Abstract
Background and purpose:
Cannabidiol (CBD) is a non-psychotomimetic compound from Cannabis sativa that induces anxiolytic- and antipsychotic-like effects in animal models. Effects of CBD may be mediated by the activation of 5-HT1A receptors. As 5-HT1A receptor activation may induce antidepressant-like effects, the aim of this work was to test the hypothesis that CBD would have antidepressant-like activity in mice as assessed by the forced swimming test. We also investigated if these responses depended on the activation of 5-HT1A receptors and on hippocampal expression of brain-derived neurotrophic factor (BDNF).
Experimental approach:
Male Swiss mice were given (i.p.) CBD (3, 10, 30, 100 mg·kg−1), imipramine (30 mg·kg−1) or vehicle and were submitted to the forced swimming test or to an open field arena, 30 min later. An additional group received WAY100635 (0.1 mg·kg−1, i.p.), a 5-HT1A receptor antagonist, before CBD (30 mg·kg−1) and assessment by the forced swimming test. BDNF protein levels were measured in the hippocampus of another group of mice treated with CBD (30 mg·kg−1) and submitted to the forced swimming test.
Key results:
CBD (30 mg·kg−1) treatment reduced immobility time in the forced swimming test, as did the prototype antidepressant imipramine, without changing exploratory behaviour in the open field arena. WAY100635 pretreatment blocked CBD-induced effect in the forced swimming test. CBD (30 mg·kg−1) treatment did not change hippocampal BDNF levels.
Conclusion and implications:
CBD induces antidepressant-like effects comparable to those of imipramine. These effects of CBD were probably mediated by activation of 5-HT1A receptors.
Keywords: cannabinoids, cannabidiol, imipramine, antidepressant, forced swimming, 5-HT1A receptor, BDNF
Introduction
Extracts of the Cannabis sativa plant elicit in humans a complex subjective experience that includes euphoria, heightened sensitivity to external stimuli and relaxation (Johns, 2001). This plant contains more than 400 different compounds, of which 66 are termed cannabinoids. Δ9-tetrahydrocannabinol (Δ9-THC), one of the major constituents of C. sativa extracts (Mechoulam, 1970), is thought to account for most of the effects of cannabis through the activation of cannabinoid CB1 receptors in the brain (Huestis et al., 2001; nomenclature follows Alexander et al., 2008). The major endogenous agonists of the CB1 receptor are anandamide and 2-arachidonoyl glycerol, referred to as endocannabinoids (Piomelli, 2003). Anandamide is removed from the synaptic space by a putative neuronal uptake mechanism (Alger, 2004) and is inactivated intracellularly by the enzyme fatty acid amide hydrolase (Di Marzo et al., 1999).
It has recently been suggested that the endocannabinoid system may be involved in the pathophysiology of depression (Hill and Gorzalka, 2005). This is supported by several pieces of evidence showing that endocannabinoids and CB1 receptors are widely distributed in brain areas that are often related to affective disorders (Devane, 1988) and that their expression is regulated by antidepressant drugs (Hill et al., 2008). Moreover, administration of inhibitors of anandamide uptake or metabolism, as well as CB1 receptor agonists induces antidepressant-like effects in different animal models (Hill and Gorzalka, 2005; Adamczyk et al., 2008). In accordance with these preclinical results, many patients report benefits from cannabis use in depressive syndromes (Gruber et al., 1996; Ware et al., 2005), although clinical trials of its use in affective disorders have yielded mixed results (Robson, 2001; Degenhardt et al., 2003).
Cannabidiol (CBD) is another major component of C. sativa that exhibits a somewhat different pharmacology compared with that of Δ9-THC (Mechoulam et al., 2007). CBD is usually described as a non-psychoactive compound that inhibits some behavioural effects of Δ9-THC, such as catalepsy in rats (Formukong et al., 1988) and psychotomimetic and anxiogenic effects in humans (Zuardi et al., 1982). CBD, however, has been shown to induce antipsychotic- and anxiolytic-like activity in preclinical and clinical studies (Zuardi et al., 1982; 2006; Guimarães et al., 1990; Resstel et al., 2006). More recently, our group showed that systemic administration of CBD was able to attenuate the development of stress-induced behavioural consequences (Resstel et al., 2009), raising the possibility that CBD could also be useful for treating psychiatric disorders thought to involve impairment of stress-coping mechanisms, such as depression.
The mechanism of action of CBD is not fully understood. This compound has a low affinity for CB receptors (Petitet et al., 1998; Thomas et al., 1998), although it may block the reuptake of anandamide (Bisogno et al., 2001) and inhibit fatty acid amide hydrolase (Watanabe et al., 1998). Moreover, Russo and colleagues (2005) reported that CBD may exhibit agonist properties at 5-HT1A receptors. In fact, recent work has shown that several CBD effects can be blocked by pretreatment with 5-HT1A receptor antagonists (Hayakawa et al., 2007; Campos and Guimarães, 2008; Resstel et al., 2009).
Although activation of 5-HT1A receptors has been consistently related to the therapeutic effect of antidepressant drugs (Savitz et al., 2009), a link between these receptors and antidepressant-like effect of CBD has not yet been investigated. Therefore, the aim of this work was to test the hypothesis that CBD would induce antidepressant-like effects in mice submitted to the forced swimming test and that this effect would involve the activation of 5-HT1A receptors. In addition, considering the recent pieces of evidence relating the effects of antidepressant drugs with increased hippocampal expression of brain derived neurotrophic factor (BDNF) (see Saaralainen et al., 2003; Duman and Monteggia, 2006) and that activation of 5-HT1A receptors regulates antidepressant-induced hippocampal BDNF expression (Ivy et al., 2003), we also investigated if CBD-induced behavioural effects in the forced swimming test would be associated with changes in BDNF expression in the hippocampus.
Methods
Animals
All animal care was in conformity with the Brazilian Society of Neuroscience and Behavior guidelines for the care and use of laboratory animals, which are in compliance with international laws. The experimental protocols were approved by the local Ethical Committee of the School of Medicine of Ribeirão Preto, University of Sao Paulo. Male Swiss mice (20–25 g) were provided by our local animal farm facility. After arriving at the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirão Preto, University of Sao Paulo, the animals were housed in groups of 6–10 animals per cage (570 cm2), in a temperature-controlled room (24 ± 1°C) under standard laboratory conditions with free access to food and water and a 12 h light/12 h dark cycle (lights on at 06:30h).
Forced swimming test
The forced swimming test was performed as described by Porsolt et al. (1977) with minor modifications. Mice were placed individually into glass cylinders (height 25 cm, diameter 17 cm) containing 10 cm of water maintained at 23–25°C. The animals were left in the cylinder for 6 min and the total duration of immobility was measured during the last 4 min period. Mice were considered to be immobile when they remained floating passively, performing only slow movements to keep their head above the water. The water was changed after each trial to avoid the influence of alarm substances (Abel and Bilitzke 1990). All experiments were videotaped and the immobility time was subsequently scored by an observer unaware of the treatments.
Exploratory activity
The test was performed according to Moreira and Guimarães (2005). Briefly, the animals were placed in a circular open field arena (40 cm in diameter with a 50 cm high Plexiglas wall) where the exploratory activity was videotaped during 6 min. The behaviour was analysed with the help of the Ethovision software (version 1.9; Noldus, the Netherlands). This software detects the position of the animal in the open field arena and calculates the distance moved.
Protein extraction and BDNF measurements
Immediately after the forced swimming test, mice were deeply anaesthetized (urethane 25%, 5 mL·kg−1), killed by decapitation and their hippocampi removed. The left and right hippocampus were homogenized in lysis buffer (NaCl 137 mM; Tris-HCl 20 mM pH 7.6; glycerol 10%) containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and, after centrifugation (5600×g, 15 min), the supernatant was stored at −80°C. Hippocampal BDNF was measured by ELISA (BDNF Emax® ImmunoAssay System kit, Promega, Madison, WI, USA) according to the manufacture's instructions. Total proteins levels were measured by the Bradford method (Bradford 1976; Sapan et al. 1999) and used to normalize the samples.
Experimental design
Experiment 1
Mice received i.p. injections of CBD (3, 10, 30, 100 mg·kg−1), imipramine or vehicles and were submitted to the forced swimming test. Independent groups of mice received CBD at the same doses or its vehicle and were submitted to the open field arena.
Experiment 2
Mice received i.p. injections of WAY100635 or saline followed, 30 min later, by a second injection of CBD (30 mg·kg−1) or vehicle and they were exposed to the forced swimming test, 30 min later.
Experiment 3
Independent groups of mice received i.p. injections of CBD (30 mg·kg−1), imipramine (30 mg·kg−1) or vehicle and were submitted to the forced swimming test as described above. Immediately afterwards, the animals were anaesthetized and killed. Their hippocampi were removed and processed for ELISA measurements of BDNF content. This time point was chosen for BDNF measurements as an attempt to correlate BDNF levels at the moment of the test with the behavioural effects induced by the drug treatments (Takeda et al., 2006; Rantamäki et al., 2007; Shieh et al., 2008).
In all experiments the animals were submitted to the behavioural test 30 min after the last drug injection.
Data analysis
The Kolmogorov-Smirnov and Levene tests were initially employed to ensure that the data satisfied the criteria for carrying out anova. The behavioural data were expressed as means ± SEM. The immobility time in the first and third experiments was analysed using one-way anova followed by Duncan's post hoc test. The distance moved in the open field arena was analysed by repeated measure analysis of variance with time (1–6 min) as the within-subjects factor and drug as the between-subjects factor. Box's epsilon function was employed to correct the degree of freedom of the repeated factors. Experiment two was analysed by two-way anova using the first (WAY100635 or saline) and the second (CBD or vehicle) injections as main factors. In case of significant interaction between factors the treatment groups were compared using a one-way anova followed by Duncan's post hoc test. For experiment three, BDNF levels were normalized to the total protein content and expressed as mean ± SEM. The results were compared using one-way anova. The significance level was set at P < 0.05.
Materials
CBD (kindly supplied by THC-Pharma, Frankfurt, Germany): 3, 10, 30, 100 mg·kg−1; imipramine hydrochloride (Sigma, St. Louis, MO, USA): 30 mg·kg−1 (dose based on Poleszak et al., 2005); WAY100635 (WAY, Sigma, St. Louis, MO, USA): 0.1 mg·kg−1 (dose based on Kaster et al., 2005). Imipramine and WAY100635 were dissolved in sterile isotonic saline solution and CBD was suspended in polyoxyethylenesorbitan monooleate (Tween 80) 2%-saline. The solutions were prepared immediately before use and injected i.p. in a volume of 10 mL·kg−1.
Results
Effects of CBD or imipramine treatment in the forced swimming test and in the open field arena
There was a significant treatment effect in the forced swimming test (F6,59= 3.89, P < 0.01), with imipramine and CBD (30 mg·kg−1) significantly reducing the immobility time compared with the vehicle group (n= 8–12, Duncan P < 0.05; Figure 1).
Figure 1.
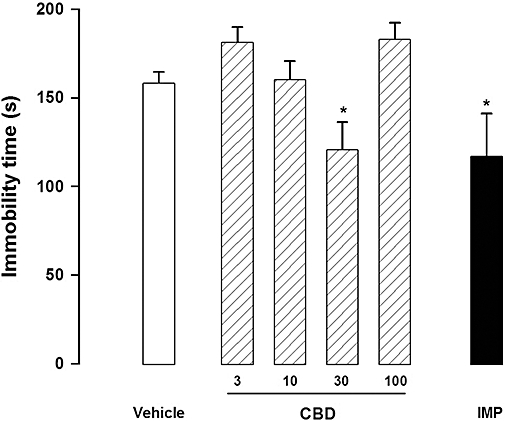
Cannabidiol (CBD, 30 mg·kg−1) and imipramine (IMP, 15 mg·kg−1) reduced immobility time in the forced swimming test. Mice (8–12/group) received i.p. injections of CBD (3–100 mg·kg−1) or imipramine (IMP) and 30 min later were submitted to the forced swim. Data represent the mean ± SEM. * indicates P < 0.05 compared with vehicle group (anova followed by Duncan).
In the open field arena the distance travelled decreased over time (F1,42= 49.12, P < 0.005), but there was no difference between drug treatment and vehicle at any dose tested (n= 8 per group; F5,42= 0.59, P > 0.05). Also, no interaction was observed between time and treatment effects (F5,42= 0.54, P > 0.05; Figure 2).
Figure 2.
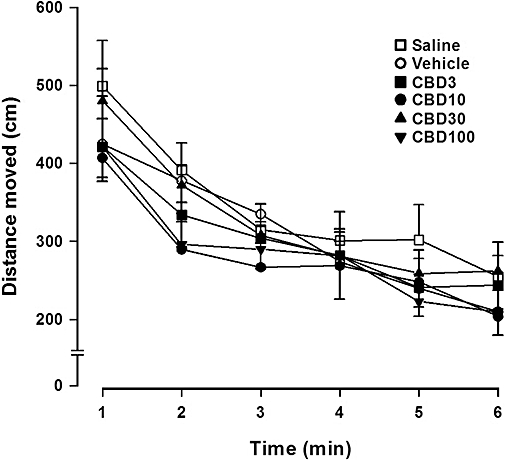
Cannabidiol (CBD, 3–100 mg·kg−1) did not induce any significant change in the exploratory activity. Mice (8 per group) received i.p. injections of CBD and 30 min later the distance moved in an open arena was analysed over 6 min. Data represent the mean ± SEM.
Effects of CBD (30 mg·kg−1), alone or in combination with WAY100635, in the forced swimming test
There was a significant interaction between the first and second injections (F1,48= 5.67, P= 0.02). Confirming results from the first experiment, saline + CBD (30 mg·kg−1, n= 11) treatment reduced immobility time in the forced swimming test when compared with the saline + vehicle group (n= 16; Duncan P < 0.05). Effects of CBD were prevented by pre-treatment with WAY100635 (n= 8, WAY100635 + CBD vs. saline + CBD: Duncan P < 0.05). WAY100635 + vehicle (n= 17) treatment did not induce significant changes in immobility time when compared with saline + vehicle group (Duncan, P > 0.05). See Figure 3.
Figure 3.
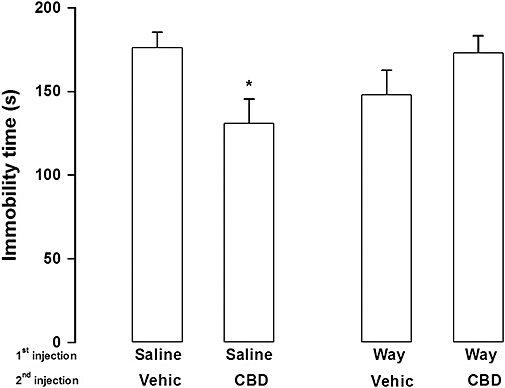
Pretreatment with WAY100635 (Way, 0.1 mg·kg−1) prevented cannabidiol (CBD, 30 mg·kg−1) effects in the forced swimming test. Mice (8–17 per group) received a first i.p. injection of WAY100635 or saline followed, 30 min later, by a second injection of CBD or vehicle (Vehic). The animals were submitted to the forced swim 30 min after the second injection. Data represents mean ± SEM. * indicates P < 0.05 compared with saline + vehicle group (anova followed by Duncan).
Effects of CBD (30 mg·kg−1) or imipramine on hippocampal BDNF levels
As observed in the previous experiments, CBD (30 mg·kg−1) and imipramine reduced the immobility time in the forced swimming test (F2,19= 5.91, P < 0.05; Figure 4A). However, these treatments failed to change hippocampal BDNF levels (F2,19= 0.013, P > 0.05; Figure 4B). Moreover, there was no correlation between immobility time and hippocampal BDNF levels (Pearson correlation, r= 0.33, n= 22, P > 0.05, data not shown).
Figure 4.
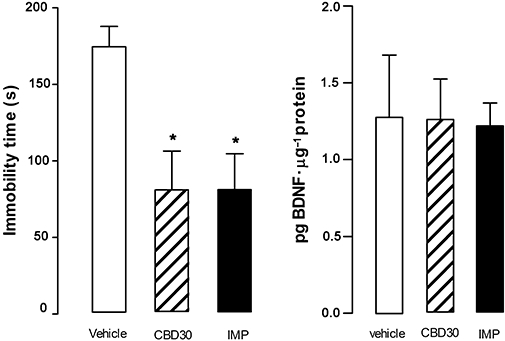
Cannabidiol-induced antidepressant-like effects were not associated with alterations in hippocampal BDNF levels. Mice (7–8 per group) received i.p. injection of cannabidiol (CBD, 30 mg·kg−1) or imipramine (IMP, 30 mg·kg−1) and 30 min later were submitted to the forced swim test (left graph). Immediately after, the animals were killed, their hippocampi removed and processed for BDNF measurements (right graph). Data represent the mean ± SEM. * indicates P < 0.05 compared with vehicle group (anova followed by Duncan).
Discussion
The present results show that CBD reduces immobility time in the forced swimming test to a similar extent as a prototype antidepressant, imipramine. Thus, this work is the first to suggest that CBD has a favourable profile in a model predictive of antidepressant-like activity (Porsolt et al., 1977). CBD, however, was effective only at the 30 mg·kg−1 dose, with smaller or higher doses producing no effect.
Considering that the forced swimming test is a paradigm based on evaluation of motor activity, drugs that induce changes in this parameter could confound data interpretation. In the present study, none of the CBD doses tested modified the distance moved in the open field arena. This datum is in agreement with previous reports showing that, at the doses tested, CBD does not induce significant motor changes (Guimarães et al., 1990; Moreira and Guimarães, 2005). This indicates that the antidepressant-like effect of CBD is not secondary to changes in motor behaviour. The present results, therefore, suggest that, in addition to anxiolytic, hypnotic and antipsychotic effects (Zuardi et al., 1982; Guimarães et al., 1990; Resstel et al., 2006), CBD could also have antidepressant properties.
The precise mechanisms underlying the effects of CBD are not well understood. CBD has a low affinity for cannabinoid CB1 receptors (Petitet et al., 1998; Thomas et al., 1998), but may indirectly affect the endocannabinoid system by blocking anandamide reuptake (Bisogno et al., 2001) and inhibiting its enzymatic hydrolysis (Watanabe et al., 1998). It has recently been proposed, in addition, that CBD could act as an agonist at 5-HT1A receptors (Russo et al., 2005). These receptors have been consistently related to the neurobiology of depression and to the mechanism of action of antidepressant drugs (Graeff et al., 1996; Joca et al., 2003; Savitz et al., 2009).
In accordance with the proposed activity of CBD on 5-HT1A receptors, the present study showed that the antidepressant-like effect of CBD is inhibited by pre-treatment with WAY100635, a selective 5-HT1A receptor antagonist (Kaster et al., 2005), at a dose that did not produce any effect by itself. This suggests that CBD effects in the forced swimming test depend on activation of 5-HT1A receptors. This is consistent with previous observation that neuroprotective (Hayakawa et al., 2007) and anxiolytic (Campos and Guimarães, 2008; Resstel et al., 2009) effects induced by CBD are sensitive to 5-HT1A receptor antagonists.
The mechanisms by which 5-HT1A receptors mediate adaptation to stress and induce antidepressant-like effects are not completely understood. 5-HT1A receptors are located presynaptically (somatodendritic autoreceptors) in 5-hydroxytryptaminergic cell bodies in the raphe nuclei of the brain stem and post-synaptically, predominantly in limbic structures such as the hippocampus, hypothalamus, prefrontal cortex and amygdala (Chalmers and Watson, 1991). It has been suggested that stress and/or genetic factors might impair post-synaptic 5-HT1A-mediated effects in limbic regions, such as the hippocampus, and predispose individuals to stress-induced behavioural consequences (Graeff et al., 1996). In fact, several studies have found reduced number and/or affinity of post-synaptic 5-HT1A receptors in the brains of depressed individuals (Sargent et al., 2000; Szewczyk et al., 2009) and of animals submitted to stress (Flugge, 1995; van Riedel et al., 2003). On the other hand, the number of 5-HT inhibitory autoreceptors is increased (Stockmeier et al., 1998). In agreement with the proposed hypothesis, chronic antidepressant treatment is shown to facilitate post-synaptic 5-HT1A receptor-mediated function (Haddjeri et al., 1998) and administration of 5-HT1A receptor agonists into the hippocampus induces antidepressant-like effects in animal models, most likely by attenuating the emotional impact of aversive stimuli (Graeff et al., 1996; Joca et al., 2003; 2007;). However, the mechanisms involved in 5-HT1A receptor-mediated antidepressant effect are still a matter of debate. One possibility is that activation of 5-HT1A receptors attenuates limbic hyperactivity, an effect that can be observed in stressed rats (Shumake et al., 2002) and depressed humans (Mayberg et al., 2000; Goldapple et al., 2004). This effect could involve attenuation of local glutamate release (Strosznajder et al., 1996).
In addition, antidepressant treatment may increase hippocampal neurogenesis and BDNF expression (see Duman and Monteggia, 2006), effects that are necessary for some of their behavioural effects (Santarelli et al., 2003; Saaralainen et al., 2003). However, it remains controversial whether their effects on stress-coping in the forced swimming test occur in parallel with increased BDNF expression. 5-HT1A receptors are thought to mediate some of the trophic actions attributed to 5-HT, such as increased neurogenesis (Brezun and Daszuta, 1999; Radley and Jacobs, 2002) and BDNF release (Ivy et al., 2003). Thus, we tested whether imipramine and CBD would increase hippocampal BDNF. However, the present study failed to detect any effect of CBD or imipramine on this variable. Despite these results, BDNF involvement in imipramine- and CBD-induced effects may not be ruled out as, even if acute effects of antidepressants on BDNF-mediated transmission have already been described (Saaralainen et al., 2003; Rantamäki et al., 2007; Dzitoyeva et al., 2008; Shieh et al., 2008), several studies have shown increased hippocampal BDNF expression only after subchronic or chronic antidepressant treatments (Castrén et al., 2007). Experimental differences between studies, other than the treatment duration, might also have contributed to the observed results. For example, the use of extracts of the whole hippocampus could have masked treatment effects on BDNF levels, as molecular and functional differences among hippocampal subregions have been described (Bannerman et al., 2004; Datson et al., 2009). Moreover, the use of tissue homogenates impairs the distinction between intracellular and released BDNF pools and it is possible that only a small and local release of BDNF is required to cause behavioural effects (Saaralainen et al., 2003).
The antidepressant-like effects of CBD were only evident at the dose of 30 mg·kg−1, with smaller or higher doses producing no effect. This inverted U-shape profile has been observed in several previous studies with CBD (Guimarães et al., 1990; Moreira et al., 2006) and is often observed with drugs that modulate the endocannabinoid system (Viveros et al., 2005). The mechanisms for this effect are not yet understood, but probably reflect the complex pharmacology of this compound. Among the possible mechanisms there is an interaction with TRPV1 vanilloid receptors. CBD can activate these receptors in µM concentrations (Bisogno et al., 2001) and they are expressed in several brain areas related to emotional responses such as the amygdala, hippocampus, prefrontal cortex and periaqueductal gray (Cristino et al., 2006). TRPV1 receptors can facilitate glutamate release (Palazzo et al. 2002) and glutamate receptor antagonists have been shown to induce antidepressant-like effects (Joca et al., 2007). Corroborating this possibility, activation of TRPV1 receptors has recently been implicated in the bell-shaped dose–response curve observed with the endocannabinoid anandamide, microinjected into the prefrontal cortex (Rubino et al., 2008).
In conclusion, the results of the present study showed that CBD induces antidepressant-like effects in the forced swimming test, suggesting for the first time that this compound may possess antidepressant properties. Moreover, this work also suggests that CBD-induced effects are probably mediated by facilitation of 5-HT1A receptor-mediated neurotransmission.
Acknowledgments
The authors wish to thank J.C. Aguiar and E.L.T. Gomes for technical support and FAPESP and CNPq for financial support. We also thank THC-Pharma for the kind supply of CBD.
Glossary
Abbreviations:
- CBD
cannabidiol
- BDNF
brain derived neurotrophic factor
- Δ9-THC
Δ9-tetrahydrocannabinol
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abel EL, Bilitzke PJ. A possible alarm substance in the forced swimming test. Physiol Behav. 1990;48:233–239. doi: 10.1016/0031-9384(90)90306-o. [DOI] [PubMed] [Google Scholar]
- Adamczyk P, Gołda A, McCreary AC, Filip M, Przegaliński E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59(2):217–228. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE. Endocannabinoids: getting the message across. Proc Natl Acad Sci USA. 2004;101:8512–8513. doi: 10.1073/pnas.0402935101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199(2):223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain–a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Steenbergen PJ, Aubert Y, Schlumbohm C, Fuchs E, et al. A molecular blueprint of gene expression in hippocampal subregions CA1, CA3, and DG is conserved in the brain of the common marmoset. Hippocampus. 2009;19(8):739–752. doi: 10.1002/hipo.20555. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: an historical overview and some recent developments. Lipids. 1999;34:S319–S325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Imbesi M, Uz T, Dimitrijevic N, Manev H, Manev R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J Neural Trans. 2008;115(6):823–827. doi: 10.1007/s00702-008-0034-7. [DOI] [PubMed] [Google Scholar]
- Flugge G. Dynamics of central nervous 5-HT1A receptors under psychosocial stress. J Neurosci. 1995;15(11):7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formukong EA, Evans AT, Evans FJ. Inhibition of the cataleptic effect of tetrahydrocannabinol by other constituents of Cannabis sativa L. J Pharm Pharmacol. 1988;40:132–134. doi: 10.1111/j.2042-7158.1988.tb05198.x. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimarães FS, Andrade GCS, Deakin JFW. Role of 5-HT in stress, anxiety and depression. Pharmaco Biochem Behav. 1996;54(1):129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, Jr, Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4:77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, De Montigny C. Long term antidepressant treatments result in a tonic activation of forebrain 5HT1a receptors. J Neurosci. 1998;18(23):10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, et al. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52(4):1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol. 2005;16(5–6):333–352. doi: 10.1097/00008877-200509000-00006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106(6):2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003;75(1):81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Joca SR, Padovan CM, Guimarães FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978(1–2):177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimarães FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10(3):227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Santos AR, Rodrigues AL. Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull. 2005;67(1–2):53–61. doi: 10.1016/j.brainresbull.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Petersa M, Murillo-Rodriguez E, Hanus LO. Cannabidiol – recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Guimarães FS. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol. 2005;512:199–205. doi: 10.1016/j.ejphar.2005.02.040. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, et al. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6. doi: 10.1016/s0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Wlaz P, Szewczyk B, Kedzierska E, Wyska E, Librowski T, et al. Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: Behavioral and pharmacokinetic studies in mice. Pharmacol Biochem Behav. 2005;81(3):524–529. doi: 10.1016/j.pbb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatment. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955(1–2):264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Moreira FA, Correa FM, Guimarães FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006;172(2):294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156(1):181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riedel E, Meijer OC, Steebergen PJ, Joels M. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience. 2003;120:649–658. doi: 10.1016/s0306-4522(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, et al. Role in Anxiety Behavior of the Endocannabinoid System in the Prefrontal Cortex. Cereb Cortex. 2008;18(6):1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Saaralainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the trkB neurotrophin receptor is induced by antidepressant drugs and requires for antidepressant-induced behavioural effects. J Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapan CV, Lundblad RL, Price NC. Colorimetric protein assay techniques. Biotechnol Appl Biochem. 1999;29(Pt 2):99–108. [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission topography with (11C) WAY100635. Arch Gen Psychiat. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh CH, Hong CJ, Huang YH, Tsai SJ. Potential antidepressant properties of cysteamine on hippocampal BDNF levels and behavioral despair in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1590–1594. doi: 10.1016/j.pnpbp.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Dissociation of septo-hippocampal metabolism in the congenitally helpless rat. Neuroscience. 2002;114(2):373–337. doi: 10.1016/s0306-4522(02)00297-x. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosznajder J, Chalimoniuk M, Samochocki M. Activation of serotonergic 5-HT1A receptor reduces Ca(2+)- and glutamatergic receptor-evoked arachidonic acid and No/cGMP release in adult hippocampus. Neurochem Int. 1996;28(4):439–444. doi: 10.1016/0197-0186(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12(2):155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Yamada T, Masuya J, Matsushita K, Tahara M, et al. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur J Pharmacol. 2006;534(1–3):115–121. doi: 10.1016/j.ejphar.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81(2):331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59:291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ogi H, Nakamura S, Kayano Y, Matsunaga T, Yoshimura H, et al. Distribution and characterization of anandamide amidohydrolase in mouse brain and liver. Life Sci. 1998;62(14):1223–1229. doi: 10.1016/s0024-3205(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39(4):421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]


