In the absence of HMPA, little or no metalated epoxide is observed (although some minor 6Li resonances are noted.)
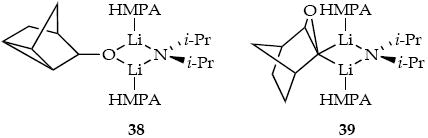
The intermediacy of 39 introduces an inordinate complexity that was unappreciated when the solution kinetics were investigated. Because we monitored the metalation using GC analysis of quenched samples, mixed dimer 39 registered as starting material rather than product. Consequently, the measured reaction orders listed in Table 2 are not easily interpreted. The small isotope effect (kH/kD = 1.3 ± 0.1) makes sense if the metalation is reversible. The observed reaction displays a net inhibition by HMPA (Figure 6), but the metalated intermediate causes us to resist further interpretation of the rate data at this point. These suspect data are archived in supporting information.
Carbamate Ortholithiation
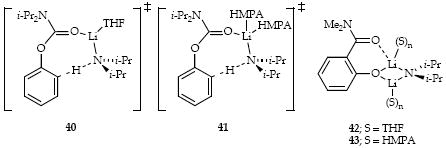
Previous studies of the LDA/THF-mediated ortholithiation of carbamate 7 show a rate-limiting metalation via monosolvated monomer (40) followed by a rapid (post-rate-limiting) anionic Fries rearrangement. Formation of mixed dimer 42 causes a marked autoinhibition when only 1.0 equiv of LDA is used. Analogous behavior is observed in the presence of HMPA: mixed dimer 43 is observed to the exclusion of any intermediate aryllithium derivatives. Under pseudo-first-order conditions a large kH/kD confirms a rate-limiting proton transfer. (The particularly large isotope effect is characteristic of ortholithiations.43) A first-order HMPA concentration dependence and a half-order LDA concentration dependence implicate disolvated monomer-based metalation (41). A zeroth-order dependence on the THF concentration shows that medium effects are unimportant.
Discussion
We studied four LDA-mediated reactions--lithiation of imine 1 (eq 1), conjugate addition to unsaturated ester 3 (eq 2), α-deprotonation of epoxide 5 (eq 3), and ortholithiation of carbamate 7 (eq 4). All are important in organic synthesis, and HMPA has played a role in controlling reactivity in each case. The results are summarized below and discussed in more detail subsequently. We must reiterate an important point: The rate studies provide only the stoichiometries of the transition structures at the rate-limiting steps;20 the three-dimensional renditions of the transition structures are based on computational studies,7,8 analogies with observable structural forms,6,21,32a and conjecture. We routinely offer this caveat in the context of rate data, but it seems especially germane in the context of LDA/HMPA-mediated reactions.
Summary
The rate studies were prefaced by structural studies showing aggregate changes throughout the reaction coordinate when moderate excesses of LDA/HMPA are used. LDA/HMPA-mediated metalation of imine 1 affords an LDA-free lithiated imine believed to be monomer 21 based on prior studies.30 Analogous reaction of epoxide 3 forms low concentrations of an intermediate lithiated epoxide as mixed dimer 39. Subsequent carbenoid-derived insertion leads to LDA-alkoxide mixed aggregate 38. Michael addition of LDA/HMPA to unsaturated ester 5 provides mixed dimer 27 as a putative E-Z mixture. Ortholithiation of aryl carbamate 7 affords an undetectable aryllithium that undergoes facile (post-rate-limiting) Fries rearrangement to give LDA-aryloxide mixed dimer 43 as the only observable product. Previous studies of mixed aggregation in LDA/HMPA mixtures are consistent with this picture of a highly salt-dependent penchant toward mixed aggregation.4c,6b,7e
Rate studies were carried out under pseudo-first-order conditions to preclude mixed aggregation effects. The intermediacy of metalated epoxide 39 proved disruptive to detailed rate studies. For the most part, however, the results are tractable and reveal considerable mechanistic diversity. The influence of THF in all LDA/HMPA-mediated reactions is discussed in a subsequent section.
Metalation of imine 1 by LDA/HMPA is mechanistically complex, displaying concentration dependencies implicating several monomer-based pathways (23-25). This complexity contrasts with analogous metalations in the absence of HMPA in which a single pathway involving a monosolvated monomer (22) is detected. LDA/HMPA-mediated conjugate addition to ester 3 proceeds via disolvated and tetrasolvated dimer-based pathways for which we depict 28 or 29 for the former and triple ion 30 for the latter. LDA/HMPA-mediated metalation of epoxide 5 was only marginally informative because of the formation of an observable lithiated epoxide (39). Even the metalation in the absence of HMPA, however, proved to be quite complex, implicating a combination of monosolvated monomer (35) and disolvated dimer (36 or 37). Last, the ortholithiation of carbamate 7 proceeds via a di-HMPA-solvated monomer (41).
Are mixed and secondary-shell solvation important?
Secondary-shell solvation--the influence of solvent as simply a medium--has received scant attention in organolithium chemistry.4,23,24 Extensive investigations of LDA-mediated metalations have generally revealed that primary-shell solvation is critical, but secondary-shell solvation is unimportant. In the case of LDA/HMPA-mediated reactions, one might presume that THF, normally a strongly coordinating solvent, would be relegated to the role of inert cosolvent. Indeed, LDA/HMPA-mediated dehydrohalogenations discussed as background (vide supra)5 and metalations of epoxide 5 and aryl carbamate 7 described herein show no influence by THF whatsoever. In short, the reaction rates in HMPA/THF/hydrocarbon mixtures are independent of the THF concentrations.
On occasion, however, supposedly inert cosolvents can influence organolithium structure and reactivity.23,24 For example, monomer-based ester enolization (see 12) is not measurably influenced by THF, whereas the putative triple-ion-based pathway (see 13) is inhibited by THF. There is no evidence that primary-shell solvation by THF is the culprit. Similarly, LDA/HMPA-mediated metalation of imine 1 via putative monomer 25 is markedly accelerated by THF whereas lower-solvated analogs 23 and 24 are not influenced by the THF concentration. By using THF/2,5-Me2THF cosolvent mixtures (rather than THF/hexane) we observe a loss of the THF concentration dependence, suggesting that the influence of the cosolvent is purely through secondary-shell (medium) effects. It is tempting, plausible, and convenient to conclude that the influence of the ethereal cosolvent is due exclusively to secondary-shell solvation. It may not be that simple, however.
The LDA/HMPA-mediated conjugate addition to ester 3 proceeds via a disolvated dimer-based pathway (28 or 29) and a tetrasolvated dimer-based pathway (30). Both have an affiliated second-order THF dependence in THF/hexane mixtures. The role of THF, however, is very strange. By holding the polarity of the medium fixed and varying the THF with 2,5-Me2THF as the cosolvent, the second-order THF dependence gives way to a first-order dependence. Given the pronounced steric demands of both LDA and HMPA and consequent buttressing, one cannot rule out primary-shell solvation by HMPA and THF. Are we suggesting that there is both primary- and secondary-shell solvation by THF? Possibly, but not with any conviction. We must confess that, despite continued efforts to distinguish primary and secondary-shell solvation effects, the latter remains largely inscrutable.
Is such mechanistic complexity unusual?
Surveying mechanisms underlying LDA/HMPA-mediated reactions one cannot help but notice the large variation of both monomer- and dimer-based pathways. An inventory of LDA/HMPA-mediated reactions reported to date includes reactions based on [(i-Pr2NLi)(HMPA)1-3]‡ and [(i-Pr2NLi)2(HMPA)2-4]‡--six total (not including putative mixed solvated forms). If each case study is viewed in isolation, the results seem reasonable. We have no reason to doubt the veracity of the data and, consequently, do not doubt the diversity of the behavior. Nevertheless, the case studies taken together afford a complexity that is daunting, vexing, and unique to LDA/HMPA-mediated reactions.
Is HMPA special?
The short answer is yes. HMPA presents a profound conflict between a marked Lewis basicity affiliated with the P=O dipole2a,44-46 and the exceptional steric demands of the splaying dimethylamino groups.7 Semiempirical computational studies of lithium amide solvation that compare HMPA with (H2N)3P=O attest to the high affinity of phosphoramides for lithium ion.7 However, the exothermicity of serial solvation tapers off gradually for (H2N)3P=O drops off markedly for HMPA. Reich and coworkers have observed this experimentally in the serial solvation of Li cations by HMPA in ethereal solvents: Three HMPA substitutions occur quantitatively; a fourth HMPA substitutes reluctantly.2 We also cannot ignore the idea expressed previously that solvation of uncoordinated HMPA could contribute significantly to its penchant for binding to lithium cations. Solvent-solvent interactions in solutions of HMPA have been discussed.23
Conclusion
LDA/HMPA elicits marked increases in mechanistic complexity compared with LDA in the absence of HMPA. This increased complexity may derive from the pronounced steric demands of both the LDA and HMPA. We have documented a plethora of mechanisms for LDA/HMPA-mediated reactions and have asked as many questions as we have answered. It is curious that the influence of HMPA on reaction rates can, as exemplified by the relative rate constants in Table 1, border on insignificant. The often marginal influence on reaction rates seems incongruent with the significant influence HMPA imparts on mechanism and its importance in organic synthesis.
Experimental Section
Reagents and Solvents
Ethereal solvents, hydrocarbons, and HMPA were vacuum transferred from calcium hydride. The hydrocarbon stills contained 1% tetraglyme to dissolve the ketyl. Imines 1 and 1-d411 and epoxides 5 and 5-d29 were recrystallized. Unsaturated ester 3 was prepared as described in Supporting Information. LDA was prepared as a solid from commercial n-BuLi and purified using a standard literature procedure.6a Air- and moisture-sensitive materials were manipulated under argon or nitrogen following standard glovebox, vacuum line, and syringe techniques.
Kinetics
The rate studies were carried out using methods based on in situ IR spectroscopy4,28 or gas chromatography9 as described in detail previously.
Supplementary Material
Table 3.
Relative rate constants for reactions of LDA.a
| substrate | Temp (°C) | kHMPA:kTHF |
|---|---|---|
| 1 | -55 | 3:1 |
| 3 | -78 | 4:125 |
| 5 | 0 | 1:6 |
| 7 | -40 | 1.5:1 |
LDA is 0.10 M in either 10.0 M THF/hexane or 0.40 M HMPA and 10.0 M THF/hexane.
Acknowledgments
We thank the National Institutes of Health for direct support of this work and Merck, Pfizer, Boehringer Ingelheim, R. W. Johnson, Sanofi-Aventis, Schering-Plough, and DuPont Pharmaceuticals (Bristol-Myers Squibb) for indirect support.
Footnotes
Supporting Information: NMR spectra, rate data, and experimental protocols (44 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References and Footnotes
- 1.Dykstra RR. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. Vol. 4. Wiley; New York: 1995. p. 2668.. (b) For a particularly interesting survey of stereo- and regioselectivity affiliated with organolithium chemistry, see: Clayden J. Organolithiums: Selectivity for Synthesis. Pergamon; New York: 2002.
- 2.Reich HJ, Kulicke KJ. J Am Chem Soc. 1995;117:6621.Reich HJ, Green DP, Medina MA, Goldenberg WJ, Gudmundsson BÖ, Dykstra RR, Philips NH. J Am Chem Soc. 1998;120:7201.Reich HJ, Sikorski WH, Gudmundsson BÖ, Dykstra RR. J Am Chem Soc. 1998;120:4035.Reich HJ, Holladay JA, Mason JD, Sikorski WH. J Am Chem Soc. 1995;117:12137.Reich HJ, Borst JP, Dykstra RR, Green DP. J Am Chem Soc. 1993;115:8728.Jantzi KL, Puckett CL, Guzei IA, Reich HJ. J Org Chem. 2005;70:7520. doi: 10.1021/jo050592+. and references cited therein.
- 3.Leading references: Reich HJ, Sanders AW, Fiedler AT, Bevan MJ. J Am Chem Soc. 2002;124:13386. doi: 10.1021/ja026915q.Reich HJ, Green DP, Phillips NH. J Am Chem Soc. 1989;111:3444.Reich HJ, Phillips NH, Reich IL. J Am Chem Soc. 1985;107:4101.Reich HJ, Dykstra RR. Angew Chem Int Ed Engl. 1993;32:1469.Reich HJ, Sikorski WH. J Org Chem. 1999;64:14. doi: 10.1021/jo981765g..
- 4.(a) Sun X, Kenkre SL, Remenar JF, Gilchrist JH, Collum DB. J Am Chem Soc. 1997;119:4765. [Google Scholar]; (b) Sun X, Collum DB. J Am Chem Soc. 2000;122:2452. [Google Scholar]; (c) Sun X, Collum DB. J Am Chem Soc. 2000;122:2459. [Google Scholar]
- 5.Ma Y, Ramirez A, Singh KJ, Keresztes I, Collum DB. J Am Chem Soc. 2006;128:15399. doi: 10.1021/ja060964b. [DOI] [PubMed] [Google Scholar]
- 6.Romesberg FE, Gilchrist JH, Harrison AT, Fuller DJ, Collum DB. J Am Chem Soc. 1991;113:5751.Romesberg FE, Collum DB. J Am Chem Soc. 1994;116:9198.. Also, see ref 4.
- 7.(a) Romesberg FE, Collum DB. J Am Chem Soc. 1992;114:2112. [Google Scholar]; (b) Armstrong DR, Mulvey RE, Walker GT, Barr D, Snaith R, Clegg W, Reed D. J Chem Soc Dalton Trans. 1988:617. [Google Scholar]; (c) Armstrong DR, Barr D, Brooker AT, Clegg W, Gregory K, Hodgson SM, Snaith R, Wright DS. Angew Chem Int Ed Engl. 1990;102:443. [Google Scholar]; (d) Romesberg FE, Collum DB. J Am Chem Soc. 1992;114:2112. [Google Scholar]; (e) Romesberg FE, Collum DB. J Am Chem Soc. 1994;116:9187. [Google Scholar]; (f) Romesberg FE, Collum DB. J Am Chem Soc. 1995;117:2166. [Google Scholar]
- 8.(a) Ramirez A, Lobkovsky E, Collum DB. J Am Chem Soc. 2003;125:15376. doi: 10.1021/ja030322d. [DOI] [PubMed] [Google Scholar]; (b) Liao S, Collum DB. J Am Chem Soc. 2003;125:15114. doi: 10.1021/ja030409z. [DOI] [PubMed] [Google Scholar]; (c) Pratt L, Robbins S. J Mol Struct (THEOCHEM) 1999;466:95. [Google Scholar]
- 9.(a) Ramirez A, Collum DB. J Am Chem Soc. 1999;121:11114. [Google Scholar]; (b) Wiedemann SH, Ramirez A, Collum DB. J Am Chem Soc. 2003;125:15893. doi: 10.1021/ja0304087. [DOI] [PubMed] [Google Scholar]; (c) Remenar JF, Collum DB. J Am Chem Soc. 1997;119:5573. [Google Scholar]
- 10.For an incisive review of lithium amides in organic synthesis, see: Eames J. Product Subclass 6: Lithium Amides. In: Snieckus V, editor. Science of Synthesis. 8a. Thieme; New York: 2006. p. 173.
- 11.Bernstein MP, Collum DB. J Am Chem Soc. 1993;115:8008. [Google Scholar]
- 12.For leading references to the organolithium chemistry of imines, see: Meyers AI. J Org Chem. 2005;70:6137. doi: 10.1021/jo050470h.
- 13.(a) Herrman JL, Kieczykowski GR, Schlessinger RH. Tetrahedron Lett. 1973:2433. [Google Scholar]; (b) Uyehara T, Asao N, Yamamoto Y. J Chem Soc Chem Commun. 1987:1410. [Google Scholar]; (c) Bellasoued M, Ennigrou R, Gaudemar M. J Organomet Chem. 1988;338:149. [Google Scholar]
- 14.Davies SG, Smith AD, Price PD. Tetrahedron: Asymmetry. 2005;16:2833. [Google Scholar]
- 15.(a) Satoh T. Chem Rev. 1996;96:3303. doi: 10.1021/cr950081v. [DOI] [PubMed] [Google Scholar]; (b) Crandall JK, Apparu M. Org React. 1983;29:345. [Google Scholar]; (c) Magnus A, Bertilsson SK, Andersson PG. Chem Soc Rev. 2002;31:223. doi: 10.1039/b104372m. [DOI] [PubMed] [Google Scholar]; (d) Hodgson DM, Gras E. Synthesis. 2002:1625. [Google Scholar]; (e) Hodgson DM, Chung YK, Nuzzo I, Freixas G, Kulikiewicz KK, Cleator E, Paris J-M. J Am Chem Soc. 2007;129:4456. doi: 10.1021/ja0672932. [DOI] [PubMed] [Google Scholar]; (f) Yanagisawa A, Yasue K, Yamamoto H. J Chem Soc Chem Commun. 1994:2103. [Google Scholar]; (g) Chemla F, Vrancken E. In: The Chemistry of Organolithium Compounds. Chap 18 Rappoport Z, Marek I, editors. Vol. 2. Wiley; New York: 2004. [Google Scholar]
- 16.(a) Morgan KM, Gronert S. J Org Chem. 2000;65:1461. doi: 10.1021/jo991619q. [DOI] [PubMed] [Google Scholar]; (b) Morgan KM, Gajewski JJ. J Org Chem. 1996;61:820. [Google Scholar]; (c) Crandall JK, Crawley LC, Banks DB, Lin L. J Org Chem. 1971;36:510. [Google Scholar]; (d) Thummel RP, Rickborn B. J Am Chem Soc. 1970;92:2064. [Google Scholar]; (e) Cope AC, Berchtold GA, Peterson PE, Sharman SH. J Am Chem Soc. 1960;82:6370. [Google Scholar]; (f) Gronert S, Lee JM. J Org Chem. 1995;60:448. [Google Scholar]
- 17.The mechanisms of epoxide metalations by chiral lithium amides have been studied extensively: Pettersen D, Diner P, Amedjkouh M, Ahlberg Per I. Tetrahedron: Asymmetry. 2004;15:1607.Yoshida T, Sakakibara K, Asami M. Chem Lett. 2003;32:150.Arvidsson Per I, Hilmersson G, Davidsson O. Helv Chim Acta. 2002;85:3814.Pettersen D, Amedjkouh M, Lill SON, Dahlen K, Ahlberg Per I. J Chem Soc Perkin Trans 2. 2001;9:1654.Hilmersson G, Malmros B. Chem Eur J. 2001;7:337. doi: 10.1002/1521-3765(20010119)7:2<337::aid-chem337>3.0.co;2-i.Olsson RI, Ahlberg Per I. Tetrahedron: Asymmetry. 1999;10:3991.
- 18.(a) Hartung CG, Snieckus V. In: Modern Arene Chemistry. Chapter 10 Astruc D, editor. Wiley-VCH; Weinheim: 2002. [Google Scholar]; (b) Snieckus V. Chem Rev. 1990;90:879. [Google Scholar]; (c) Taylor CM, Watson AJ. Curr Org Chem. 2004;8:623. [Google Scholar]
- 19.For a review of rates studies of LDA-mediated reactions, see: Collum DB, McNeil AJ, Ramirez A. Angew Chem Int Ed. 2007;49:3002. doi: 10.1002/anie.200603038.
- 20.Edwards JO, Greene EF, Ross J. J Chem Educ. 1968;45:381. [Google Scholar]
- 21.For reviews of structural studies of N-lithiated species, see: Collum DB. Acc Chem Res. 1993;26:227.Gregory K, Schleyer PvR, Snaith R. Adv Inorg Chem. 1991;37:47.Mulvey RE. Chem Soc Rev. 1991;20:167.Lucht BL, Collum DB. Acc Chem Res. 1999;32:1035..
- 22.For discussions of solvent-solvent interactions in solutions of HMPA, see: Masaguer JR, Casas JS, Sousa Fernandez A, Sordo J An Quim. 1973;69:199.Michou-Saucet MA, Jose J, Michou-Saucet C, Merlin JC. Thermochim Acta. 1984;75:85.Vandyshev VN, Serebryakova AL. Russ J Gen Chem. 1997;67:540.Kulikov MV. Russ Chem Bull. 1997;46:274.Mehta SK, Sharma AK, Bhasin KK, Parkash R. Fluid Phase Equilib. 2002;201:203.Izutsu K, Kobayashi N. J Electroanal Chem. 2005;574:197.Prado-Gotor R, Ayala A, Tejeda AB, Suarez MB, Mariscal C, Sanchez MD, Hierro G, Lama A, Aldea A, Jimenez R. Int J Chem Kinet. 2003;35:367.Salomon M. J Power Sources. 1989;26:9..
- 23.(a) Lucht BL, Collum DB. J Am Chem Soc. 1996;118:2217. [Google Scholar]; (b) Lucht BL, Bernstein MP, Remenar JF, Collum DB. J Am Chem Soc. 1996;118:10707. [Google Scholar]; (c) Reimers JR, Hall LE. J Am Chem Soc. 1999;121:3730. [Google Scholar]; (d) Wu S, Lee S, Beak P. J Am Chem Soc. 1996;118:715. [Google Scholar]; (e) Chadwick ST, Rennels RA, Rutherford JL, Collum DB. J Am Chem Soc. 2000;122:8640. [Google Scholar]; (f) Lewis HL, Brown TL. J Am Chem Soc. 1970;92:4664. [Google Scholar]; (g) Hsieh HL, Quirk RP. Anionic Polymerization: Principles and Practical Applications. Marcel Dekker; New York: 1996. [Google Scholar]
- 24.Arene-lithium interactions have been discussed extensively by Dougherty and co-workers. Ma JC, Dougherty DA. Chem Rev. 1997;97:1303. doi: 10.1021/cr9603744.
- 25.For spectroscopic studies of LDA mixed aggregates in HMPA/THF mixtures, see refs 4c and 6b. For analogous computational studies, see ref 7e.
- 26.Singh K, Collum DB. J Am Chem Soc. 2006;128:13753. doi: 10.1021/ja064655x. [DOI] [PubMed] [Google Scholar]
- 27.The concentration of LDA, although expressed in units of molarity, refers to the concentration of the monomer unit (normality). The concentrations of ethereal solvent and HMPA are expressed as total concentration of free (uncoordinated) ligand.
- 28.Rein AJ, Donahue SM, Pavlosky MA. Curr Opin Drug Discovery Dev. 2000;3:734. [PubMed] [Google Scholar]
- 29.Espenson JH. Chemical Kinetics and Reaction Mechanisms. McGraw-Hill; New York: 1995. p. 15. [Google Scholar]
- 30.Jackman LM, Scarmoutzos LM, Porter W. J Am Chem Soc. 1987;109:6524. [Google Scholar]; Jackman LM, Scarmoutzos LM, Smith BD, Williard PG. J Am Chem Soc. 1988;110:6058. doi: 10.1021/ja00226a021. [DOI] [PubMed] [Google Scholar]; Zuend SJ, Ramirez A, Lobkovsky E, Collum DB. J Am Chem Soc. 2006;128:5939. doi: 10.1021/ja060363k. [DOI] [PubMed] [Google Scholar]
- 31.We define the idealized rate law as that obtained by rounding the observed reaction orders to the nearest rational order.
- 32.(a) Remenar JF, Lucht BL, Collum DB. J Am Chem Soc. 1997;119:5567. [Google Scholar]; (b) Lucht BL, Collum DB. J Am Chem Soc. 1995;117:9863. [Google Scholar]
- 33.Alternatively, plots of kobsd versus HMPA concentration in an assortment of ethereal solvents display approximately second-order dependencies. The relative ether-dependent accelerations follow this order (with relative rates in parentheses): MeTHF (4) > 2,5-Me2THF (3) > THF (1) ≈ Et2O (1). The lack of correlation with measured binding constants (ref 32) further argues against a primary-shell effect.
- 34.Reactions of LDA with a number of substrates in THF at -78 °C display linear plots of substrate concentration versus time as well as odd sigmoidal behaviors, all of which are part of an ongoing study.
- 35.Enolates bearing a β-NH2 moiety form hexamers in solution: McNeil AJ, Toombes GES, Gruner SM, Lobkovsky E, Collum DB, Chandramouli SV, Vanasse BJ, Ayers TA. J Am Chem Soc. 2004;126:16559. doi: 10.1021/ja045144i.
- 36.Leading references to mixed solvation: Qu B, Collum DB. J Am Chem Soc. 2005;127:10820. doi: 10.1021/ja0519987.
- 37.Stanetty P, Mihovilovic MD. J Org Chem. 1997;62:1514.Aubrecht KB, Collum DB. J Org Chem. 1996;61:8674.Bernstein MP, Romesberg FE, Fuller DJ, Harrison AT, Williard PG, Liu QY, Collum DB. J Am Chem Soc. 1992;114, 5100 and references cited therein.
- 38.Morgan and coworkers investigated the elimination of cyclopentene oxide by LDA/HMPA, apparently in the absence of base-labile ethereal ligands (ref 16a,b).
- 39.For additional leading references to spectroscopic, crystallographic, and kinetic evidence of open dimers, see: Zhao P, Collum DB. J Am Chem Soc. 2003;125:14411. doi: 10.1021/ja030168v.
- 40.Bell RP. The Tunnel Effect in Chemistry. Chapman & Hall; New York: 1980. [Google Scholar]
- 41.For leading references to oxacarbenoids, see: Pratt Lawrence M, Ramachandran B. J Org Chem. 2005;70:7238. doi: 10.1021/jo050887n.Boche G, Lohrenz JCW. Chem Rev. 2001;101:697. doi: 10.1021/cr940260x.
- 42.Seebach D, Aebi JD. Helv Chim Acta. 1985;68:1507. [Google Scholar]
- 43.Singh K, Collum DB. J Am Chem Soc. 2006;128:13753. doi: 10.1021/ja064655x. [DOI] [PubMed] [Google Scholar]
- 44.For discussions of steric effects on lithium ion solvation by HMPA, see: Ishiguro S. Pure Appl Chem. 1994;66:393.Dack MRJ, Bird KJ, Parker AJ. Aust J Chem. 1975;28:955.Ishiguro S. Bull Chem Soc Jpn. 1997;70:1465.
- 45.Burford N, Royan BW, Spence REvHT, Cameron S, Linden A, Rogers RD. J Chem Soc Dalton Trans. 1990:1521. [Google Scholar]
- 46.HMPA has been estimated to bind 300 times more strongly than THF in one case (ref 2a).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


