Abstract
Background and purpose:
The endothelium-derived hyperpolarizing factor (EDHF)-type relaxation in mesenteric small arteries from 21 week old Zucker lean (ZL) and Zucker diabetic fatty (ZDF) rats was investigated using (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309), a potent activator of small-conductance, calcium-activated potassium channel (SKCa) and intermediate-conductance, calcium-activated potassium channel (IKCa).
Experimental approach:
In the presence of inhibitors of cyclooxygenase and nitric oxide synthase [indomethacin and Nω-nitro-L-arginine methyl ester (l-NAME), respectively], acetylcholine (ACh)-induced hyperpolarization and EDHF-type relaxation were investigated under isometric conditions in the wire myograph using 0.5 and 1 µM NS309 and/or selective blockers of SKCa and IKCa channels. Membrane potential was recorded with glass microelectrodes, and changes in the intracellular calcium concentration of endothelial cells were visualized by confocal microscopy. SKCa expression was assessed by Western blotting.
Key results:
In arteries from ZDF rats, ACh-induced relaxation and membrane hyperpolarization were attenuated and, compared with arteries from ZL rats, NS309 was less potent at causing relaxation. Incubation with 0.5 µM NS309 did not increase ACh-induced relaxation in arteries from ZDF rats significantly. However, 1 µM NS309 restored it (both in the absence and in the presence of indomethacin and l-NAME) without changing endothelial intracellular calcium concentration. The restored EDHF-type relaxation was more sensitive to TRAM-34 (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole) (1 µM) than to apamin. Expression of the SKCa channel was unaltered.
Conclusions and implications:
The attenuated EDHF-type relaxation in mesenteric small arteries from ZDF rats can be restored by NS309 without changes in the intracellular calcium concentration of endothelial cells. These results may have clinical implications for the treatment of endothelial dysfunction in overweight type 2 diabetic patients.
Keywords: NS309, Zucker diabetic fatty rat, ZDF, mesenteric small arteries, type 2 diabetes, endothelial dysfunction, EDHF
Introduction
Type 2 diabetes is associated with a broad variety of circulatory effects depending on the vascular bed and animal model investigated. The most commonly observed effect in the resistance arteries is a reduced responsiveness to the endothelium-dependent vasodilator acetylcholine (ACh) (De Vriese et al., 2000 and Hermans, 2007). This phenomenon is commonly referred to as endothelial dysfunction. The endothelium-dependent relaxation of small arteries is mediated through several parallel pathways, the three most important being (i) release of nitric oxide (NO) from the endothelial cells, (ii) production of prostaglandins (e.g. prostacyclin) and (iii) the endothelium-derived hyperpolarizing factor (EDHF)-type relaxation that is not dependent on NO or on prostaglandins (Feletou and Vanhoutte, 2006). These three pathways contribute differently in different vascular beds, and if one or more of these pathways are defective, the other pathways may compensate or the relaxing properties of the arteries will decrease (Desai et al., 2006). The latter will lead to a decrease in blood flow and subsequently to a reduction in the supply of oxygen and nutrition to the tissue. This is often observed in diabetic patients and leads to microvascular complications and diabetic angiopathy.
In the small mesenteric arteries, the EDHF pathway is generally considered to be of major importance, although the exact mechanism(s) through which EDHF acts is controversial. In particular, the possible role of myoendothelial gap junctions in the effects of EDHF has been the subject of much controversy (Dora et al., 2003; 2008; Matchkov et al., 2006). However, there is consensus on the key role of the small-conductance, calcium-activated potassium channel (SKCa) (SK3 or KCa2.3) and the intermediate-conductance, calcium-activated potassium channel (IKCa) (SK4 or KCa3.1), which are located on the vascular endothelial cells (Burnham et al., 2002; Bychkov et al., 2002), and pharmacological targeting of SKCa and IKCa channels has been suggested to be of clinical interest (Feletou and Vanhoutte, 2004). The SKCa channels can be selectively blocked by apamin and the IKCa channels by charybdotoxin (CbTX) or the clotrimazole derivatives (2-(2-chlorophenyl)-2,2-diphenylacetonitrile) (TRAM-39) and (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole) (TRAM-34) (Hinton and Langton, 2003). It is, therefore, generally accepted that an endothelium-dependent relaxing response that is blocked by apamin and either by CbTX, TRAM-39 or TRAM-34 resembles the EDHF response.
A new and powerful pharmacological tool in the investigation of SKCa and IKCa channels is (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309). NS309 is a positive modulator of both the SKCa and IKCa channels with a slight selectivity for IKCa over SKCa and no effect on BK channels. It is several orders of magnitude more potent than the standard reference compound 1-EBIO and has an absolute requirement for a minimum concentration of intracellular Ca2+ (Strobaek et al., 2004). It has been shown that NS309 hyperpolarizes smooth muscle cells in intact mesenteric small arteries of the rat (Absi et al., 2007), and more recently in human single, endothelial cells (Sheng et al., 2009). As the endothelium-dependent hyperpolarization has previously been shown to be reduced in resistance arteries from diabetic rats (Fukao et al., 1997; Wigg et al., 2001; Burnham et al., 2006), we hypothesized that modulation of the SKCa and IKCa channels by NS309 could increase the EDHF response in these arteries and could thereby improve the ACh-induced relaxation of mesenteric small arteries.
The Zucker diabetic fatty (ZDF) rat is a model of adult-onset type 2 diabetes mellitus derived from the insulin-resistant fatty Zucker rat (Peterson et al., 1990). Male ZDF rats consistently develop hyperglycaemia between 7 and 12 weeks of age when fed a high-energy, high-protein diet. It has previously been shown that unstimulated mesenteric small arteries from this rat model have an impaired response to EDHF (Burnham et al., 2006).
In this study, to investigate a potential role for pharmacological intervention by NS309 on the ACh-evoked EDHF-type relaxation in type 2 diabetes, we compared its effects in ZDF rats with those in Zucker lean (ZL) rats. Mesenteric small arteries from 21 week old ZDF rats were investigated under isometric conditions in a wire myograph and membrane potentials were recorded. Furthermore, changes in intracellular calcium concentrations ([Ca2+]i) of the endothelial cells were imaged by selective loading of endothelial cells in intact arteries with intracellular Ca2+-sensitive dyes, and SKCa channel expression was assessed by Western blotting.
Our findings suggest that mesenteric small arteries from ZDF rats have endothelial dysfunctions due to a decreased EDHF-type relaxation upon ACh stimulation compared to mesenteric small arteries from ZL rats. NS309 was less potent in relaxing arteries from ZDF rats, but low concentrations could restore the EDHF-type relaxation in mesenteric small arteries from ZDF rats. This strongly implicates a dysfunction of calcium-activated potassium channels for the defect in the EDHF-type response in arteries from ZDF rats.
Methods
Animals
Fifteen male ZDF rats (homozygote, ZDF/GmiCrl-fa/fa) and their respective control rats, ZDF lean (ZL) rats (heterozygote, ZDF/GmiCrl-fa/+), were purchased from Charles River Laboratories, Belgium at 10–16 weeks of age. All rats had free access to a high-energy, diabetogenic diet, Purina diet #5008 (containing 26.8% protein, 16.70% fat and 56.4% carbohydrates) from birth and throughout the experiment. The rats were housed at constant temperature (22–23°C) on a 12/12 h light/dark cycle with free access to food and tap water. Body weight and fasting blood glucose concentration were assessed before the experiments. In a subset of 10 rats, blood pressure, food intake and urine production were measured (Table 1).
Table 1.
Blood and morphological characteristics
| ZL | ZDF | |
|---|---|---|
| Weight (g) | 387.0 ± 2.5 | 386.0 ± 6.8 |
| Fasting blood glucose (mM) | 5.4 ± 0.2 | 23.9 ± 1.1* |
| Mean arterial blood pressure (mmHg) | 116.0 ± 3.0 | 118.0 ± 3.9 |
| Food intake (g·24 h−1) | 19.5 ± 1.2 | 41.4 ± 1.9* |
| Urine production (mL·24 h−1) | 9.8 ± 0.43 | 86.5 ± 10.9* |
| Normalized lumen diameter (µm) | 251.0 ± 6.0 | 287.0 ± 10.0* |
| Preconstriction to 6 µM NA (Nm−1) | 3.8 ± 0.2 | 4.3 ± 0.2 |
| Maximal precontraction (10 µM NA + 125 mM KPSS) | 4.3 ± 0.1 | 4.4 ± 0.4 |
Parameters measured in ZL and ZDF rats at the age of 21 weeks. Values are mean ± SE and were analysed by Student's t-test (n= 10–15).
Indicates P < 0.05 ZDF rats versus ZL-rats.
SE, standard error; ZDF, Zucker diabetic fatty; ZL, Zucker lean; NA, noradrenaline; KPSS, 125 mM K+-containing PSS.
For blood pressure measurements, the rats were fasted for 8–12 h before they were anaesthetized with an injection of pentobarbital sodium (50 mg·kg−1 i.p.). The left carotid artery was then surgically exposed and cannulated with a heparin (100 IU·mL−1)-treated polyethylene catheter. The catheter was attached to a pressure transducer for monitoring the mean arterial pressure, and a blood sample was taken for the measurement of the blood glucose level (Precision Xtra™, Abbott Laboratories, MediSense Products, Bedford, MA, USA). The rats were then decapitated and the mesenteric bed removed into ice-cold physiological salt solution (PSS). Experiments were performed according to the guidelines from the Danish Ethical Committee for Animal Experimentation (permission 2007/561-1265).
Isometric force measurements in mesenteric small arteries
The mesenteric bed was carefully pinned out in a Petri dish and four 2 mm long segments of third-order branches were dissected free from fat and connective tissue. The isolated arteries were threaded onto two stainless steel wires (40 µm in diameter) and were mounted as ring preparations in a quadruple wire myograph (model 610M, Danish Myo Technology, Aarhus, Denmark) for isometric force measurements. All experiments were performed simultaneously in four arteries from the same animal. Data were stored at 40 Hz on a computer (Chart 5, Powerlab, ADInstruments Ltd, Aarhus, Denmark). The bath of each myograph contained 5 mL PSS at 37°C, continuously bubbled with a gas mixture containing 5% CO2 in 21% oxygen in a nitrogen-based gas to keep pH 7.4. All experiments were performed at the same glucose concentration to ensure that ZL and ZDF responses were compared under similar conditions. After being mounted, the arteries were allowed to equilibrate for 20 min before they were stretched stepwise to characterize the passive elastic properties, as previously described by Halpern and Mulvany (1977). Experiments were performed on arteries stretched to 90% of L100, where L100 is defined as the circumference of the relaxed artery exposed to a transmural pressure of 100 mmHg. Prior to the experimental protocol, the arteries were stimulated twice with 10 µM noradrenaline (NA) dissolved in 125 mM K+-containing PSS (KPSS) for 2 min to test the viability of the vessels. NS309 was dissolved in dimethyl sulphoxide (DMSO) to give a maximal final concentration of 0.05% DMSO in the myograph bath. All compounds were added directly to the bath.
Myograph protocol
First, a cumulative concentration–response curve to NA was obtained. The vessels were then washed in PSS and rested for 30 min before being contracted with NA (6 µM), and concentration–response curves for ACh were constructed in the absence or in the presence of NS309. NS309 (0.5 or 1.0 µM) was added to the NA-constricted arteries 5 min before the first concentration of ACh was added and was present during the full concentration–response curves. In some experiments, NS309 caused relaxation during the incubation period, resulting in a lower precontraction level. However, the decision to use one identical concentration of NA in all vessels was taken, based on the premise that equal stimulation of the arteries, and thereby equal stimulation of the contractile apparatus, would be the most correct baseline for the interpretation of differences in ACh response, in the absence or in the presence of NS309. After the vessels had been washed with PSS, they were rested for 30 min before being incubated for 15 min with 100 µM Nω-nitro-L-arginine methyl ester (l-NAME), a blocker of the NO-producing endothelial nitric oxidide synthase (eNOS), and with 3 µM indomethacin, a blocker of cyclooxygenase (COX), and the concentration–response curves for ACh were repeated in the presence or in the absence of 1 µM NS309. The vessels were again washed and rested for 30 min in PSS before being incubated with 50 nM apamin and/or 1 µM TRAM-34, in the continuous presence of indomethacin and l-NAME, and the ACh concentration–response curve was repeated after 5 min incubation with 1 µM NS309. After incubation with both apamin, TRAM-34, l-NAME and indomethacin, the vessels were contracted with NA, and an NO donor S-nitroso-N-acetylpenicillamin (SNAP, 10 µM) was added. Throughout each experiment, one of the four mounted arteries remained untreated with respect to apamin and TRAM-34 to serve as a control for viable endothelial function and it was ensured that no vessels were exposed to more than five ACh concentration–response curves. In a subset of experiments, arteries were contracted with 6 µM NA, and cumulative concentration–response curves for NS309 (0.5, 1.0, 1.5, 2.0 and 5.0 µM) were obtained.
Membrane potential measurements
Microelectrodes (glass, AS100F, WPI, Sarasota, FL, USA) were prepared on a horizontal puller (Sutter P-97, Novato, CA, USA) filled with 3 M KCl and connected to an amplifier (Intra 767, WPI). Electrodes with stable resistances >30 MΩ) were used to measure membrane potential, as described previously (Mulvany et al., 1982) Recordings from smooth muscle cells of isometrically mounted normalized mesenteric arteries superfused with 10 µM HEPES in PSS, in the presence of 100 µM l-NAME and 3 µM indomethacin, were made by advancing the electrode through the adventitia into the media. Membrane potential and tension data were recorded with a PowerLab/4SP (ADInstruments Ltd.).
Endothelial cell [Ca2+]i measurements
A 3 mm long segment of a third-order mesenteric artery was mounted on two glass pipettes in an isobaric myograph containing standard PSS. To selectively load the endothelial cells of the intact artery, it was perfused for 1 h at 37°C with 2 mL PSS containing 5.7 µM Fura-Red AM and 7.9 µM Calcium Green-1 AM (Invitrogen, Carlsbad, CA, USA). The dyes were dissolved in a load mix (1 mL DMSO with 200 µL Cremophor EL and 40 mg Pluronic F 127, Sigma, St. Louis, MO, USA), and it was ensured that the final concentration of DMSO in the myograph bath was below 0.1%. After the artery had been loaded, it was transferred to an isometric wire myograph and was placed on the stage of an inverted confocal laser scanning microscope (Zeiss Axiovert 200M coupled to an LSM5 Pascal Exciter, B&M, Birkerod, Denmark). Images were acquired with a Zeiss 20 × 0.5 objective used with a wide, open pinhole. After excitation at 488 nm, the emissions signals (505–545 nm for Calcium Green-1 and 560 nm for Fura-Red) were stored on a computer and image analysis was performed with an LSM Image Browser.
Ten regions of interest (ROIs) were marked on 10 different endothelial cells and pixel values were averaged within each ROI. After normalization to 90% of L100, changes in fluorescence were measured during a cumulative ACh concentration–response curve. Hereafter, the vessels were incubated with 1 µM NS309 for about 5 min and a second ACh concentration–response curve in the presence of NS309 was obtained.
Western blotting
Western blotting was performed on protein extracted from mesenteric arteries that had been carefully dissected free of surrounding tissue. Blood was expelled from the vessel by exposing them to KPSS before they were snap-frozen in liquid nitrogen and stored at −80°C. The arteries were homogenized and, after centrifugation, the amount of protein was measured in each sample by a modified Lowry method. Sample buffers were mixed with 2 µg protein at room temperature and were applied to a Bis/Tris criterion gel and were run for 1 h at 200 V before being transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were then washed for 10 min in a Tris buffered saline Tween (TBS-T) (10 mM Tris, pH 7.5, 0.1 mM NaCl, 1 mM EDTA, 0.1% Tween 20) and were blocked in 5% skimmed milk in TBS-T for 2 h before being incubated with the primary antibody SKCa (70 kDa, rabbit, 1:200) and the loading control antibody for panactin (45 kDa, rabbit, 1:500) in TBS-T with 5% skimmed milk overnight at 4°C. The membrane was then washed in TBS-T before being incubated for 2 h in 5% skimmed milk in TBS-T with the secondary antibody, anti-rabbit (1:4000). The membrane was developed using an ECL-Plus kit (GE Healthcare, Copenhagen, Denmark) and was transferred to an X-ray film and developed. The relative amount of protein was quantified by Image Quant TL (Amersham Biosciences, Copenhagen, Denmark).
Data analysis
The contraction to NA is presented as tension with the unit N per segment length (Nm−1). The relaxation to ACh and NS309 was normalized to the NA-induced contraction ((TensionACh/Tensionprecontraction) × 100) and is given as per cent relaxation. The relaxation to ACh after incubation with NS309 was normalized to the contraction obtained after 5 min of incubation with NS309 ((TensionACh/Tensionafter NS309 incubation) × 100), and the results are given as per cent relaxation. EC50 values were calculated, based on the contraction obtained before the addition of the first concentration of ACh, by non-linear regression for variable slope using GraphPad Prism 4.01 (GraphPad Software Inc., La Jolla, CA, USA) and are presented as pD2 values (pD2=−log [EC50]).
For [Ca2+]i measurements, the ratio between changes in the mean intensity of Fura-Red and Calcium Green-1 fluorescence was taken as a measure of changes in [Ca2+]i (Peng et al., 2001).
Statistics
Up to three vessels from the same rat were used in each protocol, and n represents the number of animals; all data are presented as mean ± standard error of the mean. When appropriate, pD2 values were calculated using non-fit sigmoidal concentration–response on the initial values from each experiment and were presented as a mean pD2 value. Animal characteristics and Western blot results were compared using Student's t-test. Maximal ACh relaxation and pD2 value were compared within animal groups using one-way analysis of variance (anova) with Tukey's post test and a significance level of P < 0.05. Differences between groups were tested using Student's t-test with a significance level of P < 0.025. Changes in [Ca2+]i were evaluated by two-way anova for repeated measures followed by Bonferroni's post test. All statistical analysis was made with GraphPad Prism 4.01 (GraphPad Software Inc.).
Solutions, materials and chemicals
The composition of the standard PSS was (in mM): 119.0 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 7.0 H2O, 25.0 NaHCO3, 1.6 CaCl2, 0.026 EDTA and 5.5 glucose. KPSS was PSS in which Na+ was substituted by an equimolar concentration of K+ to reach 125 mM K+. HEPES-PSS is standard PSS with 10 mM HEPES.
The kit for the Lowry method and the Bis/Tris criterion gel were obtained from Bio-Rad, (Hercules, CA, USA). The primary antibody SKCa (# sc28621) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA); the loading control antibody for panactin (cat., #4968) was from Cell Signalling Technology Inc. (Danvers, MA, USA); and the secondary antibody, anti-rabbit (Zymed #65-26120), was from Invitrogen. The ECL-Plus kit was from GE Healthcare, and Image Quant TL was from Amersham Biosciences. NS309 was a gift from NeuroSearch A/S (Ballerup, Denmark); Fura-Red and Calcium Green-1 where purchased from Invitrogen. all other chemicals were obtained from Sigma (St. Louis, MO, USA).
The nomenclature used conforms to the British Journal of Pharmacology's ‘Guide to Receptors and Channels’ (Alexander et al., 2008).
Results
Animal characteristics
Blood values and morphological characteristics are presented in Table 1. Fasting blood glucose concentration, food intake and urine production were increased in ZDF rats compared to ZL rats. There was no difference in weight and blood pressure between the two groups, but third-order arteries from ZDF rats were larger than those from ZL rats. This is possibly due to the higher food intake creating an increased energy demand by the gut (Table 1).
NA contraction
Arteries from both ZDF and ZL rats contracted to NA concentration dependently (Figure 1A). There was no difference in the maximal response or in pD2 (5.9 ± 0.1 and 5.8 ± 0.1, n= 5; ZDF and ZL rats, respectively) between arteries from the two groups. Force development to NA in KPSS was similar in ZDF and ZL arteries (Table 1).
Figure 1.
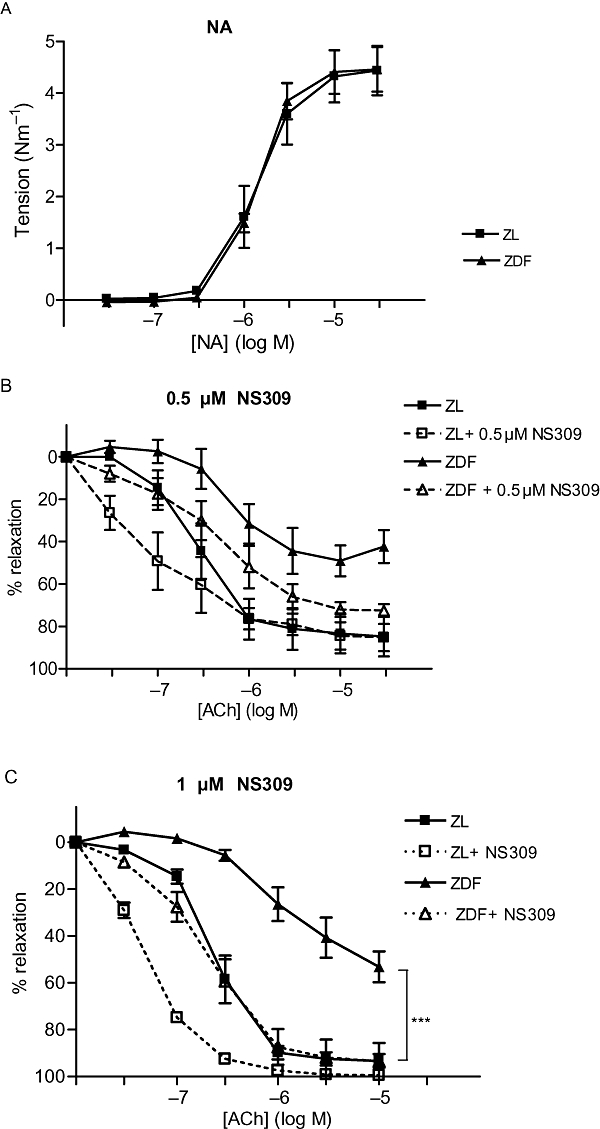
(A) Noradrenaline (NA)-induced contraction of small mesenteric arteries from Zucker lean (ZL) and Zucker diabetic fatty (ZDF) rats, under control conditions (n= 5). (B) Relative relaxation of small mesenteric arteries from ZL and ZDF rats to acetylcholine (ACh) under control conditions (n=5) and after 5 min incubation with 0.5 µM (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309) (n= 4). Arteries were precontracted with 6 µM NA. (C) Relative relaxation of small mesenteric arteries to ACh under control conditions from ZL (n= 7) and ZDF (n= 5) rats and after 5 min incubation with 1 µM NS309. Arteries were contracted with 6 µM NA. *Indicates P < 0.05, before and after NS309 treatment (one-way analysis of variance).
ACh relaxation
In arteries from both ZDF and ZL rats, ACh induced a concentration-dependent relaxation, but the maximal relaxation (relaxation to 10 µM ACh) was less and pD2 was lower (i.e. decreased sensitivity to ACh) in arteries from ZDF rats compared with arteries from ZL rats (Table 2, Figure 1B,C).
Table 2.
Maximal ACh relaxation and pD2 values under control conditions and after incubation with NS309
| Control condition |
Maximal relaxation (%) |
pD2 |
||
|---|---|---|---|---|
| ZDF (n=7) | ZL (n=5) | ZDF (n=7) | ZL (n=5) | |
| ACh | 53.1 ± 7.0* | 93.4 ± 3.0 | 5.9 ± 0.1* | 6.6 ± 0.1 |
| + INDO + l-NAME | 0.8 ± 5.0*§ | 71.1 ± 7.0 | n.d. | 6.0 ± 0.2 |
| + Apamin + INDO + l-NAME | −0.5 ± 1.0§ | 14.5 ± 8.0§ | n.d | n.d. |
| + TRAM + INDO + l-NAME | −12.4 ± 2.0§ | 3.1 ± 2.0§ | n.d. | n.d. |
| + Apamin + TRAM + INDO + l-NAME | −5.3 ± 6.0§ | −3.9 ± 2.0§ | n.d. | n.d. |
| 0.5 µM NS309 treated | – | – | – | – |
| ACh | 70.2 ± 3.0* | 86.9 ± 5.0 | 6.5 ± 0.3 | 7.6 ± 0.5 |
| 1 µM NS309 treated | – | – | – | – |
| ACh | 94.1 ± 7.0§ | 99.5 ± 8.0 | 6.7 ± 0.1*§ | 7.4 ± 0.1§ |
| + INDO + l-NAME | 55.8 ± 10.0* | 86.5 ± 5.0 | 6.4 ± 0.1 | 6.9 ± 0.1§ |
| + Apamin + INDO + l-NAME | 33.1 ± 9.0§ | 80.1 ± 5.0 | n.d. | 6.6 ± 0.1 |
| + TRAM + INDO + l-NAME | 24.8 ± 4.0§# | 29.6 ± 7.0§# | n.d. | n.d. |
| + Apamin + TRAM + INDO + l-NAME | 4.2 ± 5.0§# | 8.2 ± 4.0§# | n.d. | n.d. |
| 10 µM SNAP¤ | 46.5 ± 5.6 | 61.9 ± 5.3 | – | – |
Parameters measured in mesenteric small arteries from 21 week old ZL and ZDF rats.
In the presence of indomethacin, l-NAME, apamin, TRAM-34 and NS 309. Values are mean ± SE.
Indicates P < 0.025 ZDF rats versus ZL-rats (Student's t-test).
Indicates P < 0.05 versus control ACh arteries (one-way anova).
Indicates P < 0.05 versus NS309-, l-NAME- + indomethacin (INDO)-treated arteries (one-way anova).
n.d., not determined; anova, analysis of variance; SE, standard error; ZDF, Zucker diabetic fatty; ZL, Zucker lean; TRAM-34, (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole); ACh, acetylcholine; NS309, (6,7-dichloro-1H-indole-2,3-dione 3-oxime); l-NAME, Nω-nitro-L-arginine methyl ester; SNAP, S-nitroso-N-acetylpenicillamin.
Incubation with l-NAME and indomethacin eliminated the relaxant response to ACh in arteries from ZDF rats, but only slightly attenuated the maximal relaxation and pD2 value in arteries from ZL rats (Table 2, Figure 2A). As l-NAME and indomethacin eliminated the ACh relaxation in ZDF arteries, the addition of apamin and/or TRAM-34 as well had no effect. In arteries from ZL rats, the addition of apamin and/or TRAM-34 together with indomethacin and l-NAME significantly attenuated the maximal response to ACh (Table 2). The untreated control arteries maintained an endothelium-dependent ACh relaxation throughout the experimental period.
Figure 2.
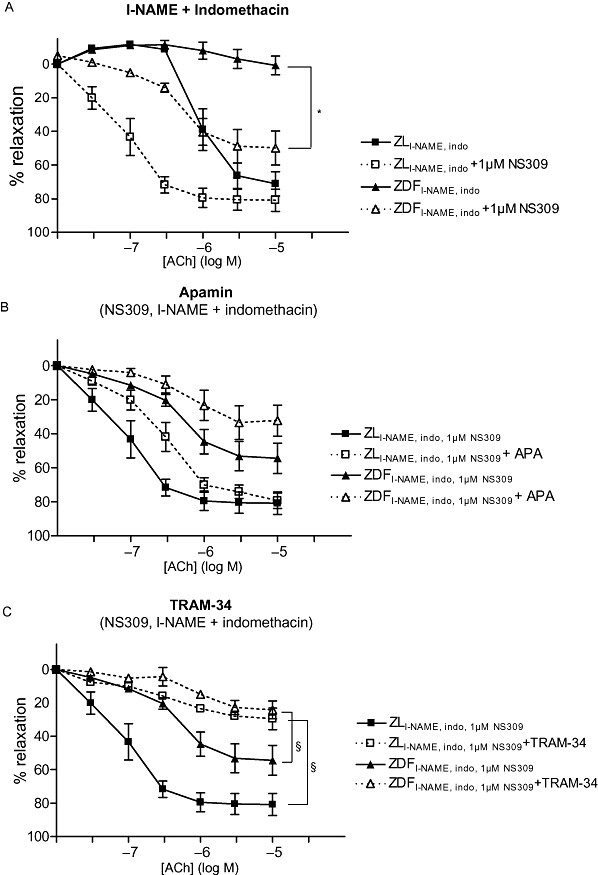
(A) Relative relaxation to acetylcholine (ACh) of small mesenteric arteries after incubation with 100 µM Nω-nitro-L-arginine methyl ester (l-NAME) and 3 µM indomethacin in Zucker lean (ZL) (n=7) and Zucker diabetic fatty (ZDF) (n= 5) rats, before and after incubation with 1 µM (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309). Arteries were precontracted with 6 µM noradrenaline (NA). (B) Relative relaxation of small mesenteric arteries precontracted with 6 µM NA to ACh in the presence of 1 µM NS309, 100 µM l-NAME and 3 µM indomethacin in ZL and ZDF rats and before and after incubation with 50 nM apamin (APA; n= 7, 5; ZDF and ZL rats respectively). (C) Relative relaxation to ACh in the presence of 1 µM NS309, 100 µM l-NAME and 3 µM indomethacin in ZL and ZDF rats before and after incubation with 1 µM (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole) (TRAM-34) (n= 7, 5; ZDF and ZL rats respectively). In (B) and (C), the relaxation to ACh without apamin and TRAM-34 is the same in the two panels and in (A). *Indicates P < 0.05, in the absence or presence of 1 µM NS309 (one-way anova). §Indicates P < 0.05 (one-way anova), before and after treatment with TRAM-34.
NS309-treated arteries
The 5 min incubation period with 0.5 µM NS309 did not change the level of NA precontraction, whereas 5 min incubation with 1 µM NS309 relaxed arteries from ZL rats by 20 ± 3% (n= 5). Incubation with 0.5 or 1.0 µM NS309 did not cause relaxation in arteries from ZDF rats. Incubation of arteries from ZDF rats with 0.5 µM NS309 appeared to improve the maximal relaxation response, although this effect was not statistically significant (Figure 1B, Table 2); 0.5 µM NS309 had no effect on the maximal ACh relaxation in arteries from ZL rats. After incubation with 1 µM NS309, the ACh concentration–response curves were shifted leftwards for both ZDF and ZL arteries (Figure 1C), and the maximal ACh-mediated relaxation and pD2 value in ZDF arteries were increased to the same level as in equally treated ZL arteries (Table 2).
In the presence of l-NAME and indomethacin, incubation with 1 µM NS309 increased the maximal ACh-mediated relaxation of ZDF arteries (Figure 2A, Table 2). Addition of apamin to arteries incubated with 1 µM NS309, l-NAME plus indomethacin did not change the maximal relaxation or pD2 value in ZDF or ZL arteries (Figure 2B, Table 2).
Incubation with TRAM-34 in the presence of 1 µM NS309, l-NAME and indomethacin reduced the maximal relaxation in arteries from both ZDF and ZL rats (P < 0.05), (Figure 2C, Table 2).
In the presence of apamin (Figure 2B) or TRAM-34 (Figure 2C) or both (not shown), 1 µM NS309 did not relax arteries from either ZDF or ZL rats. In addition, ACh relaxation was abolished in the presence of both blockers and NS309 (Table 2).
To confirm that the arteries were able to dilate in the presence of the blockers and to test the relaxation to an endothelium-independent vasodilator, the NO donor SNAP was added in the presence of 1 µM NS309, l-NAME, indomethacin, apamin and TRAM-34. Arteries from both groups relaxed to 10 µM SNAP, and there was no significant difference in the relaxation to SNAP between the two groups (Table 2).
To assess the direct vasodilator effect of NS309, concentration–response curves were obtained. NS309 was less potent [pD2 was 5.7 ± 0.03 and 6.0 ± 0.01, n= 6 (P < 0.001); ZDF and ZL rats, respectively] at relaxing the arteries from ZDF, although maximal relaxation was the same in both groups (Figure 3).
Figure 3.
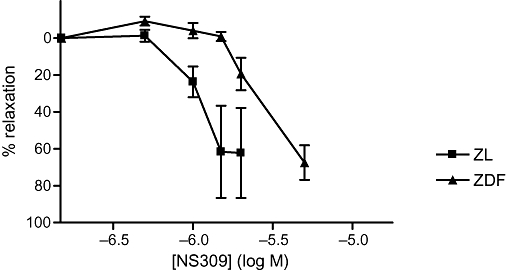
Relative relaxation of small mesenteric arteries precontracted with 6 µM noradrenaline to increasing concentrations of (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309) in Zucker lean (ZL) and in Zucker diabetic fatty (ZDF) rats (n= 3).
Membrane potential measurements
There was no difference in smooth muscle resting membrane potential between arteries from the two groups [−54.5 ± 4.9 mV and −50.3 ± 2.7 mV, n= 4 (P > 0.05); ZDF and ZL rats respectively]. The change in membrane potential to 6 µM NA was also the same in the two groups (Figure 4). However, the hyperpolarizing effect of 10 µM ACh on 6 µM NA-preconstricted arteries was significantly attenuated in ZDF but not in ZL rats (Figure 4).
Figure 4.
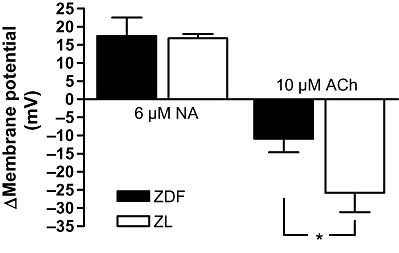
Changes in membrane potential (mV) in mesenteric small arteries from Zucker diabetic fatty (ZDF) rats and Zucker lean (ZL) rats. The arteries were contracted with 6 µM noradrenaline (NA) in the presence of indomethacin and Nω-nitro-L-arginine methyl ester; 10 µM acetylcholine (ACh) was added to the bath. The results are mean ± standard error of the mean of arteries from at least four animals in each group. *Indicates P < 0.025 (Student's t-test).
Endothelial cell [Ca2+]i imaging
Treatment with Calcium Green-1 and Fura-Red resulted in uniform loading of all endothelial cells with no apparent loading of the vascular smooth muscle cells (Figure 5). Under control conditions, incubation with NS309 had no effect on baseline [Ca2+]i in the endothelial cells (Figure 6A,B). When ACh was applied to the loaded artery, [Ca2+]i increased in the endothelial cells in a similar manner in arteries from ZDF and ZL rats (Figure 6C,D). When a concentration–response curve to ACh was obtained in the presence of NS309, there were no differences in the endothelial cell [Ca2+]i between arteries from ZDF and ZL rats (Figure 6C,D).
Figure 5.
Image showing the selective load of the endothelial cells with Calcium Green-1 and Fura-Red. The focal plane is on the endothelial cell layer, where the individual endothelial cells are clearly visible (1). Upper (2) and right (3) panels show vertical scanning layer across and along the artery showing the selective loading of the endothelial cells.
Figure 6.
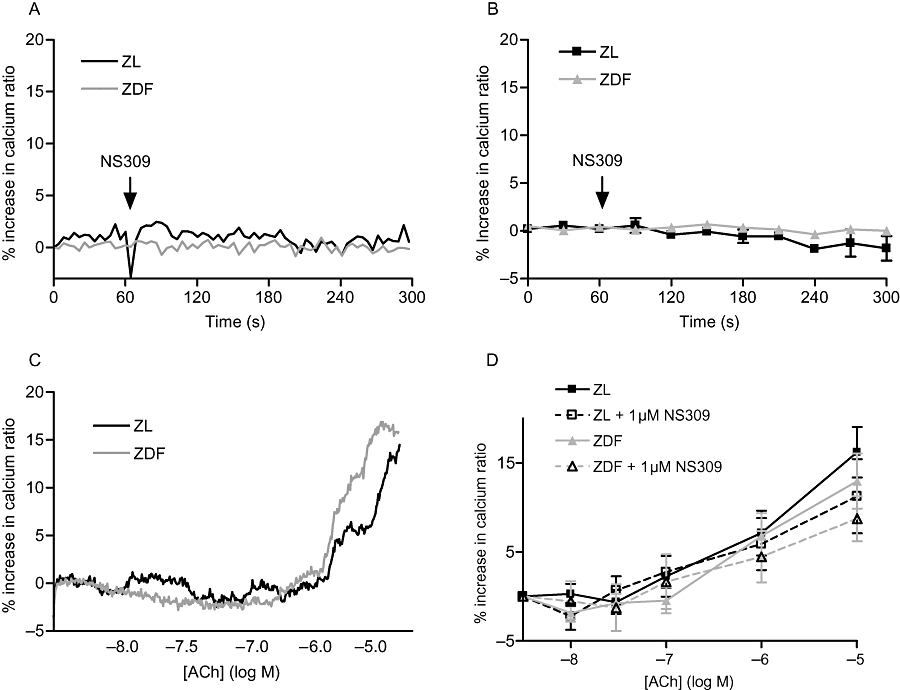
A summary of the results of the intracellular calcium ([Ca2+]i) measurements in endothelial cells from small mesenteric arteries from Zucker lean (ZL) and Zucker diabetic fatty (ZDF) rats. (A) Representative trace of changes in [Ca2+]i before and during the incubation with 1 µM (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (NS309) in ZL and ZDF rats. (B) Changes in [Ca2+]i before and during the incubation with 1 µM NS309 in ZL and ZDF rats (n= 6). (C) Original trace showing changes in [Ca2+]i in endothelial cells from ZL and ZDF rats during stimulation with cumulative concentrations of acetylcholine (ACh). (D) The [Ca2+]i in endothelial cells during stimulation with cumulative concentrations of ACh under control conditions (n= 7) and after incubation with 1 µM NS309 (n= 6) in ZL and ZDF rats. The arrow indicates the application of NS309.
Western blotting
There was no significant difference in SKCa channel protein expression in arteries from ZL compared to ZDF rats when the results were normalized for panactin content (Figure 7).
Figure 7.
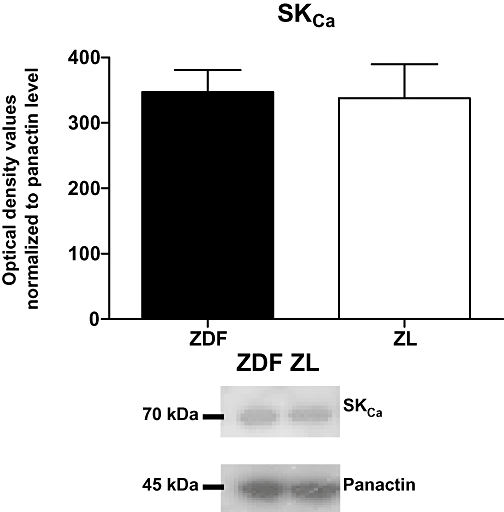
Small-conductance, calcium-activated potassium channel (SKCa) expression in mesenteric small arteries from Zucker diabetic fatty (ZDF) and Zucker lean (ZL) rats. The y-axis shows the optical density values normalized to the panactin level. Insert shows representative blots of SKCa and panactin. Results are mean ± standard error of the mean (SEM), n= 4 for both ZL and ZDF.
Discussion
In this study, we found that relaxation to ACh and to the IKCa and SKCa channel opener NS309 is impaired in mesenteric small arteries from 21 week old ZDF rats. The impaired response to ACh was also seen after inhibition of the eNOS- and COX-dependent pathways and was associated with a blunted ACh-induced hyperpolarization of the vascular smooth muscle cells. These findings suggest an attenuated EDHF-type relaxation in ZDF rats, although we cannot entirely rule out the possibility that this is caused by a defect in the vascular smooth muscle cells. However, the experiments with the endothelium-independent vasodilator SNAP did not show a significant difference between the two groups supporting the suggestion that the defect in the EDHF response resides in the endothelium. However, as the SNAP experiments were performed in the presence of indomethacin, l-NAME, TRAM-34 and after incubation with NS309, we cannot exclude a type 2 error in these experiments.
Importantly, we demonstrated that the EDHF-type relaxation could be restored by incubation with NS309. The results obtained after application of apamin and/or TRAM-34 indicated that the impaired EDHF-type relaxation was due to alterations in the IKCa channels, although SKCa channels might also be affected. The immunoblotting data suggest that the expression of the SKCa channel in arteries from ZDF is not altered compared to that in ZL rats. We did not find any differences in endothelial cell [Ca2+]i between the two groups under control conditions, during incubation with NS309 or during activation with ACh. NS309, therefore, seems to have restored the EDHF-type relaxation in a calcium-independent manner. These findings are consistent with the proposed mode of action of NS309 (Strobaek et al., 2004).
Vascular dysfunction in the ZDF rat model
The ZDF rat is a well-characterized model of type 2 diabetes displaying marked vascular dysfunction. The attenuated ACh-induced endothelium-dependent relaxation, which we found in this study, is in agreement with previous findings showing attenuated responses in epineurial arterioles (Coppey et al., 2002), in mesenteric small and coronary arteries from ZDF rats that were 16–24 or 28–40 weeks old (Oltman et al., 2006) and in mesenteric arteries of 40 week old ZDF rats (Schafer et al., 2004b) Previous reports have also shown microvascular retinopathy (Danis and Yang, 1993), increased levels of vascular reactive oxygen species (Chinen et al., 2007), and endothelial and neuronal dysfunction (Oltman et al., 2005). Interestingly, Oltman et al., (2006) found that relaxation of 40 week old ZDF coronary arteries could be markedly increased by incubation with 1 µM of the free radical scavenger tiron, indicating that oxidative stress is important for endothelial dysfunction in coronary arteries. Furthermore, Schafer et al. (2004a) found that dual inhibition of both angiotensin converting enzyme and neutral endopeptidase by 6 weeks pretreatment with a vasopeptidase inhibitor (AVE7688) increased the ACh-induced relaxation in the mesenteric arteries. Taken together, these data illustrate that the endothelial dysfunction in the ZDF rat can be accredited to a number of different factors. Thus, little is known about the role of the EDHF-type relaxation in the endothelial dysfunction of diabetic ZDF rats.
Activation of SKCa and IKCa channels
Investigation of the EDHF-type relaxation with SKCa and IKCa channel activators has previously been suggested by Feletou and Vanhoutte (2004); they also speculated that restoration of the EDHF-type relaxation could have potential pharmacological importance. However, only very few studies have used this approach in diabetic models. In a recent study by Matsumoto et al. (2006), the effects of a combination of 1-EBIO (IKCa channel activator) and riluzole (SKCa activator) were used to demonstrate impaired activity of both channels in preconstricted mesenteric arteries from the Otsuka Long-Evans Tokushima Fatty (OLETF) rat model of type 2 diabetes. This finding is in accordance with the study by Burnham et al. (2006), showing that the ACh-induced hyperpolarization is reduced in unstimulated mesenteric arteries from ZDF rats, with apamin being a more effective blocker than TRAM-39. This suggests that the EDHF response is more dependent on SKCa than the IKCa channel. In contrast, our data indicate that when isolating the EDHF system by blocking the eNOS and COX, and in the presence of NS309, the ZDF arteries have an increased sensitivity to TRAM-34 over apamin, suggesting the predominant role of the IKCa channel. The apparent discrepancy between our findings and those of Burnham et al. (2006) can probably be attributed to the fact that Burnham and colleagues investigated unstimulated arteries, while in the present study, the arteries were precontracted with NA. This is consistent with previous suggestions of differential activation of IKCa and SKCa in resting and contracted rat mesenteric arteries (Crane et al., 2003).
In the present study, NS309 was applied to activate SKCa and IKCa channels. NS309 is a more potent activator than 1-EBIO but shares the same properties, such as a slight selectivity for IKCa over SKCa, and no effect on BK channels (Strobaek et al., 2004). As with 1-EBIO, NS309 is unable to differentiate between the different subtypes of SKCa channels (SK1, SK2 and SK3) (Hougaard et al., 2007). In high concentrations, NS309 has been shown to inhibit the activity of the voltage-dependent calcium channels (VDCCs) causing smooth muscle relaxation. The IC50 of NS309 for this effect has been reported to be 10.6 ± 1.1 µM in urinary bladder smooth muscle cells from C57BL/6 mice (Morimura et al., 2006).
We found that a concentration of 0.5 µM NS309 did not increase the relaxation to ACh in arteries from ZDF rats significantly. However, 1 µM NS309, which is well below the IC50 previously found in tissue preparations, but slightly higher than the EC50 of 0.62 µM found in HEK293 cells (Pedarzani et al., 2005), proved more effective in the presence of indomethacin and l-NAME. These experiments were therefore performed at 1 µM NS309.
The 20% relaxation that we observed in ZL rats during incubation with 1 µM NS309 under control conditions was blocked by a combination of apamin and TRAM-34. We therefore excluded the inhibition of VDCC by NS309 as being important for the potentiating effect of NS309.
In this study and in previous studies in porcine retinal arterioles, we observed a direct vasodilating effect of NS309 at high concentrations (>2.5 µM) (Dalsgaard et al., 2009). Therefore, NS309 may also facilitate vasorelaxation to endothelium-independent vasodilators. However, the potentiating effect of NS309 on ACh relaxation appears to be specific, as we recently observed that when sodium nitroprusside was applied to retinal arteries, it was unable to mimic the effect of NS309 on agonist-induced endothelium-dependent relaxation (T. Dalsgaard and U. Simonsen, unpubl. obs.).
The experiments with apamin or TRAM-34 in the presence of NS309 showed that both channels are involved in the EDHF-type relaxation and that their effect is compromised in ZDF rats. This interpretation is further supported by the decreased hyperpolarization to ACh and the decreased sensitivity to NS309 of arteries from ZDF rats. The complete inhibition of the EDHF-type relaxation required a combination of the two blockers (apamin and TRAM-34), indicating that the EDHF-type relaxation is partly dependent on the simultaneous activation of both channels.
The attenuated EDHF-type relaxation in arteries from the ZDF rats could be a reflection of altered protein expression of SKCa and IKCa channels. Weston et al. (2008) reported recently that arteries from ZDF rats tended to have decreased IKCa channel expression. However, by using RT-PCR, Burnham et al. (2006) found similar mRNA levels for the IKCa channels and increased mRNA levels for the SKCa channels in mesenteric arteries from the ZDF rat. In the present study, the results obtained by immunoblotting suggested that the SKCa protein expression in mesenteric small arteries from ZDF rats was unchanged. This indicates that functional alterations rather than altered expression of calcium-activated K channels contribute to the endothelial dysfunction in arteries from ZDF rats.
In SKCa and IKCa channel knockout mice, the blood pressure increases (Taylor et al., 2003; Si et al., 2006); this is somewhat in contrast to our results showing that the blood pressure was not increased in ZDF rats where the effect of these channels is compromised. Possible explanations for this are that unlike the KO situation where the channels are completely gone, the channels are still present in the ZDF rats as evidenced by the effects of NS309, apamin and TRAM-34. Other possible explanations are differences between mice and rats or the diabetic state in ZDF, which is not present in the KO mice.
In contrast to results obtained from studies in endothelial cell cultures (Sheng and Braun, 2007), inhibition of endothelial SKCa and IKCa channels in intact arteries does not appear to change [Ca2+]i (Ghisdal and Morel, 2001; McSherry et al., 2005). In agreement with the latter observations, incubation with NS309 had no effect on [Ca2+]i in the endothelial cells in the present study. Importantly, we also found that the ACh-evoked elevation of [Ca2+]i was similar in endothelial cells from ZDF and ZL rats, both before and after incubation with NS309. This indicates that NS309 is able to potentiate the potassium channels without altering [Ca2+]i, which is consistent with the patch clamp-based characterization of NS309 (Strobaek et al., 2004). These results imply that the endothelial dysfunction of the ZDF mesenteric small arteries is not due to a difference in intracellular Ca2+ handling but rather to a reduced Ca2+ sensitivity and/or attenuated transmission of the EDHF-type response from the endothelial cells to the smooth muscle cells.
Conclusion
The present investigation suggests that (i) the endothelial dysfunction of the mesenteric small arteries from ZDF rats is, at least partly, due to a reduced EDHF-type relaxation; (ii) the EDHF-type relaxation can be restored in a [Ca2+]i-independent manner by potentiating the endothelial SKCa and IKCa channels with NS309; (iii) the reduced EDHF-type relaxation in NA-stimulated ZDF arteries is more a reflection of impaired endothelial IKCa channel function than SKCa channel function; and (iv) the altered EDHF-type response is not due to changes in endothelial cell [Ca2+]i in ZDF rat arteries.
We therefore suggest that the EDHF-type relaxation pathway is impaired in mesenteric small arteries from the ZDF type 2 diabetic rat, and that activation of SKCa and IKCa channels is of potential pharmacological interest for the treatment of microvascular complications arising from endothelial dysfunction in diabetic patients.
Acknowledgments
The authors wish to acknowledge Jørgen Andresen and Helle Zibrandtsen for their excellent technical assistance. The study was supported by the Faculty of Health Sciences, Aarhus University.
Glossary
Abbreviations:
- CbTX
charybdotoxin
- EDHF
endothelium-derived hyperpolarizing factor
- EDTA
ethylenediaminetetraacetic acid
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulphonic acid sodium salt
- IKCa
intermediate-conductance calcium-activated potassium channel
- KPSS
125 mM K+-containing PSS
- NS309
(6,7-dichloro-1H-indole-2,3-dione 3-oxime)
- PSS
physiological salt solution
- ROI
region of interest
- SKCa
small-conductance calcium-activated potassium channel
- SNAP
S-nitroso-N-acetylpenicillamin
- TRAM-34
(1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole)
- TRAM-39
(2-(2-chlorophenyl)-2,2-diphenylacetonitrile)
- ZDF
Zucker diabetic fatty
- ZL
Zucker lean
Conflict of interest
None.
References
- Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol. 2007;151(3):332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135(5):1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in type II diabetic ZDF rats. Br J Pharmacol. 2006;148(4):434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137(8):1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, et al. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148(1):160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev. 2002;18(1):49–56. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553(1):183–189. doi: 10.1113/jphysiol.2003.051896. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Bek T, Simonsen U. Role of calcium-activated potassium channels with small conductance in bradykinin-induced vasodilation of porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50(8):3819–3825. doi: 10.1167/iovs.08-3168. [DOI] [PubMed] [Google Scholar]
- Danis RP, Yang Y. Microvascular retinopathy in the Zucker diabetic fatty rat. Invest Ophthalmol Vis Sci. 1993;34(7):2367–2371. [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van d V, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai KM, Gopalakrishnan V, Hiebert LM, McNeill JR, Wilson TW. EDHF-mediated rapid restoration of hypotensive response to acetylcholine after chronic, but not acute, nitric oxide synthase inhibition in rats. Eur J Pharmacol. 2006;546(1–3):120–126. doi: 10.1016/j.ejphar.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, et al. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40(5):480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102(10):1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res. 2004;49(6):565–580. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol. 1997;121(7):1383–1391. doi: 10.1038/sj.bjp.0701258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisdal P, Morel N. Cellular target of voltage and calcium-dependent K(+) channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br J Pharmacol. 2001;134(5):1021–1028. doi: 10.1038/sj.bjp.0704348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern W, Mulvany MJ. Tension responses to small length changes of vascular smooth muscle cells [proceedings] J Physiol. 1977;265(1):21P–23P. [PubMed] [Google Scholar]
- Hermans MP. Diabetes and the endothelium. Acta Clin Belg. 2007;62(2):97–101. doi: 10.1179/acb.2007.017. [DOI] [PubMed] [Google Scholar]
- Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol. 2003;138(6):1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151(5):655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38(1):23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291(1):H357–H367. doi: 10.1152/ajpheart.00681.2005. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Kamata K. Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima fatty (OLETF) rats. Eur J Pharmacol. 2006;538(1–3):132–140. doi: 10.1016/j.ejphar.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J Pharmacol Sci. 2006;100(3):237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Nilsson H, Flatman JA. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab. 2005;289(1):E113–E122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291(4):H1780–H1787. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem. 2005;280(50):41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88(8):810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- Peterson RG, Shaw WN, Neel M, Little LA, Eichberg J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR J. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S, Linz W, Vollert H, Biemer-Daub G, Rutten H, Bleich M, et al. The vasopeptidase inhibitor AVE7688 ameliorates type 2 diabetic nephropathy. Diabetologia. 2004a;47(1):98–103. doi: 10.1007/s00125-003-1264-8. [DOI] [PubMed] [Google Scholar]
- Schafer S, Steioff K, Linz W, Bleich M, Busch AE, Lohn M. Chronic vasopeptidase inhibition normalizes diabetic endothelial dysfunction. Eur J Pharmacol. 2004b;484(2–3):361–362. doi: 10.1016/j.ejphar.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293(1):C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23(4):1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99(5):537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665(1–2):1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93(2):124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, Ruat M, et al. The expression and function of Ca(2+)-sensing receptors in rat mesenteric artery; comparative studies using a model of type II diabetes. Br J Pharmacol. 2008;154(3):652–662. doi: 10.1038/bjp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol. 2001;281(1):H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]


