Abstract
Neutrophils infiltrate systemic vasculature of women with preeclampsia, so we tested the hypothesis that factors in plasma of preeclamptic women activate endothelial cells to produce IL-8 resulting in transendothelial migration of neutrophils. Neutrophil migration was studied using the Transwell system. An endothelial cell line was grown to confluence on the inserts and treated with 10% plasma from normal nonpregnant (NNP), normal pregnant (NP) and preeclamptic (PE) women or with an oxidizing solution containing linoleic acid (OxLA). Compared to medium control, NNP plasma or NP plasma, PE plasma significantly stimulated IL-8 and neutrophil migration which was inhibited by vitamins E and C or IL-8 neutralizing antibody. Compared to medium control or LA, OxLA stimulated IL-8 and neutrophil migration which was inhibited by vitamins E and C or IL-8 antibody. Conclusion: Factors present in plasma of preeclamptic women stimulate transendothelial migration of neutrophils which is due to induction of oxidative stress and production of IL-8.
Keywords: preeclampsia, neutrophils, IL-8, oxidative stress, endothelial cells
INTRODUCTION
Neutrophils are activated in women with normal pregnancy and are further activated in women with preeclampsia 1–5. Oxidized lipids are potent activators of neutrophils 6–8 and the human placenta produces oxidized lipids and secretes them into the maternal circulation 9–11. In women with preeclampsia, placental production of oxidized lipids is significantly higher than in women with normal pregnancy 9, 11. Activation of neutrophils likely occurs as they circulate through the intervillous space and are directly exposed to oxidized lipids released by the placenta 12, 13. As the activated neutrophils return to the maternal systemic circulation, factors in the plasma of preeclamptic women could cause the neutrophils to adhere to the endothelium and infiltrate the vessel. This could cause vascular inflammation by release of neutrophil products, such as reactive oxygen species (ROS), tumor necrosis factor-alpha (TNFα), matrix metalloproteinase-8 and myeloperoxidase.
We recently reported the first direct evidence of vascular inflammation in women with preeclampsia 14, 15. We found extensive infiltration of neutrophils into the systemic vasculature of women with preeclampsia. Neutrophils were flattened and adhered onto the endothelium and they had infiltrated into the intimal space, the area between the endothelium and vascular smooth muscle. This neutrophil infiltration was associated with a significant increase in the expression of inflammation markers, such as nuclear factor-kappa B (NF-κB), cyclooxygenase-2 (COX-2) and interleukin-8 (IL-8). IL-8 is a potent neutrophil chemokine, so endothelial production of IL-8 would attract neutrophils. In the present study, we used the Transwell system to test the hypothesis that factors in plasma of preeclamptic women activate endothelial cells to produce IL-8 resulting in transendothelial migration of neutrophils. We focused on IL-8 because we had direct evidence that its expression is increased in systemic vasculature of preeclamptic women, however, other chemotactic factors may also be involved in vivo.
MATERIALS and METHODS
Blood samples used for treatments were obtained from 4 normal nonpregnant women, 4 normal pregnant women and 6 preeclamptic women by vein puncture into sodium heparin tubes. Three of the preeclamptic women had severe preeclampsia and three had mild preeclampsia. Patients were matched for age and pre-pregnancy body mass index (BMI). Pregnant women were not in labor and matched for parity and gestational age at sample collection. Blood was drawn 1–2 days before delivery. Preeclampsia was defined as sustained blood pressure of ≥ 140/90 mmHg with readings at least 6 hours apart and proteinuria (300mg/24 hr or ≥ 1+ urine dipstick). This study was approved by the Office of Research Subjects Protection, Virginia Commonwealth University.
Transendothelial Migration
The ECV-304 cell line (ATCC, Manassas, VA) was used for experiments. These cells have a cobblestone monolayer growth pattern and express endothelial biomarkers, such as intercellular adhesion molecule-1 (ICAM-1), Factor VIII, Weibel-Palade bodies and tubule formation on Matrigel. Expression of ICAM-1 allows the cells to interact with neutrophils to study migration. Cells were seeded at 20,000 cells/insert in M199 medium supplemented with 10% heat inactivated fetal bovine serum (FBS). Cells were grown for 3 days to confluence on collagen I coated Transwell inserts (0.33 cm2 area, 3.0 μm pore size, Costar, Fisher Scientific, Malvern, PA) in 24-well culture plates as previously described 16–18. Confluence was verified by evidence of a barrier to the transport of trypan blue dye from the upper chamber to the lower chamber. On the day of study, monolayers were washed with Hanks’ Balanced Salt Solution (HBSS) with 0.2% BSA and then incubated in triplicate in M-199 for 4 hours with experimental treatments. In the first experiment, plasma treatments (10%) from the following groups of women were added to the upper insert chamber containing the ECV-304 cells: 1) normal non-pregnant (NNP); 2) normal pregnant (NP); 3) preeclamptic (PE); 4) PE plus cells pretreated for 24 hours with vitamin E (VE, 50 μM) and vitamin C (VC, 100 μM); and 5) PE plus IL-8 neutralizing antibody (20 μg/ml, R&D Systems, Minneapolis, MN). A second experiment was done to directly assess the effect of oxidative stress. Treatments were: 1) Medium control; 2) linoleic acid (LA, 45 μmol/L); 3) an oxidizing solution enriched with LA (OxLA, LA plus 0.45 mM hypoxanthine + 0.005 U/ml xanthine oxidase + 50 μM ferrous sulfate); 4) OxLA plus cells pretreated for 24 hours with VE+VC; 5) OxLA plus IL-8 neutralizing antibody. Initially, a 24 hour time course experiment for IL-8 was done with LA and OxLA to determine the treatment time for subsequent experiments. IL-8 concentrations increased in a linear manner over the 24 hour period and were sufficiently elevated after 4 hours to be significantly different between OxLA and LA (109 ± 15.1 vs. 55.1 ± 6.5 pg/ml, respectively, p < 0.05, n = 4), so a 4 hour treatment period was chosen for experiments. We have previously shown that solutions composed of hypoxanthine, xanthine oxidase and LA induce oxidative stress and lipid peroxidation 7, 9, 19. Hypoxanthine, ferrous sulfate, vitamin E and vitamin C were purchased from Sigma, St. Louis, MO, xanthine oxidase from Roche Molecular BioChemicals, Indianapolis, IN, linoleic acid from Cayman Chemical, Ann Arbor, MI).
During the treatment period, neutrophils were isolated by Histopaque gradient density centrifugation with hypotonic lysis of erythrocytes as previously described 7, 8. Neutrophils were obtained from healthy non-pregnant women. The cells were pelleted by centrifugation and resuspended in 3 mL of HBSS without Ca++ or Mg++. 51Cr (2 μCi per 106 cells) was added and the cells incubated at 37°C for 1 hour in Teflon tubes using an orbital shaker. Labeling was stopped by diluting the cells to 15 mL with HBSS (no Ca++ or Mg++). Cells were centrifuged at 400 × g for 5 min at 4°C and then washed 3 times with HBSS. Cells were adjusted to a concentration of 1 ×106 cells/100 μl in HBSS - 0.2% BSA. The insert monolayers were washed with HBSS - 0.2% BSA and the transendothelial migration assay was carried out by placing 100 μl of 51Cr labeled neutrophils (106 cells) into the upper well. Neutrophils were allowed to migrate for 2 hours at 37°C. At the end of the experiment, medium was collected from the lower chamber for IL-8 measurement, the bottom of the inserts were rinsed and 500 μl from the lower wells was transferred to 12 × 75 mm glass tubes to determine transendothelial migration. The tubes were counted in a gamma counter for 1 minute and the % migration calculated based on the total amount of radioactivity added.
Purity of neutrophils was assessed by immunohistochemical staining using a mouse IgM anti-human monoclonal antibody specific for CD66b, a neutrophil antigen, as previously described 14, 15. CD66b was purchased from BD Bioscience, San Diego, CA. Zymed SuperPicture Kit (Invitrogen, Carlsbad, CA) was used for staining.
Lipid peroxidation in whole plasma samples was assessed by thiobarbituric acid reactive substances (TBARS) as previously described 9, 20. TBARS primarily reflect malondialdehyde, a break down product of lipid peroxides. The 10% plasma treatments were also analyzed for IL-8 by specific ELISA (R&D Systems, Minneapolis, MN) as previously described 19 to assure that IL-8 present in the diluted plasma was not responsible for neutrophil migration. Cell viability was assessed by trypan blue exclusion and the MTT cell proliferation/viability assay.
Patient and experimental data were analyzed using a statistical software program (GraphPad Prism 4.0 for Macintosh, Graph Pad Software, Inc., San Diego, CA, www.graphpad.com). Unpaired t-test or one-way ANOVA with Newman-Keuls post-hoc test was used to determine statistical differences. A probability level of P < 0.05 was considered statistically significant. Data are presented as mean ± SE.
RESULTS
Demographic data for normal and preeclamptic patients from whom treatment plasma samples were obtained were: Age: 28.7 ± 2.0 vs. 27.0 ± 1.7 years, P>0.7; Systolic blood pressure: 129.0 ± 9.0 vs. 178.4 ± 6.5 mmHg, P<0.05; Diastolic blood pressure: 70.0 ± 10.0 vs. 109.2 ± 5.1 mmHg, P<0.05; BMI: 30.0 ± 3.8 vs. 36.9 ± 2.1, P>0.18; Birth weight: 3277 ± 22 vs. 2696 ± 320 grams, P>0.42; Gestational age at delivery: 40.0 ± 0.6 vs. 36.3 ± 1.4 weeks, P>0.1, respectively). TBARS levels in preeclamptic plasma were significantly higher than those in normal pregnant of normal non-pregnant plasma (5.4 ± 0.8 vs. 0.9 ± 0.5 vs. 0.7 ± 0.1 μmol/L, respectively, P < 0.05). IL-8 levels were not detectable in the 10% plasma treatments.
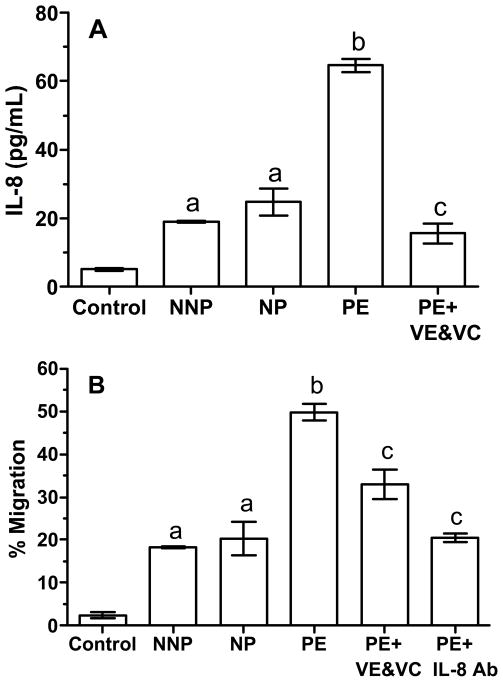
Figure 1 shows the results for the first experiment with plasma. Panel A shows the concentrations of IL-8 in the media of the lower chamber after treatment of ECV-304 cells with 10% plasma from the different patient groups. Compared to the medium control, plasma from NNP and NP patients significantly increased the concentration of IL-8 from approximately 5 pg/ml to 20 pg/ml. Plasma from PE patients caused a significant 3-fold increase in IL-8 concentrations as compared to plasma from NNP or NP patients. Pretreatment of cells with vitamins E and C reduced the effect of PE plasma on IL-8 to that of NNP and NP plasma. IL-8 was non-detectable in media treatments containing 10% plasma and so did not influence results. Panel B shows the % transendothelial migration of neutrophils to plasma treatments. The % migration paralleled the IL-8 concentrations. As compared to medium control, NNP and NP plasma increased transendothelial migration of neutrophils from approximately 2% to 15–20%. NNP and NP treatments were not different from each other. Compared to NNP and NP, PE stimulated a significant increase in neutrophil migration of over 50%. Pretreatment of cells with VE and VC significantly reduced neutrophil migration induced by PE, as did co-treatment with IL-8 neutralizing antibody.
Figure 1.
Production of IL-8 by ECV-304 cells, a cell line with endothelial cell characteristics (A), and transendothelial migration of neutrophils (B) in response to treatment with 10% plasma from normal nonpregnant (NNP), normal pregnant (NP) and preeclamptic (PE) women using the Transwell system. Cells were grown to confluence on Transwell inserts containing pores 3 microns in diameter in 24-well culture plates creating an upper chamber and lower chamber separated by the confluence of the cells on the insert. Cells were incubated with plasma treatments for 4 hours and then 51Cr-labeled neutrophils were added to the upper chamber. After 2 hours, media in the lower chamber was collected for IL-8 determination and the % of neutrophils that migrated from the upper to lower chamber was calculated. Cells incubated with PE produced more IL-8 and had more neutrophil migration than medium control or cells incubated with NNP or NP. Neutrophil migration to PE was inhibited if cells were pretreated for 24 hours with vitamins E and C or co-incubated with IL-8 neutralizing antibody. a, significantly higher than medium control, P < 0.05; b, significantly higher than NP or NNP, P < 0.01; c, significantly lower than PE, P < 0.01. Data represent mean ± SE, n = 6 independent experiments with treatments run in duplicate.
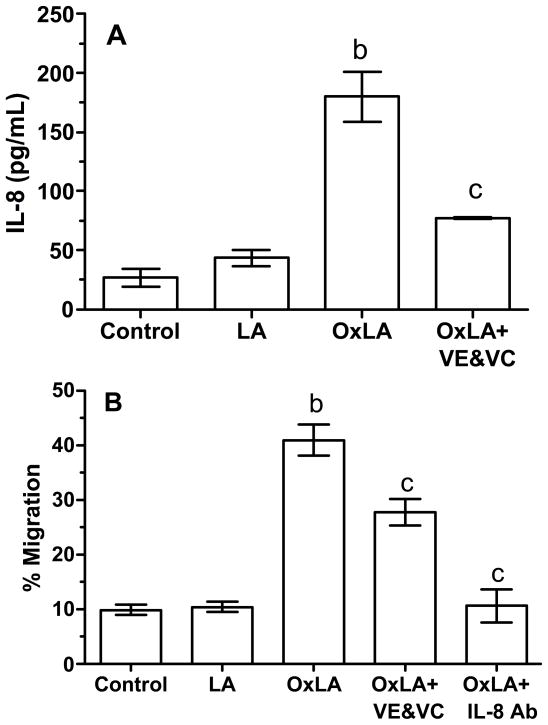
Figure 2 shows the results of the second experiment to evaluate oxidative stress. Compared to medium control and LA, OxLA significantly stimulated IL-8 production and transendothelial migration of neutrophils (panels A and B, respectfully). Pretreatment with VE and VC significantly inhibited the ability of OxLA to stimulate IL-8 and neutrophil migration. Co-treatment with IL-8 neutralizing antibody prevented OxLA from stimulating neutrophil migration.
Figure 2.
IL-8 production and % migration of neutrophils to oxidative stress using the Transwell system. Linoleic acid (LA) did not stimulate IL-8 production or transendothelial migration of neutrophils as compared to medium control, however, an oxidizing solution containing LA (OxLA) greatly stimulated IL-8 and neutrophil migration. Pretreatment of cells with VE and VC or co-incubation with an IL-8 neutralizing antibody inhibited IL-8 production and neutrophil migration induced by OxLA. b, significantly higher than medium control and LA, P < 0.01; c, significantly lower than OxLA, P < 0.01. Data represent mean ± SE, n = 7 independent experiments with treatments run in duplicate.
DISCUSSION
The cause of preeclampsia is not known, but there is good evidence that it is associated with endothelial cell activation and dysfunction 21, 22. Factors present in the blood of preeclamptic women cause endothelial cell activation. For example, exposure of endothelial cells to plasma or serum from preeclamptic women causes activation of nuclear factor-κB and increased expression of intercellular adhesion molecule-1 compared with plasma or serum from normal pregnant women 23, 24.
In this study we used the Transwell system and demonstrated that plasma obtained from preeclamptic women significantly stimulated IL-8 production by a cell line with endothelial cell characteristics and that this was associated with a significant increase in the transendothelial migration of neutrophils as compared to plasma obtained from normal nonpregnant or normal pregnant women. Oxidative stress mediated the effects of preeclamptic plasma because pre-treatment of the cells with antioxidants prevented preeclamptic plasma from stimulating IL-8 production and neutrophil migration. IL-8 was responsible for transendothelial migration because migration was prevented when preeclamptic plasma was co-incubated with IL-8 neutralizing antibody.
Transendothelial migration of neutrophils in vivo is a complicated process involving a number of cell adhesion molecules and chemotactic agents. Activated neutrophils in the circulation express adhesion molecules, such as L-selectin and integrins, which tether the neutrophils by binding to endothelial adhesion molecules, such as E-selectin and P-selectin. Neutrophils initially roll along the endothelium and then flatten and bind tightly to endothelial adhesion molecules, such as intercellular adhesion molecule-1, before migrating across the endothelium. We focused our in vitro studies on IL-8 because we had direct evidence that its expression is increased in systemic vasculature of preeclamptic women, however, other chemotactic agents, such as monocyte chemotactic protein-1, RANTES, ENA-78 and GRO-alpha, may also be involved in vivo in preeclamptic women.
Levels of linoleic acid are elevated in women with preeclampsia 25 and oxidative stress is a characteristic of preeclampsia 26, 27 so we tested the effects of LA and oxidative stress. Treatment with LA did not stimulate IL-8 production or neutrophil migration over that of the medium control, but when LA was mixed with an oxidizing solution, IL-8 and neutrophil migration were markedly stimulated similar to that of preeclamptic plasma providing evidence that oxidative stress was a mediator of neutrophil transendothelial migration.
There are a number of factors present in preeclamptic plasma that could be responsible for transendothelial migration of neutrophils. Shed membrane particles of leukocytes are one possibility. The number of neutrophils increases 2.5-fold by 30 weeks of gestation in normal pregnancy 28 and increases further in preeclampsia 29. Shed membrane particles of leukocytes are elevated in the plasma of preeclamptic women and have been shown to induce vascular inflammation 30. Treatment of vessels with membrane particles from preeclamptic women causes activation of NF-κB, increased expression of COX-2, increased formation of nitrotyrosine and production of superoxide. Preeclamptic membrane particles also stimulate production of 8-isoprostane from treated vessels, demonstrating their ability to induce oxidative stress. Leukocytes are not the only source of membrane particles, the placenta is an additional source. Syncytiotrophoblast membrane particles have been isolated in the plasma of preeclamptic women and shown to cause endothelial activation 31. Our study and those of others showing inflammatory effects of preeclamptic serum or plasma on endothelial cells could be due to leukocyte or placental membrane particles because the centrifugal force used to separate serum or plasma is not sufficient to remove membrane particles.
Lipid peroxides or their breakdown products in plasma are another possible cause of endothelial cell activation in preeclampsia. Markers of lipid peroxidation are elevated in the maternal circulation. For example, TBARS and 8-isoprostane are elevated in maternal plasma of preeclamptic women 26, 27, however, direct attempts to measure oxidized lipids or oxidized low-density lipoprotein in maternal blood have been inconsistent. Branch et al reported higher titers of autoantibodies to oxidized low-density lipoprotein 32, but other investigators have not confirmed this 33, 34. Circulating lipids per se may not be the cause of endothelial activation, but rather a breakdown product, such as malondialdehyde which is capable of inducing toxic effects on endothelial cells.
Although circulating factors in maternal blood, such as membrane particles, oxidized lipids or malondialdehyde, might activate endothelial cells to attract neutrophils, they probably would not affect the underlying vascular smooth muscle which is also inflamed in preeclamptic women 14, 15. Vascular smooth muscle dysfunction requires a further mechanism that may involve the transendothelial migration of neutrophils. Release of reactive oxygen species, tumor necrosis factor-α, and myeloperoxidase by neutrophils that have infiltrated into the intimal space could be responsible for vascular smooth muscle inflammation in preeclamptic women.
In this study we demonstrated that factors present in plasma of preeclamptic women stimulate transendothelial migration of neutrophils, and that this is due to induction of oxidative stress and production of IL-8. This may explain vascular infiltration of neutrophils observed in preeclamptic women.
Acknowledgments
Supported by a grant to SWW from the National Institutes of Health (HL069851)
The author would like to acknowledge the contributions of Amy L. Goodyear, M.S., for technical assistance in conducting experiments for this study.
References
- 1.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 2.Greer IA, Haddad NG, Dawes J, Johnstone FD, Calder AA. Neutrophil activation in pregnancy-induced hypertension. Br J Obstet Gynaecol. 1989;96:978–982. doi: 10.1111/j.1471-0528.1989.tb03358.x. [DOI] [PubMed] [Google Scholar]
- 3.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 4.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsukimori K, Maeda H, Ishida K, Nagata H, Koyanagi T, Nakano H. The superoxide generation of neutrophils in normal and preeclamptic pregnancies. Obstet Gynecol. 1993;81:536–540. [PubMed] [Google Scholar]
- 6.Gorog P. Activation of human blood monocytes by oxidized polyunsaturated fatty acids: a possible mechanism for the generation of lipid peroxides in the circulation. Int J Exp Path. 1991;72:227–237. [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan JE, Walsh SW. Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am J Obstet Gynecol. 2005;193:830–835. doi: 10.1016/j.ajog.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan JE, Walsh SW, Ford GD. Thromboxane mediates neutrophil superoxide production in pregnancy. Am J Obstet Gynecol. 2006;195:1415–1420. doi: 10.1016/j.ajog.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Walsh SW, Vaughan JE, Wang Y, Roberts LJ., II Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. Am J Obstet Gynecol. 1993;169:1462–1466. doi: 10.1016/0002-9378(93)90419-j. [DOI] [PubMed] [Google Scholar]
- 11.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab. 1995;80:1888–1893. doi: 10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 12.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregn. 1994;13:1–32. [Google Scholar]
- 14.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 15.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48.e41–48.e48. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Smart SJ, Casale TB. Interleukin-8-induced transcellular neutrophil migration is facilitated by endothelial and pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1993;9:489–495. doi: 10.1165/ajrcmb/9.5.489. [DOI] [PubMed] [Google Scholar]
- 17.Smart SJ, Casale TB. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994;266:L238–245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- 18.Wiedermann CJ, Schratzberger P, Kahler CM. Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with beta-endorphin. Brain Behav Immun. 1994;8:270–277. doi: 10.1006/brbi.1994.1025. [DOI] [PubMed] [Google Scholar]
- 19.Leik CE, Walsh SW. Linoleic acid, but not oleic acid, upregulates production of interleukin-8 by human vascular smooth muscle cells via arachidonic acid metabolites under conditions of oxidative stress. J Soc Gynecol Investig. 2005;12:593–598. doi: 10.1016/j.jsgi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Jentzsch AM, Bachmann H, Furst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 23.Endresen MJ, Morris JM, Nobrega AC, Buckley D, Linton EA, Redman CW. Serum from preeclamptic women induces vascular cell adhesion molecule-1 expression on human endothelial cells in vitro: a possible role of increased circulating levels of free fatty acids. Am J Obstet Gynecol. 1998;179:665–670. doi: 10.1016/s0002-9378(98)70061-4. [DOI] [PubMed] [Google Scholar]
- 24.Takacs P, Kauma SW, Sholley MM, Walsh SW, Dinsmoor MJ, Green K. Increased circulating lipid peroxides in severe preeclampsia activate NF-kappaB and upregulate ICAM-1 in vascular endothelial cells. FASEB J. 2001;15:279–281. doi: 10.1096/fj.00-0549fje. [DOI] [PubMed] [Google Scholar]
- 25.Lorentzen B, Henriksen T. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:33–39. doi: 10.1055/s-2007-1016250. [DOI] [PubMed] [Google Scholar]
- 26.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 28.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, et al. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77:1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- 29.Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45:229–231. doi: 10.1159/000009973. [DOI] [PubMed] [Google Scholar]
- 30.Meziani F, Tesse A, David E, et al. Shed membrane particles from preeclamptic women generate vascular wall inflammation and blunt vascular contractility. Am J Pathol. 2006;169:1473–1483. doi: 10.2353/ajpath.2006.051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 32.Branch DW, Mitchell MD, Miller E, Palinski W, Witztum JL. Pre-eclampsia and serum antibodies to oxidised low-density lipoprotein. Lancet. 1994;343:645–646. doi: 10.1016/s0140-6736(94)92639-5. [DOI] [PubMed] [Google Scholar]
- 33.Diedrich F, Renner A, Rath W, Kuhn W, Wieland E. Lipid hydroperoxides and free radical scavenging enzyme activities in preeclampsia and HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: no evidence for circulating primary products of lipid peroxidation. Am J Obstet Gynecol. 2001;185:166–172. doi: 10.1067/mob.2001.115281. [DOI] [PubMed] [Google Scholar]
- 34.Gratacos E, Casals E, Deulofeu R, et al. Serum antibodies to oxidized low-density lipoprotein in pregnant women with preeclampsia and chronic hypertension: lack of correlation with lipid peroxides. Hypertens Pregnancy. 2001;20:177–183. doi: 10.1081/PRG-100106967. [DOI] [PubMed] [Google Scholar]