Abstract
Drug-induced torsades de pointes (TdP), a rare, life-threatening, polymorphic, ventricular tachycardia associated with prolongation of the QT interval, has been the main safety reason for the withdrawal of medicines from clinical use over the last decade. Most often, drugs that prolong the action potential and delay ventricular repolarization do so through blockade of outward (repolarizing) currents, predominantly the rapid delayed rectifying potassium current, IKr. While QT interval prolongation is not a safety concern per se, in a small percentage of people, it has been associated with TdP, which either spontaneously terminates or degenerates into ventricular fibrillation. Furthermore, recent data suggest that shortening of the QT interval may also be a new safety issue waiting to surface. This review article summarizes the presentations given at a symposium entitled ‘Reducing QT liability and proarrhythmic risk in drug discovery and development’, which was part of the Federation of the European Pharmacological Societies congress, Manchester, UK, 13–17 July 2008. The objective of this symposium was to assess the effects of implementing the latest regulatory guidance documents (International Conference on Harmonization S7A/B and E14), as well as new scientific and technical trends on the ability of the pharmaceutical industry to reduce and manage the QT liability and associated potential proarrhythmic risk, and contribute to the discovery and development of safer medicines. This review outlines the key messages from communications presented at this symposium and attempts to highlight some of the key challenges that remain to be addressed.
This article is part of a themed section on QT safety. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2010
Keywords: QT interval prolongation, drug discovery and development
Introduction
Non-clinical and clinical cardiovascular toxicities remain, alongside hepatic toxicities, the main safety reason for (i) drug discontinuation throughout all stages of drug discovery and development; (ii) serious adverse events and adverse drug reactions during clinical development and post marketing; and (iii) postmarketing drug withdrawal. Cardiovascular adverse effects are diverse and in some instances can be life threatening. They are not necessarily related to the primary pharmacological target, the therapeutic class or the chemical class, although most of them are dose related and, therefore, predictable from primary, secondary or safety pharmacology screens. Cardiovascular toxicities affect any of the components of the cardiovascular system, namely, the heart, blood vessels and blood components, and can result from functional and/or structural modifications.
The QT interval begins at the onset of the QRS complex and terminates at the end of the T wave. It represents the time of ventricular depolarization and repolarization, where the QRS interval represents the time it takes for depolarization of the ventricles. Prolongation of the QT interval on the electrocardiogram (ECG) had been the reason for one-third of all drug withdrawals for the period 1990–2006 (Shah, 2005). Although this effect is not in itself a safety concern, QT interval prolongation can be associated with the potentially fatal arrhythmia called torsades de pointes (TdP). Moreover, even though TdP is extremely rare, there was evidence of TdP for most of the withdrawn drugs (Redfern et al., 2003). Considering the time and cost of developing novel, effective and safe medicines, it is not surprising that QT prolongation remains a cause for concern for regulators, clinicians, and drug discoverers and developers from academic institutions and pharmaceutical companies. This is further illustrated by the continuously growing number of adverse cardiovascular events reported to regulatory agencies (Figure 1), by the number of published articles (Figure 2) and by the regulatory guidance documents issued on this topic over the last 15 years (Figure 3).
Figure 1.
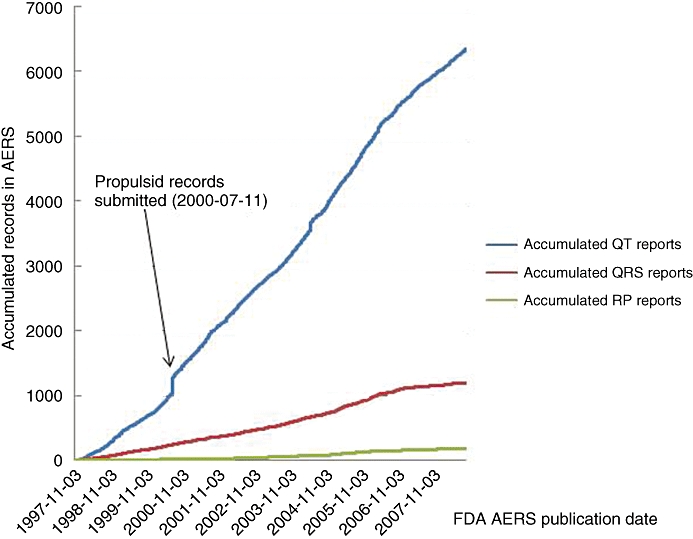
Analysis of the Adverse Events Reporting System (AERS) records for QT, QRS and PR adverse events. The AERS contains records of potential drug-induced adverse events. Data in AERS is submitted on a voluntary basis directly from health-care professionals, customers and manufacturers (who are required to report such events). Individually, AERS records are of limited value, as they cannot be used to ascribe causality between an adverse event and a particular medication. However, AERS records en masse can be used for signal detection and to identify new adverse events as they emerge for new therapies, post-marketing. AERS records were analysed from November 1997 to the end of September 2008. The total number of records for relating compounds to QT, QRS and PR adverse events were identified. Propulsid is also known as cisapride. PR, the PR interval is the time from the beginning of the P wave to the beginning of the QRS complex, it corresponds to the time lag from the onset of atrial depolarization to the onset of ventricular depolarization. QRS, the QRS interval represents the time it takes for depolarisation of the ventricles. QT, the QT interval begins at the onset of the QRS complex and terminates at the end of the T wave. It represents the time of ventricular depolarisation and repolarisation.
Figure 2.
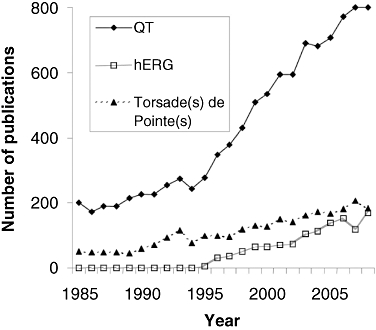
Trends in number of publications referring to ‘QT’, ‘hERG’ and ‘torsade(s) de pointe(s)’ over the last ∼25 years. The number of publications on these topics has been growing continuously, especially over the last decade. QT, duration of the QT interval of the electrocardiogram.
Figure 3.
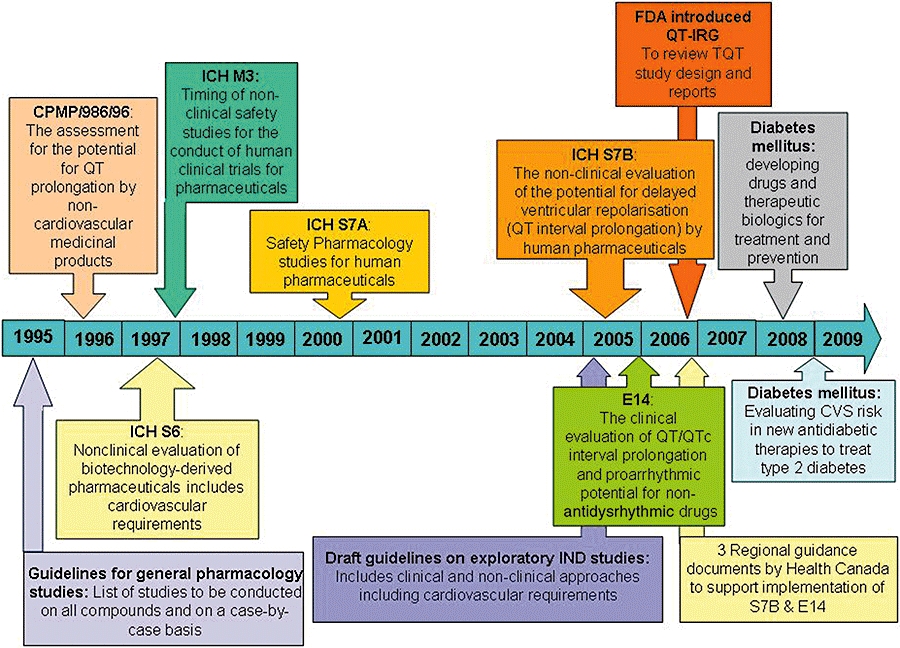
Implementation of regulatory guidance documents referring to QT, proarrhythmia, cardiac and cardiovascular assessment over the last 15 years. Over the last decade, there has been an increase in number and scope of regulatory guidelines, reflecting increasing regulatory concerns. QT, duration of the QT interval of the electrocardiogram.
This issue of the British Journal of Pharmacology contains articles based on communications presented at a symposium entitled ‘reducing QT liability and proarrhythmic risk in drug discovery and development’ that was organized under the auspice of the fifth congress of the Federation of the European Pharmacological Societies (EPHAR) held in Manchester, UK, 13–17 July 2008. The objective of this symposium was to review the effects of the latest regulatory guidance documents [International Conference on Harmonization (ICH) S7A/B and E14 (Anon., 2000; 2005a,b;] and of new, still developing, scientific and technical trends on the ability of the pharmaceutical industry to reduce, manage and mitigate QT liability and its associated proarrhythmic risk and consequently to contribute to the discovery and development of safer medicines. This mini editorial review summarizes the key messages from each communication presented at this symposium and attempts to highlight some of the key challenges that remain to be addressed. The reader is referred to each of the articles relating to this symposium that are published in this issue of the British Journal of Pharmacology.
Non-clinical approaches to assess and reduce the risk of QT interval prolongation
A generic non-clinical strategy to assess and reduce the risk of QT interval prolongation in the context of drug discovery was presented by Pollard et al. (2010). One component of this strategy is the assessment of the compound's potency at the human ether-a-go-go-related gene (hERG) that encodes the pore-forming α subunits of the channel carrying rapid delayed rectifying potassium current (IKr). Several methods of reasonable throughput, sensitivity and specificity, suitable for the ‘make-test’ cycle of early drug discovery phases, are available. These include radioligand-binding, rubidium efflux and electrophysiology-based assays. Generation of large volumes of data enables the development of in silico predictive models that allow ‘virtual screening’, thereby avoiding a waste of resources. The hERG data are supplemented with an in vivo QT/QTc assessment in relevant non-rodent species, typically the dog or the non-human primate, in line with ICH S7A/B (Anon., 2000; 2005a;). Beyond the direct inhibition of the hERG channel, other mechanisms can account for the ability of a drug to prolong the QT/QTc interval. Thus, additional non-clinical data generated from a variety of in vitro and in vivo assays can be added to the hERG and in vivo repolarization assays to further refine the integrated risk assessment. The types of assays and approaches deployed to construct this risk assessment depend upon the questions that need to be addressed, questions such as does the risk increase on repeat dosing? what is the proarrhythmic risk? why is there an in vivo QT effect although the compound was inactive at the hERG channel? why is there no effect on the QT interval, although the compound was active at the hERG channel?
Value of non-clinical repolarization assays to support drug discovery, clinical development and regulatory approval
Several scenarios of how to use non-clinical QT-related assays that are aligned to the different phases of the drug discovery and development were summarized by Valentin et al. (2010). Project examples were provided to illustrate the impact of non-clinical QT data on compound progression throughout the drug discovery and development phases, and regulatory approval. During the early discovery phases, these assays are used for hazard identification and wherever possible for hazard elimination. The collected data enable us to (i) establish structure–activity relationships and develop in silico predictive tools; (ii) influence medicinal chemistry design; (iii) solve problems earlier; (iv) provide reassurance for the project team to progress a compound; and (v) refine strategies as new scientific data and technologies emerge. For compounds progressing into preclinical development, the ‘core battery’, QT-related data enable the construction of an integrated risk assessment to (i) fulfil the regulatory requirements; (ii) assess the safety and risk-benefit for compound progression into man; (iii) define the starting dose and influence the design of the phase I clinical trials; (iv) identify clinically the relevant safety biomarkers; and (v) contribute to the patient risk management plan. After being entered into clinical development, non-clinical QT-related data can be applied in the context of risk management and risk mitigation. Additionally, the data from non-clinical ‘follow-up’ studies can be used to (i) support regulatory approval; (ii) investigate discrepancies that may have emerged within and/or between non-clinical and clinical data; (iii) understand the mechanism of an undesirable pharmacodynamic effect (e.g. arrhythmia); (iv) provide reassurance for progression into multiple dosing in humans and/or large-scale clinical trials; and (v) assess drug–drug interactions. Based on emerging data, the integrated risk assessment is then reviewed, and the benefit-risk for compound progression is reassessed.
Clinical assessment of a drug's liability to prolong the duration of the QT/QTc interval
In theory, the potential for a new chemical entity (NCE) to prolong the QT interval can be assessed at all stages of clinical drug development. The ICH E14 guidance on how to assess, clinically, a new drug's QT prolongation risk was recently adopted (Anon., 2005b). An essential piece of this guidance was the establishment of a single clinical trial, the ‘thorough QT/QTc study’, which is intended to identify, with a high degree of confidence, those drugs that may cause QT prolongation. An update on the implementation of the clinical ICH E14 guidance and on the assessment of a drug's ability to prolong the duration of the QT interval in humans was presented (Darpo, 2010). This ‘thorough QT/QTc study’ has rapidly become a standard of all clinical development programmes for systemically bioavailable NCEs. Moreover, this study is normally conducted in healthy volunteers. It includes both a positive and a negative (placebo) control and is stringently powered to exclude an effect on the QTc interval exceeding 10 ms. Because the ICH E14 guidance was intentionally not very prescriptive, it has allowed sponsors and service providers to explore new assessment methodologies such as computer-assisted algorithms for QT measurements. Additionally, regulators have worked in close collaboration with pharmaceutical industry to set standards for the design and conduct of the ‘thorough QT study’, which therefore has evolved as a key component of cardiac safety assessment of new drugs. The article in this issue by Darpo (2010) summarizes the requirements for conducting the ‘thorough QT study’, with an emphasis on the standard that has evolved based on interactions between regulators, sponsors and on the experience gained from a large number of completed studies.
Drug-induced QT/QTc shortening: an emerging safety issue?
The thorough evaluation of NCEs for their non-clinical and clinical potential to prolong the QT/QTc interval has revealed that drugs can also shorten this interval (Holbrook et al., 2009). Drug-induced QT/QTc shortening can theoretically lead to ventricular fibrillation and death. Although the clinical significance of a short QT interval is still a matter of vigorous debate (see below), the recent description of congenital short QT syndromes has disclosed parallels between the short and the long QT syndromes. As with congenital and drug-induced, long QT syndromes, proarrhythmias associated with congenital short QT syndromes (with a QTc interval < 320 ms) are beginning to raise concerns about the potential cardiac safety of QT-shortening drugs. Moreover, epidemiological evidence shows an over expression of short QT interval values in patients with idiopathic ventricular fibrillation. Therefore, as QT-shortening NCEs enter clinical development, questions about the significance of their potential to shorten ventricular action potentials will inevitably arise. Given the current clinical and regulatory environment, concerns about the proarrhythmic safety of drugs and the lack of a better understanding of the clinical significance of short QT interval, NCEs that significantly shorten the QT interval are likely to receive an unfavourable regulatory review, unless these drugs can fulfil an unmet medical need. Shah's (2010) review suggests approaches to further substantiate or confirm the safety of QT-shortening drugs. In response to Shah's article, the other side of the debate has been put forwards by Malik (2010), presenting arguments to suggest that drug-induced QT shortening might not be such a significant safety issue. While the debate continues, there is a real risk that approval of QT-shortening NCEs may be conditional upon large-scale post-marketing safety studies, with a focus on cardiac safety.
Integrated risk assessment and predictive value to humans of non-clinical repolarization assays
The main goal of non-clinical and clinical studies is to provide an integrated risk assessment for the liability of a NCE to prolong the QT interval. However, a consensus regarding the concordance between non-clinical and clinical data and how the former can reduce the demands for a human ‘thorough QT/QTc study’ has not been reached. A comprehensive review of the available literature that has attempted to assess this concordance was presented (Wallis, 2010). Prospective programmes of work sponsored by the Japanese Pharmaceutical Manufacturers Association and International Life Science Institute – The Health and Environmental Sciences Institute (ILSI-HESI) support the ability of the hERG and conscious dog assays to predict clinical outcomes for QT prolongation/TdP (Omata et al., 2005; Hanson et al., 2006). However, there were weaknesses in these studies: limited analysis of the pharmacokinetic/pharmacodynamic relationships in the in vivo studies; limited correlation with human exposure data; small numbers of drugs evaluated; and their limited range of torsadogenic propensity. Using retrospective approaches, several authors have attempted to define the value of a safety margin approach to better predict human QT prolongation/TdP liability from non-clinical studies (Webster et al., 2002; Redfern et al., 2003; De Bruin et al., 2005). Nevertheless, one of the strengths of these approaches is that the authors considered a clinically relevant end point, namely, TdP. Taken together, these studies demonstrated a good correlation between the hERG safety margin and TdP risk in humans, when the data are supported by in vivo QT data. Given that safety margins are a continuum, it would, however, be inappropriate to suggest that a given safety margin [e.g. 30-fold; Redfern et al. (2003)] would be able to distinguish between compounds that cause TdP from those that do not, as there are exceptions to the rule. Thus, a safety margin based on a combination of hERG and in vivo repolarization data is likely to provide a guide to the QT-prolonging potential of NCEs. The third approach reported by Wallis (2010) reviewed the outcomes of 19 compounds that had been evaluated in clinical studies aimed at detecting a 7- to 10-ms change in the QT interval and in non-clinical assays: an in vitro hERG assay, an in vitro Purkinje fibre repolarization assay and an in vivo QT assay in dogs. The overall conclusion from this analysis when comparing the same concentrations tested in non-clinical and clinical studies was that the effects on the hERG channel and in vivo dog QT were largely predictive of the clinical outcome for QT prolongation. Moreover, the predictive value is strengthened (i.e. >90%) if an integrative approach is undertaken rather than each assay is used in isolation; although it should be recognized that the conclusions are based on a limited data set.
Current and future challenges
Considerable scientific and regulatory advances have occurred in recent years, particularly when looking back at the proceedings of the QT-related symposia organized as part of the third EPHAR congress held in Lyon, 6–9 July 2001 (Valentin, 2002). Moreover, the number and quality of research publications published in the field of drug-induced QT-interval prolongation reflect the dynamism of this field. For example, new data in this issue by Ducroq et al. (2010) demonstrate a key role of the slow delayed rectifying potassium current, IKs, in mediating doxorubicin-induced QT-interval prolongation. Moreover, data by Abi-Gerges et al. (2010) showed the value of canine ventricular midmyocardial myocytes as a suitable model to assess drug effect on cardiac action potential and in yielding additional information about putative indicators of proarrhythmia, whereas Männikköet al. (2010) elegantly demonstrated that potencies of hERG channel blockers defined using the most common Caucasian wild-type sequence were representative of potencies for the most common single nucleotide polymorphisms across different ethnic groups. In this issue, Salvi et al., (2010) reviews the implication of the ICH E14 guidance and the changes in its interpretation since its implementation. Pharmaceutical companies are modifying the design of Phase 1 studies to increase the likelihood of detecting QTc liability early in clinical development to save time, resources and cost. With fewer drugs that prolong the QT interval reaching the licensing stage, knowing which of these drugs are torsadogenic is proving to be elusive. In these circumstances, the authors highlight the challenges facing regulatory agencies, academia and industry in identifying, developing and validating new biomarkers of proarrhythmic risk. The symposium entitled ‘Reducing QT liability and proarrhythmic risk in drug discovery and development’, presented in the fifth EPHAR Congress, highlighted some of the outstanding challenges in the field of drug-induced changes in QT interval duration and proarrhythmic risk, as summarized below.
Despite an abundant literature (see Hoffmann and Warner, 2006; Figure 2), there is still a need to understand non-hERG-mediated, drug-induced QT interval prolongation and associated proarrhythmic risk.
It is paramount to understand the translation of non-clinical repolarization assays to the clinical outcome (Wallis, 2010). It is worth mentioning some ongoing consortia efforts aimed at addressing that issue (e.g. ILSI-HESI; Trepakova et al., 2009). Such approaches should be extended well beyond repolarization assays to increase our understanding of the predictive value of non-clinical safety assays to the clinical outcome, as in the Animal Model Framework (Valentin et al., 2010).
It is important to refine our clinical assessment of drug-induced QT interval prolongation and proarrhythmic risk in circumstances where a ‘thorough QT/QTc study’ cannot be performed (e.g. oncology setting) and for compounds that prolong QTc via non-hERG-mediated mechanisms (Darpo, 2010).
As a potential safety issue, emphasis should be placed on unravelling the molecular mechanisms underpinning drug-induced QT interval shortening, its incidence and prevalence in relation to patient safety and the potential regulatory implications (Holbrook et al., 2009; Malik, 2010; Shah, 2010).
Beyond drug-induced changes in QT interval, it is necessary to increase our understanding of the molecular mechanisms underpinning drug-induced ECG changes [i.e. durations, amplitudes and morphologies of ECG intervals and waveforms such as those affecting atrial and/or ventricular depolarization, and associated proarrhythmic risks (Figure 1)], the incidence and prevalence of ECG changes in relation to patient safety, the translation from animal models to humans as well as the threshold of magnitude of changes that should be considered as a cause for concern to humans (Pettit et al., 2009; Stummann et al., 2009).
Cardiovascular liabilities remain the major reason for drug-related safety attrition and adverse drug reactions. It seems, therefore, logical to apply the learning and the philosophy of the ‘QT-story’ to other aspects of cardiovascular drug safety. Along these lines, the growing awareness and interest of the scientific and medical communities, government agencies, and industry on this topic as assessed by the recent increased number of related workshops/symposia and emerging consortia efforts are worth reporting (Park, 2008; Pettit et al., 2009; Piccini et al., 2009; Pugsley et al., 2009; Stummann et al., 2009; Valentin et al., 2010).
Addressing these issues will depend, in part, on the scientific and technological advances and regulatory challenges that drive the discovery and development of pharmaceuticals for human use. Another crucial factor will be the willingness to develop collaborative scientific research and educational programmes that contribute to the identification and resolution of cardiovascular issues that are of concern to the public, the scientific community, government agencies and industry. Finally and most critically, the success and productivity of any initiative will depend on adequate financial support.
Acknowledgments
The author wish to thank the following colleagues from AstraZeneca: Drs Najah Abi-Gerges and Chris Pollard for their helpful comments and suggestions following the review of the manuscript; Dr Dave Cook for analysing the Adverse Event Reporting System records for QT, QRS and PR adverse events and for preparing the associated Figure 1; and Claire Draper for conducting the literature search analysis and preparing the associated Figure 2.
Glossary
Abbreviations:
- ECG
electrocardiogram
- EPHAR
The Federation of European Pharmacological Societies
- hERG
human ether-a-go-go-related gene
- Ikr
rapid delayed rectifying potassium current
- ICH
International Conference on Harmonization
- NCE
new chemical entity
- PR
the PR interval is the time from the beginning of the P wave to the beginning of the QRS complex, it corresponds to the time lag from the onset of atrial depolarization to the onset of ventricular depolarization
- QRS
the QRS interval represents the time it takes for depolarization of the ventricles
- QT
the QT interval begins at the onset of the QRS complex and terminates at the end of the T wave, it represents the time of ventricular depolarization and repolarization
- QTc
rate-corrected duration of the QT interval
- SAR
structure–activity relationship
- TdP
torsades de pointes
Conflicts of interest
The author states no conflict of interest.
References
- Abi-Gerges N, Valentin JP, Pollard CE. Dog left ventricular midmyocardial myocytes for assessment of drug-induced delayed repolarization: short-term variability and proarrhythmic potential. Br J Pharmacol. 2010;159:77–92. doi: 10.1111/j.1476-5381.2009.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon. ICHS7A: Safety Pharmacology Studies for Human Pharmaceuticals. London: 2000. CPMP/ICH/539/00 16 November 2000. Available from: http://www.emea.europa.eu/pdfs/human/ich/053900en.pdf (accessed 29 October 2009. [Google Scholar]
- Anon. ICH S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization. CQT Interval Prolongation) by Human Pharmaceuticals. London: 2005a. CPMP/ICH/423/02 25 May 2005. Available from: http://www.emea.europa.eu/pdfs/human/ich/042302en.pdf (accessed 29 October 2009. [PubMed] [Google Scholar]
- Anon. ICH E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. London: 2005b. CPMP/ICH/2/04 25 May 2005. Available from: http://www.emea.europa.eu/pdfs/human/ich/000204en.pdf (accessed 29 October 2009. [PubMed] [Google Scholar]
- Darpo B. The thorough QT study four years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010;159:49–57. doi: 10.1111/j.1476-5381.2009.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. J Eur Heart. 2005;26:590–597. doi: 10.1093/eurheartj/ehi092. [DOI] [PubMed] [Google Scholar]
- Ducroq J, Moha H, Guilbot S, Dilly S, Laemmel E, Pons-Himbert C, et al. Dextrazoxane protects the heart from acute doxorubicin-induced QT-prolongation: a key role for Iks. Br J Pharmacol. 2010;159:93–101. doi: 10.1111/j.1476-5381.2009.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LA, Bass AS, Gintant G, Mittelstadt S, Rampe D, Thomas K. ILSI-HESI cardiovascular safety subcommittee initiative: evaluation of three non-clinical models of QT prolongation. J Pharmacol Toxicol Methods. 2006;54:116–129. doi: 10.1016/j.vascn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Holbrook M, Malik M, Shah RR, Valentin J-P. Drug induced shortening of the QT/QTc interval: an emerging safety issue warranting further modeling and evaluation in drug research and development? J Pharmacol Toxicol Methods. 2009;59:21–28. doi: 10.1016/j.vascn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Malik M. Facts, fancies, and follies of drug-induced QT/QTc interval shortening. Br J Pharmacol. 2010;159:70–76. doi: 10.1111/j.1476-5381.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Kasai C, Hashimoto M, Hombo T, Yamamoto K. QT PRODACT: comparison of non-clinical studies for drug-induced delay in ventricular repolarization and their role in safety evaluation in humans. J Pharmacol Sci. 2005;99:531–541. doi: 10.1254/jphs.qt-c12. [DOI] [PubMed] [Google Scholar]
- Männikkö R, Overend G, Perrey C, Gavaghan C, Valentin JP, Morten J, et al. Pharmacological and electrophysiological characterization of nine, single nucleotide polymorphisms of the hERG-encoded potassium channel. Br J Pharmacol. 2010;159:102–114. doi: 10.1111/j.1476-5381.2009.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. Medical Research Council (MRC) Centre for Drug Safety Science. 2008. Available from: http://www.liv.ac.uk/research/environment/Drug_Safety_Centre.htm (accessed 29 October 2009.
- Pettit SD, Berridge B, Sarazan RD. A call for more integrated cardiovascular safety assessment. J Pharmacol Toxicol Methods. 2009. Epub ahead of print] doi: 10.1016/j.vascn.2009.08.001. [DOI] [PubMed]
- Piccini JP, Whellan DJ, Berridge BR, Finkle JK, Pettit SD, Stockbridge N, et al. CSRC/HESI Writing Group. Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the critical path initiative. Am Heart J. 2009;158:317–326. doi: 10.1016/j.ahj.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Pollard CE, Abi-Gerges N, Bridgland-Taylor MH, Easter A, Harmer A, Hammond TG, et al. Non-clinical strategies to address QT liability and proarrhythmic risk. Br J Pharmacol. 2010;159:12–21. doi: 10.1111/j.1476-5381.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley MK, Authier S, Towart R, Gallacher DJ, Curtis MJ. Beyond the safety assessment of drug-mediated changes in the QT interval… what' next? J Pharmacol Toxicol Methods. 2009;60:24–27. doi: 10.1016/j.vascn.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Salvi V, Karnad DR, Panicker GK, Kothari S. Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol. 2010;159:34–48. doi: 10.1111/j.1476-5381.2009.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RR. Drugs, QT interval prolongation and ICH E14: the need to get it right. Drug Saf. 2005;28:115–125. doi: 10.2165/00002018-200528020-00003. [DOI] [PubMed] [Google Scholar]
- Shah RR. Drug-induced QT interval shortening: potential implications and regulatory perspectives. Br J Pharmacol. 2010;159:58–69. doi: 10.1111/j.1476-5381.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummann TC, Beilmann M, Duker G, Dumotier B, Fredriksson JM, Jones RL, et al. Report and recommendations of the workshop of the European Centre for the Validation of Alternative Methods for Drug-induced Cardiotoxicity. Cardiovasc Toxicol. 2009;9:107–125. doi: 10.1007/s12012-009-9045-3. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Koerner J, Pettit SD, Valentin JP HESI Pro-Arrhythmia Committee. A HESI consortium approach to assess the human predictive value of non-clinical repolarization assays. J Pharmacol Toxicol Methods. 2009;60:45–50. doi: 10.1016/j.vascn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Valentin JP. Proceedings of the symposia on safety pharmacology, presented during the 3rd EPHAR meeting, Lyon, 6–9 July 2001. Fund Clin Pharmacol. 2002;16:73–74. [PubMed] [Google Scholar]
- Valentin JP, Bialecki R, Ewart L, Hammond T, Leishmann D, Lindgren S, et al. A framework to assess the translation of safety pharmacology data to humans. J Pharmacol Toxicol Methods. 2010;60:152–158. doi: 10.1016/j.vascn.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Valentin JP, Pollard CE, Lainee P, Hammond T. Value of non-clinical repolarization assays in supporting the discovery and development of safer medicines. Br J Pharmacol. 2010;159:25–33. doi: 10.1111/j.1476-5381.2009.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis RM. Integrated risk assessment and predictive value to humans of non-clinical repolarisation assays. Br J Pharmacol. 2010;159:115–121. doi: 10.1111/j.1476-5381.2009.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Dev. 2002;5:116–126. [PubMed] [Google Scholar]


