Abstract
Background and purpose:
Vascular ‘denervation’ hyper-reactivity has generally been investigated 1–2 weeks after administration of chemicals that temporarily prevent transmitter release, but do not necessarily inactivate the neuronal noradrenaline transporters (NETs). We have investigated the reactivity of rat tail arteries over longer periods after removing the terminals by surgical denervation.
Experimental approach:
Two and 7 weeks after denervation, myography was used to assess contractions of isolated arterial segments to phenylephrine, methoxamine, clonidine, vasopressin and angiotensin II (AII). Denervation was confirmed by lack of tyrosine hydroxylase immunoreactive nerve terminals.
Key results:
The NET inhibitor, desmethylimipramine, increased the pEC50 for phenylephrine in control, but not denervated arteries after both 2 and 7 weeks. Relative to controls, pEC50s for phenylephrine (with desmethylimipramine), methoxamine, clonidine and vasopressin were increased at 2 but not 7 weeks after denervation. The pEC50 for phenylephrine in the absence of desmethylimipramine was greater than control after both 2 and 7 weeks' denervation. The maximum contraction to vasopressin was larger than in controls at 2 but not 7 weeks after denervation, whereas contractions to AII were markedly enhanced at both time points.
Conclusions and implications:
Increased vascular reactivity to α1- and α2-adrenoceptor agonists, and vasopressin is transient following denervation. After 7 weeks, increased reactivity to phenylephrine can be entirely accounted for by the loss of NETs. Maintained supersensitivity to AII indicates that denervation differentially and selectively affects vascular reactivity to circulating vasoconstrictor agents. This might explain persistent vasoconstriction in denervated skin of humans after nerve injuries.
Keywords: sympathetic nerve, rat tail artery, denervation, α-adrenoceptors, supersensitivity, smooth muscle, neuronal noradrenaline transporter, vasopressin, angiotensin II
Introduction
In skin, sympathetic denervation due to nerve injury or sympathectomy in both humans and animals produces an initial increase in blood flow and then a progressive and persistent decrease after a few weeks, resulting from an increase in vascular tone (i.e. active vasoconstriction; Sunderland, 1978; Kurvers et al. 1997). The skin becomes cold, cyanotic and extremely susceptible to injury, such as ulceration, due to the limited blood flow. The vasoconstriction is believed to reflect the development of supersensitivity of the denervated vasculature to circulating and locally generated vasoconstrictor agents (Sunderland, 1978; Baron and Maier, 1996). Further, reinnervation of distant vasculature by regenerating sympathetic axons is slow and incomplete (Jobling et al., 1992), often leaving distal vessels denervated for prolonged periods.
Abolition of sympathetic neuroeffector transmission in smooth muscles by silencing nerve activity or by depletion of noradrenaline stores in nerve terminals produces an increase in the muscle's sensitivity to exogenous adrenoceptor agonists (Fleming and Westfall, 1988), typically producing a leftward shift in the concentration–response curve for the agonist. However, because the change is not selective for agonists acting at neurotransmitter receptors, the increased sensitivity has been suggested to result from a generalized increase in smooth muscle reactivity (Fleming and Westfall, 1988). In addition, the loss of the neuronal transporter for noradrenaline (NET) following removal of nerve terminals can selectively increase sensitivity to adrenoceptor agonists that are substrates for this amine reuptake pump (Trendelenburg, 1966). In this case, the apparent change in sensitivity results from an increase in the concentration of agonist reaching the smooth muscle adrenoceptors.
The changes in vascular reactivity produced by disrupting neurovascular transmission have often been studied by depleting the noradrenaline stores in the perivascular nerve terminals using systemic administration of the vesicular noradrenaline uptake inhibitor, reserpine (e.g. Nasseri et al., 1985; Duckles, 1991; Taki et al., 2004). However, as reserpine may not abolish the release of co-transmitters (Brock et al., 1990) or trophic factors from the nerve terminals, the extent to which the ensuing changes in vascular reactivity mimic denervation is questionable. Systemic treatment with 6-hydroxydopamine in an adult can be used to destroy the nerve terminal plexus, but the terminals rapidly regenerate within a few weeks (Finch et al., 1973). Neither of these chemical treatments can be used to study the long-term (>2 weeks) effects of abolishing neurally released noradrenaline in a particular vascular bed because of the widespread side-effects of blocking sympathetic control of multiple body functions, as well as the unpleasant CNS effects of reserpine. Thus, these systemic interventions do not necessarily mimic the long-term effects produced by the loss of sympathetic innervation following nerve injury in humans.
Here, we have investigated the effects of surgical denervation on the rat tail artery. This artery supplies the skin of the tail and plays a role in thermoregulation similar to that of the digital arteries in man. The tail artery is innervated by sympathetic axons from the lower sacral and coccygeal paravertebral ganglia that reach the artery via four collector nerves that run the length of the tail (Sittiracha et al., 1987). We have assessed the relative contributions of changes in smooth muscle sensitivity to selective α1- and α2-adrenoceptor agonists (phenylephrine, methoxamine and clonidine) and of loss of NETs to the hyper-reactivity of the artery 2 and 7 weeks after denervation. To assess the effects of losing NETs, we compared the effects of phenylephrine, which is a substrate for the NET (Rawlow et al., 1980), in the absence and in the presence of the NET inhibitor, desmethylimipramine. To assess changes in smooth muscle sensitivity to α1- and α2-adrenoceptor agonists, we used methoxamine and clonidine, respectively; methoxamine is not a substrate for the NET (Trendelenburg et al., 1970). In a separate series of experiments, we evaluated the changes in sensitivity to vasopressin and angiotensin II (AII) after denervation.
Methods
All animal care and experimental procedures conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and were approved by the University of New South Wales Animal Care and Ethics Committee.
Female Wistar rats (7–8 weeks of age, oestrous cycle not tested) were anaesthetized with ketamine/xylazine (60 and 10 mg·kg−1 i.p.). The dorsal and ventral collector nerves were exposed via a single lateral incision on each side ∼1 cm proximal to the base of the tail and just behind the hip joints. Each of the four nerves was mobilized and lifted out of its groove, then cut and 3–5 mm of nerve trunk removed. After dusting with oxytetracycline powder, the wounds were closed using Michel clips. The animals recovered uneventfully and were maintained for 2 weeks [15 ± 1 (mean ± SD) days, n= 14) or ∼7 weeks (51 ± 4 days, n= 12)] post-operatively. In sham-operated animals, all nerves were mobilized and lifted out of their grooves but not cut; these animals were maintained for 2 (14 ± 2 days, n= 14) or ∼7 (51 ± 3 days, n= 6) weeks. We also compared responses to some agonists in arteries from unoperated rats age-matched to the 2 (n= 6) and 7 weeks (n= 6) operated animals. The responsiveness of 7 week denervated arteries to vasopressin and angiotensin was assessed relative to arteries from age-matched unoperated rats. We also assessed reactivity of the tail artery to vasopressin and AII in a group of rats where the post-ganglionic neurones supplying this vessel had been decentralized for 2 weeks (13 days) by bilaterally lesioning the sympathetic chains between the L3 and L4 ganglia (see Yeoh et al., 2004a for operation details). Arteries from age-matched unoperated rats were used as controls for the decentralized arteries.
The animals were killed by exsanguination under deep anaesthesia (pentobarbitone 100 mg·kg−1, i.p.) and the tail artery from 5 to ∼6.5 cm along the tail was removed for myography studies. The segmental nerves run distally for 3–4 cm before reaching the artery (Sittiracha et al., 1987) so that at 5–6.5 cm along the tail of nerve lesioned rats, it would be expected that the tail artery was completely denervated. The isolated arterial segments were placed in physiological saline of the following composition (in mM): 150.6 Na+, 4.7 K+, 2 Ca2+, 1.2 Mg2+, 144.1 Cl-, 1.3 H2PO4-, 16.3 HCO3- and 7.8 glucose. This solution was bubbled with carbogen gas (95% O2, 5% CO2) to achieve pH 7.25 and maintained at 36–37°C. In lesioned animals, segments of artery (∼10 mm) from immediately above and below the region used for myography studies were removed for histological assessment of the innervation. As a control for quality of immunostaining, a segment of artery was removed from the first centimetre below the base of the tail where the innervation should not have been affected and compared with that in control animals at that level. We did not assess the innervation in artery segments used for the mechanical studies because the immunoreactivity of the perivascular nerves is often abnormal after 4–5 h superfusion with saline.
Mechanical responses
Arterial ring segments (∼1.5 mm long) were mounted isometrically between stainless steel wires (50 µm diameter) in a four-chamber myograph (Multi Myograph model 610M, Danish Myo Technology, Aarhus, Denmark). Each chamber of the myograph contained 6 mL of physiological saline that was exchanged at regular intervals throughout the recording period. There was some variation in the lengths [1.45 ± 0.15 mm (mean ± SD), range 1–1.75 mm] and lumen diameters (Table 1) of the segments studied. To normalize the basal conditions, Laplace's equation was used to convert the measured force to the effective pressure exerted on the luminal surface of the artery (see Mulvany and Halpern, 1977). This is a rough estimate and assumes that the measured force is unaffected by the distorted curvature of the vessel imposed by the wires, and that the vessel wall is sufficiently thin for Laplace's equation to apply. Assuming that there are no major differences in the vessel wall properties, this normalization procedure minimizes differences in the effective distending pressure between the vessels. Initially, the effective distending pressure under basal conditions was set at ∼13.3 mN·mm−2 (∼100 mm Hg), and the vessels left to equilibrate for 30 min. After this, the basal effective distending pressure stabilized at ∼10.5 mN·mm−2 (∼80 mm Hg), which is at the peak of the vessels' length–force relationship (Yeoh et al., 2004b). The contractions to electrical stimulation and to agonists were measured as increases in wall tension (force/2 × artery segment length; see Mulvany and Halpern, 1977).
Table 1.
Lumen diameter, effective distending pressure and wall tension under basal conditions
| Lumen diameter (µm) | Basal distending pressure (mN·mm−2) | Basal wall tension (mN·mm−1) | |
|---|---|---|---|
| Two weeks control (n= 14) | 809 (731–872) | 10.6 ± 0.1 | 4.3 ± 0.1 |
| Two weeks denervated (n= 14) | 740 (704–764) | 10.4 ± 0.1 | 3.8 ± 0.1 |
| P | <0.05* | 0.24 | <0.05* |
| Seven weeks control (n= 12) | 827 ± 18 | 10.4 ± 0.1 | 4.4 ± 0.1 |
| Seven weeks denervated (n= 12) | 765 ± 24 | 10.1 ± 0.1 | 3.9 ± 0.1 |
| P | <0.001* | 0.28 | <0.001* |
The lumen diameters for 2 weeks control and 2 weeks denervated arteries are present as median and interquartile range (in parentheses). All other data are presented as means ± standard errors. P values for comparisons between control and denervated arteries were derived using either unpaired t-tests or Mann–Whitney U-tests, and
indicates significant differences.
Electrical stimulation
To confirm the absence of innervation, segments of denervated and age-matched unoperated arteries were electrically stimulated with 20 pulses at 10 Hz (0.2 ms pulse width, 25 V) through platinum plate electrodes mounted either side of the tissue. In intact tail artery preparations, 25 V is supramaximal for activating perivascular nerves (Yeoh et al., 2004b).
Chemical stimulation
In the first series of experiments, non-cumulative concentration–response curves for the α1-adrenoceptor agonists, phenylephrine (0.01–30 µM) and methoxamine (0.01–30 µM), and the α2-adrenoceptor agonist, clonidine (0.001–3 µM) were obtained by increasing the concentration of each drug in half-log increments. The tissues were exposed to each concentration for 5 min, followed by a washout period during which the tension returned to the baseline value before addition of the next concentration of agonist. In tail artery, the pA2 for the α1-adrenoceptor-selective antagonist prazosin in antagonizing the effects of phenylephrine and methoxamine is in the range 9–9.5 (Abel and Minneman, 1986; Atkinson et al., 1988) and, in preliminary experiments, we ascertained that the pA2 for the α2-adrenoceptor-selective antagonist rauwolscine in antagonising the effects of clonidine is ∼9. These pA2 values are consistent with the effects of phenylephrine and methoxamine being mediated through α1-adrenoceptors and those of clonidine being mediated through α2-adrenoceptors (Alexander et al., 2008). To determine the effects of blocking NET, concentration–response curves for phenylephrine were obtained in pairs of artery segments, one in the absence and the other in the presence of desmethylimipramine (30 nM). In preliminary experiments, we had confirmed that this concentration of desmethylimipramine produced a maximal leftward shift in the phenylephrine concentration–response curve in unoperated (innervated) arteries.
Following completion of the concentration–response curves, contractions to physiological saline containing 60 mM K+ (equimolar substitution of K+ for Na+) were recorded in the presence of the α1-adrenoceptor antagonist, prazosin (0.01 µM), and the α2-adrenoceptor antagonist, idazoxan (0.1 µM), to inhibit the actions of any noradrenaline released from the sympathetic nerves. In control tissues, the combined application of these concentrations of antagonists reduced contractions evoked by trains of perivascular stimuli at 1 and 10 Hz by ∼95% (see Yeoh et al., 2004b).
In the second series of experiments, non-cumulative concentration–response curves for vasopressin (0.0001–1 µM), and AII (0.001–10 µM) were obtained by increasing the concentration of each drug in log increments. The tissues were exposed to each concentration of vasopressin and AII for 5 min, followed by a 30 min washout period; this minimized the possibility of tachyphylaxis.
Immunohistochemistry
Artery segments taken from 1–2, 4–5 and 6–7 cm along the tail were fixed at their in vivo length overnight in Zamboni's fixative, washed with phosphate-buffered saline and infiltrated with 30% sucrose before being blocked together and frozen so that longitudinal sections (20 µm thick) could be cut. After permeabilizing with 50% ethanol, sections were incubated at room temperature overnight in a solution containing mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (ImmunoStar Inc, Hudson, WI, USA). After washing, the sections were incubated for 2 h in CY3-labelled donkey anti-mouse IgG antibody (Jackson ImmunoResearch Inc, Baltimore, PA, USA) at room temperature in the dark. The sections were washed briefly and cover slipped in anti-fade mounting medium (AF1: Citifluor Ltd, London, UK) and examined in an Olympus fluorescence microscope fitted with a Chroma filter 31002 (wavelength: excitation 515–550 nm, emission 575–615 nm).
Data analysis
Groups of artery segments from rats with nerve lesions are referred to as ‘denervated arteries’ or ‘decentralized arteries’, and those from sham-operated rats or unoperated rats (used for assessing changes in reactivity to vasopressin and AII in 7 weeks denervated and 2 weeks decentralized arteries) rats are referred to as ‘control arteries’.
The output from the myograph was recorded and analysed using a PowerLab data acquisition system and the program Chart (ADInstruments, Bella Vista, NSW, Australia). The peak amplitudes of the contractions to phenylephrine, methoxamine, clonidine, vasopressin, AII and 60 mM K+ were measured. The EC50s were estimated by fitting the data to the Hill equation using non-linear regression analysis (Prism 4, GraphPad software, Inc., San Diego, CA, USA).
All statistical comparisons were made using SPSS 13 (SPSS Inc., Chicago, IL, USA). Comparisons between the concentration–response curves were made using repeated measures analysis of variance with a single independent variable (for between-group comparisons). Other comparisons were made either with Student's unpaired t-tests or with Mann–Whitney U-tests if there was unequal variance between the groups of data (assessed using Levene's test of homogeneity of variance). The data compared using Mann–Whiney U-tests are presented as median and interquartile range (IQR). All other data are presented as mean ± SEM. P values <0.05 were considered as indicating a significant difference. In all cases, n indicates the number of animals studied.
Drugs
The drug/molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008). R-(-)-phenylephrine HCl, methoxamine HCl, clonidine HCl, prazosin HCl, (±)-idazoxan HCl, desmethylimipramine HCl and AII were purchased from Sigma Chemical Company (Castle Hill, NSW, Australia). [Arg 8]-vasopressin was purchased from Auspep (Parkville, Victoria, Australia). Prazosin was prepared as a 1 mM stock solution in 10% (vol·vol−1) dimethyl sulphoxide in water, and all other drugs were prepared as stock solutions in water. The final concentration of dimethyl sulphoxide was 0.0001% (vol·vol−1), and this was without effect on the responses of the isolated tail artery.
Results
Criteria for denervation
To include data for arteries in the denervated group, we required that their electrically evoked contractions be <5% of those in control arteries and that there was histological verification of lack of noradrenergic nerve terminals.
In arteries from the 26 rats that we accepted as denervated, stimulation with 20 pulses at 10 Hz produced a negligible contraction (peak amplitude: 2 weeks denervated 0.3 ± 0.1 mN·mm−1, n= 14; 7 weeks denervated 0.3 ± 0.1 mN·mm−1, n= 12). In comparison, arteries from age-matched control rats produced a robust contraction to this stimulus (peak amplitude: 2 weeks 8.9 ± 0.7 mN·mm−1, n= 6; 7 weeks 7.7 ± 0.9 mN·mm−1, n= 6). The small residual contraction in denervated arteries may be explained by the direct activation of smooth muscle; this was confirmed in six cases by resistance to tetrodotoxin (0.5 µM).
In all denervated arteries, there were no detectable TH-positive axons in the segment of artery taken from immediately below the region used to assess changes in reactivity to α-adrenoceptor agonists (∼7 cm from the base of the tail). Immediately above this region (4–5 cm from base of tail), 15 arteries had no detectable TH-positive axons, but in the remaining 11 arteries (six 2 weeks and five 7 weeks after denervation) occasional single TH-positive axons were present, usually at the proximal end of the segment.
Basal conditions for the contraction studies with and without denervation
Table 1 shows the estimated lumen diameter, the effective pressure exerted on the luminal surface and wall tension under basal conditions for the control and denervated groups of arteries. The lumen diameters of both the 2 and 7 weeks denervated arteries were smaller than those of their control arteries. However, there were no differences in the basal effective pressure exerted on the luminal surface between control and denervated arteries at either time point, although the basal wall tension was significantly smaller for the denervated arteries. This reduction in wall tension is in accord with Laplace's law, as the decrease in lumen diameter will increase the pressure that the denervated arteries exert on the luminal contents for a given level of wall tension.
Effects of denervation on concentration–response curves to phenylephrine in the absence and in the presence of desmethylimipramine
The effects of blocking neuronal uptake with desmethylimipramine on the concentration–response curves to phenylephrine were evaluated to determine whether sensitivity to this α1-adrenoceptor agonist was affected by the absence of NETs in denervated arteries. In the absence of desmethylimipramine, phenylephrine concentration–response curves for both 2 and 7 weeks denervated arteries were shifted to the left of those for their controls (Figure 1A,C). Thus, the pEC50 for phenylephrine was significantly larger in denervated arteries than in their control arteries (Table 2). The contractions to the maximum concentration of phenylephrine tested (30 µM) were larger in the 2 weeks denervated arteries than in their controls (P < 0.01). This difference may be because the concentration–response curve to phenylephrine in 2 weeks control arteries had not fully plateaued (see Figure 1A). The maximum contraction to phenylephrine did not differ between the 7 weeks control and denervated arteries (P= 0.69; Figure 1C).
Figure 1.
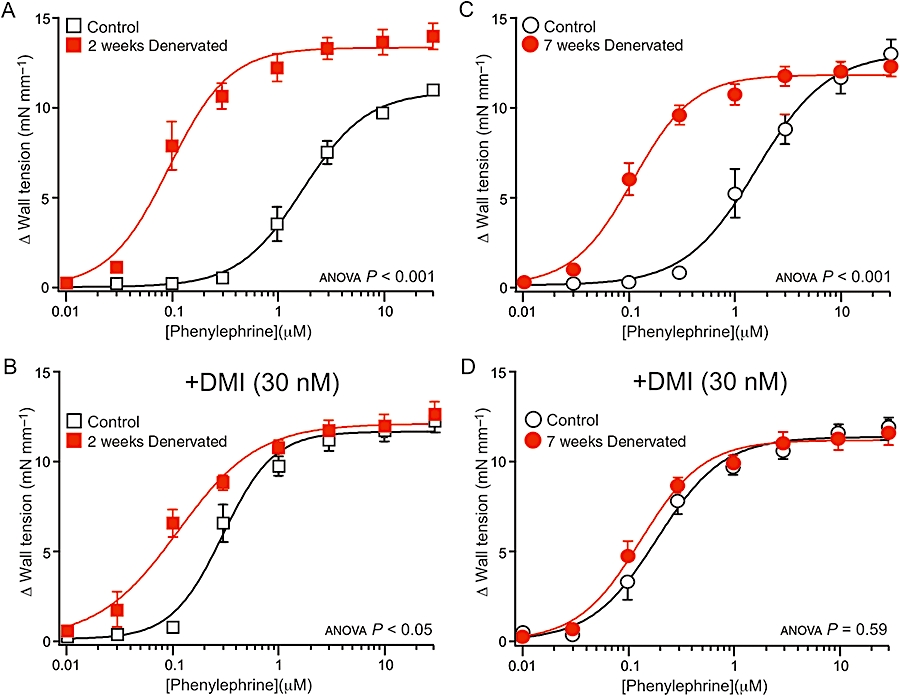
Effects of denervation on concentration–response curves for the α1-adrenoceptor agonist, phenylephrine. (A–D) Data are shown for arteries denervated for 2 weeks (A,B) and 7 weeks (C,D), and their control arteries (from sham-operated rats), in the absence (A,C) and in the presence (B,D) of the neuronal noradrenaline uptake inhibitor desmethylimipramine (DMI, 30 nM). In the absence of desmethylimipramine, the phenylephrine concentration–response curves for denervated arteries were shifted to the left of those for the control arteries. Desmethylimipramine shifted the phenylephrine concentration–response curves to the left in control arteries, but did not change those in denervated arteries. In the presence of desmethylimipramine, the phenylephrine concentration–response curve for 2 weeks denervated arteries lay to the left of that for their controls, but the curve for 7 weeks denervated arteries overlay the control curve. P values indicate the significance of the differences between the curves for control and denervated arteries (assessed by analysis of variance). For each group of arteries, n= 6.
Table 2.
Effects of denervation on the pEC50 for phenylephrine with and without desmethylimipramine (DMI, 30 nM), and for methoxamine and clonidine
|
Phenylephrine |
Phenylephrine+DMI |
Methoxamine |
Clonidine |
|
|---|---|---|---|---|
| pEC50 | pEC50 | pEC50 | pEC50 | |
| Two weeks control (n= 6) | 5.76 ± 0.10 | 6.58 (6.38–6.63) | 5.92 ± 0.05 | 7.08 (6.92–7.13) |
| Two weeks denervated (n= 6) | 7.05 ± 0.09 | 7.03 (7.00–7.07) | 6.29 ± 0.04 | 7.85 (7.60–8.14) |
| P | <0.01* | <0.01* | <0.01* | <0.01* |
| Seven weeks control (n= 6) | 5.81 ± 0.10 | 6.74 ± 0.10 | 6.25 ± 0.08 | 7.33 ± 0.12 |
| Seven weeks denervated (n= 6) | 6.98 ± 0.06 | 6.94 ± 0.06 | 6.50 ± 0.16 | 7.73 ± 0.15 |
| P | <0.01* | 0.13 | 0.20 | 0.08 |
The 2 weeks data for phenylephrine in the presence of desmethylimipramine and for clonidine are presented as medians and interquartile ranges (in parentheses). All other data are presented as means ± standard errors. P values for comparisons between control and denervated arteries were derived using either unpaired t-tests or Mann–Whitney U-tests, and
indicates significant differences.
In control arteries, addition of desmethylimipramine (30 nM) produced leftward shifts in the phenylephrine concentration–response curves (Figure 1B,D) whereas in both 2 and 7 weeks denervated arteries, the addition of desmethylimipramine did not shift these relationships (c.f. Figure 1A–D).
After the addition of desmethylimipramine, the phenylephrine concentration–response curve for 2 weeks denervated arteries lay to the left of that of their controls (Figure 1B), but this difference was not present for 7 weeks denervated arteries (Figure 1D). Comparison of pEC50 values confirmed that this difference was significant only at 2 weeks (Table 2). The maximum response to phenylephrine in the presence of desmethylimipramine did not differ between control and denervated arteries at either time point (P > 0.1 for both comparisons; Figure 1B,D).
The pEC50 for phenylephrine in the presence or in the absence of desmethylimipramine did not change over time. There was no difference between the values for 2 and 7 weeks control arteries (phenylephrine, P= 0.76; phenylephrine + desmethylimipramine, P= 0.09) or between 2 and 7 weeks denervated arteries (phenylephrine, P= 0.53; phenylephrine + desmethylimipramine, P= 0.63).
Concentration–response curves to methoxamine
Methoxamine is not a substrate for NET (Trendelenburg et al., 1970). Two weeks post-operatively, the concentration–response curve for the denervated arteries lay to the left of that of their controls (Figure 2A), and the pEC50 for methoxamine was significantly larger in the denervated arteries than in the control arteries (Table 2). However, there was no difference in the maximum response for methoxamine (control 12.8 ± 0.3 mN·mm−1, denervated 12.3 ± 0.7 mN·mm−1, P= 0.55) at this time point.
Figure 2.
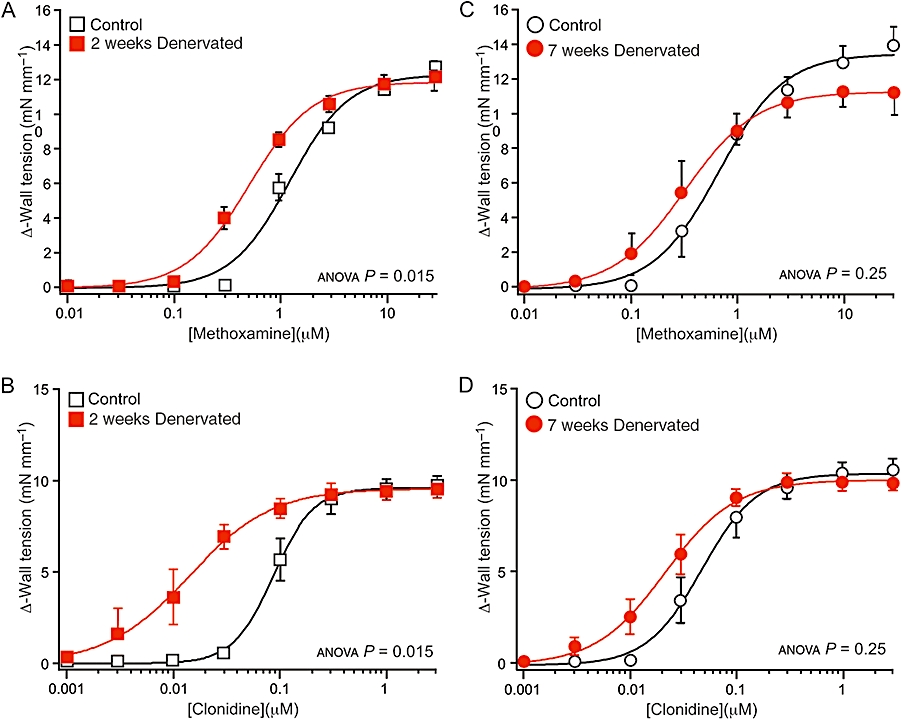
Effects of denervation on concentration–response curves for the α1-adrenoceptor agonist methoxamine (A,C) and the α2-adrenoceptor agonist clonidine (B,D). (A–D) Data are shown for arteries denervated for (A,B) 2 weeks and (C,D) 7 weeks and their controls (from sham-operated rats). The methoxamine and clonidine concentration–response curves for 2 weeks denervated arteries were shifted to the left of those for their controls, but the concentration–response curves for both drugs in the arteries denervated for 7 weeks did not differ from those of their controls. P values indicate the significance of the differences between the curves for control and denervated arteries (assessed by analysis of variance). For each group of arteries, n= 6.
Seven weeks post-operatively, there were no significant differences in the concentration–response curves (Figure 2C), the pEC50 values (Table 2) or the maximum responses (control 14.2 ± 1.1 mN·mm−1, denervated 11.8 ± 1.0 mN·mm−1, P= 0.14) between control and denervated arteries.
The pEC50 for methoxamine was slightly lower in 2 weeks control than in 7 weeks control arteries (P < 0.01), but did not differ significantly between 2 and 7 weeks denervated arteries (P= 0.25).
Comparison of EC50 ratios for phenylephrine and methoxamine
Two weeks post-operatively, the EC50 ratios (control : denervated) for phenylephrine in the presence of desmethylimipramine and for methoxamine were 2.9 and 2.2 respectively. These ratios measure the magnitude of the denervation-induced increase in sensitivity of the vascular smooth muscle to α1-adrenoceptor agonists. In the absence of desmethylimipramine, the EC50 ratio for phenylephrine 2 weeks post-operatively was 30.1, indicating that the major factor contributing to increased reactivity to this agent following denervation is loss of neuronal uptake. Seven weeks post-operatively, the EC50 ratios for phenylephrine in the absence and in the presence of desmethylimipramine were 14 and 1.6 respectively. The EC50 ratio for methoxamine was 1.7 at this time point. These findings again emphasize that the primary cause of the denervation-induced increased reactivity to phenylephrine is loss of neuronal uptake, but, in addition, show that smooth muscle hypersensitivity to α1-adrenoceptor agonists is transient.
Concentration–response curves to clonidine
In 2 weeks denervated arteries, the concentration–response curve for clonidine was shifted to the left of that for their control arteries (Figure 2B) and the pEC50 was significantly increased (Table 2). However, after 7 weeks denervation, while there was a tendency for a leftward shift in concentration–response curve for clonidine, the curves did not differ significantly (Figure 2D). The pEC50 for clonidine also did not differ significantly between the 7 weeks control and denervated arteries (Table 2). At both time points, the maximum contractions to clonidine did not differ between denervated and control arteries (2 weeks P= 0.89, 7 weeks P= 0.54; Figure 2B,D). Two and 7 weeks post-operatively, the EC50 ratios between the control and denervated arteries were, respectively, 5.2 and 2.0, indicating that smooth muscle hypersensitivity to clonidine also does not persist.
The pEC50 to clonidine did not differ significantly between 2 and 7 weeks control arteries (P= 0.07) or between 2 and 7 weeks denervated arteries (P= 0.32).
Responses to 60 mM K+
The contractions of 2 weeks denervated arteries to 60 mM K+ varied considerably in amplitude, but did not differ significantly from those of their control arteries (P= 0.33, Figure 3A). However, the contractions of 7 weeks denervated arteries to 60 mM K+ were significantly smaller than those of their controls (Figure 3B).
Figure 3.
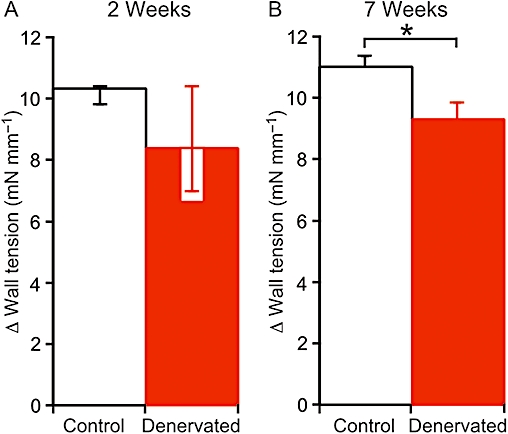
Effect of denervation on the amplitude of contractions evoked by increasing the K+ concentration to 60 mM. (A,B) Data are shown for arteries denervated for (A) 2 weeks and (B) 7 weeks and their controls (from sham-operated rats). There was no significant difference between control and denervated arteries after 2 weeks, but, after 7 weeks, the K+ evoked contraction of denervated arteries was smaller than that of their controls. Data in (A) are presented as medians and interquartile ranges, and compared using Mann–Whitney U-tests. Data in (B) are presented as means and SEMs, and compared using unpaired t-tests. Significant differences indicated *P < 0.05. For each group of arteries, n= 6.
Changes in reactivity to vasopressin and AII
Concentration–response curves for vasopressin differed significantly between control and denervated arteries at both time points (Figure 4A,C), but the curves were shifted to the left after 7 weeks denervation much less than after 2 weeks denervation. In 2 weeks denervated arteries, both the pEC50 for this agent (control 8.45 ± 0.18; denervated 9.53 ± 0.26; P < 0.01) and the maximum contraction (control 9.2 mN·mm−1, IQR 7.2–10.1, denervated 11.6 mN·mm−1, IQR 10.7–12.3, P < 0.01) were increased. However, in 7 weeks denervated arteries, the differences between the pEC50s (control 8.26 ± 0.04; denervated 8.48 ± 0.09; P= 0.06) and the maximum contractions (control 9.0 mN·mm−1, IQR 8.0–10.0; denervated 10.6 mN·mm−1, IQR 10.4–11.1; P= 0.08) did not reach statistical significance. Furthermore, the pEC50 for vasopressin was significantly larger in 2 weeks than in 7 weeks denervated arteries (P < 0.01). Two and 7 weeks after denervation, the EC50 ratios between control and denervated arteries for vasopressin were 8.2 and 1.6, respectively, demonstrating that hypersensitivity to this agent also decreases with time.
Figure 4.
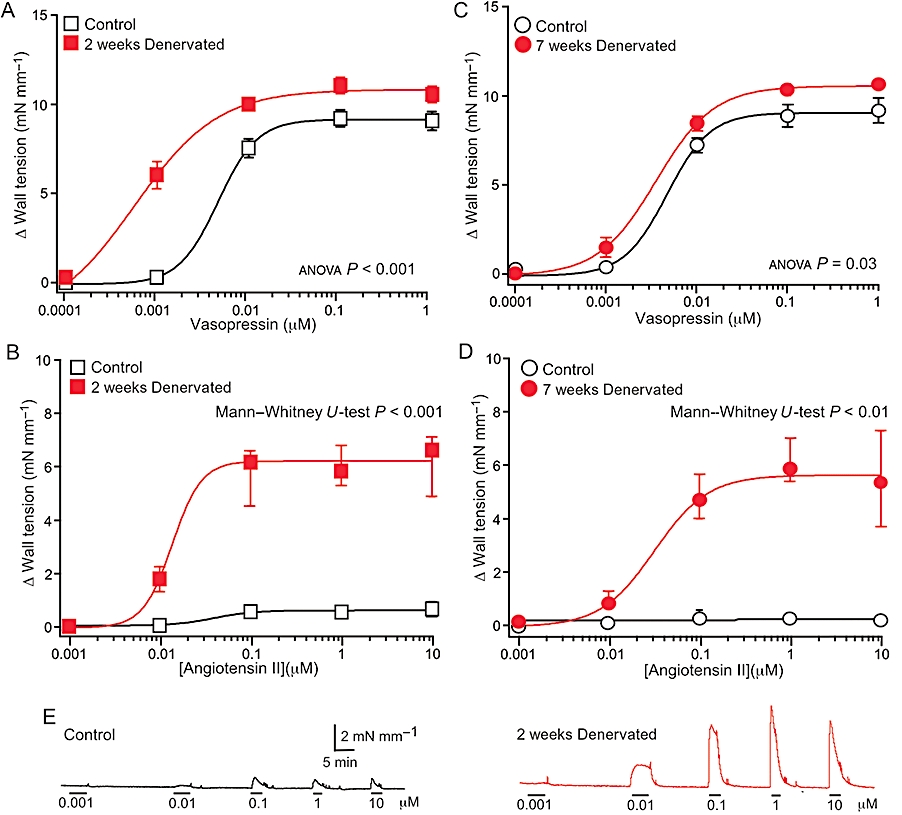
Effects of denervation on concentration–response curves for the physiological vasoconstrictor agents, vasopressin (A,C) and angiotensin II (AII; B,D). Data are shown for arteries denervated for 2 weeks (A,B), and 7 weeks (C,D) and their controls (from 2 weeks sham-operated and unoperated animals aged-matched to the 7 weeks denervated rats). At both time points, the concentration–response curves for vasopressin in the denervated arteries differed significantly from those of their controls, but the magnitude of this difference was small after 7 weeks. At both time points, the maximum contractions to AII in the denervated arteries were markedly larger than in their controls. (E) Traces showing representative contractions to increasing concentrations of AII in control and 2 weeks denervated arteries. In A and C, data are presented as means ± SEMs, and P values indicate the significance of the differences between the curves for control and denervated arteries (assessed by analysis of variance). In B and D, data are presented as medians and interquartile ranges, and P values indicate the significance of the difference between the maximum contractions to AII (assessed by Mann–Whitney U-tests). For the 2 weeks groups, n= 8, whereas for the 7 weeks groups, n= 6.
AII produced only very small contractions in control arteries (Figure 4B,D), and it was not possible to fit the data to the Hill equation. In contrast, in both 2 and 7 weeks denervated arteries, AII-induced contractions were many times larger than those in their controls (Figure 4B,D). The pEC50 for AII in 7 weeks denervated arteries (7.53 ± 0.10) was similar to that of 2 weeks denervated arteries (7.55 ± 0.33; P= 0.97).
Denervation versus decentralization
Decentralization, like denervation, abolishes activation of smooth muscle adrenoceptors by neurally released noradrenaline but, in contrast to denervation, does not cause loss of perivascular sympathetic axons. We have previously assessed changes in sensitivity of decentralized arteries and demonstrated a transient increase in the sensitivity of the smooth muscle to α-adrenoceptor agonists (Yeoh et al., 2004a). In decentralized arteries, as observed in denervated arteries, the sensitivity of the smooth muscle to phenylephrine and clonidine was increased 1–2 weeks post-operatively, and this hypersensitivity was absent 7 weeks post-operatively.
Here, we investigated whether the effects of decentralization on the sensitivity of the tail artery to vasopressin and AII were similar to those of denervation. Figure 5 shows concentration–response curves for vasopressin and AII in arteries from 2 weeks decentralized and unoperated control animals. At this time point, the effects of denervation and decentralization on reactivity to vasopressin and AII were similar (cf. Figures 5A,B with 4A,B). In decentralized arteries, as observed in denervated arteries, both the pEC50 for vasopressin (control 8.30, IQR 8.23–8.39, decentralized 8.61, IQR 8.53–8.95, P < 0.01) and the maximum contraction (control 9.5 ± 0.6 mN·mm−1; decentralized 12.6 ± 0.7 mN·mm−1, P < 0.01) evoked by this agent were increased. After decentralization, the contractions to AII were also markedly increased in size (Figure 5B).
Figure 5.
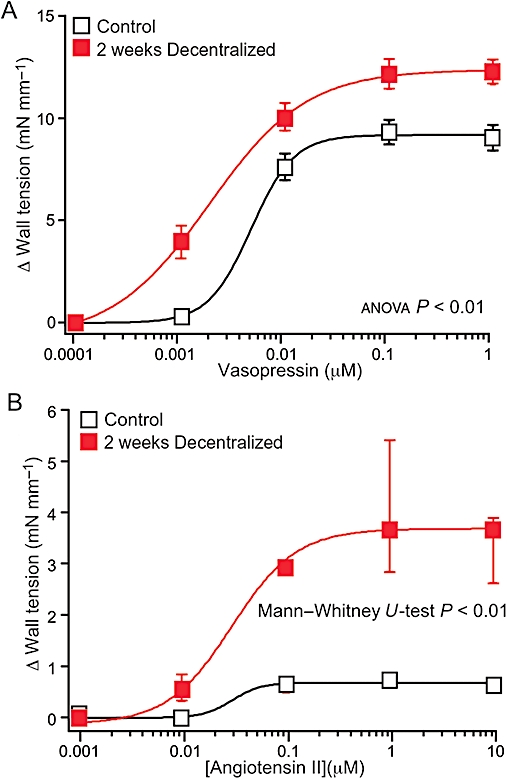
Effects of decentralization on concentration–response curves for the physiological vasoconstrictor agents, vasopressin (A) and angiotensin II (AII; B). Data are shown for arteries decentralized for 2 weeks and their controls (from unoperated rats). The concentration–response curves for vasopressin in the decentralized arteries differed significantly from their controls. The maximum contractions to AII in the decentralized arteries were markedly larger than in their controls. In A, data are presented as means ± SEMs, and P values indicate the significance of the differences between the curves for control and denervated arteries (assessed by analysis of variance). In B, data are presented as medians and interquartile ranges, and the P value indicates the significance of the difference between the maximum contractions to AII (assessed by Mann–Whitney U-tests). For both groups, n= 6.
Unoperated versus sham-operated animals
For α-adrenoceptor agonists, the concentration–response curves in sham-operated (control) arteries were shifted slightly to the right, and the pEC50s were slightly smaller at 2 weeks than at 7 weeks, although only the pEC50s for methoxamine were significantly different. We compared equivalent measurements for sham-operated and unoperated animals age-matched to each post-operative period. In comparison with arteries from unoperated rats, those from 2 weeks sham-operated rats showed no significant differences in sensitivity to clonidine (pEC50: unoperated 7.25 ± 0.14; sham operated 7.03 ± 0.06; P= 0.23) or vasopressin (pEC50: unoperated 8.75 ± 0.21; sham-operated 8.45 ± 0.18; P= 0.28), but they were less sensitive to methoxamine (unoperated 6.14 ± 0.05; sham-operated 5.92 ± 0.06; P < 0.01). This difference had disappeared after 7 weeks (pEC50 for methoxamine: unoperated 6.16 ± 0.06; sham-operated 6.25 ± 0.06; P= 0.42). In all control groups (i.e. sham and unoperated), the contractions to AII were very small (Figures 4B,D and 5B). These data suggest that the operation itself had a small effect for at least 2 weeks afterwards on muscle reactivity.
Discussion
This study has demonstrated that surgical removal of the sympathetic innervation to the tail artery produces only a transient increase in sensitivity of the vascular smooth muscle to α1- and α2-adrenoceptor agonists. Furthermore, the increased sensitivity to phenylephrine in the denervated arteries is primarily due to loss of its removal by NETs and, in the longer term, this mechanism entirely explains increased sensitivity to this agent. While hypersensitivity of the vascular smooth muscle to α-adrenoceptor agonists and to vasopressin decreased with time after denervation, the greatly augmented contractions to AII persisted, indicating that changes in the behaviour of smooth muscle can be maintained. In further support of this, contractions to 60 mM K+ were reduced in 7 weeks denervated arteries. Together, these findings demonstrate that reactivity to vasoconstrictor agents is differentially affected by denervation.
The arteries included in this study were tested for both structural and functional evidence of denervation. Denervation was confirmed functionally by absent or residual (<5% of maximum) contractions to electrical stimulation (that can be explained by direct activation of smooth muscle) and histologically by the paucity of TH-positive axons in the segment of artery immediately proximal to that used for mechanical assessments and the absence of TH in the segment isolated from immediately below. In the denervated arteries, blockade of neuronal uptake of phenylephrine with desmethylimipramine did not change sensitivity to this agent, confirming the absence of functional sympathetic nerve terminals. In contrast, desmethylimipramine produced a large leftward shift of the concentration–response curves for phenylephrine in control arteries (see Figure 1).
The changes in sensitivity of the tail artery 2 weeks after denervation are similar to those observed previously when reserpine treatment was used to deplete the catecholamine stores in sympathetic nerve terminals (e.g. Nasseri et al., 1985; Duckles, 1991; Slovut et al., 2004; Taki et al., 2004). In this model, in which the nerve terminals remain intact, treatment with reserpine for 1 week increased vascular muscle sensitivity to phenylephrine two- to threefold in the presence of desmethylimipramine (Nasseri et al., 1985; Duckles, 1991). This enhanced sensitivity to phenylephrine is similar to that observed here for 2 weeks denervated arteries. On the other hand, if reserpine treatment was continued for 2 weeks, sensitivity to phenylephrine increased 10-fold in tissues in which the NET had not been blocked (Taki et al., 2004). This larger increase may be explained by the chronic exposure to reserpine which, unlike a single exposure (Iversen et al., 1965), may reduce NET activity (Antonaccio and Smith, 1974). The fivefold increase in sensitivity to clonidine reported for tail arteries from rats after 3 days of treatment with reserpine (Abe et al., 1989) is also similar to that observed here for 2 weeks denervated arteries.
By 7 weeks after surgical denervation, vascular muscle sensitivity to α1- and α2-adrenoceptor agonists returned to control levels. The increase in sensitivity of the vascular muscle to α1- and α2-adrenoceptor agonists was similarly transient when activity in the post-ganglionic neurones supplying the tail artery was stopped by decentralization (Yeoh et al., 2004a). This implies that hypersensitivity does not depend on the loss of nerve terminals, but rather on the absence of sympathetic activity. At 2 weeks, both denervated and decentralized arteries also showed increased responsiveness to vasopressin and AII (Figure 5), so that the modified response to these circulating vasoconstrictor agents also does not depend on the loss of innervation, but on the absence of activation by noradrenaline and/or other transmitters.
Careful inspection of the data presented in Figures 1 and 2 reveals that the relative decline in hyper-reactivity of the smooth muscle to α-adrenoceptor agonists after 7 weeks denervation reflects, in part, a greater sensitivity of the arteries from 7 weeks sham-operated animals compared to that of the 2 weeks sham-operated animals. This difference only reached significance for methoxamine and suggests an effect of the operation per se in the early post-operative weeks.
Evidence that increased sensitivity of smooth muscle to α1-adrenoceptor agonists is associated with increased α1-adrenoceptor expression is limited. In reserpine-pretreated tail arteries, ligand binding studies failed to show changes in the density or affinity of binding of α1-adrenoceptor ligands [125I]BE 2254 (Nasseri et al., 1985) or [3H]prazosin (Taki et al., 2004). However, while displacement of [3H]prazosin binding by the α1A-adrenoceptor selective antagonist KMD-3213 was unaffected, the α1D-adrenoceptor selective antagonist, BMY 8378, inhibited high affinity binding of [3H]prazosin in reserpine-pretreated arteries. Increased expression of α1D-adrenoceptors was confirmed by a selective increase in their mRNA (Taki et al., 2004; see also Kamikihara et al., 2007). However, nerve-evoked contractions of the tail artery are almost completely blocked by α1A-adrenoceptor selective antagonists (Sulpizio and Hieble, 1991; Brock and Tripovic, unpublished observations). Therefore, interrupting neurovascular transmission in tail arteries does not increase the expression of adrenoceptors that are normally activated by nerve-released noradrenaline.
Other studies of surgical denervation on vascular reactivity to α1-adrenoceptor agonists are consistent with changes in sensitivity to these agents being entirely explained by the loss of NETs. In canine saphenous vein (Flavahan et al., 1987) and in arteries of the porcine nasal mucosa (Lacroix and Lundberg, 1989), increased sensitivity to noradrenaline was primarily explained by the loss of NETs and, when these amine pumps were blocked, the smooth muscle was supersensitive to α2-adrenoceptor agonists but not to α1-adrenoceptor agonists. The porcine spleen is the only organ where surgical denervation has been studied in arterial vessels that are exclusively activated by α1-adrenoceptor agonists (Lundberg et al., 1988). In this organ, hyper-reactivity to noradrenaline 2 weeks after denervation was entirely accounted for by loss of NETs (Lundberg et al., 1988).
The hyper-responsiveness of smooth muscle to a wide range of contractile agents after interruption of sympathetic neuroeffector transmission led to the suggestion that supersensitivity reflects a generalized increase in smooth muscle reactivity (see Fleming and Westfall, 1988). A number of factors have been identified following denervation of various smooth muscles. Thus, depolarization of muscle cells associated with decreased plasma membrane Na+-K+ ATPase (Wong et al., 1981; Hershman et al., 1995) can sensitize the muscle to agents that evoke contraction by opening voltage-dependent Ca2+ channels (Urquilla et al., 1978). However, surgical denervation of the tail artery does not decrease resting membrane potential (Jobling et al., 1992), and reactivity to 60 mM K+ was not enhanced (see Figure 3). In addition, increased expression of the gap junction protein, connexin-43, within a few days of interrupting of sympathetic neurovascular transmission in the rat tail artery with reserpine (Slovut et al., 2004) might increase coupling between smooth muscle cells and thereby enhance their reactivity to contractile agents, but whether this change in connexin-43 is maintained in the longer term is not known. The transient increase in sensitivity of denervated vascular smooth muscle to α-adrenoceptor agonists and to vasopressin might be explained by some common mechanisms. However, the maintained increase in contractions to AII must depend on a different mechanism, possibly an increase in AII receptor expression and/or a change in the intracellular signalling to the contractile mechanism.
Denervation has also been reported to produce structural changes in the arterial wall, resulting in narrowing of the vessel lumen and changes in smooth muscle phenotype (e.g. Dimitriadou et al., 1988; Shweta et al., 2005; Kacem and Sercombe, 2006). However, in the present study, the reduced lumen diameter of denervated arteries (Table 1) was not associated with any change in maximum contractions to phenylephrine (in the presence of desmethylimipramine) (Figure 1), methoxamine or clonidine (Figure 2). Further, the structural change persisted when the heightened reactivity did not. Therefore, it is unlikely that changes in the dimensions of the vessel wall contribute to the increased reactivity of 2 weeks denervated arteries.
For clinical assessments of changes in vascular reactivity to α-adrenoceptor agonists, it has often been assumed that phenylephrine is not a substrate for the NET. For example, hand veins in insulin-dependent diabetic patients showed increased sensitivity to noradrenaline, but not to phenylephrine; this was interpreted as indicating a loss of neuronal uptake (i.e. sympathetic denervation) with no change in smooth muscle reactivity (Eichler et al., 1992). However, phenylephrine is a substrate for the NET in human vascular nerves (Stevens and Moulds, 1980). Therefore, a more likely explanation for the selective increase in reactivity to noradrenaline, which activates both α1- and α2-adrenoceptors, is an increased vascular sensitivity to α2-adrenoceptor agonists (Bodmer et al., 1995). The use of methoxamine to test the α1-adrenoceptor sensitivity of human hand veins would be more appropriate (e.g. Abdelmawla et al., 1995).
In summary, the view that the effects of interrupting sympathetic neurovascular transmission can be explained by a generalized increase in vascular smooth muscle reactivity needs to be revised. Denervation induces a marked increase in reactivity to exogenous α-adrenoceptor agonists that are substrates for the NET, which is largely accounted for by its absence. In comparison, there was a relatively small and transient increase in smooth muscle sensitivity to α1- and α2-adrenoceptor agonists, similar to that observed after decentralization of the post-ganglionic supply (Yeoh et al., 2004a), implying that hyper-reactivity results from the interruption of neurovascular transmission. These changes might follow either the lack of activation of smooth muscle receptors or the absence of another substance normally released by nerve terminal activity. In the longer term, hypersensitivity to α-adrenoceptor agonists was due only to the absence of NETs. This should be borne in mind when interpreting data from humans with denervated vasculature. Further, while the maximum contractions to other vasoconstrictor agents was unaffected in 7 weeks denervated arteries, there was a marked and maintained increase in the maximum contraction to AII, indicating that denervation differentially and selectively affects the reactivity of the vascular smooth muscle to particular circulating vasoconstrictor agents. It may be that the enhanced vasoconstriction seen in denervated skin reflects this selective sensitivity to AII.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (350903 and 350904).
Glossary
Abbreviations:
- AII
angiotensin II
- NET
neuronal norepinephrine transporter
- TH
tyrosine hydroxylase
Statement of conflicts of interest
None.
References
- Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of desipramine on noradrenaline- and methoxamine-evoked venoconstriction in man. Br J Clin Pharmacol. 1995;40:445–451. [PMC free article] [PubMed] [Google Scholar]
- Abe K, Saito H, Matsuki N. Potentiation by treatment with reserpine of α2-adrenoceptor-mediated contractions of rat tail artery. Eur J Pharmacol. 1989;171:59–67. doi: 10.1016/0014-2999(89)90429-9. [DOI] [PubMed] [Google Scholar]
- Abel PW, Minneman KP. Alpha1 adrenergic receptor binding and contraction of rat caudal artery. J Pharmacol Exp Ther. 1986;239:678–686. [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonaccio MJ, Smith CB. Effects of chronic pretreatment with small doses of reserpine upon adrenergic nerve function. J Pharmacol Exp Ther. 1974;188:654–667. [PubMed] [Google Scholar]
- Atkinson J, Trescases N, Benedek C, Boillat N, Fouda AK, Krause F, et al. Alpha1 and α2 adrenoceptor agonists induce vasoconstriction of the normotensive rat caudal artery in vitro by stimulation of a heterogeneous population of α1 adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1988;1338:529–535. doi: 10.1007/BF00179325. [DOI] [PubMed] [Google Scholar]
- Baron R, Maier C. Reflex sympathetic dystrophy: skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain. 1996;67:317–326. doi: 10.1016/0304-3959(96)03136-3. [DOI] [PubMed] [Google Scholar]
- Bodmer CW, Schaper NC, Janssen M, De Leeuw PW, Williams G. Selective enhancement of α2-adrenoceptor-mediated vasoconstriction in insulin-dependent diabetic patients with microalbuminuria. Clin Sci (Lond) 1995;88:421–426. doi: 10.1042/cs0880421. [DOI] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC, Starke K, Wardell CF. Alpha2-adrenoceptor-mediated autoinhibition of sympathetic transmitter release in guinea-pig vas deferens studied by intracellular and focal extracellular recording of junction potentials and currents. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:45–52. doi: 10.1007/BF00178971. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Aubineau P, Taxi J, Seylaz J. Ultrastructural changes in the cerebral artery wall induced by long-term sympathetic denervation. Blood Vessels. 1988;25:122–143. doi: 10.1159/000158727. [DOI] [PubMed] [Google Scholar]
- Duckles SP. Reserpine-induced supersensitivity in rat caudal artery: influence of age. J Pharmacol Exp Ther. 1991;256:513–518. [PubMed] [Google Scholar]
- Eichler HG, Blaschke TF, Kraemer FB, Ford GA, Blochl-Daum B, Hoffman BB. Responsiveness of superficial hand veins to α-adrenoceptor agonists in insulin-dependent diabetic patients. Clin Sci (Lond) 1992;82:163–168. doi: 10.1042/cs0820163. [DOI] [PubMed] [Google Scholar]
- Finch L, Haeusler G, Kuhn H, Thoenen H. Rapid recovery of vascular adrenergic nerves in the rat after chemical sympathectomy with 6-hydroxydopamine. Br J Pharmacol. 1973;48:59–72. doi: 10.1111/j.1476-5381.1973.tb08222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan NA, Miller VM, Aarhus LL, Vanhoutte PM. Denervation augments α2 but not α1 adrenergic responses in canine saphenous veins. J Pharmacol Exp Ther. 1987;240:589–593. [PubMed] [Google Scholar]
- Fleming WW, Westfall DP. Adaptive supersensitivity. In: Trendelenburg U, editor. Handbook of Experimental Pharmacology, Vol. 90: Catecholamines. Berlin: Springer Verlag; 1988. pp. 509–559. [Google Scholar]
- Hershman KM, Taylor DA, Fleming WW. Adaptive supersensitivity and the Na+/K+ pump in the guinea pig vas deferens: time course of the decline in the alpha 2 subunit. Mol Pharmacol. 1995;47:726–729. [PubMed] [Google Scholar]
- Iversen LL, Glowinski J, Axelrod J. The uptake and storage of H3-norepinephrine in the reserpine-pretreated rat heart. J Pharmacol Exp Ther. 1965;150:173–183. [PubMed] [Google Scholar]
- Jobling P, McLachlan EM, Janig W, Anderson CR. Electrophysiological responses in the rat tail artery during reinnervation following lesions of the sympathetic supply. J Physiol. 1992;454:107–128. doi: 10.1113/jphysiol.1992.sp019256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Sercombe R. Differing influence of sympathectomy on smooth muscle cells and fibroblasts in cerebral and peripheral muscular arteries. Auton Neurosci. 2006;124:38–48. doi: 10.1016/j.autneu.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kamikihara SY, Mueller A, Lima V, Akinaga J, Nojimoto FD, Castilho A, et al. Alpha1-adrenoceptors in proximal segments of tail arteries from control and reserpinised rats. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:117–126. doi: 10.1007/s00210-007-0176-4. [DOI] [PubMed] [Google Scholar]
- Kurvers HA, Tangelder GJ, De Mey JG, Slaaf DW, Beuk RJ, van den Wildenberg FA, et al. Skin blood flow abnormalities in a rat model of neuropathic pain: result of decreased sympathetic vasoconstrictor outflow? J Auton Nerv Syst. 1997;63:19–29. doi: 10.1016/s0165-1838(96)00127-0. [DOI] [PubMed] [Google Scholar]
- Lacroix JS, Lundberg JM. Adrenergic and neuropeptide Y supersensitivity in denervated nasal mucosa vasculature of the pig. Eur J Pharmacol. 1989;169:125–136. doi: 10.1016/0014-2999(89)90824-8. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hemsen A, Rudehill A, Harfstrand A, Larsson O, Sollevi A, et al. Neuropeptide Y- and α-adrenergic receptors in pig spleen: localization, binding characteristics, cyclic AMP effects and functional responses in control and denervated animals. Neuroscience. 1988;24:659–672. doi: 10.1016/0306-4522(88)90359-4. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nasseri A, Barakeh JF, Abel PW, Minneman KP. Reserpine-induced postjunctional supersensitivity in rat vas deferens and caudal artery without changes in alpha adrenergic receptors. J Pharmacol Exp Ther. 1985;234:350–357. [PubMed] [Google Scholar]
- Rawlow A, Fleig H, Kurahashi K, Trendelenburg U. The neuronal and extraneuronal uptake and deamination of 3H-(-)-phenylephrine in the perfused rat heart. Naunyn Schmiedebergs Arch Pharmacol. 1980;314:237–247. doi: 10.1007/BF00498545. [DOI] [PubMed] [Google Scholar]
- Shweta A, Denton KM, Kett MM, Bertram JF, Lambert GW, Anderson WP. Paradoxical structural effects in the unilaterally denervated spontaneously hypertensive rat kidney. J Hypertens. 2005;23:851–859. doi: 10.1097/01.hjh.0000163155.29740.d2. [DOI] [PubMed] [Google Scholar]
- Sittiracha T, McLachlan EM, Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987;21:647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Slovut DP, Mehta SH, Dorrance AM, Brosius FC, Watts SW, Webb RC. Increased vascular sensitivity and connexin43 expression after sympathetic denervation. Cardiovasc Res. 2004;62:388–396. doi: 10.1016/j.cardiores.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Moulds RF. Are neuronal uptake mechanisms responsible for the difference in sensitivity to noradrenaline between human arteries and veins? Clin Exp Pharmacol Physiol. 1980;7:139–145. doi: 10.1111/j.1440-1681.1980.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Sulpizio A, Hieble JP. Lack of a pharmacological distinction between α1-adrenoceptors mediating intracellular calcium-dependent and independent contractions to sympathetic nerve stimulation in the perfused rat caudal artery. J Pharmacol Exp Ther. 1991;257:1045–1052. [PubMed] [Google Scholar]
- Sunderland S. Nerves and Nerve Injuries. Edinburgh: Churchill Livingstone; 1978. [Google Scholar]
- Taki N, Tanaka T, Zhang L, Suzuki F, Israilova M, Taniguchi T, et al. Alpha1D-adrenoceptors are involved in reserpine-induced supersensitivity of rat tail artery. Br J Pharmacol. 2004;142:647–656. doi: 10.1038/sj.bjp.0705817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg U. Mechanisms of supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1966;18:629–640. [PubMed] [Google Scholar]
- Trendelenburg U, Maxwell RA, Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of l-norepinephrine in the cat's nictitating membrane. J Pharmacol Exp Ther. 1970;172:91–99. [PubMed] [Google Scholar]
- Urquilla PR, Westfall DP, Goto K, Fleming WW. The effects of ouabain and alterations in potassium concentration on the sensitivity to drugs and the membrane potential of the smooth muscle of the guinea-pig and rat vas deferens. J Pharmacol Exp Ther. 1978;207:347–355. [PubMed] [Google Scholar]
- Wong SK, Westfall DP, Fedan JS, Fleming WW. The involvement of the sodium–potassium pump in postjunctional supersensitivity of the guinea-pig vas deferens as assessed by [3H]ouabain binding. J Pharmacol Exp Ther. 1981;219:163–169. [PubMed] [Google Scholar]
- Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol. 2004a;561:583–596. doi: 10.1113/jphysiol.2004.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh M, McLachlan EM, Brock JA. Tail arteries from chronically spinalized rats have potentiated responses to nerve stimulation in vitro. J Physiol. 2004b;556:545–555. doi: 10.1113/jphysiol.2003.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]


