Abstract
Background and purpose:
5-HT1B receptors may have a role in pulmonary hypertension. Their relationship with the activity of BKCa, a T-type voltage-operated calcium channel (VOCC) and cyclic nucleotide-mediated relaxation was examined.
Experimental approach:
Ring segments of bovine pulmonary arteries were mounted in organ baths in modified Krebs–Henseleit buffer (37oC) under a tension of 20 mN and gassed with 95% O2/5% CO2. Isometric recordings were made using Chart 5 software.
Key results:
Contractile responses to 5-HT (10 nM–300 µM) were inhibited similarly by the 5-HT1B receptor antagonist SB216641 (100 nM) and the T-type VOCC blockers mibefradil (10 µM) and NNC550396 (10 µM) with no additive effect between SB216641 and mibefradil. Inhibition by SB216641 was prevented by the potassium channel blocker, charybdotoxin (100 nM). 5-HT1B receptor activation and charybdotoxin produced a mibefradil-sensitive potentiation of responses to U46619. Bradykinin (0.1 nM–30 µM), sodium nitroprusside (0.01 nM–3 µM), zaprinast (1 nM–3 µM), isoprenaline (0.1 nM–10 µM) and rolipram (1 nM–3 µM) produced 50% relaxation of arteries constricted with 5-HT (1–3 µM) or U46619 (30–50 nM) in the presence of 5-HT1B receptor activation, but full relaxation of arteries constricted with U46619, the 5-HT2A receptor agonist 2,5 dimethoxy-4 iodoamphetamine (1 µM) or 5-HT in the presence of 5-HT1B receptor antagonism. Enhanced relaxation of 5-HT-constricted arteries by cGMP-dependent pathways, seen in the presence of the 5-HT1B receptor antagonist, was reversed by charybdotoxin whereas cAMP-dependent relaxation was only partly reversed by charybdotoxin.
Conclusions and implications:
5-HT1B receptors couple to inhibition of BKCa, thus increasing tissue sensitivity to contractile agonists by activating a T-type VOCC and impairing cGMP-mediated relaxation. Impaired cAMP-mediated relaxation was only partly mediated by inhibition of BKCa.
Keywords: pulmonary arteries, 5-hydroxytryptamine, 5-HT1B receptor, T-type voltage-operated calcium channels, BKCa, bradykinin, isoprenaline, cAMP, cGMP
Introduction
5-Hydroxytryptamine (5-HT) is a pulmonary vasoconstrictor implicated in the development of pulmonary hypertension (Hervéet al., 1995; Egermayer et al., 1999; MacLean et al., 2000). Both 5-HT2A and 5-HT1B receptors (nomenclature follows Alexander et al., 2008) are involved in mediating pulmonary artery vasoconstriction in the normal lung (MacLean et al., 2000). While the 5-HT2A receptor is clearly contractile, the 5-HT1B receptor is often reported to be ‘silent’ requiring pharmacological synergy, an agonist-induced tone, to expose its contractile properties (MacLean, 1999; Banes and Watts, 2001; Wang, 2001). The 5-HT1B receptor couples to Gi/o (Hoyer et al., 1994); however, the transduction pathways and mechanisms by which this receptor influences vascular tone are not well understood.
We have investigated the role of voltage-operated calcium channels (VOCC) in the contractile response to 5-HT and the thromboxane A2 mimetic U46619 in bovine pulmonary arteries (BPA) (Alapati et al., 2007). In endothelium-intact BPA the contractile response to 5-HT is sensitive to verapamil and mibefradil but is insensitive to nifedipine. Verapamil is a non-selective L-type VOCC blocker, inhibiting both medium (Cav 1.3), and high voltage-activated L-type channels (Cav 1.1 and 1.2; nomenclature follows Alexander et al., 2008), whereas nifedipine is a selective blocker of the high voltage-activated L-type channels, which suggests that the inhibitory action of verapamil is due to inhibition of Ca2+ entry via the medium voltage-activated channel. As mibefradil has a high specificity for each of the low voltage-activated T-type channels (Cav 3.1–3.3, Alexander et al., 2008) its inhibitory action is due to inhibition of Ca2+ entry via a T-type VOCC. This may indicate that the contractile response mediated by 5-HT involves activation of low/medium voltage-activated L- (Cav 1.3) and T-type VOCC.
In contrast to 5-HT, the contractile response mediated by the thromboxane A2 mimetic U46619 in the endothelium-intact BPA (Alapati et al., 2007) and rat pulmonary arteries (McKenzie et al., 2009) is insensitive to VOCC blockade. This response, however, is potentiated by the K+ channel blockers, 4-aminopyridine and charybdotoxin, inhibitors of voltage-activated potassium channels (KV) and large conductance calcium-activated potassium channels (BKCa,) respectively (McKenzie et al., 2009), indicating that both KV and BKCa exert an inhibitory influence on the U46619 contractile response. Interestingly, the potentiation associated with inhibition of KV is nifedipine-sensitive whereas inhibition of BKCa evokes a mibefradil-sensitive potentiation (McKenzie et al., 2009). This latter observation suggests that inhibition of BKCa may be associated with activation of a mibifradil-sensitive T-type VOCC and raises the possibility that the mibefradil-sensitive component of the 5-HT-induced contraction may be due to a 5-HT receptor-linked inhibition of BKCa.
The role of BKCa in regulating vascular tone remains to be fully understood. Its activation following agonist-induced increases in [Ca2+]i or depolarization is thought to be important in limiting the contractile response (Jaggar et al., 1998; Brayden, 2002; Nilsson and Aalkjaer, 2003) and, in pulmonary arteries, increased IBK is associated with several vasoconstrictors including noradrenaline, histamine and endothelin (Wang and Large, 1992; Salter and Kozlowski, 1995; Wang et al., 1997). BKCa can also be activated by cyclic nucleotide-dependent kinases, to provide a hyperpolarizing stimulus that contributes to cyclic nucleotide-mediated relaxation (Robertson et al., 1993; Archer et al., 1994; Schubert et al., 1999; White et al., 2000; Barman et al., 2003).
In bovine pulmonary conventional arteries, activation of the 5-HT1D/1B receptor does not directly induce a contractile response but does potentiate the contractile response mediated by the 5-HT2A receptor (Shaw et al., 2000). The purpose of the present study was to investigate the hypothesis that the 5-HT1D/1B receptor in BPA may activate a T-type VOCC and inhibit cyclic nucleotide-mediated relaxation by linking to inhibition of BKCa.
Methods
Tissue preparation
Bovine lungs were obtained from a local abattoir within 50 min of slaughter. Ring segments were dissected from 4th–5th generation conventional arteries (3–4 mm diameter and 3–4 mm in length) and freed of surrounding connective tissue. Care was taken not to damage the luminal surface of the preparation. The vessels were suspended between two stainless steel wire hooks in 10 mL Linton vessel chambers containing modified Krebs–Henseleit physiological salt solution of the following composition (mM): NaCl (119), KCl (4.7), NaHCO3 (24.8), MgSO4 (1.2), KH2PO4 (1.2), CaCl (2.5), glucose (11.1). Tissues were maintained at 37°C under a tension of 20 mN, and gassed with a mixture of 95% O2/5% CO2. Solutions of high K+ physiological salt solution (60 mM) were made by equimolar replacement of NaCl with KCl. Changes in isometric tension were recorded using Chart 5 software.
Experimental protocols
The tissues were allowed to equilibrate for 60 min before each experiment. Rings were initially contracted with KCl (60 mM). Agonists were added to the organ baths cumulatively in 0.5 log unit to construct cumulative log concentration–response curves. Agonist responses are expressed as a percentage of the initial KCl response. Where necessary, the integrity of the endothelium was assessed using the endothelium-dependent vasodilator bradykinin as described in Tracey et al. (2002).
Investigations into the influence of 5-HT1D/5-HT1B receptors, the effect of charybdotoxin and the involvement of a T-type VOCC on contractile responses
The involvement of the 5-HT1D and 5-HT1B receptors on contractile responses to 5-HT was assessed by examining the effect of the mixed 5-HT1D/1B receptor antagonist GR127935 (100 nM) (Skingle et al., 1996) and subtype selective antagonists (Price et al., 1997) BRL15572 (5-HT1D) and SB216641 (5-HT1B) on the concentration–response curves to 5-HT (1 nM–300 µM). BRL15572 and SB216641 were used at 100 nM, which produced maximal effects. Antagonists were pre-incubated for 40 min before the addition of the agonist.
The involvement of VOCC in the contractile responses to 5-HT was assessed using the L-type VOCC blocker verapamil (Cav1.1, 1.2, 1.3) and the T-type VOCC blockers (Huang et al., 2004) mibefradil and NNC550396 (Cav 3.1–3.3). Both verapamil and mibefradil were used at 10 µM, which produced maximal effects. Although mibefradil is selective for the T-type channels, its metabolites may also block L-type channels (Wu et al., 2000). For this reason we included the non-hydrolysable analogue of mibefradil, NNC550396 (10 µM) in these studies.
To assess the relationship between BKCa on T-type VOCC activation, concentration–response curves to U46619 were constructed in the absence and presence of the BKCa/IKCa inhibitor charybdotoxin (ChTx, 100 nM, Félétou and Vanhoutte, 2006) alone and in the presence of mibefradil (10 µM) or NNC550396 (10 µM). The effect of charybdotoxin was also examined on concentration–response curves to 5-HT in the presence and absence of the 5-HT1B receptor antagonist SB216641.
To assess the influence of 5-HT1 receptor and T-type VOCC activation, concentration–response curves for U46619 were constructed in the absence and presence of 5-HT (5 µM) with the 5-HT2A receptor antagonist ritanserin (1 µM) alone and in the presence of mibefradil (10 µM).
Studies investigating the effect of the 5-HT1D or 5-HT1B receptor and charybdotoxin on relaxation mediated by cyclic nucleotides
Arteries were constricted to a similar extent by 5-HT or U46619; mean values 41.6 ± 2.3 and 44.1 ± 1.9 mN for 5-HT (1–3 µM) and U46619 (30–50 nM) respectively. Thereafter, concentration–response curves were constructed for relaxation using agents known to relax vascular smooth muscle by elevating intracellular cAMP (isoprenaline, rolipram) or cGMP [bradykinin, sodium nitroprusside (SNP), zaprinast]. To assess the influence of 5-HT1 receptor subtypes on the relaxation of 5-HT-constricted vessels responses were repeated in the presence of GR127935 (100 nM), BRL15572 (100 nM) or SB216641 (100 nM).
The influence of the 5-HT1 receptor on relaxation by isoprenaline and bradykinin was further assessed by comparing the relaxation in vessels contracted with 5-HT, the selective 5-HT2A agonist 2,5 dimethoxy-4 iodoamphetamine (DOI, 1 µM) or U46619 in the absence and presence of the 5-HT1 agonist 5-carboxamidotryptamine (5-CT, 1 µM) or the selective 5-HT1B agonist CP93129 (1 µM).
To assess the involvement of IK in cyclic nucleotide-mediated relaxation responses to isoprenaline and bradykinin in some experiments were examined in high (25 mM) [K+]o and compared with the response in normal (5.9 mM) [K+]o. The involvement of BKCa in cyclic nucleotide-mediated relaxation was assessed by comparing concentration–response curves for relaxation to isoprenaline, bradykinin, SNP, zaprinast and rolipram of U46619-constricted vessels in the absence and presence of charybdotoxin (ChTx, 100 nM). Similar experiments, using charybdotoxin were performed with vessels constricted with 5-HT in the absence and presence of the 5-HT1B receptor antagonist SB216641 (10 µM). In some experiments the involvement of IKCa in cyclic nucleotide-mediated relaxation was assessed using TRAM34 (1 µM).
Drug incubation
Unless otherwise stated all drugs, or an equal volume of the drug vehicle, in control tissues, were incubated for approximately 40 min before the addition of U46619 or 5-HT.
Data analysis
All data were collected using Chart for Windows (ADInstruments). Maximum contractile responses to agonists were calculated as a percentage of the contraction produced by KCl (60 mM) and are expressed as the means ± SEM. Relaxations were expressed as % decrease in the U46619- or 5-HT-induced contraction and expressed as means ± SEM.
The mean log concentration–response curves to agonists were analysed by fitting to a four-parameter logistic equation (giver below) using nonlinear regression (Graph Pad Prism):
where X is the logarithm of the molar concentration of agonist and Y is the response. The upper limit is the maximum contraction (or relaxation) (Emax) and log EC50 is the agonist concentration that produces 50% of the maximum response. The lower limit is the resting tone in the absence of any contractile agonist (or the pre-contraction used for relaxation). Comparison between mean sensitivity (pEC50) or maximum contraction (Rmax) were carried out using unpaired Student's t-test (two-tailed). Where necessary multiple comparisons were conducted using one-way anova with Tukey post test. All differences were considered as statistically significant when P < 0.05. In all cases, n= the number of arteries from different animals
Materials
U46619 was purchased from Biomol Research Laboratories Inc. 5-HT, mibefradil, NNC550396, verapamil hydrochloride, charybdotoxin and SNP were purchased from Sigma-RBI. TRAM 34, 4-AP (4-aminopyridine), isoprenaline, BRL15572, GR127935, SB216641, zaprinast, rolipram and CP93129 were purchased from Tocris Bioscience. U46619, verapamil, TRAM 34, BRL15572 and rolipram were dissolved in ethanol. SNP and zaprinast were dissolved in DMSO. All other drugs were dissolved in distilled water.
Results
Effect of the 5-HT1 receptor antagonists GR127935 (5-HT1D/1B), SB216641 (5-HT1B), BRL15572 (5-HT1D) and the VOCC blockers verapamil, mibefradil and NNC550396 on the concentration–response curve to 5-HT
The concentration–response curve for 5-HT-induced contraction was shifted to the right by the 5-HT1B receptor antagonist SB216641 (100 nM) and the mixed 5-HT1D/1B receptor antagonist GR127935 (100 nM), but not by the 5-HT1D receptor antagonist BRL15572 (100 nM) (Figure 1A, Table 1). Mibefradil (10 µM), its non-hydrolysable analogue NNC550396 (10 µM) and verapamil (10 µM) produced similar rightward shifts of the 5-HT concentration–response curve (Figure 1B and C, Table 1). Their effects were maximal at this concentration (data not shown). The shift produced when SB126641 was combined with mibefradil was not different from that produced by either drug alone (Figure 1B, Table 1). In contrast, when SB216641 was combined with verapamil (10 µM) a much greater inhibition was observed (Figure 1C, Table 1).
Figure 1.
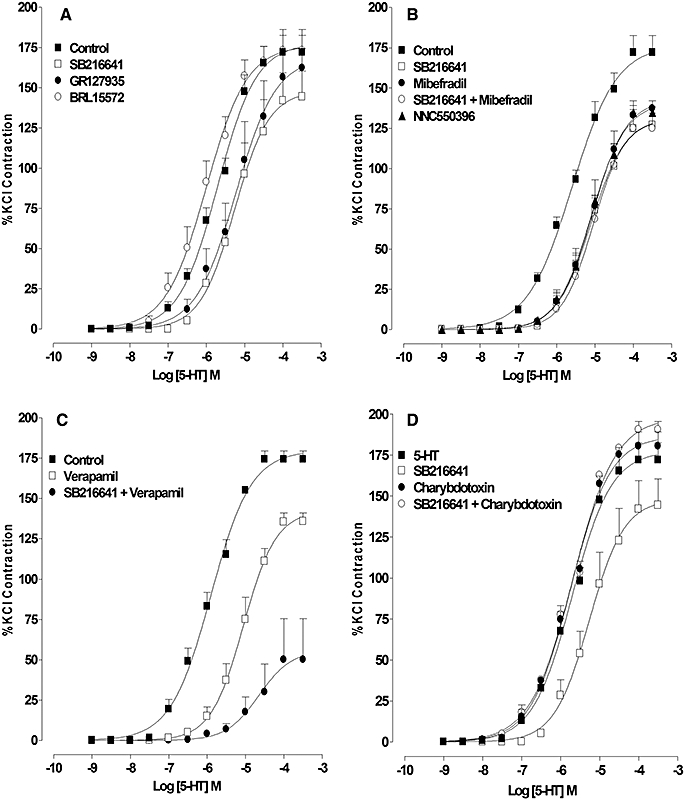
Effect of the 5-HT1D (BRL15572), 5-HT1B (SB216641) or the mixed 5-HT1D/1B (GR127935) receptor antagonists, mibefradil, a T-type voltage-operated calcium channel (VOCC) blocker or verapamil, an L-type VOCC blocker on the concentration–response curve for 5-HT-induced contraction. (A) Response to 5-HT in the absence (control) and presence of SB216641 (100 nM), BRL15572 (100 nM) and GR127935 (1 µM). (B) Response to 5-HT in the absence (control) and presence of SB216641 (100 µM), NNC550396 (10 µM), mibefradil (10 µM) and mibefradil combined with SB216641. (C) Response to 5-HT in the absence (control) and presence of verapamil (10 µM) and verapamil combined with SB216641. (D) Response to 5-HT in the absence (control) and presence of SB216641 (100 nM), charybdotoxin (100 nM) and charybdotoxin combined with SB216641. Results are the means ± SEM from four to five experiments (number of arteries from different animals).
Table 1.
Effect of 5-HT1B receptor antagonism, the voltage-operated calcium channel blockers verapamil, mibefradil, NNC550396 and the BKCa blocker charybdotoxin (ChTx) on the 5-HT-induced concentration–response curve
| pEC50 | Rmax | n | |
|---|---|---|---|
| 5-HT (control) | 5.60 ± 0.07 | 176.6 ± 6.7 | 5 |
| 5-HT + SB216641 | 5.11 ± 0.09* | 131.6 ± 7.1* | 5 |
| 5-HT + mibefradil | 5.08 ± 0.09* | 142.8 ± 7.9* | 5 |
| 5-HT + SB216641 + mibefradil | 5.05 ± 0.09* | 130.4 ± 7.1* | 5 |
| 5-HT + NNC550396 | 5.09 ± 0.05* | 141.2 ± 4.1* | 4 |
| 5-HT (control) | 5.90 ± 0.05 | 179.7 ± 4.0 | 5 |
| 5-HT + verapamil | 5.06 ± 0.07* | 142.3 ± 5.9 | 4 |
| 5-HT + verapamil + SB216641 | 4.65 ± 0.41*§ | 55.8 ± 16.9*§ | 4 |
| 5-HT (control) | 5.71 ± 0.06 | 177.5 ± 5.5 | 5 |
| 5-HT + SB216641 | 5.27 ± 0.12* | 148.0 ± 10.2* | 5 |
| 5-HT + ChTx | 5.75 ± 0.04 | 186.6 ± 3.8 | 5 |
| 5-HT + SB216641 + ChTx | 5.69 ± 0.04# | 198.0 ± 3.7# | 5 |
Significantly different (0.00 < P < 0.05, anova with Tukey post test)
from 5-HT control;
from 5-HT + verapamil;
from 5-HT + SB216641. n= number of arteries from different animals.
Effect of 5-HT1-receptor activation on basal tone and on U46619-induced contraction
In the presence of the 5-HT2A receptor antagonist ritanserin, 5-HT did not alter the basal tone (data not shown), but produced a leftward shift of the concentration–response curve to U46619 (0.1 nM–3 µM) and increased the maximum response. This effect of 5-HT was absent when repeated in the presence of mibefradil (10 µM) (Figure 2A, Table 2).
Figure 2.
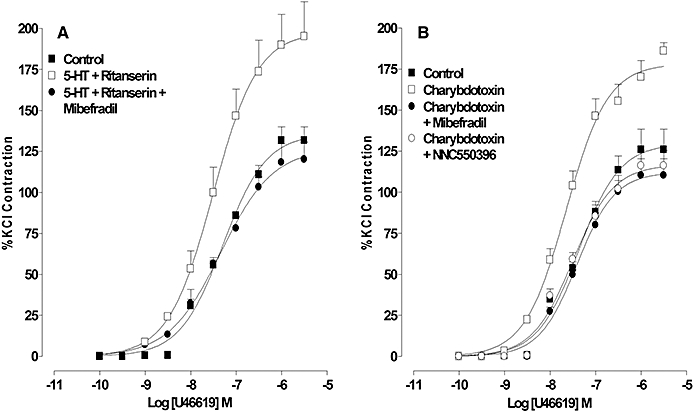
Effect of 5-HT1 receptor activation and charybdotoxin on the contractile response to U46619. (A) The response to U46619 in the absence (control) and presence of 5-HT and ritanserin alone or with mibefradil. (B) The response to U46619 in the absence and presence of charybdotoxin (ChTx) alone or in combination with mibefradil or NNC550396 (10 µM). Results are the means ± SEM from four to five experiments (number of arteries from different animals).
Table 2.
Effect of charybdotoxin (ChTx) or activation of the 5-HT1 receptor on the concentration–response curve for U46619-induced contraction in the absence and presence of the T-type voltage-operated calcium channel blockers mibefradil and NNC550396
| pEC50 | Rmax | n | |
|---|---|---|---|
| U46619 (control) | 7.29 ± 0.07 | 135.7 ± 5.4 | 5 |
| U46619 + 5-HT + ritanserin | 7.51 ± 0.10 | 197.9 ± 10.6* | 4 |
| U46619 + 5-HT + ritanserin + mibefradil | 7.33 ± 0.10 | 126.7 ± 6.2# | 4 |
| U46619 (control) | 7.37 ± 0.09 | 129.6 ± 6.1 | 5 |
| U46619 + ChTx | 7.65 ± 0.06* | 178.2 ± 5.4* | 5 |
| U46619 + ChTx + mibefradil | 7.41 ± 0.08§ | 112.1 ± 4.4§ | 4 |
| U46619 + ChTx + NNC550396 | 7.49 ± 0.08§ | 116.7 ± 4.7§ | 4 |
Significantly different (0.00 < P < 0.05, anova with Tukey post test)
from U46619 control;
from U46619 with 5-HT1 receptor activation;
from U46619 in the presence of ChTx. n= number of arteries from different animals.
Effect of charybdotoxin on (i) the concentration–response curve to 5-HT in the absence and presence of 5-HT1B receptor antagonism, (ii) the contractile response to the 5-HT2A receptor agonist DOI and (iii) the concentration–response curve to U46619 in the absence and presence of T-type channel blockade
Charybdotoxin (100 nM) did not affect the concentration–response curve for 5-HT; however, the rightward shift normally produced by SB216641 (100 nM) was absent in the presence of charybdotoxin (Figure 1D, Table 1). The contractile response induced by DOI (1 µM) was increased from 98 ± 11% to 133 ± 4% of the KCl contraction (n= 4). Charybdotoxin shifted the concentration–response curve for U46619 to the left and increased the maximum response (Figure 2B, Table 2) but, in the presence of either mibefradil (10 µM) or NNC550396 (10 µM), the charybdotoxin-mediated potentiation was absent (Figure 2B, Table 2).
Influence of the 5-HT1B receptor on cGMP- (bradykinin) or cAMP- (isoprenaline) mediated relaxations
In rings constricted with U46619 (30–50 nM), bradykinin (0.01 nM–30 µM) or isoprenaline (0.1 nM–10 µM) induced a concentration-dependent relaxation that almost fully reversed the pre-constriction (Figure 3A and B, Table 3). In contrast, in rings that were constricted to a similar extent by 5-HT (1–3 µM), the maximum relaxation induced by isoprenaline or bradykinin was approximately half of the pre-constriction (Figure 3A and B, Table 3) but, when repeated in the presence of the selective 5-HT1B antagonist SB216641 (100 nM) or the mixed 5-HT1D/1B receptor antagonist GR127935 (100 nM), but not the 5-HT1D antagonist BRL15572 (100 nM), bradykinin or isoprenaline induced almost full relaxation (Figure 3A and B, Table 3).
Figure 3.
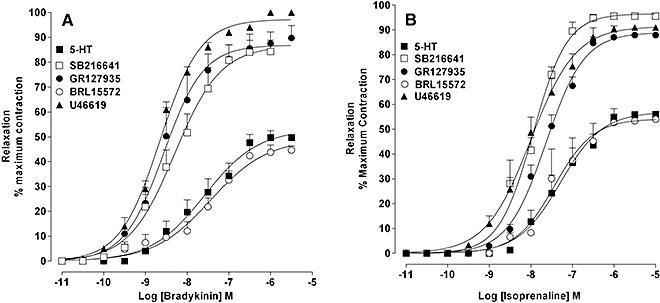
Relaxation induced by bradykinin and isoprenaline of artery rings constricted with U46619 or 5-HT: effect of 5-HT1B and 5-HT1D receptor antagonism. Concentration–response curves for (A) bradykinin- and (B) isoprenaline-induced relaxation of artery rings constricted to a similar extent with U46619 and 5-HT. In 5-HT-constricted rings responses to bradykinin and isoprenaline were examined in the absence and presence of SB216641 (100 nM), GR127935 (1 µM) and BRL15572 (100 nM). Results are the means ± SEM from four to six experiments (number of arteries from different animals).
Table 3.
Relaxation by bradykinin and isoprenaline of artery rings constricted with U46619 or 5-HT alone or in the presence of either 5-HT1B or 5-HT1D receptor antagonists or the mixed 5-HT1D/1B antagonist
| Pre-constriction |
Bradykinin |
Isoprenaline |
||||
|---|---|---|---|---|---|---|
| pEC50 | Rmax | n | pEC50 | Rmax | n | |
| U46619 | 8.65 ± 0.01 | 97.2 ± 0.5 | 8 | 8.00 ± 0.01 | 91.4 ± 0.4 | 6 |
| 5-HT | 7.58 ± 0.03 | 52.9 ± 0.8 | 6 | 7.29 ± 0.03 | 56.9 ± 0.7 | 4 |
| 5-HT + SB216641 | 8.29 ± 0.02* | 86.5 ± 0.7* | 7 | 7.95 ± 0.02* | 96.5 ± 0.9* | 4 |
| 5-HT + GR127935 | 8.56 ± 0.02* | 86.9 ± 0.6* | 4 | 7.64 ± 0.02* | 88.9 ± 0.6* | 4 |
| 5-HT + BRL15572 | 7.44 ± 0.06 | 49.1 ± 1.3 | 5 | 7.39 ± 0.03 | 54.1 ± 0.6 | 4 |
Significantly different (0.001 < P < 0.05, Student's t-test) from 5-HT. n= number of arteries from different animals.
In arteries constricted with the selective 5-HT2A agonist DOI (1 µM), a supramaximal concentration of bradykinin (10 µM) and isoprenaline (5 µM) induced about twice as much relaxation as that induced in rings constricted to a similar extent by 5-HT (n= 4–6, P < 0.001; Figure 4A and B).
Figure 4.
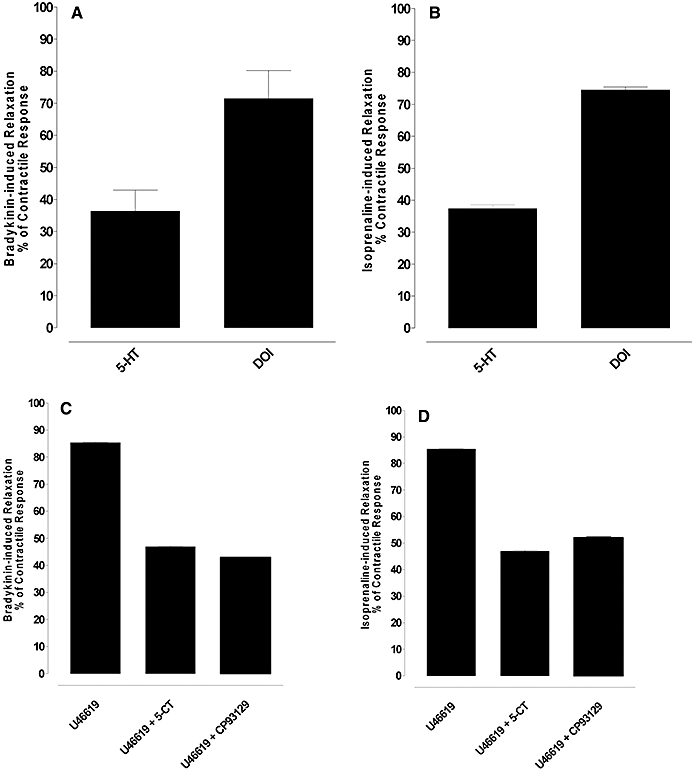
Relaxation induced by bradykinin and isoprenaline of artery rings constricted with 5-HT, the 5-HT2A selective agonist 2,5 dimethoxy-4 iodoamphetamine (DOI) (1 µM) or U46619 alone or in the presence of activation of the 5-HT1B receptor. (A and B) Relaxation induced by bradykinin and isoprenaline in rings contracted to a similar extent by 5-HT or DOI. (C and D) Relaxation induced by bradykinin and isoprenaline in rings contracted to a similar extent by U46619 and U46619 with 5-CT (1 µM) or CP93129 (1 µM). Results are the means ± SEM from four to six experiments (number of arteries from different animals).
In arteries constricted by U46619, a supramaximal concentration of isoprenaline (5 µM) and bradykinin (10 µM) induced about 80% relaxation (Figure 4C and D) and these relaxations were reduced to about 40% by the non-selective 5-HT1 agonist 5-CT (1 µM) or the selective 5-HT1B agonist CP93129 (1 µM) (n= 4–5, P < 0.001; Figure 4C and D). CP93129 did not affect the basal tone (results not shown). The mean level of constriction for U46619 alone, U466619 in the presence of CP93129 and U46619 in the presence of 5-CT was 44.1 ± 1.9, 43 ± 0.9 and 43.8 ± 1 mN.
In artery rings constricted by 5-HT, the isoprenaline- and bradykinin-induced relaxation was unaffected by increasing [K]o from 5.9 (normal) to 25 mM (high [K]o); however, the enhanced relaxation normally produced by SB216641 for both agents was not observed in [K]o= 25 mM (Figure 5A and B).
Figure 5.
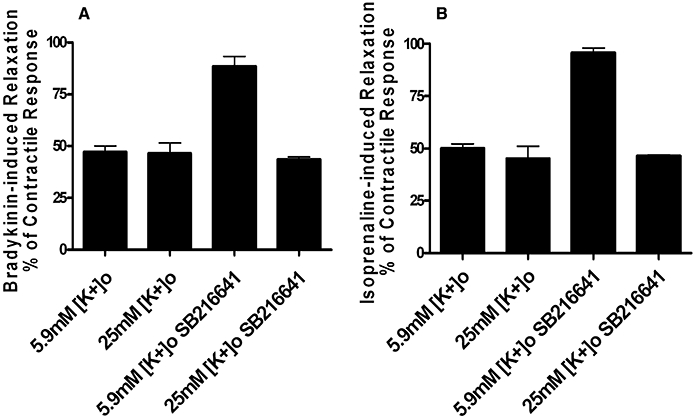
Effect of increasing [K]o to 25 mM on isoprenaline and bradykinin-induced relaxation of rings pre-constricted with 5-HT or 5-HT in the presence of SB216641. In 5-HT constricted rings relaxation to isoprenaline and bradykinin was unaffected by high [K]o, but the relaxation in the presence of SB216641 was reduced by approximately 40–50%. Results are the means ± SEM from four to six experiments (number of arteries from different animals).
Effect of charybdotoxin on cyclic nucleotide-mediated relaxation of rings pre-constricted with U46619 or 5-HT in the absence and presence of 5-HT1B receptor antagonism
In rings pre-constricted with U46619 (30–50 nM), bradykinin, SNP, zaprinast (Figure 6A, C and E, Table 4), isoprenaline and rolipram (Figure 6A, G and I, Table 5) produced almost full relaxation of the pre-constriction. In the presence of charybdotoxin, the concentration–response curves for relaxation by bradykinin (0.1 nM–30 µM), SNP (0.01 nM–3 µM) and zaprinast (1 nM–3 µM) were shifted to the right and the maximum relaxation reduced by approximately 40–50% (Figure 6A, C and E, Table 4). Charybdotoxin produced a small rightward shift of the isoprenaline (0.1 nM–10 µM) and rolipram (1 nM–3 µM) concentration–response curves but did not change the maximum response (Figure 6G and I, Table 5).
Figure 6.
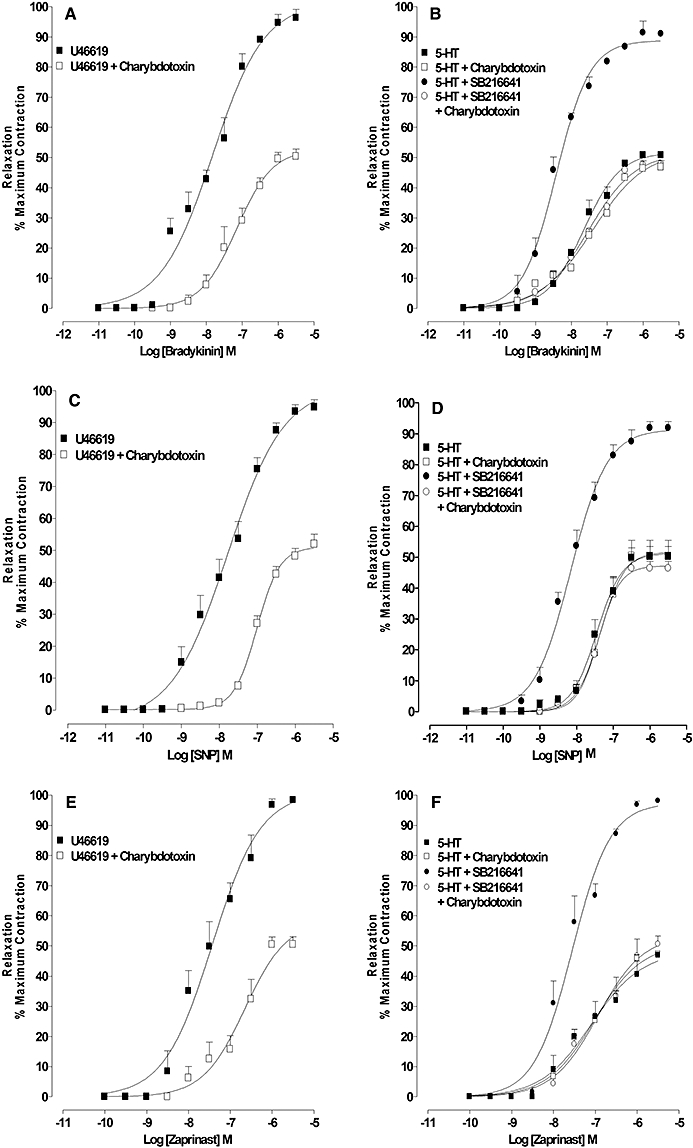
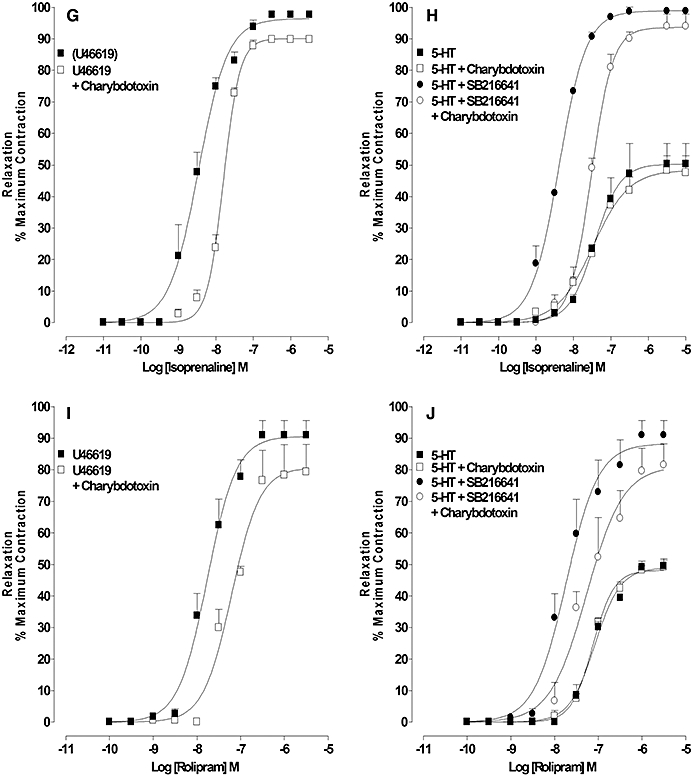
Effect of charybdotoxin on relaxation, by agents that increase cGMP and cAMP, of artery rings constricted with U46619, 5-HT or 5-HT in the presence of 5-HT1B receptor antagonism: concentration–response curves for (A) bradykinin-, (C) SNP-, (E) zaprinast-, (G) isoprenaline-, (I) rolipram-induced relaxation of U46619-constricted rings in the absence and presence of charybdotoxin (100 nM); concentration–response curves for (B) bradykinin-, (D) SNP-, (F) zaprinast-, (H) isoprenaline-, (J) rolipram-induced relaxation of 5-HT-constricted rings in the absence and presence of charybdotoxin (100 nM), SB216641 (100 nM) and charybdotoxin combined with SB216641. Results are the means ± SEM from four to five experiments (number of arteries from different animals).
Table 4.
Influence of charybdotoxin (ChTx) on relaxation, by agents that increase cGMP, of artery rings constricted with U46619, 5-HT or 5-HT in the presence of 5-HT1B receptor antagonism
| Pre-constriction |
Bradykinin |
SNP |
Zaprinast |
||||||
|---|---|---|---|---|---|---|---|---|---|
| pEC50 | Rmax | n | pEC50 | Rmax | n | pEC50 | Rmax | n | |
| U46619 | 7.83 ± 0.11 | 100 ± 4.4 | 5 | 7.78 ± 0.10 | 100 ±1.6 | 5 | 7.43 ± 0.11 | 100 ± 5.6 | 4 |
| U46619 + ChTx | 7.15 ± 0.10* | 52.8 ± 0.1* | 5 | 7.01 ± 0.04* | 51.0 ± 0.6* | 5 | 6.65 ± 0.18* | 58.4 ± 6.9* | 4 |
| 5-HT | 7.66 ± 0.06 | 51.7 ± 1.6 | 5 | 7.45 ± 0.06 | 51.2 ± 1.7 | 5 | 7.10 ± 0.15 | 48.1 ± 3.9 | 4 |
| 5-HT + ChTx | 7.40 ± 0.11 | 52.0 ± 2.6 | 5 | 7.40 ± 0.06 | 47.3 ± 1.8 | 5 | 7.02 ± 0.11 | 50.7 ± 3.1 | 4 |
| 5-HT + SB216641 | 8.42 ± 0.05* | 88.8 ± 1.8* | 5 | 8.16 ± 0.05* | 91.4 ± 2.0* | 5 | 7.55 ± 0.08 | 97.4 ± 3.8* | 4 |
| 5-HT + SB216641 + ChTx | 7.53 ± 0.06# | 51.5 ± 1.3# | 5 | 7.35 ± 0.08# | 51.8 ± 2.6# | 5 | 6.94 ± 0.16# | 53.6 ± 5.1# | 4 |
Significantly different (0.00 < P < 0.05, anova with Tukey post test)
from U46619 or 5-HT controls;
from 5-HT in the presence of the 5-HT1B receptor antagonist SB216641. n= number of arteries from different animals.
Table 5.
Influence of charybdotoxin (ChTx) on relaxation, by agents that increase cAMP, of artery rings constricted with U46619, 5-HT or 5-HT in the presence of 5-HT1B receptor antagonism
| Pre-constriction |
Isoprenaline |
Rolipram |
||||
|---|---|---|---|---|---|---|
| pEC50 | Rmax | n | pEC50 | Rmax | n | |
| U46619 | 8.47 ± 0.05 | 96.4 ± 1.9 | 5 | 7.76 ± 0.06 | 90.6 ± 2.7 | 4 |
| U46619 + ChTx | 7.80 ± 0.02§ | 90.1 ± 3.9 | 5 | 7.21 ± 0.08§ | 80.7 ± 4.1 | 4 |
| 5-HT | 7.44 ± 0.08 | 50.3 ± 2.2 | 5 | 7.08 ± 0.04 | 48.9 ± 1.4 | 4 |
| 5-HT + ChTx | 7.48 ± 0.07 | 48.4 ± 1.6 | 5 | 7.13 ± 0.03 | 48.1 ± 0.9 | 4 |
| 5-HT + SB216641 | 8.39 ± 0.02* | 98.9 ± 1.0* | 5 | 7.73 ± 0.08* | 88.3 ± 3.9* | 4 |
| 5-HT + SB216641 + ChTx | 7.52 ± 0.03# | 93.6 ± 1.4* | 5 | 7.27 ± 0.11# | 81.1 ± 5.3* | 4 |
Significantly different (0.00 < P < 0.05, anova with Tukey post test)
from U46619
or 5-HT controls;
from 5-HT in the presence of the 5-HT1B receptor antagonist SB216641. n= number of arteries from different animals.
In rings pre-constricted with 5-HT (1–3 µM), bradykinin, SNP, zaprinast, isoprenaline and rolipram produced concentration-dependent relaxations with maximum relaxations that were approximately 40–50% of the pre-constriction. These responses were unaffected by charybdotoxin (100 nM) (Figure 6B, D, F, H and J, Tables 4 and 5). In rings constricted with 5-HT in the presence of SB216641 (100 nM), but not SB216641 with charybdotoxin, the concentration–response curves for relaxation by bradykinin, SNP and zaprinast were shifted to the left and the maximum relaxation increased to approximately 90% of the pre-constriction (Figure 6B, D and F, Table 4). In the presence of SB216641, the concentration–response curves for relaxation to isoprenaline and rolipram were shifted to the left and the maximum relaxation increased to approximately 90% of the pre-constriction. The further addition of charybdotoxin reduced the leftward shift but not the maximum relaxation (Figure 6H and J, Table 5).
Effect of TRAM34 on bradykinin- and isoprenaline-induced relaxation of rings pre-constricted with U46619 or 5-HT in the absence and presence of 5-HT1B receptor antagonism
In rings contracted with 5-HT (1-3 µM) the concentration–response curves for relaxation to bradykinin (0.01 nM–30 µM) and isoprenaline (0.1 nM–10 µM) in the absence and presence of SB216641 (100 nM) were unaffected by TRAM 34 (1 µM) (Figure 7A and B).
Figure 7.
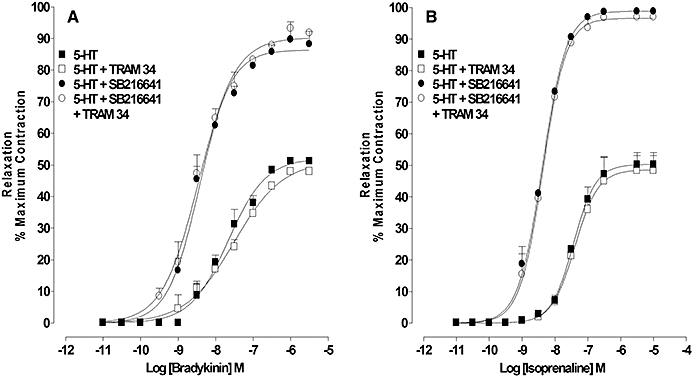
Influence of IKCa on relaxation, by agents that increase cGMP and cAMP, of artery rings constricted with 5-HT or 5-HT in the presence of 5-HT1B receptor antagonism: concentration–response curves for (A) bradykinin- and (B) isoprenaline-induced relaxation of 5-HT-constricted rings in the absence and presence of TRAM 34 (1 µM), SB216641 (100 nM) and TRAM 34 combined with SB216641. Results are the means ± SEM from four to five experiments (number of arteries from different animals).
Effect of mibefradil on the reduced relaxation to SNP seen in the presence of charybdotoxin
In artery rings pre-constricted with U46619 (30–50 nM), SNP-induced relaxation in the presence of charybdotoxin (100 nM) was unaffected by mibefradil (10 µM) (Figure 8).
Figure 8.
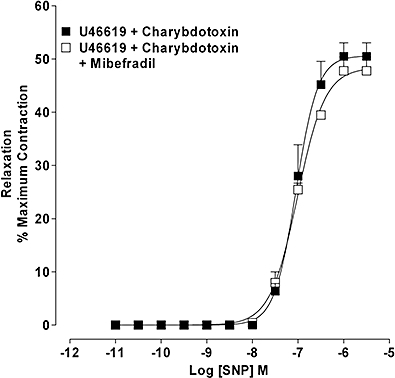
Effect of T-type voltage-operated calcium channel (VOCC) blockade on SNP-induced relaxation in the presence of BKCa blockade. Concentration–response curve for SNP-induced relaxation of U46619-constricted arteries in the presence of charybdotoxin (100 nM) in the absence and presence of mibefradil (10 µM). Results are the means ± SEM from four experiments (number of arteries from different animals).
Discussion
Based on the observations that the contractile response of BPA to 5-HT is sensitive to mibefradil (Alapati et al., 2007), a selective T-type VOCC blocker (Perez-Reyes, 2002), that blockade of BKCa in rat pulmonary artery, produced a mibefradil-sensitive potentiation of the contractile response to U46619 (McKenzie et al., 2009) and that relaxation by cyclic nucleotides is mediated, in part, by BKCa activation (Robertson et al., 1993; Archer et al., 1994; Schubert et al., 1999; White et al., 2000; Barman et al., 2003), the present study examined the possibility that a 5-HT receptor may inhibit BKCa, which contributes to the contractile response by both activating a T-type VOCC and impairing the inhibitor influence of cyclic nucleotides.
Previous attempts to investigate the role of the 5-HT1 receptor in BPA showed that a reduced sensitivity was associated with the mixed 5-HT1D/1B receptor antagonist GR127935 (Shaw et al., 2000). The present study confirmed this observation and demonstrated that selective antagonism of the 5-HT1B receptor but not the 5-HT1D receptor, reduced the tissue sensitivity. This indicates that activation of the 5-HT1B receptor increases the tissue sensitivity to 5-HT.
5-HT1B receptor and the mibefradil-sensitive contractile component of the 5-HT response
Both mibefradil and verapamil have previously been shown to produce a similar partial inhibition of the contractile response to 5-HT but, in combination, at concentrations that individually produce maximal effects, they markedly inhibit the contractile response to 5-HT (Alapati et al., 2007). As verapamil is a non-selective inhibitor of the L-type VOCC and as the contractile response is insensitive to nifedipine, an inhibitor of the high voltage-activated L-Type VOCC, it has been suggested that the inhibitory action of verapamil is mediated through inhibition of the nifedipine-insensitive medium voltage-activated L-Type VOCC (CaV 1.3) (Alapati et al., 2007). Mibefradil is reported to act as a selective blocker of each of the low voltage-activated T-type VOCCs (Perez-Reyes, 2002); however, recently it has been reported that metabolites of mibefradil block other non-T-type VOCC (Wu et al., 2000). The observation that NNC550396, a non-hydrolysable analogue of mibefradil (Huang et al., 2004), has a similar inhibitory profile to mibefradil suggests that the inhibitory action of mibefradil is mediated through inhibition of a T-type channel. These observations could indicate that 5-HT induces a membrane depolarization that is sufficient to activate low medium voltage-activated channels.
The observations that the tissue sensitivity to 5-HT was reduced to a similar extent by 5-HT1B receptor antagonists or mibefradil and that no additive effect was observed when these two agents were combined could indicate that inhibition of the T-type channel and antagonism of the 5-HT1B receptor have similar effects. This view is further supported by the observation that in the presence of verapamil, 5-HT1B receptor antagonism produced a marked inhibition of the contractile response, similar to the inhibition reported for mibefradil in the presence of verapamil (Alapati et al., 2007). Moreover, the observation that activation of the 5-HT1B receptor produces a mibefradil-sensitive potentiation of the U46619 contractile response indicates that this receptor is linked to increased conductance of a T-type calcium channel.
In rat pulmonary arteries charybdotoxin does not affect the basal tone but produces a mibefradil-sensitive potentiation of the U46619-induced contractile response (McKenzie et al., 2009). Charybdotoxin is reported to block BKCa and IKCa (Félétou and Vanhoutte, 2006), suggesting that inhibition of one or both channels underlies the activation of the T-type channel. In the present study charybdotoxin did not affect the basal tone of the BPA but, unlike TRAM 34, a selective IKCa blocker (Félétou and Vanhoutte, 2006), produced a mibefradil-sensitive potentiation of the U46619 concentration–response curve. These observations suggest that charybdotoxin-sensitive potassium channels, BKCa or possibly BKCa and IKCa, are normally active following TP receptor stimulation in rat and BPA and that inhibition of these channels is linked to increased conductance of the T-type calcium channel.
In contrast to U46619 the present study shows that charybdotoxin did not affect the concentration–response curve to 5-HT, which could indicate that charybdotoxin-sensitive channels are not normally activated by 5-HT. As antagonism of the 5-HT1B receptor however, reduced the tissue sensitivity to 5-HT and because this was prevented by charybdotoxin, this might indicate that charybdotoxin-sensitive K+ channels were activated when the 5-HT1B receptor was blocked, implying that activation of the 5-HT1B receptor normally inhibits conductance through these channels. This interpretation would explain why mibefradil, which normally reduces the tissue sensitivity to 5-HT to a similar extent as 5-HT1B receptor antagonism, has no inhibitory effect in the presence of the 5-HT1B receptor antagonist, unless charybdotoxin is also present. These observations suggest that 5-HT1B receptor activation couples to inhibition of BKCa, which in turn provides the depolarizing stimulus for activation of the mibefradil-sensitive T-type VOCC.
The 5-HT1B agonists RU24969 (Shaw et al., 2000) and CP3129 do not affect the basal tone of bovine pulmonary conventional arteries, which suggests that stimulation of the 5-HT1B receptor in these arteries does not directly induce a contractile response. Because charybdotoxin, in both rat pulmonary artery (McKenzie et al., 2009) and BPA, also does not affect the resting tone, it would seem that BKCa conductance does not greatly influence resting Em. This view is consistent with the observations that activation of BKCa follows stimulation of several contractile receptors. In rat pulmonary artery endothelin and noradrenaline (Salter and Kozlowski, 1995; Wang et al., 1997) and in rabbit pulmonary artery histamine and noradrenaline (Wang and Large, 1992) induce an outward calcium-activated charybdotoxin-sensitive current. In the present study, the contractile response to 5-HT is only sensitive to charybdotoxin following blockade of the 5-HT1B receptor, which indicates that activation of the 5-HT2A receptor is also linked to increased conductance of BKCa. This view is supported by the observation that the contractile response to the 5-HT2A agonist DOI is potentiated by charydotoxin. These observations suggest that the contractile response associated with activation of the 5-HT1B receptor is seen only in the presence of agonist receptors that activate BKCa and this may explain why the 5-HT1B receptor is often reported to be ‘silent’ requiring agonist-induced tone to contribute to contraction. The mechanism by which the 5-HT1B receptor inhibits BKCa is unclear; however, in human coronary arteries 5-HT is reported to inhibit BKCa via activation of the tyrosine kinase c-Src (Alioua et al., 2002).
In addition to stimulating BKCa several studies suggest that contractile agonists also stimulate ClCa (Salter and Kozlowski, 1995) and the observation in rat pulmonary artery that the charybdotoxin-induced, mibefradil-sensitive, potentiation of the U46619-induced contractile response is absent in the presence of chloride channel blockade (Mckenzie et al., 2009) suggests that reduced IBK together with the stimulated ICl underlies the depolarizing stimulus for T-type VOCC activation. The involvement of Cl- in the contractile response to U46619 contrasts with the contractile response to 5-HT, which is insensitive to chloride channel blockade or depletion of extracellular chloride (Alapati et al., 2007) and suggesting that stimulated ICl is not required for T-type VOCC activation by 5-HT.
5-HT1B receptor and cyclic nucleotide-mediated relaxation
Elevating the level of cyclic nucleotides in smooth muscle leads to relaxation mediated by multiple mechanisms. For example, cAMP, via A-Kinase, can phosphorylate and inhibit MLCK (Conti and Adelstein, 1981;Kerrick and Hoar, 1981) whereas cGMP, via G-kinase, can phosphorylate and activate MLCP (Wu et al., 1996; Lee et al., 1997), resulting in dephosphorylation of myosin and reduced interaction with actin. Both cyclic nucleotides can stimulate heat shock protein 20 (HSP20), which prevents interaction between actin and myosin without affecting myosin phosphorylation (Flynn et al., 2005). Cyclic nucleotides can also evoke a membrane hyperpolarization by phosphorylating and activating BKCa (Robertson et al., 1993; Archer et al., 1994; Fukao et al., 1999; Schubert et al., 1999; White et al., 2000; Barman et al., 2003) or specific BKCa subtypes (Zhou et al., 2001), KV (Zhao et al., 1997; 1998;) and cAMP can activate KATP (Quayle et al., 1997;Brayden, 2002)
In BPA pre-constricted with U46619, elevating cyclic nucleotides with agonists such as bradykinin (cGMP) and isoprenaline (cAMP), SNP, which activates guanylyl cyclase, or by inhibiting the breakdown of cyclic nucleotides with the phosphodiesterase inhibitors rolipram (type IV, cAMP) and zaprinast (type V, cGMP) produces full relaxation. In contrast, in arteries pre-constricted to a similar extent by 5-HT, the maximum relaxation produced by all of these agents is only around half of that seen in the U46619 constriction. The impaired relaxation is associated with activation of the 5-HT1B receptor because in arteries constricted by activating the 5-HT2A receptor alone with the selective 5-HT2A receptor agonist DOI or 5-HT in the presence of either the mixed 5-HT1D/1B receptor antagonist GR127935 or the 5-HT1B receptor antagonist SB216641, but not the 5-HT1D receptor antagonist BRL15572, these vasodilators induced full relaxation. That the impaired relaxation is linked to the 5-HT1B receptor is further supported by the observation that in U46619-constricted arteries the relaxation by bradykinin and isoprenaline could be markedly impaired by activating the 5-HT1B receptor with the non-selective 5-HT1 agonist 5-CT or the selective 5-HT1B agonist CP93129.
The concentration of 5-CT or CP93129 used in this study was maximal; higher concentration did not produce further inhibition of the relaxation; therefore, a component of the relaxation, approximately half, is insensitive to the 5-HT1B receptor. These observations show that activation of the 5-HT1B receptor impairs relaxation mediated through both cAMP-dependent and cGMP-dependent pathways to a similar extent. The mechanism by which the 5-HT1B receptor inhibits cyclic nucleotide-mediated relaxation is unclear. Because 5-HT1B receptor activation is associated with activation of a T-type VOCC, it is possible that the impaired relaxation is due to the contribution of this channel to the contraction. However, as relaxation of U46619-constricted rings by SNP in the presence of charybdotoxin was unaffected by mibefradil, this would indicate that the involvement of the T-type VOCC in the contraction is not the reason for the impaired relaxation. As the relaxation induced by bradykinin or isoprenaline of U46619- or 5-HT- (in the presence of 5-HT1B receptor antagonism) was reduced by approximately half by increasing [K]o to 25 mM and, as high [K]o did not affect the relaxation in 5-HT-constricted rings, this suggests that the 5-HT1B receptor-sensitive component is associated with a hyperpolarisation.
As the contractile studies indicate that the 5-HT1B receptor links to inhibition of BKCa and, as cGMP (Robertson et al., 1993; Archer et al., 1994; Peng et al., 1996; Saqueton et al., 1999) and cAMP (Soria, 1988; Minami et al., 1993; Schubert et al., 1999) via their respective kinases are reported to phosphorylate and stimulate conductance through BKCa, then the part of the cyclic nucleotide-mediated relaxation that is inhibited by 5-HT1B receptor activation could be explained by its ability to inhibit BKCa. This explanation is consistent with the findings for cGMP-dependent relaxation because the enhanced bradykinin-, SNP- and zaprinast-induced relaxation produced by antagonism of the 5-HT1B receptor is prevented completely by charybdotoxin, suggesting that it operates through increased conductance of BKCa. In contrast, however, the cAMP-mediated relaxation that is sensitive to the 5-HT1B receptor is only partly sensitive to charybdotoxin. Thus 5-HT1B receptor activation must inhibit cAMP-mediated relaxation by a mechanism(s) additional to inhibition of a charybdotoxin-sensitive BKCa.
In conclusion, the 5-HT1B receptor is implicated in the development of pulmonary hypertension (MacLean et al., 2000; Keegan et al., 2001; Wang et al., 2001) and may also have a role in systemic arterial hypertension (Banes and Watts, 2001). The present study shows that in bovine pulmonary conventional arteries, the 5-HT1B receptor did not exhibit direct contractile activity but in the presence of 5-HT2A receptor activation or TP receptor activation it coupled, by an unknown mechanism, to inhibition of BKCa, which increased the tissue sensitivity to the contractile receptor by activating a T-type VOCC and facilitated contractile responses by opposing physiological antagonism of the contractile response by cyclic nucleotides. While the impaired cGMP-mediated relaxation appeared to be fully explained by 5-HT1B receptor-linked inhibition of BKCa, this mechanism cannot fully explain the impaired cAMP-mediated relaxation, indicating that 5-HT1B receptors inhibit cAMP-mediated relaxation by a mechanism additional to inhibition of BKCa. In order to further dissect the effect of BKCa blockade on the activation of T-type channels, it would be necessary to provide direct evidence that a T-type channel is expressed at the mRNA level and to measure changes in K+ activity using electrophysiological techniques.
Glossary
Abbreviations:
- 4-AP
4-aminopyridine
- BKCa
large conductance voltage and calcium-activated potassium channel
- BRL15572
1-(3-chlorophenyl)-4-[3,3-diphenyl (2-(s,r)hydroxypropanyl) piperazine]hydrochloride
- ChTx
charybdotoxin
- GR127935
N-[4-methoxy-3-(4-methyl-1-piperazinyl-O-phenyl]2′-methyl-4′(5-methyl-1,2.4-oxadiazol-3-yl[1,1,-biphenyl]-4-carboxamide hydrochloride monohydrate
- CP93129
1,4-dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrro l[3,2-b]pyridin-5-one dihydrochloride)
- KATP
ATP-activated potassium channel
- Kv
voltage-activated potassium channel
- mibefradil
(1S,2S)-2-[2[[3-(2-benzimidazolylpropyl]methylamino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphthyl methoxyacetate dihydrochloride hydrate
- NS1619
1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-9trifluoromethyl)-2H-benzyimidazol-2-one
- NNC550396
(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphthyl cyclopropanecarboxylate dihydrochloride
- PNU37883
N-cyclohexyl-N′-tricyclo[3.3.1.13,7]dec-1-yl-4-morpholinecarboximidamine hydrochloride
- SB216641
N-[3-[3-(dimethylamino)ethoxy]-4-methoxyphenyl]-2′-meth yl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)-[1,1′-biphenyl]-4-carboxamide hydrochloride
- SNP
sodium nitroprusside
- TRAM 34
(1-[(2-chlorophenyl)diphenylmethyl]-1H pyrazole)
- U46619
9,11-dideoxy-9α, 11α-methanoepoxy prostaglandin F2α
Conflicts of interest
None.
References
- Alapati VR, McKenzie C, Blair A, Kenny D, MacDonald A, Shaw AM. Mechanisms of U46619- and 5-HT-induced contraction of bovine pulmonary arteries: role of chloride ions. Br J Pharmacol. 2007;151:1224–1234. doi: 10.1038/sj.bjp.0707338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels, 1st edition. Br J Pharmacol. 2008;153:S115. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Huang JMC, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol. 2003;286:L149–L155. doi: 10.1152/ajplung.00207.2003. [DOI] [PubMed] [Google Scholar]
- Banes AKL, Watts SW. Enhanced contraction to 5-hydroxytryptamine is not due to ‘unmasking’ of 5-hydroxytryptamine1B receptors in the mesenteric artery of the deoxycorticosterone acetate-salt rat. Hypertension. 2001;38:891–895. doi: 10.1161/hy1001.091779. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- Conti MA, Adelstein RS. The relationship between calmodulin binding and phosphorylation of smooth muscle kinase by the catalytic subunit of 3′:5′-cAMP dependent protein kinase. J Biol Chem. 1981;256:3178–3181. [PubMed] [Google Scholar]
- Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax. 1999;54:161–168. doi: 10.1136/thx.54.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF the Complete Story. New York: Taylor & Francis; 2006. [Google Scholar]
- Flynn CR, Brophy CM, Furnish EJ, Komalavilas P, Tessier D, Thresher J, et al. Transduction of phosphorylated heat shock-related protein 20, HSP20, prevents vasospasm of human umbilical artery smooth muscle. J Appl Physiol. 2005;98(5):1836–1845. doi: 10.1152/japplphysiol.01043.2004. [DOI] [PubMed] [Google Scholar]
- Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274(16):10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- Hervé P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Humphrey PPA. International Union of Pharmacology Classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, et al. NNC55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-,1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: A new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Weliman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, et al. Ca2+ channels, ryanodine receptors and Ca2+-activated K channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension-Converging evidence using 5-HT1B-receptor knockout mice and 5-HT1B/1D-receptor antagonist GR127935. Circ Res. 2001;89:1231–1239. doi: 10.1161/hh2401.100426. [DOI] [PubMed] [Google Scholar]
- Kerrick WG, Hoar PE. Inhibition of smooth muscle tension by cyclic AMP-dependent protein kinase. Nature. 1981;292:253–255. doi: 10.1038/292253a0. [DOI] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- MacLean MR. Pulmonary hypertension, anorexigens and 5-hydroxytryptamine: pharmacological Synergism in action? Trends Pharmacol Sci. 1999;20:490–495. doi: 10.1016/s0165-6147(99)01389-9. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, Adnot A. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie C, MacDonald A, Shaw AM. Mechanisms of U46619-induced contraction of rat pulmonary arteries in the presence and absence of the endothelium. Br J Pharmacol. 2009;157:581–596. doi: 10.1111/j.1476-5381.2008.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Fukuzawa K, Nakaya Y, Xeng XR, Inoue I. Mechanism of activation of Ca-activated K+ channel by cyclic AMP in cultured porcine coronary artery smooth muscle cells. Life Sci. 1993;53:1129–1135. doi: 10.1016/0024-3205(93)90549-i. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3:79–89. doi: 10.1124/mi.3.2.79. [DOI] [PubMed] [Google Scholar]
- Peng W, Karwande SV, Hoildal JR, Farrukh IS. Potassium currents in cultured human pulmonary arterial smooth muscle cells. J Appl Physiol. 1996;80:1187–1196. doi: 10.1152/jappl.1996.80.4.1187. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2002;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, et al. SB-216641 and BRL-15572—compounds to pharmacologically discriminate 5-HT1B and 5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and Inwardly Rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Salter KJ, Kozlowski RZ. Endothelin receptor coupling to potassium and chloride channels in isolated rat pulmonary arterial myocytes. J Pharmacol Exp Ther. 1995;279:1053–1062. [PubMed] [Google Scholar]
- Saqueton R, Noack T, Serebryakov VN. NO causes perinatal pulmonary vasodilation through K+ channel activation and intracellular calcium release. Am J Physiol. 1999;276:L925–L932. doi: 10.1152/ajplung.1999.276.6.L925. [DOI] [PubMed] [Google Scholar]
- Schubert R, Noark T, Serebryabov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- Shaw AM, Bunton DC, Brown T, Irvine J, MacDonald A. Regulation of sensitivity to 5-hydroxytryptamine in pulmonary supernumerary but not conventional arteries by a 5-HT1D-like receptor. Eur J Pharmacol. 2000;408:69–82. doi: 10.1016/s0014-2999(00)00757-3. [DOI] [PubMed] [Google Scholar]
- Skingle M, Beattie DT, Scopes DIC, Starkey SJ, Connor HE, Feniuk W, et al. GR127935; a potent and selective 5-HT1D receptor antagonist. Behav Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- Soria B. The biophysical basis of K channel pharmacology. In: Soria B, Ceva V, editors. Ion Channel Pharmacology. New York: Oxford University Press; 1988. pp. 167–185. [Google Scholar]
- Tracey A, Bunton D, Irvine J, MacDonald A, Shaw AM. Relaxation to bradykinin in bovine pulmonary superunerary arteries can be mediated by both a nitric oxide-dependent and -independent mechanism. Br J Pharmacol. 2002;137:538–544. doi: 10.1038/sj.bjp.0704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dong X, Zhang X, Xing J. 5-HT1B receptor augmented 5-HT vasoconstrictor response of pulmonary artery in monocrotaline-induced pulmonary hypertensive rats. Acta Pharmacol Sin. 2001;22(3):269–273. [PubMed] [Google Scholar]
- Wang Q, Large WA. Action of histamine on single smooth muscle cells dispersed from the rabbit pulmonary artery. J Physiol. 1992;468:125–139. doi: 10.1113/jphysiol.1993.sp019763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang Y-X, Yu M, Kotlikoff MI. Ca2+ -activated Cl– currents are activated by metabolic inhibition in rat pulmonary artery smooth muscle cells. Am J Physiol. 1997;273:C520–C530. doi: 10.1152/ajpcell.1997.273.2.C520. [DOI] [PubMed] [Google Scholar]
- White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ Res. 2000;86:897–905. doi: 10.1161/01.res.86.8.897. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhang M, Vest PA, Bhattacharjee A, Liu L, Li M. A mibefradil metabolite is a potent intracellular blocker of L-type Ca2+ currents in pancreatic β-cells. J Pharmacol Exp Ther. 2000;292:939–949. [PubMed] [Google Scholar]
- Wu X, Somlyo AV, Somlyo AP. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Com. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Wang J, Rubin LJ, Yuan XJ. Inhibition of K(V) and K(Ca) channels antagonizes NO-induced relaxation in pulmonary artery. Am J Physiol. 1997;272(2):H904–H912. doi: 10.1152/ajpheart.1997.272.2.H904. Pt 2. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Wang J, Rubin LJ, Yuan XJ. Roles of K+ and Cl- channels in cAMP-induced pulmonary vasodilation. Exp Lung Res. 1998;24(1):71–83. doi: 10.3109/01902149809046055. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, et al. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem. 2001;276(46):43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]


