Abstract
The human lung produces considerable amounts of H2O2. In the normal uninflamed epithelium of both the airways and the alveoli, mucosal release of H2O2 is readily detected both in cell cultures in vitro and in the exhaled breath of humans. The dual oxidases DUOX1 and DUOX2 are the H2O2-producing isoforms of the NADPH oxidase family found in epithelial cells. The DUOXs are prominently expressed at the apical cell pole of ciliated cells in the airways and in type II cells of the alveoli. Recent studies focused on the functional consequences of H2O2 release by DUOX into the lung lining fluid. In the airways, a major function of DUOX is to support lactoperoxidase (LPO) to generate bactericidal OSCN−, and there are indications that the DUOX/LPO defense system is critically dependent on the function of the CFTR Cl− channel, which provides both SCN− (for LPO function) and HCO3− (for pH adjustment) to the airway surface liquid. Although DUOX is also functional in the alveolar epithelium, no comparable heme peroxidase is present in the alveolus, and thus DUOX-mediated H2O2 release by alveolar cells may have other functions, such as cellular signaling. Antioxid. Redox Signal. 11, 2453–2465.
Introduction
The epithelium of the airways and the alveoli is continuously exposed to high levels of oxygen and, at the same time, epithelial cells express mechanisms to generate reactive oxygen species (ROS). For a long time, the release of ROS by intracellular mechanisms (such as the mitochondrial electron transport chain) was considered to cause oxidative stress and pathology in lung tissue (48, 90). With the identification of the NADPH oxidase (NOX) family of membrane proteins that allow for a regulated and measured production of superoxide (O2•−) and H2O2, the physiological release of H2O2 at the mucosa of lung epithelial cells was recognized and the DUOX isoforms of the NOX family were found to be expressed and functional at the apical membrane of both airway and alveolar epithelial cells (37, 43, 98).
The epithelia found in the lung are tissues of innate defense, and in this review I will focus largely on the innate defense aspect of DUOX biology. At first the structure and cellular composition of the epithelia found in the lung is introduced to highlight their different functions. Then H2O2 release by the human lung is reviewed to estimate the relation of H2O2 produced by epithelial cells compared to leukocytes. Finally, the cellular role, regulation, and mechanisms of DUOX function are reviewed. Current hypotheses of the role of DUOX in innate defense and its dependence on CFTR function are discussed. Other cellular functions of DUOX in signaling and maintenance of barrier function have been reviewed recently by van der Vliet (113).
Structure of the Airway and Alveolar Epithelium
In the classic model of human lung anatomy (118), the lung consists of 24 generations of dichotomously branching airway tubes where each airway divides to form two smaller airways [although variations to this scheme have been reported (54)]. Starting at the trachea (generation 0) and terminating at the alveolar sac (generation 23), the function and cellular composition of the epithelium that lines the lung changes continuously. From the trachea to approximately generation 10 (dependent on the anatomical location within the lung) the airways are called bronchi and are lined with a moderately-tight [∼300–400 Ω·cm2, (122)] pseudo-stratified surface epithelium. Epithelial tightness is a functional feature that determines the ability of the epithelium to maintain concentration gradients between the airway surface liquid (ASL, the thin fluid film that lines the airways) and the serosa. The airway surface epithelium consists of three major cell types: (a) ciliated cells, (b) mucus-producing goblet cells, and (c) small basal cells found between them on the basal membrane (Fig. 1). Basal cells are considered to be the progenitor cells for the surface epithelium (53). Ciliated cells account for 60–80% of the mucosal surface (64). The thickness of the proximal epithelium is 10–50 μm (decreasing with increasing generation of branching) and its major function is to conduct air, filter particles, and kill microbes. The mucosal surface of the airways is lined with a thin fluid film that consist of the periciliary layer in which the cilia beat, and an overlying mucus blanket of variable height (dependent on the amount of mucus produced). In normal airways the volume of the ASL is ∼1 μl/cm2 (120). The small volume is a determinant of the concentrations of defense factors secreted by the epithelium into this fluid film.
FIG. 1.
Overview of human airways. The proximal airways (trachea, bronchi, conducting bronchioles) are lined with a ciliated surface epithelium whose main surface cells are ciliated, goblet, and basal cells. The function of the proximal airways is to conduct gases and filter particles. There is ∼1 gland per square millimeter surface epithelium. The distal respiratory bronchioles are characterized by numerous alveoli, whose epithelium consists of type I and type II alveolar cells. The major function of the alveoli is gas exchange.
Seromucous glands are found all along the trachea and bronchi at a density of ∼1 per square millimeter epithelial area (15, 110). Glands contain serous tubules and acini that secrete salt, water, mucus, and antimicrobial factors (8, 9). Although the surface epithelium also secretes water and mucus, the volume of gland secretions (123) exceeds that of the surface epithelium (57) ∼10-fold and, correspondingly, most protein content of the ASL originates from glands.
The number and size of glands declines with airway generations, and they disappear completely at approximately generation 10, after which the airways are called conducting bronchioles up to generation 16. From generation 17 on, the airways become respiratory bronchioles with an increasing number of alveoli budding off, until generation 23, where the airways terminate in an alveolar sac (diameter roughly 0.25 mm). The alveoli are the site of gas exchange. In contrast to the epithelium of the upper airways, the alveolar epithelium is very tight [transepithelial resistances are >2000 Ω·cm2 (37, 70)] and thus it can maintain large concentration gradients between the lumen and the serosa. It is composed of large but thin (0.05‱0.2 μm) type I cells (20, 45), and small cuboidal type II cells (Fig. 1), which are characterized by lamellar bodies as the storage organelle of surfactant. Alveoli are lined with an extremely thin fluid film with a volume of ∼10–20 nl/cm2 and a corresponding height of 0.1–0.2 μm. This is by far the thinnest fluid film on any epithelium and as a result, factors secreted by alveolar cells result in high luminal concentrations.
Leukocytes are continuously present in the airspaces. In normal uninflamed lungs the vast majority are macrophages. For example, in bronchoalveolar lavage fluid obtained from healthy subjects, a recent typical study found 94% macrophages, 4% lymphocytes, 1% neutrophils, and 0.7% eosinophils (104). Macrophages are mainly found in the alveolar region [∼2 macrophages per alveolus (20)] and represent the major phagocytic defense against infectious agents that escape the defenses of the conducting airways.
The lung is an organ of innate defense. Owing to the lung's prominently large and well-ventilated surface, the epithelium continuously encounters a vast array of infectious particles. All sections of the airways and the alveoli express a number of parallel, partially redundant, and functionally unrelated defense mechanisms. These include mechanical clearance by the mucociliary escalator, resident phagocytic immune cells, and a number of diverse factors that are secreted by the epithelium into the lung lining fluid (for review see refs. 41, 74, 95, 106). Oxidative processes based on the DUOX/lactoperoxidase (LPO) system have only recently been recognized as a defense factor in the airways. Its importance in comparison to the earlier described lung defenses are currently under investigation. It was found that a disruption of the DUOX/LPO system resulted in inhibition of bacterial killing by cultured airway epithelia (17, 78), suggesting a significant role of the DUOX/LPO mechanism in airway defenses.
The Lung Continuously Generates and Releases H2O2
In vivo measurements of H2O2 release
Oxygen has been considered as a pulmonary toxin for over a century (101). The identification of superoxide metabolism in mammalian cells (73) and the eventual recognition of superoxide dismutase activity in the lung (19) provided indications of in vivo generation of O2•− and dismutation to H2O2 in lung tissue. Initially, phagocytic white blood cells in the lung parenchyma were considered as the major source of H2O2 production in the lung, however, with the identification of the NADPH oxidase gene family, a number of other lung cells, and in particular epithelial cells, were identified as vigorous producers of H2O2. Although excessive production of oxidants is detrimental to tissues, the regulated release of H2O2 by lung cells is implicated in normal cellular physiology. Owing to the primary function of lung epithelial cells in innate defense, the observed production of H2O2 by lung cells was initially largely considered as related to lung defenses (43). However, additional roles in cellular signaling and barrier function have been considered recently (99, 119).
In recent years, the release of H2O2 by the human lung has been identified by measuring the H2O2 content of exhaled breath condensate. Initial studies focused on inflammatory airway diseases but with improvements in the technique, H2O2 release from normal uninflamed lungs was also detected. In the first study of its kind, Baldwin et al. (7) measured H2O2 in the exhaled breath of patients with acute respiratory distress syndrome (ARDS), a severe condition characterized by diffuse inflammation of the lung parenchyma, neutrophil invasion, and massive release of inflammatory mediators. Patients with ARDS were found in this and similar studies to exhale high levels of H2O2 (Table 1) and in later studies with improved sampling and detection, uninflamed controls were found to exhale low but measurable levels of H2O2 of ∼95 nM (Table 1) (61). These initial studies established that normal lungs generate low amounts of H2O2 and inflammation greatly increased the H2O2 concentration in exhaled breath.
Table 1.
H2O2 Concentrations in Exhaled Breath Condensate
| Condition | Control H2O2 (nM) | Disease H2O2 (nM) | Reference |
|---|---|---|---|
| ARDS | |||
| 1,680 | 7 | ||
| ND | 2,400 | 107 | |
| 95 | 552 | 61 | |
| Asthma | |||
| 1,140 | 1 | ||
| 192/2381 | 29 | ||
| 200 | 670 | 68 | |
| 10 | 260 | 4 | |
| 270 | 720 | 55 | |
| 24 | 127 | 35 | |
| 30 | 540 | 3 | |
| 250 | 810 | 28 | |
| COPD | |||
| 120 | 500 | 21 | |
| 29 | 205/6002 | 27 | |
| 410 | 60 | ||
| 50 | 480 | 81 | |
| 320/420 | 112 | ||
| 2903 | 280 (ns) | 111 | |
| CF | |||
| 280/1604 | 58 | ||
| 89 | 64 (ns) | 51 | |
| Bronchiectasis | |||
| 260 | 870 | 69 | |
| 300 | 800/1,6005 | 67 | |
| Smoking | |||
| 190 | 410 | 80 | |
| 50 | 240 | 79 | |
| Common cold | |||
| 90 | 200 | 59 | |
| Pneumonia | |||
| 160 | 590 | 72 |
Data are in nM for control and disease group of studies cited in the reference column. Some studies had no control group. Superscript numbers indicate additional disease or confounding factors: 1, allergic asthmatics; 2, acute exacerbation; 3, control group included smokers; 4, on IV antibiotics; 5, Pseudomonas-infected. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis; ND, not detected; ns, not significantly different from respective control.
Owing to the potential use of H2O2 as a marker of airway inflammation, H2O2 was measured in exhaled breath in several inflammatory airway diseases (Table 1). Most studies found that inflammation of the airways [as in asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, and others] was associated with increases in levels of H2O2 in the exhaled breath condensate. Normal controls exhaled ∼90 nM H2O2 (median of Table 1 controls), and ranged from undetectable to 300 nM. The relatively large variability between control groups of individual studies may be related to a number of previously described confounding factors of this technique, including circadian periodicity of exhaled levels of H2O2 (80, 111), the effect of flow rate during breath collection on the measured H2O2 concentration (94), differences between H2O2 levels exhaled by males and females (79), or technical differences in condensate processing. In most studies the disease group exhaled 4–5 times higher levels of H2O2 (∼420 nM, median of disease group in Table 1). It was found that the level of inflammation, the white blood cell count, and disease exacerbation correlated positively with exhaled breath H2O2 (27, 68, 72), suggesting a significant role of invading leukocytes in H2O2 production during airway inflammation.
Although it is reasonable to assume that the number of leukocytes correlates with exhaled H2O2 levels, this was not found in studies in cystic fibrosis (CF) patients, despite the fact that CF lung disease is characterized by neutrophil invasion of the airways. This puzzling observation may be related to an inhibition of DUOX-mediated H2O2 production in CF airways by an acidic airway pH, as discussed below (see Lack of bacterial killing in CF lungs).
Cell type-specific generation of H2O2
The cellular source of H2O2 in the normal uninflamed lung has been a matter of debate for many years. The presence of resident macrophages in the alveolar spaces of normal lungs and the parallel increase of white blood cell counts with exhaled H2O2 indicated white blood cells as the source of a large fraction of the H2O2 released by the lung. Table 2 compiles studies where H2O2 release by different lung cells was measured. The results are grouped by species (human, rat, guinea pig, and human cell lines) and by the major cell or tissue type (macrophages, alveolar type II cells, and airway epithelial cells). In the first study of lung epithelial H2O2 production, Kinnula et al. (63) used freshly isolated rat alveolar type II cells. When investigating the release of H2O2 by type II cells in suspension, a continuous rate of 3.2 fmole·h−1·cell−1 was found, which was much less than the rates found in macrophages obtained from the same preparation (66 fmole·h−1·cell−1). However, it was noted that alveolar type II cells produced H2O2 continuously, while H2O2 release from macrophages was transient, peaked at ∼15 min and then decayed (63). Later studies on rat or guinea pig type II cells (114, 116) found somewhat lower rates of H2O2 release (Table 2), which may have been related to improved cell isolation techniques with fewer contaminating macrophages. Further studies focused on the polarized release of type II cells into the apical medium. When using polarized tight cultures of a relatively pure human primary type II cell preparation that included culture conditions to remove contaminating cell types, a rate of 0.2 fmole·h−1·cell−1 of H2O2 release into the apical medium was found (37). This rate is relatively small compared to previous measurements, possibly because only the apical, blocker-sensitive fraction of H2O2 release was determined, while previous studies measured the total (including the serosal) H2O2 release. Nevertheless, these studies established the ability of alveolar cells to continuously secrete H2O2 into the alveolar lining fluid suggesting that the H2O2 found in exhaled breath of normal subjects at least partially originates from alveolar epithelial cells.
Table 2.
H2O2 Production by Lung Cells
| Species Cell type | Rate of H2O2 release (fmole·cell−1·h−1) | Reference |
|---|---|---|
| Human | ||
| Macrophage | 14 | 47 |
| Type II cell | 0.2 | 37 |
| Airway epithelium | 1.5/12* | 78 |
| Airway epithelium | 0.2–1.0 | 119 |
| Airway epithelium | 0.014/0.1* | 88 |
| Airway epithelium | 2.5 | # |
| Airway epithelium | 0/4.3* | 83 |
| Rat | ||
| Macrophage | 66 | 63 |
| Type II cell | 3.2 | 63 |
| Type II cell | 0.9 | 114 |
| Type II cell | 0.7 | 86 |
| Airway epithelium | 7.8/27* | 78 |
| Guinea pig | ||
| Macrophage | 4.3 | 116 |
| Type II cell | 1.2 | 116 |
| Airway epithelium | 1.3 | 62 |
| Human lung cell lines | ||
| CFBE41o- | 0.25 | 96 |
| JME/CF15 | 0.24 | 97 |
| Calu-3 | 0.11 | # |
| NCI-H292 | 0/2.1* | 83 |
| A549 | 0/0.12* | 83 |
Rates of H2O2 release from lung cells are expressed on a per-cell basis in fmole·cell−1·h−1. When values in the referenced publication were reported per cm2 epithelial area, then 106 cells per cm2 were assumed; when values were reported per amount of DNA, then 6 pg DNA per cell was assumed; when values were reported per amount of protein, then 0.15 pg/μm3 and a volume of ciliated cells of (5,000 μm3), macrophages (rat, 1,150 μm3; guinea pig, 1,500 μm3; human, 4200 μm3), and type II cells (500 μm3) was assumed. *rate after stimulation with Ca2+-agonist (ATP, ionomycin, or thapsigargin).
#H. Fischer, unpublished.
A549, human type II cell-like; Calu-3, human serous gland cell-like; CFBE41o-, human bronchial epithelial origin of CF genotype; JME/CF15, human nasal epithelial origin of CF genotype; NCI-H292, human lung epitheloid.
The airway epithelium continuously releases mucosal H2O2 and rates of release tend to be higher than those found in alveolar preparations (Table 2). In several studies it was shown that H2O2 release could be stimulated by Ca2+-agonists resulting in rates of up to 4.3 fmole·cell−1·h−1 in human cells and even higher in rat airway cultures (Table 2). For comparison, Table 2 also lists several human lung epithelial cell lines that are in common use in research studies.
Functional Characteristics of DUOX
The two large NOX homologues DUOX1 and DUOX2 were originally identified and cloned from the epithelium of the thyroid gland (11, 23, 31). Further investigations found that the DUOXs are widely expressed in epithelial tissues, including epithelia of the airways (39, 43, 98), alveoli (37), intestinal tract (34, 43), salivary glands (43), and prostate (117).
Human DUOX1 and DUOX2 are highly similar transmembrane proteins (77% identical and 83% similar) that have a predicted domain structure as shown in Fig. 2A. DUOX is comprised of a gp91phox homology domain typical for all members of the NOX family, intracellular EF hand-type Ca2+ binding pockets, and an extracellular domain that is not found in other NOXs in humans (Fig. 2A). The gp91phox homology domain (ranging from transmembrane domain 2 to the C terminus) contains the electron (e−) transport machinery that is found in all NOX family members that includes binding sites for intracellular NADPH and FAD, and two hemes in the transmembrane region. One cycle of NADPH oxidation releases two electrons and two protons that are both used to finally generate one extracellular H2O2. The electrons are passed from the primary e− donor NADPH [with a midpoint potential −320 mV, as determined for NOX2, (25)] along its electrochemical gradient to FAD, the two hemes, and to oxygen (midpoint potential −160 mV). This large electrochemical gradient from NADPH to O2 of 160 mV indicates that e− flux is always outward (see also discussion in ref. 25), resulting in the generation of extracellular O2•− and H2O2.
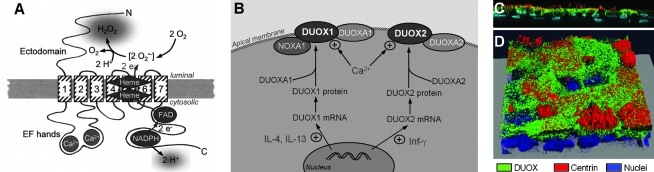
FIG. 2.
DUOX in the plasma membrane. (A) Model of DUOX in the membrane. The transmembrane domains are numbered 1 to 7. The NOX-typical gp91phox homology domain stretches from transmembrane domain 2 to the C terminus and contains the electron (e−) transport chain, including binding sites for NADPH, FAD, and two hemes. O2•− forms as an intermediate and is internally dismutated to the final release product H2O2. DUOX releases cytosolic H+ during NADPH oxidation. Two intracellular EF hands bind Ca2+, and an ectodomain of unclear function might be involved in H2O2 metabolism. The products of DUOX function are extracellular H2O2 and intracellular H+. (B) Cellular regulation of DUOX1 and DUOX2 and currently identified interacting proteins. DUOX1 is specifically upregulated by IL-4 and IL-13 by ∼4-fold, while DUOX2 expression is highly induced by 20-fold by treatment of cells with interferon-γ [Inf-γ, (50)]. Both DUOXs interact with their respective ER-resident chaperone DUOXA1 and DUOXA2 (46), and the DUOXA proteins may be present and functional in the plasma membrane (76). In the apical membrane the function of the DUOXs is upregulated by intracellular Ca2+. NOXA1 has been shown to interact with and inhibit DUOXA1 in the plasma membrane in a Ca2+-dependent fashion (83). Circled + indicates stimulatory effect. (C and D) DUOX protein localizes to the apical pole of airways. Primary human airway epithelial culture was stained for DUOX (green), centrin (red), and nuclei (blue), as described (98). (C) Side view with apical aspect pointing up. DUOX is localized at the apical pole of ciliated, centrin-labeled cells. Nuclei at the bottom of the image are from basal cells, which do not stain for DUOX. (D) 3-D reconstructed confocal image stack at angled view onto the mucosa. Cells that do not stain for centrin (nonciliated cells, likely goblet cells) also do not stain for DUOX (Images in C and D by J. Tseng and H. Fischer, DUOX antibody kindly provided by F. Miot, centrin 20H5 antibody kindly provided by J.L. Salisbury.) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The primary product of the small NOX homologs is O2•−, however, natively expressed DUOX has consistently been found to release H2O2, and not O2•− (22, 37, 39, 43). Intermediate O2•− formation could be shown during H2O2 generation of DUOX-expressing thyroid membrane fractions, suggesting an internal dismutation reaction to H2O2. Ameziane–El-Hassani et al. (2) investigated the role of the large ectodomain (that comprises residues 1‱583 in DUOX1 and 1‱596 in DUOX2) in H2O2 production. Experiments using mutations or partial deletions in the ectodomain of DUOX2 suggested a function of the ectodomain in the dismutation reaction (2). On the other hand, owing to the structural similarity of the ectodomain to other heme peroxidases, it had been proposed earlier that the ectodomain functions as an H2O2-utilizing peroxidase, hence the “dual oxidase” (DUOX) nomenclature (32). However, Donko et al. (30) argued that this domain lacks significant residues necessary for heme binding, and no detectable peroxidase activity could be found in an otherwise functional recombinant DUOX expression system (76). Currently, the role of the ectodomain of DUOX is not well understood, but it may be involved in H2O2 generation (2, 30, 76) or in H2O2 utilization (32,49).
Initial attempts to reconstitute functional DUOX in recombinant expression systems resulted in ER retention of immature DUOX. It was proposed early on that DUOX forms complexes or interacts with other protein factors (117). Expression of functional DUOX1 and DUOX2 in the plasma membrane was then found to be critically dependent on the respective ER-resident maturation factors DUOXA1 and DUOXA2 (46), which support ER-to-Golgi transfer of DUOX1 and DUOX2 (Fig. 2B). In addition it was recently shown that the respective DUOX/DUOXA complexes are present and form stable functional complexes in the plasma membrane (76). This observation is supported by the finding of a parallel upregulation of expression of DUOX and DUOXA in native lung epithelial cells (37). On the other hand, when DUOX was recombinantly expressed at increasing levels while maintaining a constant and comparably low expression of recombinant DUOXA, Rigutto et al. (92) found that H2O2 production increased with increasing DUOX expression at the plasma membrane, which argues against a simple 1:1 DUOX/DUOXA plasma membrane complex.
Other proteins have also been described to functionally interact with DUOX. p22phox and EF-hand binding protein 1 (EFP1) were found to interact with DUOX but without a measurable functional consequence (117), and both p22phox and EFP1 are expressed in airway epithelium (98, and C. Schwarzer and H. Fischer, unpublished). In addition, NOXA1 [a regulatory subunit of the NOX1 complex (42)] was found to be expressed in airways and to interact and inhibit DUOX1 function (83). The DUOX1/NOXA1 complex was localized in the plasma membrane and could be dissociated in presence of high Ca2+ concentrations (83), indicating a role for NOXA1 in the Ca2+-dependent regulation of DUOX1 (Fig. 2B). These reports suggest that DUOX associates with other proteins and forms functional complexes both during maturation and trafficking, and in the plasma membrane.
Expression and Function of DUOX in Lung Epithelium
Airway surface epithelium
Cell type-specific expression
Polarized epithelial airway cultures express DUOX at the apical cell pole of ciliated cells (98). Other epithelial cell types (mainly basal and goblet cells) have not been investigated in detail, however, we found recently that neither nonciliated cells nor basal cells express DUOX at a level that can be detected using immunocytochemistry. Figure 2C and D show a side view (Fig. 2C) and a 3D reconstruction of a confocal image stack (Fig. 2D) of a confluent primary airway epithelial culture with the apical aspect pointing up. Culture was immunostained for DUOX (green), centrin (red, a marker for ciliated cells), and nuclei (blue). This culture contains ciliated cells (identified by centrin staining), nonciliated cells (likely goblet cells), and basal cells (identified by the nuclei at the base of the culture visible in Fig. 2C). DUOX appears to be expressed only in ciliated surface cells, but not in nonciliated cells nor in basal cells. Interestingly, goblet cells (and also glands) are the sites for LPO release (18), indicating that H2O2 production (from ciliated cells) and H2O2 utilization by LPO (from goblet cells and glands) are physically separated, although the reason for this is currently unclear.
Basal cells are generally considered as the progenitor stem cells of the airway epithelium (53). There is no appreciable expression of DUOX in basal cells, suggesting that during maturation from the basal to the ciliated phenotype, expression of DUOX is induced. For comparison, in fetal alveolar type II cells, the expression of DUOX1 is developmentally regulated during maturation to an adult phenotype in vivo, and also in vitro during hormone-induced maturation (37). Similarly, in vitro airway epithelial cultures increase the amount of DUOX expressed over time in culture (88). Although these observations may be related to both increased expression of DUOX per cell and an increase in the number of ciliated cells in the culture, the sum of these observations indicates a role for increased expression of DUOX during epithelial maturation.
The observation that only ciliated (and not goblet) cells express DUOX likely affects the bactericidal activity of the airways in inflammatory airway diseases. For example, in CF, asthma, and chronic bronchitis, the airway epithelium undergoes histological changes to a de-differentiated squamous or mucous phenotype, resulting in a loss of ciliated cells. At the same time these airway diseases are characterized by poor bacterial killing by the airway epithelium and chronic airway infections. Since DUOX expression and function has been strongly indicated in airway defense (78), a reduction in ciliated cells in a de-differentiated phenotype is expected to deprive the epithelium of an important defense mechanism.
Regulation of DUOX by intracellular Ca2+ in airways
The airway epithelium releases H2O2 continuously and its release is further stimulated by mediators that increase intracellular Ca2+ concentrations (Cai). A first indication for the Ca2+-dependence of H2O2 release was noticed by Kinnula et al. (62) when performing experiments in isolated airway cells in Ca2+/Mg2+ free experiments. Cai agonists, such as ATP, thapsigargin, or ionomycin are now widely used to activate H2O2 release from airway cultures (39, 43, 78, 83). Two mechanisms have been identified that mediate Cai-dependent regulation of DUOX function: (a) the intracellular EF hand Ca2+ binding sites, and (b) Cai-dependent binding of NOXA1 to DUOX1 (Fig. 2A). Site-directed mutation of the predicted Ca2+-binding glutamates to glutamine in the EF hands of DUOX1 (E839Q and E875Q) or DUOX2 (E843Q and E879Q) resulted in an almost total loss of function by the recombinantly expressed mutants (92). In addition, the regulation of DUOX by Cai was modified by phosphorylation of DUOX by protein kinases A and C. However, Ca2+ and intact EF hands were required for kinase-dependent regulation (92), indicating that Ca2+ binding to DUOX is necessary and sufficient for H2O2 production. In addition, NOXA1 was recently shown to interact with and inhibit DUOX1 in a Ca2+-dependent fashion (Fig. 2B) possibly involving the C terminal region of DUOX1 (83). Whether the EF hands of DUOX1 are involved in the Ca2+-regulated NOXA1/DUOX1 interaction is unclear. An analysis of the Ca2+-dependence of these mechanisms, such as the determination of the role of the EF hands in the NOXA1/DUOX1 interaction, could help to functionally dissect these mechanisms.
On the other hand, the regulation of the Cai signal has been extensively studied in airways. Physiological stimuli of airway surface cells are ATP and acetylcholine, which are released in an autocrine fashion, and are in turn stimulated by mucosal stressors such as mechanical or chemical stimulation (84). Airway epithelial cells express purinergic receptors for extracellular ATP on both their apical and the basolateral membranes. The Cai signal evoked by addition of apical or basolateral ATP was shown to remain highly localized to the respective cell pole (84, 91). Thus, stimulation of the activity of apically localized DUOX is likely mediated by an apical stimulus. Another typical characteristic of the Cai signal is its short-lived and transient nature (∼2 min), although a sustained, slightly elevated intracellular Cai remains present during agonist stimulation (84, 91). Whether the physiologically obtained Cai are sufficient to maintain elevated H2O2 release by DUOX, or whether H2O2 release physiologically follows similar short-lived transients have not been investigated.
Are there distinct roles for DUOX1 and DUOX2 in airways?
Despite the high structural similarity of the two DUOX isoforms, it was found that the H2O2 production by DUOX2 is larger compared to DUOX1 in cell-free systems [∼5X higher (2)] or in intact cells after recombinant expression [∼2X higher, (92)]. However, based on semiquantitative RT-PCR measurements, the normal airway epithelium expresses DUOX1 at ∼5X higher levels than DUOX2 (50, 98), suggesting that the release of H2O2 by DUOX1 and DUOX2 might be similar in normal airways.
However, the level of expression of DUOX1 and DUOX2 is selectively regulated by cytokines (Fig. 2B). In a seminal paper, Harper and colleagues (50) investigated the regulation of gene expression by inflammatory cytokines and found that DUOX1 mRNA was specifically but moderately increased (by approximately fourfold) by the Th2 cytokines IL-4 and IL-13, while DUOX2 mRNA levels were upregulated by the Th1 cytokine interferon-γ by ∼20-fold. The authors suggested that the highly inducible DUOX2 is involved in responses to infection and inflammation, while the more steady expression of DUOX1 suggested a constitutive role in normal, noninflamed airways, including defense, signaling (119), and mucus production (99).
Defense function of DUOX in the airways
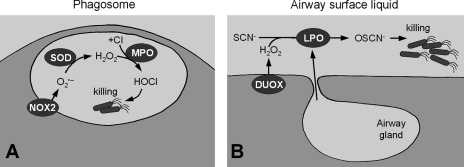
The fact that NOX2 is involved in bacterial killing and that the primary function of the airway epithelium is innate defense indicated that DUOX in the airways is part of a defense mechanism. A model of bacterial killing at the airway surface is now widely considered that is similar to the mechanism of NOX2-based killing in phagocytes (65) (Fig. 3A). There, the NOX2-based NADPH oxidase generates O2•− inside the phagosome, which is dismutated to H2O2 (by phagosomal SOD) and further converted to bactericidal HOCl by myeloperoxidase (MPO) (Fig. 3A) (26,105). A similar oxidative process was proposed for the airway epithelium by Conner and colleagues who initially identified lactoperoxidase (LPO) in sheep airway secretions (44) where LPO was found to comprise ∼1% of soluble airway protein content (18).
FIG. 3.
Similar mechanisms of innate defense in phagocytes and the airways. (A) In phagocytic cells, the NOX2 complex produces O2•− inside the phagosome, which is dismutated to H2O2 by superoxide dismutase (SOD) and further reduced to bactericidal HOCl by myeloperoxidase (MPO). (B) In the airways, DUOX of the surface epithelium releases H2O2 into the airway surface liquid and lactoperoxidase (LPO), secreted mainly by submucosal glands (but also by surface goblet cells), produces bactericidal OSCN−.
LPO is one of the three closely related, heme-containing peroxidases that produce bactericidal ROS (the others are MPO and eosinophil peroxidase). LPO is frequently found in glandular secretions (e.g., in saliva or in milk). In the airways, LPO has been localized to the submucosal glands and to goblet cells (18, 44). The initially proposed model of LPO-mediated killing lacked a verified source of H2O2 (18, 33, 121), but once DUOX was found to be expressed in airways and to release H2O2 into the ASL, a model for an antibacterial mechanism based on the DUOX/LPO system was proposed (39, 43). It is based on the observations that normal, uninflamed airways continuously release H2O2 into the ASL where LPO generates antibacterial OSCN− from H2O2 and SCN− (Fig. 3B). Note that H2O2 by itself shows little antibacterial activity (89). The SCN− concentration was recently identified as the rate-limiting factor during generation of OSCN− (17). SCN− concentrations in normal airway secretions (∼460 μM, ref. 121) were determined to be sufficient to maintain a continuous LPO activity in the airways. A key functional difference between the airway DUOX/LPO system and the phagocytic NOX2/MPO system is that the system in phagocytes is active only during the respiratory burst (lifetime ∼2 min, ref. 24) whereas DUOX generates H2O2 continuously.
Despite the presence of LPO in the ASL, normal subjects continuously exhale H2O2. This suggests that the production of H2O2 by the airways overwhelms the available LPO activity. However, when assuming that one ciliated airway cell generates 2.5 fmole H2O2 per hour (Table 2) and there are ∼2 × 109 ciliated cells in a lung, then the airways produce H2O2 at a stunning rate of ∼5 μmole/h. In comparison, the H2O2 concentration found in the exhaled breath is 90 nM (Table 1) and is exhaled at a rate of ∼0.7 nmole/h (94), indicating that only a very small fraction of the produced H2O2 escapes the airways. Although this is a rough estimate that does not consider resident white blood cells (which increases this rate) or buffering by other antioxidants (which decreases the final concentrations), this suggests that almost all of the produced H2O2 is utilized by the airways, likely by LPO, and only a minute amount is exhaled.
Lack of bacterial killing in CF lungs
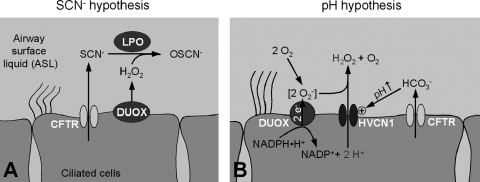
CF is caused by mutations in the CFTR Cl− channel, resulting in dysfunction or total lack of CFTR in the plasma membrane. Normal CFTR is expressed in the apical membrane of airway epithelial cells. Mutated CFTR affects the function of various organs, but the major clinical problem is the chronic infection of the airways owing to a reduced ability to kill bacteria at the airway surface. Although a number of mechanisms have been proposed for the airway disease in CF (115), a causal relation of CFTR to an innate defense process in the airways has been lacking. Currently there are two mechanistic hypotheses that require normal CFTR function for the DUOX/LPO system in the airways to work properly (Fig. 4). Both are based on the nonselective conduction of small anions by CFTR: first, the SCN− hypothesis is based on CFTR's conduction of SCN−, which is necessary for the generation of bactericidal OSCN− in the ASL (Fig. 4A). Second, the pH hypothesis is based on CFTR's ability to conduct HCO3− and alkalinize the pH of the ASL, which affects the generation of H2O2 by DUOX (Fig. 4B). Both proposed mechanisms are not mutually exclusive and can be expected to operate in parallel.
FIG. 4.
Proposed relations of CFTR to DUOX function. (A) SCN− hypothesis. CFTR as an SCN− conductor is required to supply the DUOX/LPO system with SCN− for the conversion into bactericidal OSCN−. Availability of SCN− is the rate-limiting factor indicating that this system is inhibited in CF (17, 78). (B) pH hypothesis. CFTR as a HCO3− conductor is required to alkalinize the pH of the ASL to allow the HVCN1 H+ channel to release intracellular H+ produced by DUOX from NADPH. Stoichiometry is given for one NADPH oxidation cycle resulting in two intracellular H+ to generate one extracellular H2O2. Note, however, that HVCN1 does not discriminate between H+ of different intracellular origin and has been found to conduct a substantially higher H+ flux than expected from NADPH oxidation alone (98). Since both H+ and HCO3− are driven by the pH gradient, H+ flux only occurs into an alkaline ASL, while HCO3− is released into an acidic ASL (crossover point is approximately at pH6.9, H. Fischer, unpublished). Circled + indicates activation.
The basis for the SCN− hypothesis was introduced into the airway field by Conner and colleagues (18) and proposes that SCN− transport by CFTR is necessary to maintain LPO function and bacterial killing (Fig. 4A). It is based on the observations that the concentration of SCN− in the ASL is a rate-limiting factor for the DUOX/LPO system and that LPO is specific for SCN− (17). Thus, a continuous source of SCN− is required to maintain the SCN− concentration in the ASL necessary for LPO function.
Previously, CFTR was shown to conduct SCN− fairly well at a rate of ∼60% of that found for Cl− (56, 66), which made CFTR a good candidate to support SCN− secretion into the ASL. However, several initial observations argued against transepithelial SCN− secretion via CFTR: first, the low concentrations of SCN− compared to other anions and the known anion competition and tighter binding of SCN− inside the CFTR pore (102, 108) suggested low SCN− throughput. Second, the unknown electrochemical driving forces at the apical membrane for SCN−, and the unfavorable SCN− concentration gradient across the epithelium (mucosal, [SCN−] =460 μM; serosal [SCN−] = 50 μM, ref. 40) did not support the idea of passive SCN− currents across CFTR. Then, in a break-through study, Fragoso et al. (40) found that the airway epithelium utilizes a Na+-driven basolateral transporter to move SCN− transepithelial against a significant gradient, which was identified as the Na+/I– symporter that also transports SCN− with a high affinity. Measured rates of SCN− transport by airway epithelia were found to be 2–10 fmole·h−1·cell−1, which compares well to the rates of H2O2 produced by airway cells (Table 2). SCN− transport was stimulated by CFTR activation or blocked by CFTR inhibition and was greatly diminished in CF airways (40, 85). At the same time, bacterial killing by airways in presence of LPO was shown to be directly related to the availability of SCN− (17, 78, 85). These investigations gave strong support for the role of the DUOX/LPO system as a defense mechanism of the airways that requires normal CFTR function. Thus, this mechanism links CFTR function immediately to innate defense.
The pH hypothesis (Fig. 4B) suggests a relation between the pH of the ASL and the NADPH oxidase activity of DUOX (36). It is based on the function of CFTR as a HCO3− conductor (38, 87) and its role in alkalinizing the pH of the ASL (16) while DUOX produces intracellular acid that is released into the ASL across the HVCN1 H+ channel. The pH hypothesis proposes that CFTR is required to alkalinize the pH of the ASL to allow the HVCN1 H+ channel to release intracellular H+ produced by DUOX.
NADPH oxidation by DUOX generates intracellular H+ according to NADPH·H+. NADP+ + 2 e− + 2 H+ (Fig. 4B) and acidifies the cytosol (98). The only apical mechanism in airways that efficiently releases intracellular H+ is the HVCN1 H+ channel (for a detailed discussion of other H+ transporters see (36, 38)). The inside-to-outside H+ gradient both activates HVCN1 in the apical membrane and drives H+ across it into the ASL. Activation of HVCN1 by the pH gradient is a key characteristic to support the apical release of H+ from the cell during NADPH oxidation (14, 77). The HCO3− buffer of the ASL maintains the inside-to-outside H+ gradient (52, 103) and HCO3− is supplied to the ASL by CFTR. HCO3− is conducted by CFTR at a rate of ∼25% compared to Cl− (56, 87). HCO3− conduction across CFTR into the ASL is the major mechanism of the epithelium to alkalinize the ASL (38). This model predicts that efficient H2O2 production is dependent on the inside-to-outside H+ gradient that is maintained by CFTR activity.
In cultured CF airway epithelia in vitro, ASL pH and ASL HCO3− concentrations are reduced (16, 100, 103) and ASL pH in CF patients is too acidic in most studies (10, 75, 82, 109). As a result it is expected that HVCN1 is inactive, intracellular H+ is released inefficiently, and NADPH oxidation is blocked [as shown for NOX2, (77)]. In support of the pH hypothesis, exhaled H2O2 concentrations measured in CF patients were surprisingly low (Table 1). Although the observations in human subjects might result from a number of other mechanisms, they support the general notion that an acidic ASL inhibits DUOX function. Thus, the pH hypothesis links CFTR function as a HCO3− channel to innate defense governed by DUOX.
Airway submucosal glands
Submucosal glands of the airways are the main source of antibacterial factors (including LPO) that are found in the ASL. However, there is no indication that submucosal glands express DUOX. For comparison, salivary glands express DUOX2 along their ducts (43), and rectal glands express DUOX2 at their bottom (43), although another study did not find rectal glands or colonic and small intestinal crypts to express DUOX (34). Owing to the difficulty in investigating glands because they are deeply embedded in the submucosal tissue, the Calu-3 cell line has been used to investigate DUOX in a model airway serous gland cell. Calu-3 were found to express no DUOX1 and little DUOX2 in one report (98), and some DUOX1 and no DUOX2 in another (71). On the other hand, Calu-3 have been reported to lack chromosome 15 (see www.atcc.org) where both DUOX1 and DUOX2 are normally localized (23, 31). This indicates major rearrangements in the localization of DUOX in this cell line, suggesting that Calu-3 is not a sensible model for the investigation of the function of DUOX in glands. Thus, despite the prominent role of airway glands in the release of defense factors there is currently no indication for DUOX expression there, pointing to a function of DUOX solely at the surface epithelium.
Alveolar cells
Although alveoli are mainly made up of type I cells, there is currently no information available about their role in H2O2 production. Type II cells have been investigated recently. Alveolar type II cells express DUOX1, which, as in airway cells, is found at the apical cell pole. No significant expression of DUOX2 was found (37). In functional studies of human alveolar type II cells, H2O2 and H+ production was found, but rates of release of both H2O2 and H+ are generally low compared to the airway epithelium (37).
For comparison, in the type II-like cell line A549, DUOX1 mRNA could not be detected, but DUOX2 mRNA was lowly expressed (71), and measured H2O2 output (after stimulation with ionomycin, a Ca2+ agonist) was similar to primary human type II cells (Table 2). When considering the extremely low volume of the alveolar lining fluid (10‱20 nl/cm2), even low secretory rates may result in considerable effects in the alveolus.
Cai regulation in alveolar cells has been intensely investigated in relation to the release of surfactant by lamellar bodies of type II cells (5). Regulation of Cai is largely governed by mechanical triggers, such as lung expansion. In addition, type II cells also express purinergic and adrenergic receptors that couple to Cai stimulation (13), suggesting that H2O2 release by DUOX in alveolar cells is regulated by these external stimuli.
Nevertheless, the function of DUOX remains obscure in alveoli. Alveolar type II cells have their own arsenal of defense factors, most notably the surfactant proteins A and D, which directly interact and kill microorganisms (74). However, in other innate defense cells, H2O2 is converted by heme peroxidases to form bactericidal ROS. For example, myeloperoxidase in neutrophils generates HOCl, the peroxidase of eosinophils forms HOBr or OSCN−, and on mucosal surfaces LPO generates OSCN−. In contrast, there is no complementary heme peroxidase in alveoli, rather, the selenium-containing glutathione peroxidase is found in the alveolar lining fluid (6), which is structurally unrelated to the heme peroxidases and reduces H2O2 to water in the presence of the high concentrations of glutathione found in the alveolar lining fluid (12). Thus, the function of DUOX in the alveoli might not be in mucosal defense and may not have a mucosal target because released H2O2 is degraded to water by the alveolar glutathione peroxidase.
Conclusions
Since the identification of DUOX in the epithelia of the lung, and the associated measured release of H2O2, the view of the role of ROS in the lung has changed considerably. Although massive release of oxidants during lung inflammation is still considered detrimental to the surrounding tissue, the regulated release of H2O2 by DUOX now appears associated with normal lung epithelial function. The quantitative distinction between detrimental and functionally supportive ROS production is still murky, but likely it is related to the equilibrium of the rates of ROS production and utilization by the lung.
Previously, a number of antibacterial factors have been identified in the ASL, including lysozyme, lactoferrin, β-defensins, and several others (95). The expression of DUOX in the airway epithelium represents a novel antibacterial mechanism that resembles the phagocytic NOX2-based mechanism. Both phagocytes and airway epithelial cells secrete strikingly similar defense factors (including all the ones mentioned above), suggesting that both cell types utilize comparable mechanisms for innate defense. However, there are unique airway-typical characteristics; most notably, the DUOX-based NADPH oxidase operates in parallel to the CFTR Cl− channel in the apical membrane.
The function of CFTR in the airways and its relation to airway disease in CF has been investigated intensely for many years; however, a direct relation of CFTR to an airway defense mechanism has been elusive. The permeation of several types of small, physiologically available anions across CFTR has been noted (56, 66), but a physiological function or a relation to CF airway disease was unclear. In particular, the permeability to SCN− appeared puzzling because it has few known physiological functions. However, with the identification of LPO in the airways (44, 93), an SCN−-utilizing enzyme had been identified and a role for CFTR as an SCN− conductor in support of LPO function in airway defense could be established (17, 78).
At the same time, HCO3− permeation across CFTR was shown to alkalinize the ASL (16, 87), while the consequence of an alkalinized ASL was puzzling. However, an overly acidic ASL was noted in CF airways (38). Only after DUOX had been identified as an acid producer, together with the realization that the HVCN1 H+ channel required an alkalinized ASL to function properly, a mechanistic testable model could be laid out where H2O2 production by DUOX depends on CFTR as a HCO3− channel (36). Thus, there are indications that normal CFTR function is required for both the production of H2O2 by DUOX and the support of LPO to form bactericidal OSCN−. CFTR appears intimately related to the innate defense mechanism provided by DUOX and it is tempting to speculate that the CFTR-HVCN1-DUOX-LPO system is the functional complex that provides the oxidative defenses to the airway surface.
Abbreviations Used
- ARDS
acute respiratory distress syndrome
- ASL
airway surface liquid
- Cai
intracellular Ca2+ concentration
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- COPD
chronic obstructive pulmonary disease
- DUOX
dual oxidase
- e−
electron
- EFP1
EF-hand binding protein 1
- ER
endoplasmic reticulum
- HVCN1
voltage-dependent H+ channel
- LPO
lactoperoxidase
- MPO
myeloperoxidase
- NOX
NADPH oxidase
- ROS
reactive oxygen species
Acknowledgments
My thanks go to Mauri Krouse, Gregory Conner, Richart Harper, Albert van der Vliet, and Terry Machen for stimulating discussions and suggestions, and to Françoise Miot for providing the DUOX antibody. The author's laboratory is funded by the National Institutes of Health (HL089196, HL86323), Philip Morris USA, and the Cystic Fibrosis Foundation (FISCHE07G0).
References
- 1.Al–Obaidy A. Al–Samarai A. Exhaled breath condensate pH and hydrogen peroxide as non-invasive markers for asthma. Saudi Med J. 2007;28:1860–1863. [PubMed] [Google Scholar]
- 2.Ameziane–El-Hassani R. Morand S. Boucher J–L. Frapart Y–M. Apostolou D. Agnandji D. Gnidehou S. Ohayon R. Noel–Hudson M–S. Francon J. Lalaoui K. Virion A. Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;2280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 3.Antczak A. Kurmanowska Z. Kasielski M. Nowak D. Inhaled glucocorticosteroids decrease hydrogen peroxide level in expired air condensate in asthmatic patients. Respir Med. 2000;94:416–421. doi: 10.1053/rmed.1999.0801. [DOI] [PubMed] [Google Scholar]
- 4.Antczak A. Nowak D. Shariati B. Krol M. Piasecka G. Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–1241. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- 5.Ashino Y. Ying X. Dobbs LG. Bhattacharya J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol. 2000;279:L5–13. doi: 10.1152/ajplung.2000.279.1.L5. [DOI] [PubMed] [Google Scholar]
- 6.Avissar N. Finkelstein JN. Horowitz S. Willey JC. Coy E. Frampton MW. Watkins RH. Khullar P. Xu YL. Cohen HJ. Extracellular glutathione peroxidase in human lung epithelial lining fluid and in lung cells. Am J Physiol Lung Cell Mol Physiol. 1996;270:L173–182. doi: 10.1152/ajplung.1996.270.2.L173. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin S. Simon R. Grum C. Ketai L. Boxer L. Devall L. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986;1:11–14. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- 8.Basbaum C. Jany B. Finkbeiner W. The serous cell. Annu Rev Physiol. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- 9.Basbaum C. Madison J. Sommerhoff C. Brown J. Finkbeiner W. Receptors on airway gland cells. Am Rev Respir Dis. 1990;141:S141–144. doi: 10.1164/ajrccm/141.3_Pt_2.S141. [DOI] [PubMed] [Google Scholar]
- 10.Bodini A. D'Orazio C. Peroni D. Corradi M. Folesani G. Baraldi E. Assael B. Boner A. Piacentini G. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol. 2005;40:494–499. doi: 10.1002/ppul.20336. [DOI] [PubMed] [Google Scholar]
- 11.Caillou B. Dupuy C. Lacroix L. Nocera M. Talbot M. Ohayon R. Deme D. Bidart J-M. Schlumberger M. Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab. 2001;86:3351–3358. doi: 10.1210/jcem.86.7.7646. [DOI] [PubMed] [Google Scholar]
- 12.Cantin AM. North SL. Hubbard RC. Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 13.Chander A. Fisher AB. Regulation of lung surfactant secretion. Am J Physiol Lung Cell Mol Physiol. 1990;258:L241–253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 14.Cherny V. Markin V. DeCoursey T. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi H. Finkbeiner W. Widdicombe J. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197:361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coakley RD. Grubb BR. Paradiso AM. Gatzy JT. Johnson LG. Kreda SM. O'Neal WK. Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conner G. Wijkstrom–Frei C. Randell S. Fernandez V. Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner GE. Salathe M. Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med. 2002;166:57S–61. doi: 10.1164/rccm.2206018. [DOI] [PubMed] [Google Scholar]
- 19.Crapo J. Tierney D. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974;226:1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- 20.Crapo JD. Barry BE. Gehr P. Bachofen M. Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;125:740–745. doi: 10.1164/arrd.1982.125.6.740. [DOI] [PubMed] [Google Scholar]
- 21.De Benedetto F. Aceto A. Dragani B. Spacone A. Formisano S. Cocco R. Sanguinetti CM. Validation of a new technique to assess exhaled hydrogen peroxide: Results from normals and COPD patients. Monaldi Arch Chest Dis. 2000;55:185–188. [PubMed] [Google Scholar]
- 22.De Deken X. Wang D. Dumont J. Miot F. Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 23.De Deken X. Wang D. Many M-C. Costagliola S. Libert F. Vassart G. Dumont JE. Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 24.DeCoursey T. Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCoursey TE. Interactions between NADPH oxidase and voltage-gated proton channels: Why electron transport depends on proton transport. FEBS Lett. 2003;555:57–61. doi: 10.1016/s0014-5793(03)01103-7. [DOI] [PubMed] [Google Scholar]
- 26.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 27.Dekhuijzen P. Aben K. Dekker I. Aarts L. Wielders P. van Herwaarden C. Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 28.Dohlman A. Black H. Royall J. Expired breath hydrogen peroxide is a marker of acute airway inflammation in pediatric patients with asthma. Am Rev Respir Dis. 1993;148:955–960. doi: 10.1164/ajrccm/148.4_Pt_1.955. [DOI] [PubMed] [Google Scholar]
- 29.Doniec Z. Nowak D. Tomalak W. Kurzawa R. Exhaled hydrogen peroxide H2O2 in allergic and non-allergic stable mild asthmatic children. Przegl Lek. 2005;62:1343–1345. [PubMed] [Google Scholar]
- 30.Donko A. Peterfi Z. Sum A. Leto T. Geiszt M. Dual oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuy C. Ohayon R. Valent A. Noel–Hudson M–S. Deme D. Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 32.Edens WA. Sharling L. Cheng G. Shapira R. Kinkade JM. Lee T. Edens HA. Tang X. Sullards C. Flaherty DB. Benian GM. Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol. 2001;154:879–892. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El–Chemaly S. Salathe M. Baier S. Conner GE. Forteza R. Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med. 2003;167:425–430. doi: 10.1164/rccm.200206-531OC. [DOI] [PubMed] [Google Scholar]
- 34.El Hassani RA. Benfares N. Caillou B. Talbot M. Sabourin J-C. Belotte V. Morand S. Gnidehou S. Agnandji D. Ohayon R. Kaniewski J. Noel–Hudson M-S. Bidart J–M. Schlumberger M. Virion A. Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastro-intest Liver Physiol. 2004;288:933–942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 35.Emelyanov A. Fedoseev G. Abulimity A. Rudinski K. Fedoulov A. Karabanov A. Barnes PJ. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- 36.Fischer H. Airway surface liquid pH and innate defense by the NADPH oxidase. Ped Pulmonol. 2007;30:175–177. [Google Scholar]
- 37.Fischer H. Gonzales LK. Kolla V. Schwarzer C. Miot F. Illek B. Ballard PL. Developmental regulation of Duox1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1506–L1514. doi: 10.1152/ajplung.00029.2007. [DOI] [PubMed] [Google Scholar]
- 38.Fischer H. Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forteza R. Salathe M. Miot F. Forteza R. Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 40.Fragoso MA. Fernandez V. Forteza R. Randell SH. Salathe M. Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol Lond. 2004;561:183–194. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109:693–697. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geiszt M. Lekstrom K. Witta J. Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 43.Geiszt M. Witta J. Baffi J. Lekstrom K. Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 44.Gerson C. Sabater J. Scuri M. Torbati A. Coffey R. Abraham JW. Lauredo I. Forteza R. Wanner A. Salathe M. Abraham WM. Conner GE. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez R. Yang YH. Griffin C. Allen L. Tigue Z. Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L179–189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- 46.Grasberger H. Refetoff S. Identification of the maturation factor for dual oxidase: Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 47.Greening A. Lowrie D. Extracellular release of hydrogen peroxide by human alveolar macrophages: The relationship to cigarette smoking and lower respiratory tract infections. Clin Sci Lond. 1983;65:661–664. doi: 10.1042/cs0650661. [DOI] [PubMed] [Google Scholar]
- 48.Halliwell B. Gutteridge JMC. Oxford: Oxford University Press; 1999. Free radicals in biology and medicine. [Google Scholar]
- 49.Harper R. Xu C. McManus M. Heidersbach A. Eiserich J. Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium. FEBS Lett. 2006;580:5150–5154. doi: 10.1016/j.febslet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 50.Harper RW. Xu C. Eiserich J. Chen Y. Kao C–Y. Thai P. Setiadi H. Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Ho LP. Faccenda J. Innes JA. Greening AP. Expired hydrogen peroxide in breath condensate of cystic fibrosis patients. Eur Respir J 1999. 1999;13:103–106. doi: 10.1183/09031936.99.13110399. [DOI] [PubMed] [Google Scholar]
- 52.Holma B. Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Sci Total Environ 1985; 1985;41:101–123. doi: 10.1016/0048-9697(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 53.Hong KU. Reynolds SD. Watkins S. Fuchs E. Stripp BR. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 54.Horsfield K. Cumming G. Morphology of the bronchial tree in man. J Appl Physiol. 1968;24:373–383. doi: 10.1152/jappl.1968.24.3.373. [DOI] [PubMed] [Google Scholar]
- 55.Horvath I. Donnelly LE. Kiss A. Kharitonov SA. Lim S. Fan Chung K. Barnes PJ. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158:1042–1046. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- 56.Illek B. Tam AW–K. Fischer H. Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelia. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- 57.Jiang C. Finkbeiner WE. Widdicombe JH. McCray PB. Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science. 1993;262:424–427. doi: 10.1126/science.8211164. [DOI] [PubMed] [Google Scholar]
- 58.Jobsis Q. Raatgeep H. Schellekens S. Kroesbergen A. Hop W. de Jongste J. Hydrogen peroxide and nitric oxide in exhaled air of children with cystic fibrosis during antibiotic treatment. Eur Respir J. 2000;16:95–100. doi: 10.1034/j.1399-3003.2000.16a17.x. [DOI] [PubMed] [Google Scholar]
- 59.Jobsis RQ. Schellekens SL. Fakkel–Kroesbergen A. Raatgeep RH. de Jongste JC. Hydrogen peroxide in breath condensate during a common cold. Mediators Inflamm. 2001;10:351–354. doi: 10.1080/09629350120102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasielski M. Nowak D. Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respir Med. 2001;95:448–456. doi: 10.1053/rmed.2001.1066. [DOI] [PubMed] [Google Scholar]
- 61.Kietzmann D. Kahl R. Muller M. Burchardi H. Kettler D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS. Intensive Care Med. 1993;19:78–81. doi: 10.1007/BF01708366. [DOI] [PubMed] [Google Scholar]
- 62.Kinnula VL. Adler KB. Ackley NJ. Crapo JD. Release of reactive oxygen species by guinea pig tracheal epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 1992;262:L708–712. doi: 10.1152/ajplung.1992.262.6.L708. [DOI] [PubMed] [Google Scholar]
- 63.Kinnula VL. Everitt JI. Whorton AR. Crapo JD. Hydrogen peroxide production by alveolar type II cells, alveolar macrophages, and endothelial cells. Am J Physiol Lung Cell Mol Physiol. 1991;261:L84–91. doi: 10.1152/ajplung.1991.261.2.L84. [DOI] [PubMed] [Google Scholar]
- 64.LeDizet M. Beck JC. Finkbeiner WE. Differential regulation of centrin genes during ciliogenesis in human tracheal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1145–1156. doi: 10.1152/ajplung.1998.275.6.L1145. [DOI] [PubMed] [Google Scholar]
- 65.Leto T. Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1556. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 66.Linsdell P. Tabcharani JA. Rommens JM. Hou YX. Chang XB. Tsui LC. Riordan JR. Hanrahan JW. Permeability of wild-type and mutant CFTR chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loukides S. Bouros D. Papatheodorou G. Lachanis S. Panagou P. Siafakas NM. Exhaled H2O2 in steady-state bronchiectasis: Relationship with cellular composition in induced sputum, spirometry, and extent and severity of disease. Chest. 2002;121:81–87. doi: 10.1378/chest.121.1.81. [DOI] [PubMed] [Google Scholar]
- 68.Loukides S. Bouros D. Papatheodorou G. Panagou P. Siafakas NM. The relationships among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severity. Chest. 2002;121:338–346. doi: 10.1378/chest.121.2.338. [DOI] [PubMed] [Google Scholar]
- 69.Loukides S. Horvath I. Wodehouse T. Cole PJ. Barnes PJ. Elevated levels of expired breath hydrogen peroxide in bronchiectasis. Am J Respir Crit Care Med. 1998;158:991–994. doi: 10.1164/ajrccm.158.3.9710031. [DOI] [PubMed] [Google Scholar]
- 70.Lubman RL. Crandall ED. Polarized distribution of Na+-H+ antiport activity in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1994;266:L138–147. doi: 10.1152/ajplung.1994.266.2.L138. [DOI] [PubMed] [Google Scholar]
- 71.Luxen S. Belinsky SA. Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 72.Majewska E. Kasielski M. Luczynski R. Bartosz G. Bialasiewicz P. Nowak D. Elevated exhalation of hydrogen peroxide and thiobarbituric acid reactive substances in patients with community acquired pneumonia. Respir Med. 2004;98:669–676. doi: 10.1016/j.rmed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 73.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 74.McCormack FX. Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McShane D. Davies JC. Davies MG. Bush A. Geddes DM. Alton EWFW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21:37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 76.Morand S. Ueyama T. Tsujibe S. Saito N. Korzeniowska A. Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan D. Cherny VV. Murphy R. Katz BZ. DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol Lond. 2005;569:419–431. doi: 10.1113/jphysiol.2005.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moskwa P. Lorentzen D. Excoffon KJDA. Zabner J. McCray PB., Jr. Nauseef WM. Dupuy C. Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2006;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nowak D. Antczak A. Krol M. Pietras T. Shariati B. Bialasiewicz P. Jeczkowski K. Kula P. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur Respir J. 1996;9:652–657. doi: 10.1183/09031936.96.09040652. [DOI] [PubMed] [Google Scholar]
- 80.Nowak D. Kalucka S. Bialasiewicz P. Krol M. Exhalation of H2O2 and thiobarbituric acid reactive substances (TBARs) by healthy subjects. Free Radic Biol Med. 2001;30:178–186. doi: 10.1016/s0891-5849(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 81.Nowak D. Kasielski M. Antczak A. Pietras T. Bialasiewicz P. Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: No significant effect of cigarette smoking. Respir Med. 1999;93:389–396. doi: 10.1053/rmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- 82.Ojoo JC. Mulrennan SA. Kastelik JA. Morice AH. Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60:22–26. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pacquelet S. Lehmann M. Luxen S. Regazzoni K. Frausto M. Noack D. Knaus UG. Inhibitory action of Noxa1 on Duox activity in airway cells. J Biol Chem. 2008;36:24649–24658. doi: 10.1074/jbc.M709108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paradiso AM. Mason SJ. Lazarowski ER. Boucher RC. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- 85.Pedemonte N. Caci E. Sondo E. Caputo A. Rhoden K. Pfeffer U. Di Candia M. Bandettini R. Ravazzolo R. Zegarra–Moran O. Galietta LJV. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: Role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 86.Piotrowski W. Marczak J. Dinsdale D. Kurmanowska Z. Tarasow Y. Komos J. Nowak D. Release of hydrogen peroxide by rat type II pneumocytes in the prolonged culture. Toxicol In Vitro. 2000;14:85–93. doi: 10.1016/s0887-2333(99)00080-6. [DOI] [PubMed] [Google Scholar]
- 87.Poulsen JH. Fischer H. Illek B. Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rada B. Lekstrom K. Damian S. Dupuy C. Leto T. The pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reeves EP. Nagl M. Godovac–Zimmermann J. Segal AW. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J Med Microbiol. 2003;52:643–651. doi: 10.1099/jmm.0.05181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Repine JE. Pulmonary oxygen toxicity: Current assessment of the contributions of oxygen metabolites and neutrophils. In: Taylor AE, editor; Matalon A, editor; Ward PA, editor. Physiology of Oxygen Radicals. Bethesda, MD: American Physiological Society; 1986. pp. 119–130. [Google Scholar]
- 91.Ribeiro CMP. Paradiso AM. Livraghi A. Boucher RC. The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia. J Gen Physiol. 2003;122:377–387. doi: 10.1085/jgp.200308893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rigutto S. Hoste C. Grasberger H. Milenkovic M. Communi D. Dumont JE. Corvilain B. Miot F. De Deken X. Activation of dual oxidases (Duox1 and Duox2): Differential regulation mediated by PKA and PKC-dependent phosphorylation. J Biol Chem. 2009;284:6725–6734. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salathe M. Holderby M. Forteza R. Abraham WM. Wanner A. Conner GE. Isolation and characterization of a peroxidase from the airway. Am J Respir Cell Mol Biol. 1997;17:97–105. doi: 10.1165/ajrcmb.17.1.2719. [DOI] [PubMed] [Google Scholar]
- 94.Schleiss MB. Holz O. Behnke M. Richter K. Magnussen H. Jorres RA. The concentration of hydrogen peroxide in exhaled air depends on expiratory flow rate. Eur Respir J. 2000;16:1115–1118. doi: 10.1034/j.1399-3003.2000.16f16.x. [DOI] [PubMed] [Google Scholar]
- 95.Schutte B. McCray P. Beta-defensins in lung host defense. Annu Rev Physiol. 2002;264:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 96.Schwarzer C. Fischer H. Kim E. Barber K. Mills A. Kurth M. Gruenert D. Suh J. Machen T. Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl− transport in human bronchial epithelial cells. Free Radic Biol Med. 2008;245:1653–1662. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwarzer C. Illek B. Suh JH. Remington SJ. Fischer H. Machen TE. Organelle redox of CF and CFTR-corrected airway epithelia measured with roGFP1. Free Radic Biol Med. 2007;43:300–316. doi: 10.1016/j.freeradbiomed.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwarzer C. Machen TE. Illek B. Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 99.Shao MXG. Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith JJ. Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith JL. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol Lond. 1899;24:19–35. doi: 10.1113/jphysiol.1899.sp000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith SS. Steinle ED. Meyerhoff ME. Dawson DC. Cystic fibrosis transmembrane conductance regulator: Physical basis for lyotropic anion selectivity patterns. J Gen Physiol. 1999;114:799–818. doi: 10.1085/jgp.114.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song Y. Salinas D. Nielson DW. Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290:C741–749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 104.Starosta V. Rietschel E. Paul K. Baumann U. Griese M. Oxidative changes of bronchoalveolar proteins in cystic fibrosis. Chest. 2006;129:431–437. doi: 10.1378/chest.129.2.431. [DOI] [PubMed] [Google Scholar]
- 105.Steinberg BE. Grinstein S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 106.Strieter RM. Belperio JA. Keane MP. Cytokines in innate host defense in the lung. J Clin Invest. 2002;109:699–705. doi: 10.1172/JCI15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sznajder J. Fraiman A. Hall J. Sanders W. Schmidt G. Crawford G. Nahum A. Factor P. Wood L. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest. 1989;96:606–612. doi: 10.1378/chest.96.3.606. [DOI] [PubMed] [Google Scholar]
- 108.Tabcharani JA. Rommens JM. Hou YX. Chang XB. Tsui LC. Riordan JR. Hanrahan JW. Multi-ion pore behaviour in the CFTR chloride channel. Nature. 1993;366:79–82. doi: 10.1038/366079a0. [DOI] [PubMed] [Google Scholar]
- 109.Tate S. MacGregor G. Davis M. Innes JA. Greening AP. Airways in cystic fibrosis are acidified: Detection by exhaled breath condensate. Thorax. 2002;57:926–929. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tos M. Development of the tracheal glands in man. Acta Pathol Microbiol Scand. 1966;185:1–130. [PubMed] [Google Scholar]
- 111.van Beurden W. Dekhuijzen P. Harff G. Smeenk F. Variability of exhaled hydrogen peroxide in stable COPD patients and matched healthy controls. Respiration. 2002;69:211–216. doi: 10.1159/000063622. [DOI] [PubMed] [Google Scholar]
- 112.van Beurden W. Harff G. Dekhuijzen P. van der Poel–Smet S. Smeenk F. Effects of inhaled corticosteroids with different lung deposition on exhaled hydrogen peroxide in stable COPD patients. Respiration. 2003;70:242–248. doi: 10.1159/000072004. [DOI] [PubMed] [Google Scholar]
- 113.van der Vliet A. NADPH oxidases in lung biology and pathology: Host defense enzymes, and more. Free Rad Biol Med. 2008;44:938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Klaveren RJ. Roelant C. Boogaerts M. Demedts M. Nemery B. Involvement of an NAD(P)H oxidase-like enzyme in superoxide anion and hydrogen peroxide generation by rat type II cells. Thorax. 1995;52:465–471. doi: 10.1136/thx.52.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verkman AS. Song Y. Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 116.Wallaert B. Aerts C. Gressier B. Gosset P. Voisin C. Oxidative inactivation of alpha 1-proteinase inhibitor by alveolar epithelial type II cells. J Appl Physiol. 1993;75:2376–2382. doi: 10.1152/jappl.1993.75.6.2376. [DOI] [PubMed] [Google Scholar]
- 117.Wang D. De Deken X. Milenkovic M. Song Y. Pirson I. Dumont JE. Miot F. Identification of a novel partner of Duox: EFP1, a thioredoxin-related protein. J Biol Chem. 2005;280:3096–3103. doi: 10.1074/jbc.M407709200. [DOI] [PubMed] [Google Scholar]
- 118.Weibel ER. Morphometry of the Human Lung. Heidelberg: Springer-Verlag; 1963. [Google Scholar]
- 119.Wesley UV. Bove PF. Hristova M. McCarthy S. van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 120.Widdicombe JH. Volume of airway surface liquid in health and disease. Am J Respir Crit Care Med. 2002;2165:1566. doi: 10.1164/ajrccm.165.11.165111. [DOI] [PubMed] [Google Scholar]
- 121.Wijkstrom–Frei C. El–Chemaly S. Ali–Rachedi R. Gerson C. Cobas MA. Forteza R. Salathe M. Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29:206–212. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 122.Willumsen NJ. Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 1989;256:C1054–1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- 123.Wu JV. Krouse ME. Wine JJ. Acinar origin of CFTR-dependent airway submucosal gland fluid secretion. Am J Physiol Lung Cell Mol Physiol. 2007;2007;292:L304–311. doi: 10.1152/ajplung.00286.2006. [DOI] [PubMed] [Google Scholar]