Abstract
The ICH E14 guidance on how to clinically assess a new drug's liability to prolong the QT interval was adopted in May 2005. A centre-piece of the guidance was the establishment of one single trial, the ‘thorough QT/QTcstudy’, intended to confidently identify drugs that may cause QT prolongation. Initially perceived as a great challenge, this study has rapidly become a standard component of all clinical development programs for new molecular entities. The study is normally conducted in healthy volunteers, includes both a positive and a negative (placebo) control and is stringently powered to exclude an effect on the QTc interval exceeding 10 ms. The E14 guidance was intentionally not very prescriptive and allowed sponsors and service providers to explore new methodologies. This has allowed for a rapid development of new methods during the first years after the guidance's implementation, such as computer-assisted algorithms for QT measurements. Regulators have worked in close collaboration with pharmaceutical industry to set standards for the design and conduct of the ‘thorough QT/QTc study’, which therefore has evolved as a key component of cardiac safety assessment of new drugs. This paper summarizes the requirements on the ‘thorough QT/QTc study’ with emphasis on the standard that has evolved based on interactions between regulators and sponsors and the experience from a large number of completed studies.
This article is part of a themed section on QT safety. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2010
Keywords: QT prolongation, ICH E14, thorough QT/QTc study, ECG methodology
Introduction
QT prolongation and proarrhythmias have been one of the most common reasons for withdrawal of drugs from the market (Shah, 2002a,b;) and the list of compounds includes most therapeutic areas (Darpo, 2001; Yap and Camm, 2003). In addition, QT prolongation has also resulted in many drugs (e.g. moxifloxacin, vardenafil, alfuzosin and ziprasidone) having precautionary statements in the label (Strnadova, 2005). The incidence of drug-induced proarrhythmias with non-cardiovascular drugs is very low and estimates have ranged from approximately one in 100 000 patients for cisapride to substantially lower numbers (Wysowski and Bacsanyi, 1996; Darpo, 2001; Barbey et al., 2002). Even though a rare event, the potential outcome is serious and includes sudden death and even a small risk is therefore unacceptable for drugs intended to treat benign conditions. A drug with the propensity to prolong the QT interval may still be approved for more serious conditions as the small risk can be balanced by a benefit not otherwise achieved. It is, however, increasingly apparent that even for these drugs, including oncology agents, there is an expectation that the risk of QT prolongation should be adequately addressed during development (Sarapa and Britto, 2008; Rock et al., 2009).
The ‘International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use’ (ICH) issued the E14 clinical guidance in May 2005: ‘The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs’ (ICH Harmonized Tripartite Guideline E14, 2005). The guidance was quickly implemented in Europe and in the United States but has yet to be implemented in Japan. The centre-piece of the guidance is the ‘thorough QT/QTc study’ (TQT), which is a dedicated study with the primary objective to quantify the effect of a new molecular entity (NME) on the QT interval. In 2007, the US Food and Drug Administration (FDA) formed an Internal Review Team (IRT) with responsibility to oversee the clinical assessment of QT prolongation for all drugs that the agency reviewed. The IRT acts in an advisory function to the therapeutic divisions and reviews all protocols and reports for TQT studies and advises on QT assessment in other clinical trials. In addition to interactions with the sponsors on individual drugs or on methodologies, individual members of the IRT have shared their experience at various meetings (e.g. DIA meetings) and through publications (see e.g. Garnett et al., 2008). A similar advisory function that industry can interact with on a continuous basis does in practice not exist in Europe or in Japan. To date, the IRT has reviewed more than 100 TQT studies (n= 112, 9 April 2009) and obtained an unparalleled experience. The establishment of the ‘FDA ECG warehouse’ (Sarapa, 2007), to which all electrocardiograms (ECGs) from TQT studies should be submitted as annotated xml files, has also further facilitated FDA's analyses across studies and some of the results thereof have been shared publicly.
Based on the experience from a large number of completed studies, of which only a small fraction has been published (Table 1), and on the interactions between regulators and sponsors, a standard for TQT studies has evolved since the implementation of the E14 guidance 4 years ago. In line with this, clarifications to the guidance have been issued by the E14 Implementation Working Group as a Questions & Answers document (ICH E14 Q&A, 2008). It should be clearly acknowledged that there is more than one way to conduct a perfectly acceptable TQT study and that there still are some important areas, which are debated and for which there is no simple answer at this stage. We can therefore expect further changes to the way these studies are conducted. With this in mind, this paper describes the standard for TQT studies that has evolved since E14's implementation 4 years ago.
Table 1.
Published thorough QT studies March 2009
| Reference | Drug | SD/MD | Subjects# | Design | Positive control |
|---|---|---|---|---|---|
| Serra et al., 2005 | Darifenacin | MD | 188 | Pl | Moxi |
| Extramiana et al., 2005 | Alfuzosin | SD | 48 | XO | Moxi |
| Malhotra et al., 2007 | Tolterodine | MD | 48 | XO | Moxi |
| Hulhoven et al., 2007 | Levocetirizine | SD | 52 | XO | Moxi |
| Sarapa et al., 2008 | Ritonavir | SD | 65 | XO | Moxi |
| Dixon et al., 2008 | Lamotrigine | MD | 153 | Pl | Moxi |
| Malik et al., 2008 | Rotigotine | MD | 66 | Pl | Moxi |
| Rosillon et al., 2008 | Brivaracetam | MD | 184 | Pl | Moxi |
| Hulhoven et al., 2008 | Levetiracetam | SD | 52 | XO | Moxi |
| Iwamoto et al., 2008 | Raltegravir | SD | 31 | XO | Moxi |
| Kubitza et al., 2008 | Rivaroxaban | SD | 50 | XO | Moxi |
| Damle et al., 2009 | Nelfinavir | MD | 68 | XO | Moxi |
Total number of enrolled subjects.
* Submitted.
Moxi, moxifloxacin; Pl, parallel-designed; SD/MD, single dose/multiple dose; XO, crossover.
The thorough QT/QTc study
The objective of the TQT study is to confidently exclude that a drug prolongs the QTc interval 10 ms or more at the one-sided upper 95% confidence limit. It is not to establish to which extent a drug is proarrhythmic but to identify those drugs, which need a more careful assessment of this liability in the targeted patient population. Drugs with a positive TQT study, that is, for which an effect exceeding 10 ms cannot be exclude, therefore need additional ECG monitoring in patients (Darpo et al., 2006b; Vik et al., 2008).
Timing of the TQT study
There is no request per se on when the TQT study should be performed but it is advisable to conduct the TQT study well before pivotal trials are undertaken to establish whether additional ECG monitoring is needed. It is also imperative to have sufficient knowledge of the pharmacokinetic profile of the drug, as the exposure in the TQT study should substantially exceed that observed in patients, including patients with impaired clearance of the drug (see below). The timing of the TQT study within a clinical development program will also be influenced by other factors, such as:
The outcome of the non-clinical safety assessment: In line with ICH S7B, all candidate drugs undergo a careful non-clinical evaluation, which at a minimum includes an in-vitro hERG channel assay and an in-vivo evaluation of ECG effects in, for example, dogs on non-human primates (for a detailed discussion, see Pugsley et al., 2008). At present, a negative non-clinical package does not obviate the need for a TQT study, even though this may change based on ongoing initiatives to evaluate the predictive value of non-clinical assays performed at today's standards (Hanson et al., 2006; Trepakova et al., 2009). For these drugs, it will in most cases be sufficient to perform the TQT studies during the latter part of phase 2. For a drug with an unambiguous non-clinical signal, it may be important to exclude that a non-clinical signal translates into a clinical effect relatively earlier, to avoid costly investments into a program that may become non-viable with a clinical QT liability;
Severity of indication: A small QT effect may be acceptable for life-threatening disorders, particularly when no other therapy exists. In these cases, it may be preferable to conduct the TQT once some data on the clinical benefit of the drug has been obtained.
Previous experience with the pharmacological class versus novel mode-of-action: Clearly, there have been advancements in the ability to design out hERG inhibition, which primarily is related to the chemistry of the NME (Leeson and Springthorpe, 2007). Despite this, NMEs from certain pharmacological classes, for example fluoroquinolone antibiotics, still often demonstrate QT liability. For such classes, it may be advisable to perform the TQT relatively early in the program.
The E14 guidance describes which inclusion and exclusion criteria that should be used before the results from the TQT study are available and the impact of these results on the subsequent development program. The guidance leaves to the sponsor to decide the timing of the TQT study and this has not been changed by practice or by clarifications.
Role of the positive control (PC)
The role of the PC is to demonstrate the study's ability to detect a small effect on the QT interval. In an overwhelming majority of TQT studies, moxifloxacin, a fluoroquinolone antibiotic with a mild QT prolonging effect (Culley et al., 2001; Bloomfield et al., 2008) has been used, even though individual examples of other drugs, such as low-dose infusion of ibutilide and sparfloxacin, exist. In most studies, moxifloxacin has caused a larger peak effect than 5 ms, more in the range of 8 to 15 ms (Garnett, 2008). How assay sensitivity should be established based on the QTc effect caused by moxifloxacin has been widely discussed and was clarified through the E14 Q&A document (ICH E14 Q&A, 2008). The answer on Question 1 states that there are two conditions that need to be fulfilled to establish assay sensitivity: the first is that the PC must cause a significant effect on the QTc interval, that is, the lower bound of the one-sided 95% confidence interval (CI) of the placebo-corrected QTcF must be above 0 ms. This confirms that the TQT study can detect an increase in QTc, which is essential for the conclusion that a negative finding for the NME is meaningful. The second condition is that the study should be able to detect an effect of about 5 ms (the QTc threshold of regulatory concern), in case there is such an effect. Two approaches can thereby be taken: (i) the most widely used approach has been to use of a PC with an effect size greater than 5 ms, such as moxifloxacin, whereby an effect significantly greater than 5 ms must be demonstrated (i.e. lower bound of one-sided 95% CI should be above 5 ms; Figure 1). The document, however, cautions against using PCs with too large an effect, which may lead to the questioning of the study's ability to detect a 5 ms QTc prolongation. If this is the case, the effect of the PC can be examined at times other than the peak effect to determine whether an effect close to the threshold of regulatory concern could be detected. (ii) The other approach is to use a PC with a peak effect close to 5 ms, in which cases the lower bound of the one-sided 95% CI must be >0. When using this approach, the sponsor needs to be confident that this small effect can be repeatedly reproduced, that is, to have a reasonably precise estimate of the drug's usual effect. Lastly and importantly, the answer states that the effect of the PC should be ‘reasonably similar to its usual effect’ in other TQT studies, in terms of peak effect (size and time point) and time course of the effect. If, as an example, the peak effect of moxifloxacin is around 5 ms, which would suggest an underestimation of the effect, questions regarding assay sensitivity can be raised and the interpretability of a negative finding for the NME could be questioned.
Figure 1.
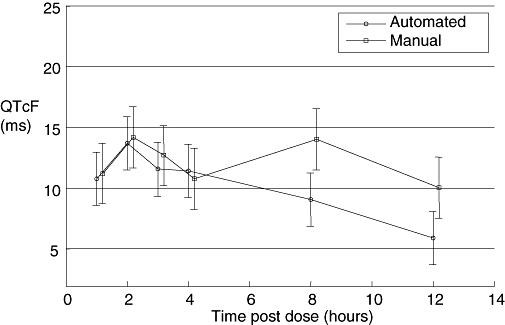
Examples of the effect induced by moxifloxacin: Baseline-adjusted, placebo-corrected QTcF after oral dosing of 400 mg moxifloxacin in a TQT study, measured with an automated and a fully manual measurement technique. With both techniques a peak effect of approximately 14 ms was seen 2 h after dosing, at which time the lower bound of the confidence interval clearly exceeded 5 ms. With the automated technique, QTcF thereafter declined, whereas with the manual, a second peak was seen at 8 h. Bars represent two-sided 90% confidence interval. QTcF, QT heart rate corrected according to Fridericia; TQT, thorough QT/QTc.
In practice, the IRT has adopted a pragmatic approach in terms of assay sensitivity based on the moxifloxacin response. Assay sensitivity based on the QTc effect of 400 mg oral moxifloxacin has been established if:
the largest baseline-adjusted, placebo-corrected effect on QTcF is about 8 to 15 ms;
the peak effect is observed between 1 and 3 h post-dose;
the lower bound of the one-sided 95% CI of the peak effect exceeds 5 ms; and
the QTcF thereafter declines.
If these criteria are fulfilled, a negative result for the NME can be accepted.
Importantly, the same definition has been used and accepted when novel methodologies for, for example, interval measurements (see below) have been reviewed and discussed by the IRT. The consistent use of one PC, moxifloxacin, has thereby facilitated the conduct of and the comparison across several TQT studies and has supported the emergence of novel, more efficient ECG methodologies.
Design considerations
Treatment arms/groups
As within-subject variability is lower than between-subject, a crossover-designed TQT study is more efficient than a parallel-designed and requires a smaller sample size to exclude an effect. This was pointed out in the E14 guidance and has been underlined by the IRT on several occasions (Zhang, 2008). In certain instances, such as with drugs that need to be dosed for a week or more either to obtain steady state or because of dose-titration, a parallel-designed study is, however, preferable. The most stringent approach is then to have a separate treatment group for both placebo and the PC, which initially was requested by the IRT. This is, however, an ineffective design in which subjects in two full treatment groups are not dosed with the NME, which corresponds to 50% of participating subjects if a therapeutic and a supra-therapeutic dose is used (resulting in four treatment groups of the same size). Alternative designs have therefore been used, such as dosing the PC either early or late in the placebo-arm (see e.g. Zhang et al., 2007). A downside with, for example, lead-in dosing with the PC is that assay sensitivity is not established on the same day as the effect of the NME. Furthermore, and this also applies to dosing of the PC at the end of the placebo-treatment period, assay sensitivity is established as ΔQTc (QTc on PC – QTc on placebo either on preceding day or at baseline), whereas the effect of the NME is based on ΔΔQTc for the drug (baseline-adjusted, placebo-corrected). Recently, Joanne Zhang, lead statistician on the IRT, therefore suggested a ‘nested crossover’ design for these studies with a joint placebo/PC group, which will achieve a substantial reduction of the sample size (Zhang, 2009). In half of the subjects in the placebo/PC group, the PC is dosed on the first day of treatment and in the other half on the day after the last treatment day. In the example given in Figure 2B, double-blind treatment with either the NME or placebo is administered for 14 days between Day 1 and Day 14. The PC is therefore dosed on Day 1 and on Day 15 in each half of the subjects in the placebo group.
Figure 2.

Schematic outline of a ‘nested crossover’ design in a parallel TQT study. Panel A shows a parallel-designed TQT study with separate treatment group for a supra-therapeutic and a therapeutic dose of the NME, for placebo and for the positive control (PC), resulting in four groups. The NME and placebo are administered for 14 days (Day 1 through Day 14) and the primary assessment is performed on Day 14. In group 4, placebo is given on Day 1 through Day 13 and the PC (moxifloxacin, PC) on Day 14. QTc on Day 14 is baseline-adjusted by subtracting values on Day 0 in all groups. Four treatment groups with equal allocation results in 4 × 40 = 160 subjects. Panel B shows the ‘nested crossover’ design applied to the same example, in which the effect of the NME is evaluated after 14 days of dosing. In Group 1 and 2, the NME is given on Day 1 to Day 14. The joint placebo–PC treatment group (Group 3) is divided into two arms with half the subjects in each. In the PC/placebo arm, the PC is given on Day 1 followed by placebo to Day 15. In the placebo/PC arm (bottom), placebo is given on Day 1 to Day 14 and the PC on Day 15. NME, new molecular entity; PC, positive control; TQT, thorough QT/QTc.
Calculation of ΔΔQTc for moxifloxacin (demonstration of assay sensitivity) is then performed as follows: for the half, in which the PC is dosed on Day 15 (placebo/PC arm), the change-from-baseline (ΔQTc) for the PC is calculated as:
QTcDay15 minus QTcDay1.
The placeboΔQTc for this half is calculated as:
QTcDay0 minus QTcDay14.
For the other half, in which the PC is dosed on Day 1 (PC/placebo arm), the ΔQTc is calculated as:
QTcDay1 minus QTcDay15.
The placeboΔQTc for this half is calculated as:
QTcDay14 minus QTcDay0.
The baseline-adjusted, placebo-corrected QTc (ΔΔQTc) for the PC is then calculated as the average of the two arms.
Calculation of ΔΔQTc for the NME is performed the same way as with a traditional parallel-designed study: in this example, QTc on Day 14 is compared with values on baseline (Day 0) for both the NME and placebo.
This design has not yet been widely tested, but seems to represent a useful approach that achieves the same sample size reduction as the ‘lead-in design’ but without this design's weaknesses. It should be noted that there is a prize for the reduction of the sample size: the number of ECGs in each treatment group is roughly doubled (two additional assessment days) and the PC has to be blinded to avoid unblinding of the placebo treatment, which otherwise is not any longer a requirement (see below).
Blinding of the PC
The experimental conditions of the TQT study must be stringently controlled and study procedures identical between treatment arms/groups. As an example, blood draws (which always should be performed immediately after the ECG recording to avoid confounding stress) should be performed in all treatment periods, even though the samples from the placebo and PC may not be analysed. A rationale for storing samples from the PC arm can be that pharmacokinetic data sometimes can help explain unexpected results, such as a small moxifloxacin effect based on low peak plasma levels (as has been the case in some studies utilizing encapsulation of the drug). Awareness of treatment may introduce a confounding effect on the QT interval and double-blind administration of placebo/NME is therefore an absolute requirement that has remained. Initially, the IRT also mandated blinding of the PC. As an illustration of the analyses that FDA can perform across TQT studies, Christine Garnett from the IRT presented data from TQT studies that had used blinded or open-label moxifloxacin at the April 2008 DIA meeting (Garnett, 2008). The conclusion from this analysis was that assay sensitivity using moxifloxacin could be achieved with either approach; that is, the QTc effect was very similar. In some cases with over-encapsulated moxifloxacin, the QTc effect was, however, unexpectedly small, which likely was caused by altered pharmacokinetics of the drug due to the encapsulation. The IRT has thereafter accepted the use of open-label moxifloxacin in TQT studies and the same recommendation was later made in the Q&A document by the ICH E14 Implementation Working Group (IWG) [Question 7 (ICH E14 Q&A, 2008)]. Exceptions will exist, where the PC needs to be blinded, such as the nested crossover design discussed above, in which open-label moxifloxacin would effectively also unblind the placebo group.
Sample size
When calculating the sample size for the TQT study, it is important to evaluate at the variability of similar studies preferably performed at the same clinical site using the same ECG techniques and central ECG laboratory. It also seems prudent to assume that the NME has some effect on the QTc interval, for example 2 to 3 ms and to use 90% power. With these assumptions and the variability obtained from a trained clinical site and an experienced central ECG laboratory using a standard measurement technique, sample sizes between 32 and 48 subjects for a crossover study or per group in a parallel-designed TQT study has often been sufficient.
ECG assessment
As pointed out, pharmacokinetic properties of an NME should be well known when the TQT study is conducted and ECG acquisition and blood samples should be timed to ensure that the peak plasma concentration of the drug (and of the PC) and major metabolites are covered. In many cases, this can be achieved with six to eight time points, and it is worth bearing in mind that the objective of the study is to exclude a QTc effect: the likelihood of false positive findings increases with the number of assessed time-points, which should be factored into the decision.
Although there may theoretically be an advantage to demonstrate assay sensitivity at the same time of the day as when the peak effect of the NME is expected, this has not been a requirement. In most TQT studies, moxifloxacin has been dosed in the morning and the peak effect has been observed 1 to 3 h thereafter. ECG recordings must be performed identically in all treatment groups/arms, but analysis of the moxifloxacin induced effect can be restricted to time points covering the peak effect and a few time points thereafter, for example from 1 h to 6 h post-dosing.
Averaging replicates of ECG recordings from each time point is today standard as it substantially reduces the variability of the QT measurement. There are several data sets that demonstrate that this reduction of variability is pronounced up to triplicates and then levels off (an example is given in Patterson et al., 2005) and most sponsors today use triplicate ECG recordings at each time point.
In the answer to Question 2 in the E14 Q&A document (ICH E14 Q&A, 2008) it is recommended that ECGs are measured blindly in regard to subject, time and treatment to reduce potential bias. More recently, the IRT has asked that all ECGs from an individual are measured by a single reader on 1 day. Analysis of T wave morphological changes, which sometimes is performed by comparison with baseline, can be performed after the QT analysis and can also be blinded to treatment.
Study population
The concept that underlies the TQT study is that if a drug has a QT prolonging effect in patients (and consequently may be proarrhythmic), then this effect can be demonstrated in healthy volunteers, if sufficiently high doses of the NME are given. The intention of the TQT is not to study whether a QTc effect is larger in any specific subpopulation, such as females or certain ethnicities. There is therefore no expectation on gender balance or inclusion of different ethnicities in the TQT study. It is well described that women are at a higher risk for the development of proarrhythmias caused by drugs with an effect on cardiac repolarization (Makkar et al., 1993; Ebert et al., 2000; Bednar et al., 2002). Women have a somewhat longer QTc interval than men (Burke et al., 1997; Rautaharju and Zhang, 2002; Sarapa et al., 2004) and it has been shown that the degree of drug-induced QTc prolongation may vary in different phases of the menstrual cycle (Rodriguez et al., 2001). It would therefore seem reasonable to assume that women also react with a larger degree of QTc prolongation than men at the same plasma exposure of a drug. There are a few examples in which this has been demonstrated (Benton et al., 2000; Rodriguez et al., 2001; Shin et al., 2007) but the overall pattern is less consistent. As an example, no difference in concentration – QTc effect profile by gender was observed in a recent analysis of a large number of patients treated with dofetilide (personal communication, Dr P Wicker, previously Pfizer). Based on the variable drug response that women may exhibit during different phases of the menstrual cycle, it can even be argued that the precision of the TQT study would increase by using men only. This topic could quite easily be studied by any sponsor that has performed a number of TQT studies, but other considerations, such as the feasibility of recruiting healthy volunteers are often more important than a slight reduction of the number of enrolled subjects.
Baseline assessment
In crossover-designed TQT studies, a baseline assessment can be made either through time-matched recordings on a full baseline day before each treatment period (time-matched baseline) or through a limited number of recordings (e.g. three time points) before each period (predose baseline). Based on the experience from several sponsors (Bloomfield et al., 2008), core ECG laboratories and the IRT (Zhang, 2008), it is usually not necessary to use a time-matched baseline in crossover studies, because adjustments for subject- and study-specific diurnal variation are accounted for in the assessment of time-matched drug-placebo differences in QT/QTc effect. The ‘pre-dose’ baseline is therefore regarded as adequate for crossover studies [Question 6 in E14 Q&A document (ICH E14 Q&A, 2008)]. For parallel-designed TQT studies, a full baseline day is still the most widely used approach and baseline-adjusted ΔQTc is then calculated by comparing the QTc value for each time point at baseline and post-dosing (‘time-matched’). There are some data (Darpo, 2008), however, suggesting that results would be the same and the variability lower if baseline was generated through averaging of all values from a full baseline day. More research and analyses across TQT studies can be expected on this topic.
Dose
A high, supratherapeutic dose of the NME, which results in an exposure in excess of what would be observed in patients with impaired clearance of the drug, should be used in the TQT study. In most cases, a therapeutic and a supratherapeutic dose have been studied. An interesting design was recently used in a TQT study with raltegravir, a HIV-1 integrase inhibitor, in which a single, supratherapeutic dose only was used (Iwamoto et al., 2008). This approach will obviously be sufficient if the result is clearly negative. Most sponsors tend, however, to also include the therapeutic dose, in case the high dose is ‘slightly positive’. It can be argued that the effect of the therapeutic dose can be estimated by concentration effect modeling, but this has so far not gained wide acceptance.
There is no requirement per se on dosing to steady state of the NME and single-doses can be used in the TQT study, provided that a sufficiently high exposure of both parent and major metabolites can be achieved. If there are slowly appearing metabolites, which require many days of dosing to obtain steady state or sufficiently high exposure, multiple-dosing is warranted. The same applies to NMEs that need to be dose-titrated to reach targeted dose-levels due to tolerability issues.
There are instances when supratherapeutic or even therapeutic doses cannot be tested in healthy volunteers due to toxicity of the drug (e.g. chemotherapeutics) or tolerability issues (e.g. neuroleptics or dopamine agonists). These drugs are not waived from the requirement of a careful clinical QT assessment, which then has to be performed in patients (Sarapa and Britto, 2008; Rock et al., 2009), either as a designated study or as part of a larger one. The challenges associated with TQT studies performed in patients are numerous and are related to many factors, including number of sites, training of investigators, higher incidence of cardiovascular disease and thereby larger variability of ECG measurements. There are a few examples, however, of successful ‘TQT-like’ studies which could be conducted in a relatively limited patient population (66 patients on drug and 64 on placebo) due to stringently controlled experimental conditions (Malik et al., 2008).
Heart rate correction
There are numerous ways of correcting for changes in heart rate to obtain the corrected QT interval, QTc and no clear consensus on which is the preferred algorithm (Malik, 2001). The limitations of Bazett's QT correction (QTcB) are widely acknowledged, as this algorithm overcorrects the QT interval with increasing heart rate, thereby producing false positive QTc prolongation. Even so, it is still a requirement that QTcB should be reported to allow for comparison with historical data. For drugs without clear effect on the heart rate, it has been the experience of the IRT and of many sponsors that QTcF works well (Zhang, 2008). For these drugs, there does not seem to be much of an advantage to use the subject-specific QTcI, which is derived from the QT/RR slope from each individual in the drug-free state. Furthermore, the derivation of QTcI is sometimes used to justify an additional full baseline day in crossover-designed studies, which is difficult to defend if there is no added value of using this algorithm.
Drugs with an inherent effect on the heart rate are much more challenging to study in TQT studies and there is not yet much experience with these and no firm guidance. The Cardiac Safety Research Consortium (http://www.cardiac-safety.org/) is a collaborative think tank with members from the FDA, academicians and pharmaceutical industry, has initiated a white paper on the topic, which will summarize current knowledge and outline future areas of research. Methods for heart rate correction of the QT interval that will be described in the white paper include ‘Holter-bin’ (Badilini and Maison-Blanche, 2005; Extramiana et al., 2005; Malik, 2005), QTcI derived from a broad range of QT/RR pairs through continuous Holter recordings at baseline, beat-to-beat analysis (Fossa et al., 2005; 2006;), PK/PD modeling with heart rate as a covariate (Li, 2008) and assessment of the QT interval at a fixed heart rate through, for example, submaximal exercise (Demolis et al., 1996; 2000; 2003;). Ideally, these and potentially other methods should be tested on common datasets derived from TQT studies with NMEs with an effect on the heart rate and with and without effect on cardiac repolarization. Looking ahead, this could potentially be achieved through the FDA ECG warehouse, if data on the NMEs were to be de-identified and released.
Emerging methods for QT interval measurements
The use of ECG interval measurement methods, which are either ‘fully manual’ or use some degree of ‘manual adjudication’, that is, measurements made by an computer-based algorithm are reviewed and corrected as needed, are currently recommended for trials in which QT assessment is an important objective, for example the TQT study [see Question 4 A and B in Q&A document for summary of methods and recommendations (ICH E14 Q&A, 2008)]. For NMEs with a positive TQT study, the same methods are also recommended for ECG assessment from a subset of patients in late stage trials (see also Section 2.3 in E14 document). When the TQT study is negative, it is deemed adequate to perform the routine ECG monitoring in subsequent trials with automated ECG methods.
Evidently, automated measurement techniques, provided these are proven reliable for the detection of small QT changes, would represent an important opportunity to conduct TQT studies more efficiently, as both ‘fully manual and manually adjudicated’ techniques are laborious and resource intensive. The utility of fully or partially automated measurement techniques has been compared with manual techniques in a number of studies and these techniques have been shown to produce similar results when tested on drugs with a QT prolonging effect (Azie et al., 2004; Sarapa et al., 2004; Darpo et al., 2006a; Fosser et al., 2009; Sarapa et al., 2009a). It has also been shown that different techniques generate different absolute QT intervals (Kligfield et al., 2006; Kligfield et al., 2007), and that some automated techniques consistently demonstrate the same QTc effect measured as change from baseline as manual techniques. The absolute QT interval is of interest in clinical assessment of, for example, QT prolongation but less important when change from one time point to another is the main objective, as in TQT studies. Even so, concern exists regarding the ability of automated methods to identify individual patients with drug-induced changes in T-wave morphology. The E14 Q&A document (Question 4B) therefore recommends that if a fully automated technique without visual assessment of the T-wave morphology is used, the technique should undergo validation studies, which must include ECGs with T-wave abnormalities. In this context, it can be noted that automated algorithms in fact are able to improve the detection of subtle T-wave changes, induced by, for example, moxifloxacin (Couderc et al., 2008). Access to ECG waveforms from TQT studies from the FDA ECG warehouse has facilitated the testing of novel, highly automated algorithms for QT interval measurements. Using these data sets, emerging, methods have been shown to reproduce the moxifloxacin QTc effect in a way that is consistent with the E14 requirements (Couderc and Zareba, 2009). Some methods also support the reader to determine which QT interval should be overread, through ‘confidence scores’, which identifies T-waves that fall outside templates that are produced on baseline recordings (Sarapa et al., 2009b; Strachan et al., 2009). This is a highly dynamic area and it can be foreseen that the role of automated QT algorithms will dramatically increase during the next coming years, not only to replace manual QT measurements but also to improve the detection of subtle T-wave changes.
Conclusion
Four years have now elapsed since the ICH E14 guidance on the clinical assessment of QT prolongation was adopted. The guidance was quickly implemented in the United States and in Europe and substantial experience with these studies has now been gained by regulators and sponsors. The total number of TQT studies, which have been submitted to the FDA ECG warehouse today exceeds 100. The requirements on the TQT study have been modified and clarified through iterative interactions between the FDA's IRT and sponsors and through a Q&A process driven by the IWG. Modifications to the initial E14 guidance include that the PC does not need to be blinded; the PC does not need to be in a separate treatment group in parallel-designed studies and how assays sensitivity is defined by the moxifloxacin-induced QTc response.
Glossary
Abbreviations:
- CI
confidence interval
- E14
ICH Harmonized Tripartite Guideline E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs
- ICH
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IRT
FDA's Internal Review Team
- IWG
ICH E14 Implementation Working Group
- NME
new molecular entity
- PC
positive control
- ΔQTc
baseline-adjusted QTc
- ΔΔQTc
baseline-adjusted, placebo-corrected QTc
- QTc
heart rate-corrected QT interval
- QTcB
QT heart rate corrected according to Bazett
- QTcF
QT heart rate corrected according to Fridericia
- QTcI
QT subject-specific heart rate correction
- TQT
thorough QT/QTc
References
- Azie NE, Adams G, Darpo B, Francom SF, Polasek EC, Wisser JM, et al. Comparing methods of measurement for detecting drug-induced changes in the QT interval: implications for thoroughly conducted ECG studies. Ann Noninvasive Electrocardiol. 2004;9:166–174. doi: 10.1111/j.1542-474X.2004.92542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badilini F, Maison-Blanche P. Holter monitoring for QT: the RR bin method in depth. In: Morganroth J, Gussak I, editors. Cardiac Safety of Noncardiac Drugs: Practical Guidelines for Clinical Research and Drug Development. Totowa, NJ: Humana Press, Inc; 2005. pp. 167–185. [Google Scholar]
- Barbey JT, Lazzara R, Zipes DP. Spontaneous adverse event reports of serious ventricular arrhythmias, QT prolongation, syncope, and sudden death in patients treated with cisapride. J Cardiovasc Pharmacol Ther. 2002;7:65–76. doi: 10.1177/107424840200700202. [DOI] [PubMed] [Google Scholar]
- Bednar MM, Harrigan EP, Ruskin JN. Torsades de pointes associated with nonantiarrhythmic drugs and observations on gender and QTc. Am J Cardiol. 2002;89:1316–1319. doi: 10.1016/s0002-9149(02)02337-8. [DOI] [PubMed] [Google Scholar]
- Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67:413–418. doi: 10.1067/mcp.2000.105761. [DOI] [PubMed] [Google Scholar]
- Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84:475–480. doi: 10.1038/clpt.2008.33. [DOI] [PubMed] [Google Scholar]
- Burke JH, Ehlert FA, Kruse JT, Parker MA, Goldberger JJ, Kadish AH. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol. 1997;79:178–181. doi: 10.1016/s0002-9149(96)00707-2. [DOI] [PubMed] [Google Scholar]
- Couderc JP, Zareba W. An update on QT measurement and interpretation methodologies. Ann Noninvasive Electrocardiol. 2009;14(Suppl. 1):S1–S2. doi: 10.1111/j.1542-474X.2008.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc JP, McNitt S, Hyrien O, Vaglio M, Xia X, Polonsky S, et al. Improving the detection of subtle I(Kr)-inhibition: assessing electrocardiographic abnormalities of repolarization induced by moxifloxacin. Drug Saf. 2008;31:249–260. doi: 10.2165/00002018-200831030-00006. [DOI] [PubMed] [Google Scholar]
- Culley CM, Lacy MK, Klutman N, Edwards B. Moxifloxacin: clinical efficacy and safety. Am J Health Syst Pharm. 2001;58:379–388. [PubMed] [Google Scholar]
- Damle B, Fosser C, Ito K, Tran A, Clax P, Uderman H, et al. Effects of Standard and Supratherapeutic Doses of Nelfinavir on Cardiac Repolarization: A Thorough QT Study. J Clin Pharmacol. 2009;49:291–300. doi: 10.1177/0091270008329551. [DOI] [PubMed] [Google Scholar]
- Darpo B. Spectrum of drugs prolonging the QT interval and the incidence of torsades de pointes. Eur Heart J. 2001;3(Suppl. K):70–80. [Google Scholar]
- Darpo B. Key issues with TQT studies. Presentation at the DIA meeting. 2008. ‘3rd annual cardiac safety conference’. Barcelona December 2008.
- Darpo B, Agin M, Kazierad DJ, Layton G, Muirhead G, Gray P, et al. Man versus machine: is there an optimal method for QT measurements in thorough QT studies? J Clin Pharmacol. 2006a;46:598–612. doi: 10.1177/0091270006286900. [DOI] [PubMed] [Google Scholar]
- Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use E14 guideline. J Clin Pharmacol. 2006b;46:498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- Demolis JL, Charransol A, Funck-Brentano C, Jaillon P. Effects of a single oral dose of sparfloxacin on ventricular repolarization in healthy volunteers. Br J Clin Pharmacol. 1996;41:499–503. doi: 10.1046/j.1365-2125.1996.03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demolis JL, Kubitza D, Tenneze L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658–666. doi: 10.1067/mcp.2000.111482. [DOI] [PubMed] [Google Scholar]
- Demolis JL, Vacheron F, Cardus S, Funck-Brentano C. Effect of single and repeated oral doses of telithromycin on cardiac QT interval in healthy subjects. Clin Pharmacol Ther. 2003;73:242–252. doi: 10.1067/mcp.2003.4. [DOI] [PubMed] [Google Scholar]
- Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, et al. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008;66:396–404. doi: 10.1111/j.1365-2125.2008.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert SN, Liu X, Woosley RL. Gender as a risk factor for acquired torsades de pointes. In: Franz MR, editor. Monophasic Action Potentials: Bridging Cell and Bedside. Armonk, NY: Futura Publishing Company, Inc; 2000. pp. 659–676. [Google Scholar]
- Extramiana F, Maison-Blanche P, Cabanis MJ, Ortemann-Renon C, Beaufils P, Leenhardt A. Clinical assessment of drug-induced QT prolongation in association with heart rate changes. Clin Pharmacol Ther. 2005;77:247–258. doi: 10.1016/j.clpt.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Fossa AA, Wisialowski T, Magnano A, Wolfgang E, Winslow R, Gorczyca W, et al. Dynamic beat-to-beat modeling of the QT-RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a-go-go-related gene inhibition. J Pharmacol Exp Ther. 2005;312:1–11. doi: 10.1124/jpet.104.073288. [DOI] [PubMed] [Google Scholar]
- Fossa AA, Wisialowski T, Crimin K. QT prolongation modifies dynamic restitution and hysteresis of the beat-to-beat QT-TQ interval relationship during normal sinus rhythm under varying states of repolarization. J Pharmacol Exp Ther. 2006;316:498–506. doi: 10.1124/jpet.105.095471. [DOI] [PubMed] [Google Scholar]
- Fosser C, Duczynski G, Agin M, Wicker P, Darpo B. Comparison of manual and automated measurements of the QT interval in healthy volunteers: an analysis of five thorough QT studies. Clin Pharmacol Ther. 2009 doi: 10.1038/clpt.2009.34. (Epub ahead of print); doi: 10.1038/clpt.2009.34. [DOI] [PubMed] [Google Scholar]
- Garnett C. Moxifloxacin needs to be given in a double-blind manner in the TQT study – counterpoint. Presentation at the DIA meeting ‘QT and arrhythmia issues in drug development. 2008. Washington DC April 2008.
- Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, et al. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol. 2008;48:13–18. doi: 10.1177/0091270007307881. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Bass AS, Gintant G, Mittelstadt S, Rampe D, Thomas K. ILSI-HESI cardiovascular safety subcommittee initiative: evaluation of three non-clinical models of QT prolongation. J Pharmacol Toxicol Methods. 2006;54:116–129. doi: 10.1016/j.vascn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hulhoven R, Rosillon D, Letiexhe M, Meeus MA, Daoust A, Stockis A. Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study. Eur J Clin Pharmacol. 2007;63:1011–1017. doi: 10.1007/s00228-007-0366-5. [DOI] [PubMed] [Google Scholar]
- Hulhoven R, Rosillon D, Bridson WE, Meeus MA, Salas E, Stockis A. Effect of levetiracetam on cardiac repolarization in healthy subjects: a single-dose, randomized, placebo- and active-controlled, four-way crossover study. Clin Ther. 2008;30:260–270. doi: 10.1016/j.clinthera.2008.02.002. [DOI] [PubMed] [Google Scholar]
- ICH E14 Q&A. E14 Implementation working group questions & answers. 2008. Available at: http://www.ich.org/LOB/media/MEDIA4719.pdf (accessed 4 June 2008.
- ICH Harmonized Tripartite Guideline E14. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-antiarrhythmic Drugs. 2005. Available at: http://www.ich.org/cache/compo/276-254-1.html.
- Iwamoto M, Kost JT, Mistry GC, Wenning LA, Breidinger SA, Marbury TC, et al. Raltegravir thorough QT/QTc study: a single supratherapeutic dose of raltegravir does not prolong the QTcF interval. J Clin Pharmacol. 2008;48:726–733. doi: 10.1177/0091270008318007. [DOI] [PubMed] [Google Scholar]
- Kligfield P, Hancock EW, Helfenbein ED, Dawson EJ, Cook MA, Lindauer JM, et al. Relation of QT interval measurements to evolving automated algorithms from different manufacturers of electrocardiographs. Am J Cardiol. 2006;98:88–92. doi: 10.1016/j.amjcard.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Kligfield P, Tyl B, Maarek M, Maison-Blanche P. Magnitude, mechanism, and reproducibility of QT interval differences between superimposed global and individual lead ECG complexes. Ann Noninvasive Electrocardiol. 2007;12:145–152. doi: 10.1111/j.1542-474X.2007.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitza D, Mueck W, Becka M. Randomized, double-blind, crossover study to investigate the effect of rivaroxaban on QT-interval prolongation. Drug Saf. 2008;31:67–77. doi: 10.2165/00002018-200831010-00006. [DOI] [PubMed] [Google Scholar]
- Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- Li J. PK/PD modelling technique to correct for heart-rate induced QT perturbations. Presentation at the DIA meeting. 2008. ‘3rd annual cardiac safety conference’. Barcelona December 2008.
- Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- Malhotra BK, Glue P, Sweeney K, Anziano R, Mancuso J, Wicker P. Thorough QT study with recommended and supratherapeutic doses of tolterodine. Clin Pharmacol Ther. 2007;81:377–385. doi: 10.1038/sj.clpt.6100089. [DOI] [PubMed] [Google Scholar]
- Malik M. Problems of heart rate correction in the assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:412–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- Malik M. Assessment of drug-induced QT prolongation: to bin or not to bin? Clin Pharmacol The. 2005;77:241–246. doi: 10.1016/j.clpt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Malik M, Andreas JO, Hnatkova K, Hoeckendorff J, Cawello W, Middle M, et al. Thorough QT/QTc study in patients with advanced Parkinson's disease: cardiac safety of rotigotine. Clin Pharmacol Ther. 2008;84:595–603. doi: 10.1038/clpt.2008.143. [DOI] [PubMed] [Google Scholar]
- Patterson S, Agin M, Anziano R, Burgess T, Chuang-Stein C, Dmitrienko A, et al. Investigating drug-induced QT and QTc prolongation in the clinic: a review of statistical design and analysis considerations. Report from Pharmaceutical Research and Manufacturer of America QT statistics expert team. Drug Information J. 2005;39:243–265. [Google Scholar]
- Pugsley MK, Authier S, Curtis MJ. Principles of safety pharmacology. Br J Pharmacol. 2008;154:1382–1399. doi: 10.1038/bjp.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaharju PM, Zhang ZM. Linearly scaled, rate-invariant normal limits for QT interval: eight decades of incorrect application of power functions. J Cardiovasc Electrophysiol. 2002;13:1211–1218. doi: 10.1046/j.1540-8167.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- Rock EP, Finkle J, Fingert HJ, Booth BP, Garnett CE, Grant S, et al. Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157:827–836. doi: 10.1016/j.ahj.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- Rosillon D, Astruc B, Hulhoven R, Meeus MA, Troenaru MM, Watanabe S, et al. Effect of brivaracetam on cardiac repolarisation – a thorough QT study. Curr Med Res Opin. 2008;24:2327–2337. doi: 10.1185/03007990802278453. [DOI] [PubMed] [Google Scholar]
- Sarapa N. Quality assessment of digital annotated ECG data from clinical trials by the FDA ECG warehouse. Expert Opin Drug Saf. 2007;6:595–607. doi: 10.1517/14740338.6.5.595. [DOI] [PubMed] [Google Scholar]
- Sarapa N, Britto MR. Challenges of characterizing proarrhythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opin Drug Saf. 2008;7:305–318. doi: 10.1517/14740338.7.3.305. [DOI] [PubMed] [Google Scholar]
- Sarapa N, Morganroth J, Couderc JP, Francom SF, Darpo B, Fleishaker JC, et al. Electrocardiographic identification of drug-induced QT prolongation: assessment by different recording and measurement methods. Ann Noninvasive Electrocardiol. 2004;9:48–57. doi: 10.1111/j.1542-474X.2004.91546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapa N, Nickens DJ, Raber SR, Reynolds RR, Amantea MA. Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies. Clin Pharmacol Ther. 2008;83:153–159. doi: 10.1038/sj.clpt.6100263. [DOI] [PubMed] [Google Scholar]
- Sarapa N, Francom SF, Azzam SM, Wickremasingha PK, Tyl B. Detection of clinically significant QTc prolongation in phase I studies in healthy participants: comparison of 2 semiautomated QT measurement methods. J Clin Pharmacol. 2009a;49:103–108. doi: 10.1177/0091270008326717. [DOI] [PubMed] [Google Scholar]
- Sarapa N, Gussak I, Vajdic B, George S, Hadzievski L, Francom SF, et al. Comparison of QTinno, a fully automated electrocardiographic analysis program, to semiautomated electrocardiographic analysis methods in a drug safety study in healthy subjects. J Electrocardiol. 2009b;42:358–366. doi: 10.1016/j.jelectrocard.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Serra DB, Affrime MB, Bedigian MP, Greig G, Milosavljev S, Skerjanec A, et al. QT and QTc interval with standard and supratherapeutic doses of darifenacin, a muscarinic M3 selective receptor antagonist for the treatment of overactive bladder. J Clin Pharmacol. 2005;45:1038–1047. doi: 10.1177/0091270005279010. [DOI] [PubMed] [Google Scholar]
- Shah RR. Drug-induced prolongation of the QT interval: regulatory dilemmas and implications for approval and labelling of a new chemical entity. Fundam Clin Pharmacol. 2002a;16:147–156. doi: 10.1046/j.1472-8206.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- Shah RR. Drug-induced prolongation of the QT interval: why the regulatory concern? Fundam Clin Pharmacol. 2002b;16:119–124. doi: 10.1046/j.1472-8206.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- Shin JG, Kang WK, Shon JH, Arefayene M, Yoon YR, Kim KA, et al. Possible interethnic differences in quinidine-induced QT prolongation between healthy Caucasian and Korean subjects. Br J Clin Pharmacol. 2007;63:206–215. doi: 10.1111/j.1365-2125.2006.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan IG, Hughes NP, Poonawala MH, Mason JW, Tarassenko L. Automated QT analysis that learns from cardiologist annotations. Ann Noninvasive Electrocardiol. 2009;14(Suppl. 1):S9–21. doi: 10.1111/j.1542-474X.2008.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnadova C. The assessment of QT/QTc prolongation in clinical trials: a regulatory perspective. Drug Information J. 2005;39:407–433. [Google Scholar]
- Trepakova ES, Koerner J, Pettit SD, Valentin JP. A HESI consortium approach to assess the human predictive value of non-clinical repolarization assays. J Pharmacol Toxicol Methods. 2009;60:45–50. doi: 10.1016/j.vascn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Vik T, Pollard C, Sager P. Early clinical development: evaluation of drug-induced torsades de pointes risk. Pharmacol Ther. 2008;119:210–214. doi: 10.1016/j.pharmthera.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Bacsanyi J. Cisapride and fatal arrhythmia (letter) N Eng J Med. 1996;335:290–291. doi: 10.1056/NEJM199607253350416. [DOI] [PubMed] [Google Scholar]
- Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. More lessons learned for TQT studies – FDA perspectives. Presentation at the DIA meeting ′QT and arrhythmia issues in drug development. 2008. Washington DC April 2008.
- Zhang J. Can moxifloxacin be given in a placebo arm during one day of a parallel designed study? Presentation at the DIA meeting. 2009. ‘3rd annual cardiac safety conference’. Barcelona December 2008.
- Zhang L, Chappell J, Gonzales CR, Small D, Knadler MP, Callaghan JT, et al. QT effects of duloxetine at supratherapeutic doses: a placebo and positive controlled study. J Cardiovasc Pharmacol. 2007;49:146–153. doi: 10.1097/FJC.0b013e318030aff7. [DOI] [PubMed] [Google Scholar]


