Abstract
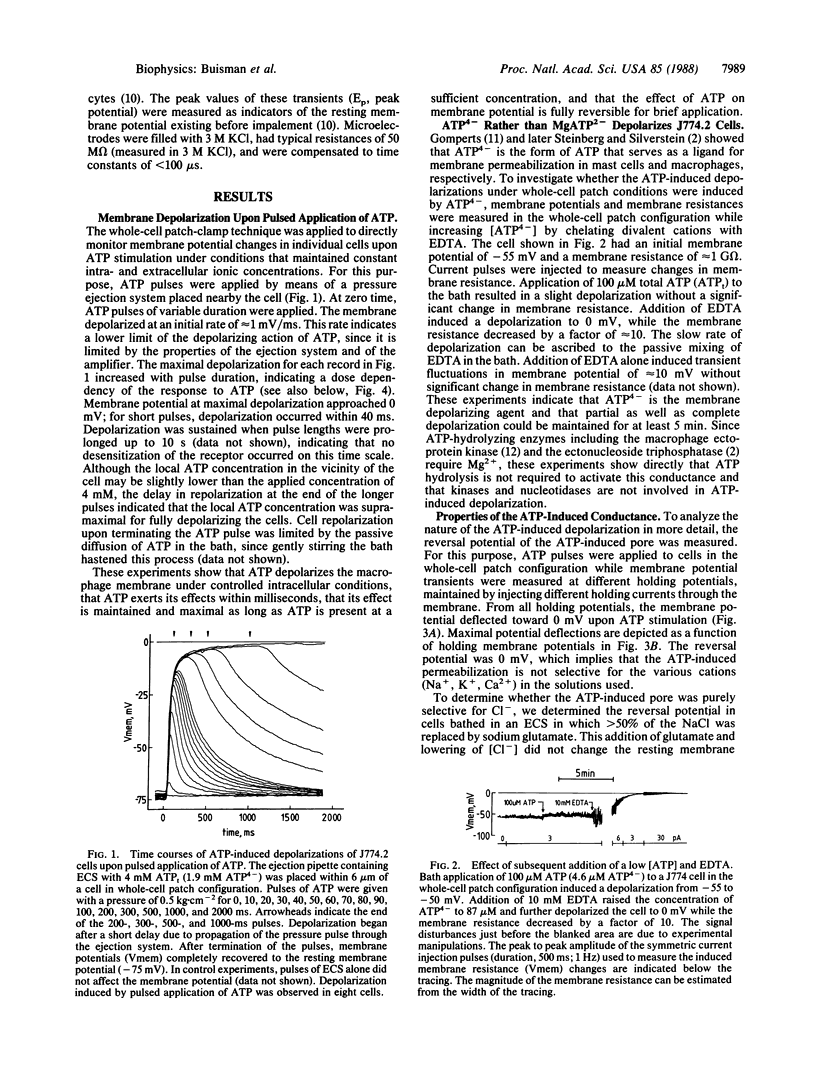
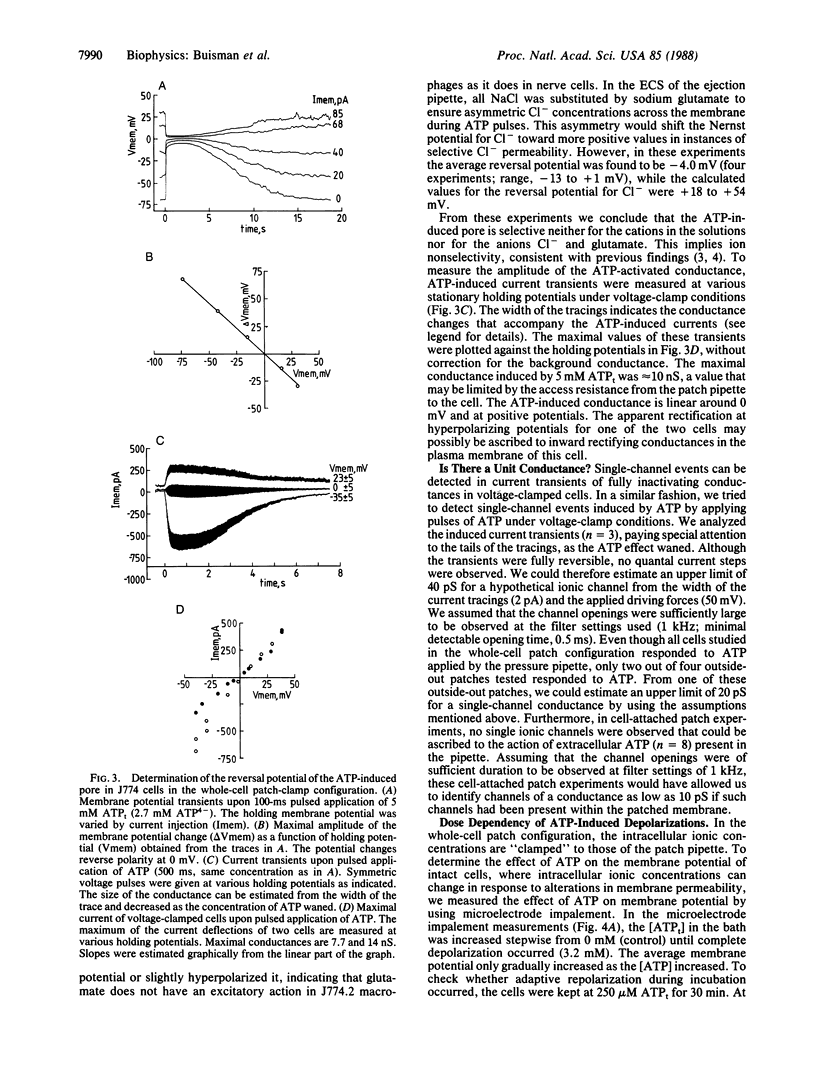
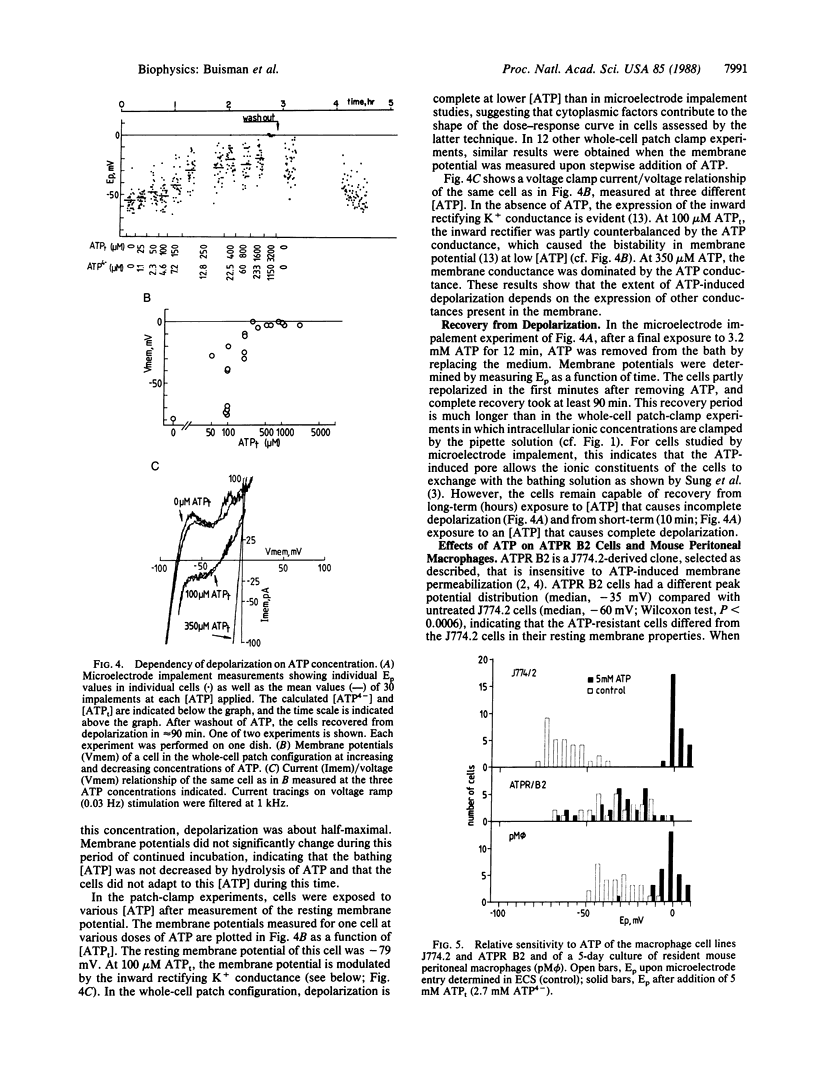
Extracellular ATP in its tetra-anionic form (ATP4-) induces ion fluxes and membrane depolarization in the mouse macrophage-like cell line J774.2 and in resident mouse macrophages. We analyzed the effects of extracellular ATP4- by both patch-clamp and intracellular microelectrode techniques. Whole-cell patch-configuration membrane potential measurements on J774.2 cells revealed that ATP4- -induced depolarization occurred within 40 ms of pulsed application of ATP and was completely reversible. The depolarizations were accompanied by a dramatic increase in membrane conductance and showed no sign of adaptation to ATP over a period of 30 min. At 5 mM total ATP (ATPt) the whole-cell conductance was approximately 10 nS, and an upper limit of 20 pS for a single-channel conductance has been established. The reversal potential associated with the ATP-induced depolarization at asymmetric K+, Na+, Ca2+, and Cl- concentrations across the membrane was 0 mV. In patch-clamped cells depolarization was complete at 20 microM ATP4-, and repolarization from full depolarization occurred in approximately 5 s. In contrast, in intact cells measured by microelectrode impalement, complete depolarization occurred at approximately 2 mM ATP4- and repolarization was much slower (approximately 100 min). These findings indicate that the changes in intracellular ionic composition that occur after ATP treatment affect the rate of cell repolarization. At lower concentrations of ATP, potassium conductances modulated the depolarizing effect of ATP. ATP also depolarized mouse peritoneal macrophages, but a variant cell line (ATPR B2), derived from J774.2 cells by prolonged exposure to ATP, was insensitive to ATP. Our results provide a membrane electrophysiological description and analysis of a large nonselective plasma membrane conductance of macrophages induced by extracellular ATP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano F., Kitagawa T., Akamatsu Y. Protein kinase activity on the cell surface of a macrophage-like cell line, J774.1 cells. Biochim Biophys Acta. 1984 Mar 23;803(3):163–173. doi: 10.1016/0167-4889(84)90006-5. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981 Aug;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gallin E. K., Livengood D. R. Inward rectification in mouse macrophages: evidence for a negative resistance region. Am J Physiol. 1981 Jul;241(1):C9–17. doi: 10.1152/ajpcell.1981.241.1.C9. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Sheehy P. A. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. J Physiol. 1985 Dec;369:475–499. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ince C., Leijh P. C., Meijer J., Van Bavel E., Ypey D. L. Oscillatory hyperpolarizations and resting membrane potentials of mouse fibroblast and macrophage cell lines. J Physiol. 1984 Jul;352:625–635. doi: 10.1113/jphysiol.1984.sp015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., van Bavel E., van Duijn B., Donkersloot K., Coremans A., Ypey D. L., Verveen A. A. Intracellular microelectrode measurements in small cells evaluated with the patch clamp technique. Biophys J. 1986 Dec;50(6):1203–1209. doi: 10.1016/S0006-3495(86)83563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., van Dissel J. T., Diesselhoff M. M. A teflon culture dish for high-magnification microscopy and measurements in single cells. Pflugers Arch. 1985 Mar;403(3):240–244. doi: 10.1007/BF00583594. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975 Oct 2;257(5525):393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Steinberg T. H., Silverstein S. C. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987 Mar 5;262(7):3118–3122. [PubMed] [Google Scholar]
- Sung S. S., Young J. D., Origlio A. M., Heiple J. M., Kaback H. R., Silverstein S. C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985 Nov 5;260(25):13442–13449. [PubMed] [Google Scholar]
- Ypey D. L., Clapham D. E. Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc Natl Acad Sci U S A. 1984 May;81(10):3083–3087. doi: 10.1073/pnas.81.10.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


