Abstract
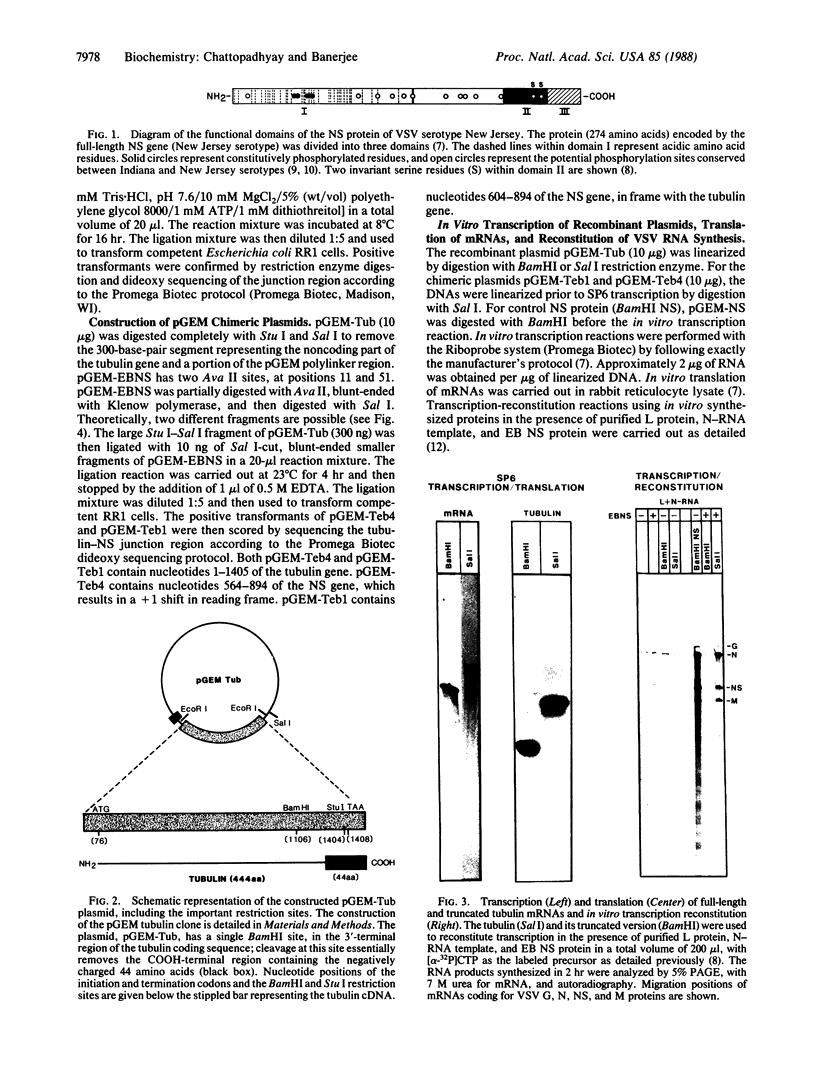
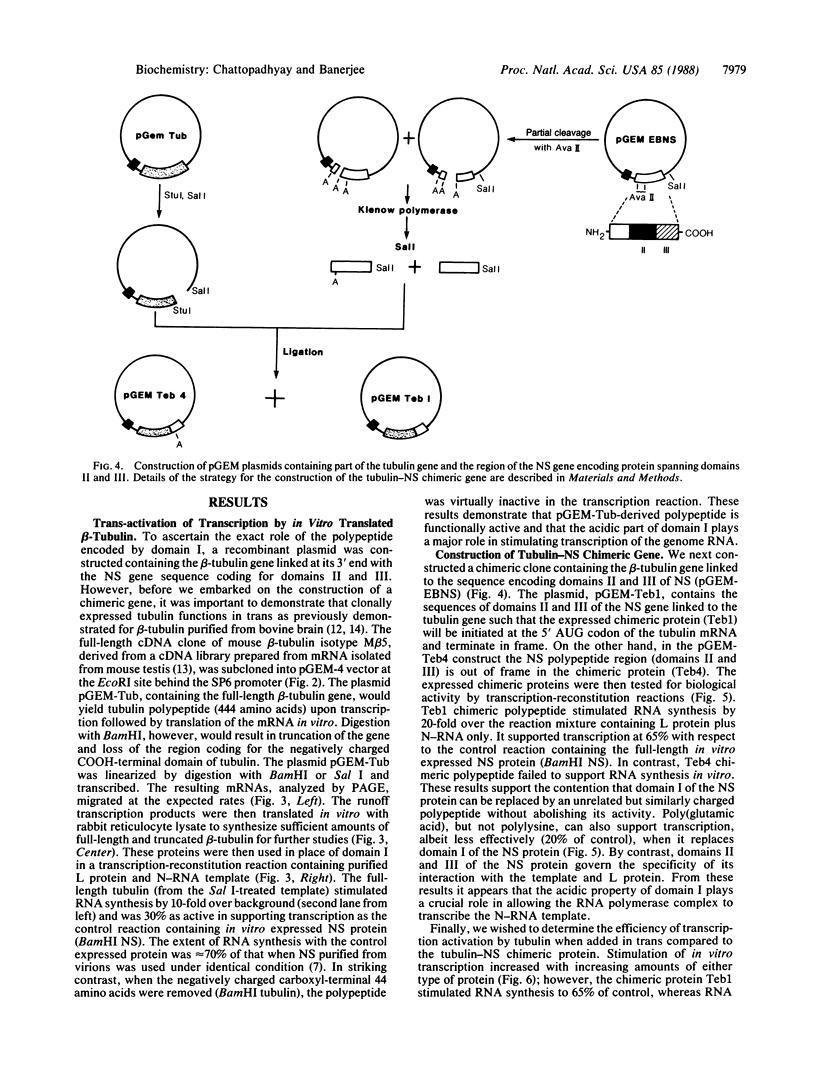
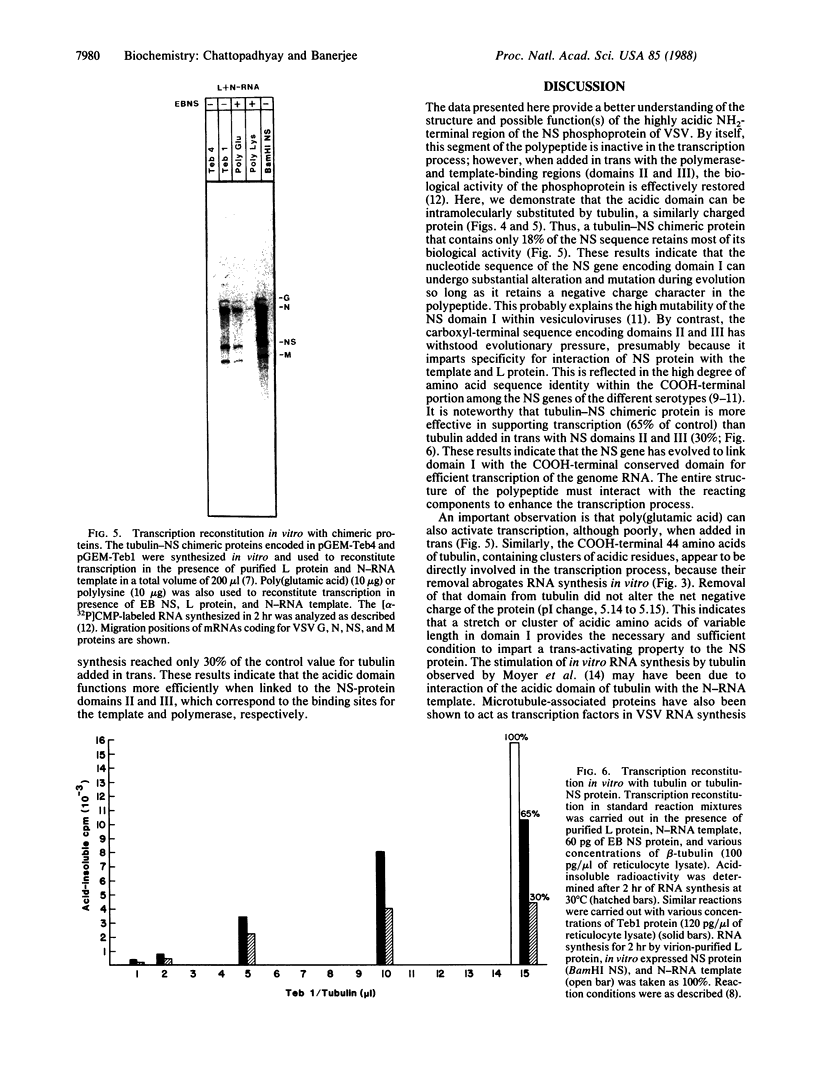
The phosphoprotein (NS) of vesicular stomatitis virus is an indispensable subunit of the virion-associated RNA polymerase (L). NS consists of a highly acidic NH2-terminal domain and a basic COOH-terminal domain. Unlike the latter, the amino acid sequences of the NH2-terminal regions are highly dissimilar among different viral serotypes, although they share structural similarities. We have cloned an NS gene into the SP6 transcription vector and replaced the 5'-terminal 80% by a full-length gene for beta-tubulin, which contains an acidic COOH-terminal domain. Here we present evidence that the chimeric tubulin-NS protein is biologically active and that the acidic region in tubulin directly affects the transcription reaction. These observations indicate that NS probably functions as an activator protein in which the acidic domain stimulates transcription of the viral genes by interacting with the RNA polymerase as observed for eukaryotic cellular transcription activators.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. The transcription complex of vesicular stomatitis virus. Cell. 1987 Feb 13;48(3):363–364. doi: 10.1016/0092-8674(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Two separate domains within vesicular stomatitis virus phosphoprotein support transcription when added in trans. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8932–8936. doi: 10.1073/pnas.84.24.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Requirements and functions of vesicular stomatitis virus L and NS proteins in the transcription process in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):40–49. doi: 10.1016/0006-291x(85)90568-6. [DOI] [PubMed] [Google Scholar]
- Dillon P. J., Gupta K. C. Early steps in the assembly of vesicular stomatitis virus nucleocapsids in infected cells. J Virol. 1988 May;62(5):1582–1589. doi: 10.1128/jvi.62.5.1582-1589.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Vesicular stomatitis virus NS proteins: structural similarity without extensive sequence homology. J Virol. 1985 Jul;55(1):60–66. doi: 10.1128/jvi.55.1.60-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Chattopadhyay D., Banerjee A. K. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Harmon S. A., Summers D. F. Stimulation of vesicular stomatitis virus in vitro RNA synthesis by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5410–5413. doi: 10.1073/pnas.83.15.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W., Murti K. G. Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis. J Virol. 1979 Oct;32(1):304–313. doi: 10.1128/jvi.32.1.304-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L. D., Condra C., Lazzarini R. A. Cloning and expression of a viral phosphoprotein: structure suggests vesicular stomatitis virus NS may function by mimicking an RNA template. J Gen Virol. 1986 Aug;67(Pt 8):1571–1579. doi: 10.1099/0022-1317-67-8-1571. [DOI] [PubMed] [Google Scholar]
- Kakidani H., Ptashne M. GAL4 activates gene expression in mammalian cells. Cell. 1988 Jan 29;52(2):161–167. doi: 10.1016/0092-8674(88)90504-1. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Thornton B. J., Emerson S. U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Sequences of Chandipura virus N and NS genes: evidence for high mutability of the NS gene within vesiculoviruses. Virology. 1987 Apr;157(2):298–306. doi: 10.1016/0042-6822(87)90272-8. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Rae B. P., Elliott R. M. Characterization of the mutations responsible for the electrophoretic mobility differences in the NS proteins of vesicular stomatitis virus New Jersey complementation group E mutants. J Gen Virol. 1986 Dec;67(Pt 12):2635–2643. doi: 10.1099/0022-1317-67-12-2635. [DOI] [PubMed] [Google Scholar]
- Rae B. P., Elliott R. M. Conservation of potential phosphorylation sites in the NS proteins of the New Jersey and Indiana serotypes of vesicular stomatitis virus. J Gen Virol. 1986 Jul;67(Pt 7):1351–1360. doi: 10.1099/0022-1317-67-7-1351. [DOI] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Villasante A., Lewis S. A., Cowan N. J. The mammalian beta-tubulin repertoire: hematopoietic expression of a novel, heterologous beta-tubulin isotype. J Cell Biol. 1986 Nov;103(5):1903–1910. doi: 10.1083/jcb.103.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster N., Jin J. R., Green S., Hollis M., Chambon P. The yeast UASG is a transcriptional enhancer in human HeLa cells in the presence of the GAL4 trans-activator. Cell. 1988 Jan 29;52(2):169–178. doi: 10.1016/0092-8674(88)90505-3. [DOI] [PubMed] [Google Scholar]




