Abstract
Electrically assisted delivery is noninvasive and has been investigated in a number of ocular drug delivery studies. The objectives of this study were to examine the feasibility of electrically assisted delivery of macromolecules such as small interfering RNA (siRNA) into the corneal epithelium, to optimize the iontophoresis and electroporation methods, and to study the mechanisms of corneal iontophoresis for macromolecules. Anodal and cathodal iontophoresis, electroporation and their combinations were the methods examined with mice in vivo. Cyanine 3 (Cy3) glyceraldehyde 3-phosphate dehydrogenease (GAPDH) siRNA and fluorescein isothiocyanate (FITC)-dextran of different molecular weights (4 to 70 kDa) were the macromolecules studied. Microscopy and histology after cryostat sectioning were used to analyze and compare the delivery of the macromolecules to the cornea. Iontophoresis was effective in delivering siRNA and dextran up to 70 kDa into the cornea. The electroporation method studied was less effective than that of iontophoresis. Although both iontophoresis and electroporation alone can deliver the macromolecules into the cornea, these methods alone were not as effective as the combination of iontophoresis and electroporation (iontophoresis followed by electroporation). The significant enhancement of dextran delivery in anodal iontophoresis suggests that electroosmosis can be a significant flux enhancing mechanism during corneal iontophoresis. These results illustrate the feasibility of electrically assisted delivery of macromolecules such as siRNA into the cornea.
Keywords: macromolecule, siRNA, iontophoresis, electroporation, cornea
1. Introduction
Many eye diseases such as macular degeneration and glaucoma have genetic components (Blair-Parks et al., 2002) and are chronic in nature. The use of gene therapy to treat these diseases is therefore attractive due to the potential of its long term effect and ability to target genetic mutations (Bainbridge et al., 2006; Fattal and Bochot, 2006; Klausner et al., 2007). Although recent progress in genetic engineering and molecular biology has provided new therapeutic entities for the treatment of eye disease, the development of a noninvasive and effective method to deliver macromolecules in gene therapy has lagged behind. In addition to improving therapy, an effective method to noninvasively deliver macromolecules into the eye can be a useful research tool in ocular research.
Since the first demonstration of the silencing effect of the small interfering RNA (siRNA) in mammal cells in vitro (Elbashir et al., 2001) and in vivo (McCaffrey et al., 2002), siRNA technology has emerged as a potential tool for treating various diseases (Dykxhoorn et al., 2006). siRNA is a double-stranded RNA of around 21–23 base-paired nucleotides. It is highly negatively charged (~40 negative charges) and has molecular weight of approximately 13 kDa (Gilmore et al., 2006; Kim and Kim, 2009). The in vitro and in vivo delivery of siRNA into different cells using viral and non-viral vector carrier systems have been studied (de Fougerolles, 2008). The key factors influencing successful delivery of siRNA are cell uptake and retention of siRNA in the cytoplasm where the silencing effect of siRNA takes place. Electrically assisted delivery is believed to be particularly useful for the delivery of siRNA due to the multiple charges on the molecule.
The feasibility of gene delivery to corneal cells in vitro has been investigated previously (Kao, 2002; Jun and Larkin, 2003). Effective gene delivery to corneal cells has been demonstrated in vivo. For example, gene gun was used to disrupt cell membranes and tight junction barriers to introduce foreign genes into intact corneal epithelium (Tanelian et al., 1997; Shiraishi et al., 1998; Wang et al., 2002). Intrastromal injection (Jani et al., 2007; Singh et al., 2007), the combination of intrastromal injection and electroporation (Blair-Parks et al., 2002; Oshima et al., 2002; Zhou and Dean, 2007) or ultrasound (Sonoda et al., 2006; Yamashita et al., 2007), and intracameral injection to the anterior chamber followed by electroporation (Oshima et al., 1998; Sakamoto et al., 1999) have also been investigated. However, noninvasive and effective corneal delivery of macromolecules in vivo remains a challenge. It has been hypothesized that electrically assisted drug delivery such as iontophoresis and electroporation and/or their combination can be used to achieve noninvasive and effective delivery of macromolecules to the cornea.
Transcorneal iontophoresis is the use of an electric field to enhance the delivery of compounds into and across the cornea. The adverse effects of ocular iontophoresis have been studied and reviewed (Halhal et al., 2004; Myles et al., 2005; Eljarrat-Binstock and Domb, 2006). Transcorneal iontophoresis has been investigated previously for the delivery of macromolecules such as oligonucleotides into the eye (Asahara et al., 2001; Voigt et al., 2002; Berdugo et al., 2003). This technique has also been studied extensively for the delivery of small molecules such as ciprofloxacin (Vaka et al., 2008), dexamethasone phosphate (Eljarrat-Binstock et al., 2005), miconazole (Yoo et al., 2002), tobramycin (Maurice, 1989), and vancomycin (Choi and Lee, 1988). Positively charged fluorescent nanoparticles demonstrated better penetration into rabbit eyes than negatively charged nanoparticles in transcorneal iontophoresis (Eljarrat-Binstock et al., 2008). Although these studies have shown the feasibility of transcorneal iontophoresis, the mechanisms of iontophoretic drug delivery to the cornea have not been fully understood. The mechanisms of iontophoretic transport across a mucosal membrane such as the cornea are believed to be the direct interaction of the electric field with the charge of the ionic permeant (electrophoresis or electromigration), convective solvent flow that affects the transport of both neutral and ionic compounds (electroosmosis), and electric field-induced pore formation in the membrane (electroporation or electro-permeabilization) (Bejjani et al., 2007). To optimize iontophoretic delivery of macromolecules into the cornea, the contributions of these mechanisms in iontophoretic transport in the cornea should be investigated.
The objectives of the present study were to (a) examine the feasibility of delivering a model siRNA into the corneal epithelium with iontophoresis and electroporation, (b) study the mechanisms of corneal iontophoresis for macromolecules, and (c) identify a protocol that was effective in delivering the siRNA without tissue damages. Table 1 summarizes the experimental protocols in the present study.
Table 1.
Summary of the experiments.a
| Permeant | Protocol | Delivery and enhancing mechanism |
Purpose |
|---|---|---|---|
| Dextran | 2 mA anodal or cathodal iontophoresis for 10 s |
Electroosmosis and electro- permeabilization |
Determine the effect of electroosmosis |
| Dextran | 0.5 mA anodal iontophoresis for 1 min |
Electroosmosis and electro- permeabilization |
Determine the effect of electric current |
| Dextran, GAPDH siRNA |
Passive delivery for 1 min | Diffusion, effect of the solution, device physical effect |
Passive control |
| GAPDH siRNA | 0.5 mA anodal or cathodal iontophoresis for 1 min |
Electrophoresis, electroosmosis, and electro-permeabilization |
Determine the effect of electrophoresis; examine the delivery over 24 hr |
| GAPDH siRNA | 20 V (8 pulses, 50 ms length, 1 s interval) anodal or cathodal electroporation |
Electroporation and diffusion | Examine the delivery over 24 hr; compare electroporation with iontophoresis |
| GAPDH siRNA | Combined 0.5 mA cathodal iontophoresis and 20 V anodal (or cathodal) electroporation |
Electrophoresis, electroosmosis, electro-permeabilization, and diffusion |
Examine the delivery over 24 hr |
n ≥ 3 replicates in each experimental condition.
2. Methods
2.1. Materials
Phosphate-buffered saline (PBS) of pH 7.4 (consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride) was prepared by dissolving PBS tablets (Sigma-Aldrich, St. Louis, MO) in distilled, deionized water. Fluorescein isothiocyanate (FITC)-labeled dextrans with average molecular weights of 4, 20, and 70 kDa (degree of substitution 0.004–0.005 mol FITC/mol of glucose) were purchased from Sigma-Aldrich (St. Louis, MO). Dextran solutions (2 mg/mL) were prepared by dissolving appropriate amounts of dextran powder in PBS. Cyanine 3 (Cy3)-labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) siRNA was purchased from Ambion (Austin, TX). The siRNA has the following sequences: sense strand 5’-GGU CAU CCA UGA CAA CUU UTT-3’ and antisense strand 5’-AAA GUU GUC AUG GAU GAC CTT-3’. The siRNA powder was reconstituted in ribonuclease (RNase)-free water provided by the manufacturer at 50 μM. Paraformaldehyde (PFA) solution of 4% (w/v) was prepared by diluting 16% (w/v) PFA solution (EM grade, Electron Microscopy Sciences, Hatfield, PA) in PBS. 4′,6′-Diamidino-2-phenylindole (DAPI) was from Sigma-Aldrich (St. Louis, MO). Sucrose (EM Science, Gibbstown, NJ) solutions of 10%, 20%, and 30% (w/v) were prepared in distilled, deionized water. The dextrans and siRNA were checked for purity using size exclusion chromatography (SEC) and gel electrophoresis. Briefly, SEC was performed using a Shimadzu high performance liquid chromatography system (SIL-20A autosampler, SPD-20A Prominence UV/VIS detector, LC-20AT pump) and TSKgel™ G4000 PWXL column (300 mm × 7.8 mm, particle size 10 µm, pore size 500 Å, Tosoh Bioscience, LLC., Montgomeryville, PA). Gel electrophoresis experiments were performed with 0.1% (w/v) agar gel at 150 V for 30 min and the bands were assayed with Epichem Darkroom UVP bioimaging system (Upland, CA). All the dextrans and siRNA had a purity of > 97%. All materials were used as received.
2.2. Animals
Mice (Male, 4–6 weeks, C57BL/6) supplied from Harlan Sprague Dawley (Indianapolis, IN) were used in the study. Some preliminary and confirmation studies were conducted with mice (wild type) obtained from other studies at the University of Cincinnati after they were released from the investigators. All experiments were conducted in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and with approval of the Institutional Animal Care and Use Committee at the University of Cincinnati.
2.3. Animal corneal delivery in vivo
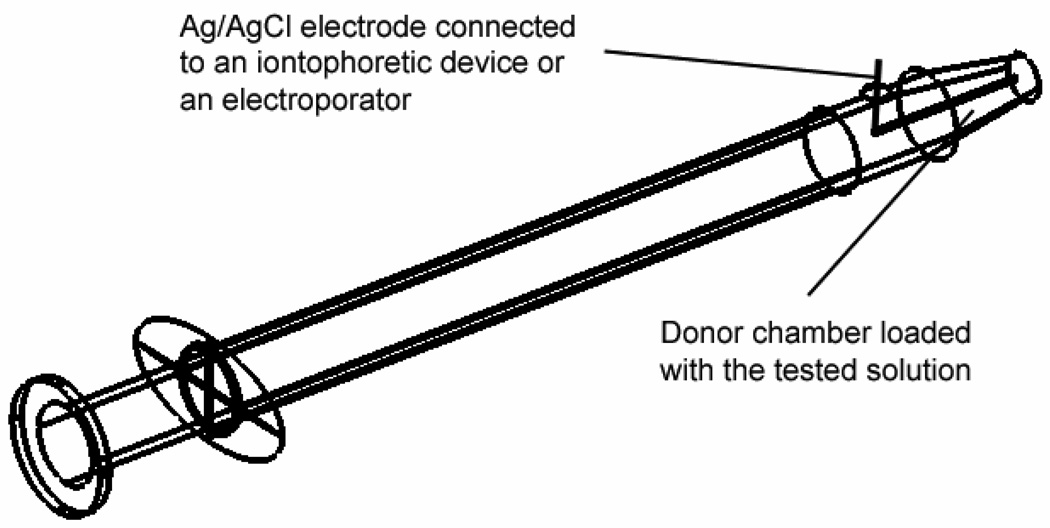
The mice were anesthetized by intraperitoneal injection of 10 mg/kg xylazine (Phoenix Pharmaceutical, Inc., St. Joseph, MO) and 80 mg/kg ketamine (Phoenix Pharmaceutical, Inc., St. Joseph, MO) before the experiments. The iontophoresis and electroporation protocols are described in the next section. The test solution (dextran and siRNA solutions) was applied on the cornea of a mouse with the aid of a custom-designed dosing device (Fig. 1). The device was constructed from a BD Tuberculin SlipTip™ 1-mL syringe (BD Medical, Franklin Lakes, NJ), which had an orifice of 2 mm2 at the open end of the tube after the removal of the needle attached. A small hole was drilled on the wall of the syringe barrel around the 0.05-mL mark. Ag/AgCl electrode (as a driving electrode) was inserted in the syringe through this hole such that one end of the electrode was close to the orifice of the syringe and the other end of the electrode was outside the hole to connect to either the positive or negative lead (depending on the study) of a constant current iontophoretic device (Phoresor II Auto, Model PM 850, Iomed, Inc., Salt Lake City, UT) or an electroporator (Electro Square Porator, ECM 830, BTX, Inc., Holliston, MA). The tip of the device (the 2 mm2 opening) was then loaded with 20 µL test solution, gently placed on the cornea surface, and held for a predetermined period depending on the delivery protocols. An alligator clip (as a reference electrode) connecting to the Phoresor or the electroporator was clamped on PBS saturated Kimwipes® on the ear opposite to the treated eye to complete the electric circuit. The electric current and/or electric pulses were applied and the voltage across the eye (i.e., two electrodes) was measured with a multimeter (Fluke 73III, Everett, WA) or portable oscilloscope (Fluke ScopeMeter 123). The eye was rinsed with PBS after the applications of the test solution. The cornea was observed under a fluorescence stereomicroscope (Stemi SV 11 Apo, Carl Zeiss MicroImaging, Inc., Thornwood, NY) with either a green or red color filter to assess the location and intensity of the fluorescence. Unless otherwise stated, similar levels of fluorescence (or relatively reproducible results) were observed for all eyes treated under the same delivery protocol.
Fig. 1.
Schematic diagram of the dosing device.
2.4. Iontophoresis and electroporation protocols
Iontophoresis, electroporation, and their combinations were tested for the capability to deliver neutral dextrans and negatively charged siRNA into the cornea. Anodal (anode electrode in the delivering chamber) and cathodal (cathode electrode in the delivering chamber) iontophoresis of 0.5 mA for 1 min and 2 mA for 10 s were the iontophoresis protocols used in the present study, corresponding to electric current densities of approximately 25 and 100 mA/cm2, respectively. These protocols provided total electric current doses of approximately 32 and 50 mA·s, respectively, due to the time required for the iontophoresis device to reach the target electric current—the ramping time was about 8 s from 0 to 0.5 mA and about 30 s from 0 to 2 mA. Eight electric pulses at 20 V with a pulse length of 50 ms and a pulse interval length of 1 s were the electroporation protocol. Both anodal (anode electrode in the delivering chamber) and cathodal (cathode electrode in the delivering chamber) electroporation protocols were tested. In the combination protocol, 0.5 mA iontophoresis was applied on the cornea for 1 min, followed by eight 20 V electric pulses (the same electroporation protocol as described above). The same combined iontophoresis and electroporation treatments using only PBS was the control. Passive delivery experiments in which the same device was applied on the cornea for 1 min (without current or electric pulses applied) were the other control.
2.5. Histology study
The mice were sacrificed after the examination of the cornea under the fluorescence stereomicroscope. The whole eyes were immediately fixed in 4% PFA solution overnight at 4°C for cryosectioning. The fixed eyes were transferred and soaked in sucrose solutions of gradually increased concentrations of 10%, 20%, and then 30% (w/v) over 1 day to allow for equilibration. The eyes were embedded in a sample block containing optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc., Torrance, CA) under a stereomicroscope (Stemi DV 4, Carl Zeiss MicroImaging, Inc., Thornwood, NY) and stored at −80°C until cryostat sectioning. The sample block was sectioned into 10-µm sections with a cryostat microtome (Microm HM560, Micron Instruments, Inc., San Marcos, CA). The sections were stained with DAPI for histology examination by fluorescence microscopy (Nikon Eclipse E800, Nikon Instruments, Inc., Melville, NY).
3. Results
3.1. Dextran delivery
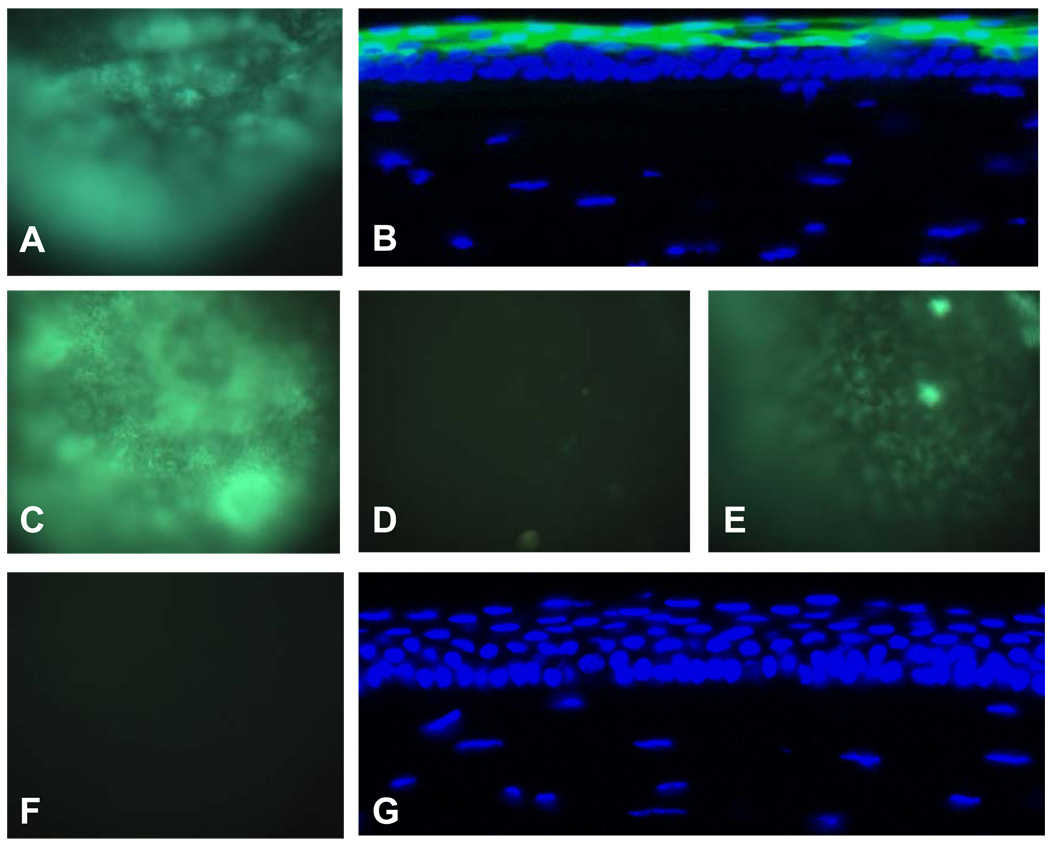
Figs. 2A and 2B show the images of the cornea treated with 2 mA anodal iontophoresis of FITC-labeled dextran of 70 kDa, obtained in vivo and after cryostat sectioning, respectively. All the eyes treated (n = 3) showed the same high fluorescence level in the cornea in vivo (Fig. 2A), suggesting that dextran was successfully delivered into the cornea. The green fluorescence from FITC-labeled dextran overlapped with the blue fluorescence from DAPI in the corneal epithelium (Fig. 2B). No detectable dextran was delivered into the corneal stroma. Similar results were also obtained with dextrans of 4 and 20 kDa (data not shown). These results suggest the feasibility of delivering macromolecules up to 70 kDa into the corneal epithelium with anodal iontophoresis.
Fig. 2.
Representative images of the cornea after iontophoretic and passive delivery of FITC-labeled dextrans (n ≥ 3). The fluorescence images were acquired immediately after the treatments. A: in vivo image, 2 mA anodal iontophoresis of dextran 70 kDa; B: cryostat section image, 2 mA anodal iontophoresis of dextran 70 kDa; C: in vivo image, 2 mA anodal iontophoresis of dextran 20 kDa; D: in vivo image, 2 mA cathodal iontophoresis of dextran 20 kDa; E: in vivo image, 0.5 mA anodal iontophoresis of dextran 20 kDa; F: in vivo image, passive delivery of dextran 20 kDa; and G: cryostat section image, passive delivery of dextran 20 kDa. All in vivo images: magnification 20×; all cryostat section images: magnification 400×.
Figs. 2C and 2D compare the in vivo images of the cornea after 2 mA anodal and cathodal iontophoresis of 20 kDa FITC-labeled dextran. The image taken after anodal iontophoresis in Fig. 2C (n = 5) shows stronger fluorescence intensity than that after cathodal iontophoresis in Fig. 2D (n = 5), suggesting that dextran was more effectively delivered by anodal iontophoresis than cathodal iontophoresis at the same current density. Because dextran is uncharged, electrophoresis is not the flux enhancing mechanism in dextran iontophoretic transport. The results of Figs. 2C and 2D suggest that electroosmosis facilitates the delivery of neutral macromolecules during anodal iontophoresis and is a significant flux enhancing mechanism in corneal iontophoretic delivery. This result is consistent with the negatively charged nature of the cornea at pH 7.4 (Monti et al., 2003).
Fig. 2E shows the in vivo images taken after 0.5 mA anodal iontophoresis (n = 5). When the anodal current intensity was decreased from 2 to 0.5 mA, the voltage output of the Phoresor decreased from approximately 18 to 9 V. A drastic decrease in the fluorescence intensity was observed in the image taken after the 0.5 mA treatment (Fig. 2E) compared with that after the 2 mA treatment (Fig. 2C) even though the electric current doses in the 2 mA treatment were only ~50% higher than that in the 0.5 mA protocol (50 vs. 32 mA·s). Enhanced delivery of neutral permeants due to iontophoresis is greater when higher electric current is applied.
In the passive control experiments, FITC-labeled dextran (e.g., 70 kDa) solutions were applied for 1 min without iontophoresis or electroporation treatments (passive controls). No fluorescence (n = 3) was detected in the cornea in these control experiments (Figs. 2F and 2G, in vivo and cryostat, respectively), indicating no significant dextran delivered into the cornea by passive delivery.
3.2. siRNA delivery
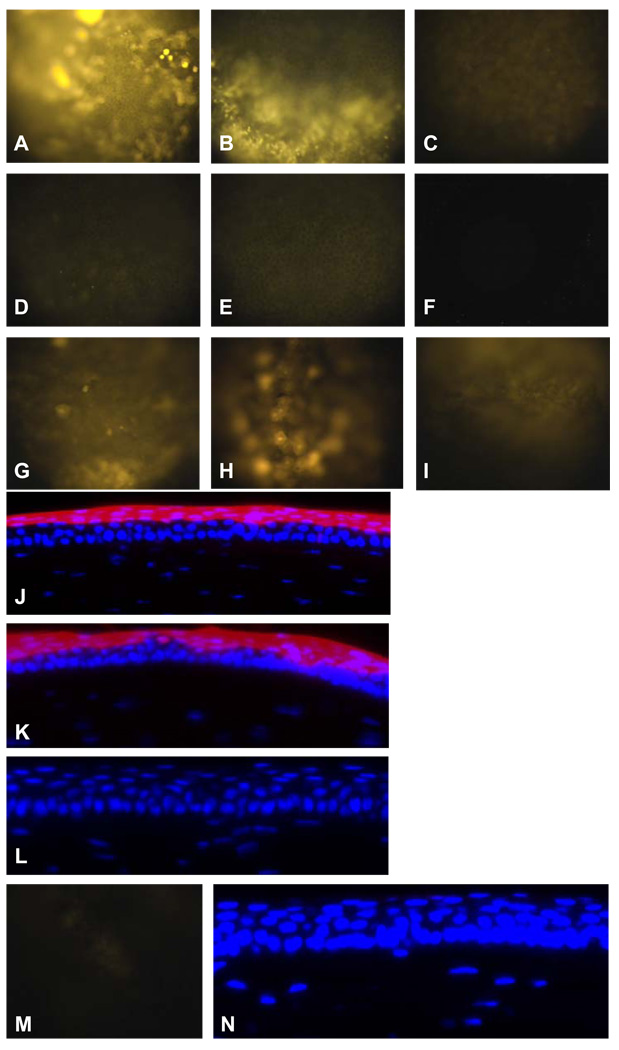
Figs. 3A–3C present the 6-hr images of the corneas treated with the combined protocol of cathodal iontophoresis and anodal electroporation, 0.5 mA cathodal iontophoresis protocol, and 20 V anodal electroporation protocol, using Cy3-labeled GAPDH siRNA. The images taken immediately after the treatments (0 hr data) are not included in Fig. 3 because the images after cathodal iontophoresis and the combination protocol were overexposed at the exposure time of 200 ms used in all the experiments. Generally, the fluorescence intensity is strongest in the eye treated with the combination protocol (Fig. 3A, n = 9) and is weakest in the eye with the anodal electroporation protocol (Fig. 3C, n = 3). Among the nine corneas treated with the combination protocol, six corneas showed the same high level of fluorescence and three showed slightly weaker fluorescence. Anodal electroporation is less effective than cathodal iontophoresis to deliver the siRNA (Fig. 3B, n = 3). Figs. 3D–3F present the 24-hr images of the corneas treated with the combined protocol, 0.5 mA cathodal iontophoresis, and 20 V anodal electroporation. At 24 hr after the treatments, the fluorescence intensity greatly decreased—dimmer but detectable level of fluorescence was observed in the cornea with both the combination (Fig. 3D, n = 5) and the iontophoresis (Fig. 3E, n = 3) protocols. No detectable fluorescence was observed in the eye with the anodal electroporation protocol at 24 hr after the treatment (Fig. 3F, n = 3). Among the five corneas treated with the combination protocol, four corneas showed the same level of fluorescence as shown in the figure and one showed higher level of fluorescence than the other four. The combination of iontophoresis and electroporation is better than either cathodal iontophoresis or anodal electroporation alone for the retention of siRNA in the cornea. It is interesting to note that the voltage output of the Phoresor in the 0.5 mA siRNA cathodal iontophoresis study was similar to that in the 0.5 mA dextran anodal iontophoresis (~9 V) even though the concentrations of the ions in the siRNA and dextran solutions were different.
Fig. 3.
Representative images of the cornea after iontophoresis, electroporation, and passive delivery of Cy3-labeled GAPDH siRNA (n ≥ 3). A: 6-hr in vivo image, combination of 0.5 mA cathodal iontophoresis and 20 V anodal electroporation; B: 6-hr in vivo image, 0.5 mA cathodal iontophoresis: C: 6-hr in vivo image, 20 V anodal electroporation; D: 24-hr in vivo image, combination of 0.5 mA cathodal iontophoresis and 20 V anodal electroporation; E: 24-hr in vivo image, 0.5 mA cathodal iontophoresis; F: 24-hr in vivo image, 20 V anodal electroporation; G: 0-hr in vivo image, 20 V anodal electroporation; H: 0-hr in vivo image, 20 V cathodal electroporation; I: 0-hr in vivo image, 0.5 mA anodal iontophoresis; J: 0-hr cryostat section image, combination of 0.5 mA cathodal iontophoresis and 20 V anodal electroporation; K: 6-hr cryostat section image, combination of 0.5 mA cathodal iontophoresis and 20 V anodal electroporation; L: 24-hr cryostat section image, combination of 0.5 mA cathodal iontophoresis and 20 V anodal electroporation; M: 0-hr in vivo image, passive delivery; N: 0-hr cryostat section image, passive delivery. All in vivo images: magnification 20×; all cryostat section images: magnification 400×.
Figs. 3G–3I show the in vivo images of the cornea immediately after the treatments of 20 V anodal electroporation, 20 V cathodal electroporation, and 0.5 mA anodal iontophoresis, respectively (0 hr data). Much weaker fluorescence image was observed in the eye treated with these protocols compared to those after the combined protocol of cathodal iontophoresis and electroporation and cathodal iontophoresis alone. Comparing Figs. 3G and 3H, greater siRNA was delivered by cathodal electroporation (Fig. 3H, n = 3) than by anodal electroporation (Fig. 3G, n = 3). This suggests the contribution of electrophoresis to the delivery of the negatively charged siRNA during the cathodal electroporation treatment. Fig. 3I shows weak but detectable fluorescence (n = 4). Therefore, electroosmosis and electro-permeabilization could enhance the delivery of the siRNA (compared with passive delivery). The weaker siRNA delivery by anodal iontophoresis (Fig. 3I) than that of electroporation (Figs. 3G and 3H) suggests a significant contribution of electrophoresis. Although the 0-hr image of the eye treated with 20 V cathodal electroporation (Fig. 3H) showed stronger fluorescence than that of 20 V anodal electroporation (Fig. 3G), no drastic difference in the fluorescence intensity was observed in the 6-hr images of the eyes treated with the combination protocol of cathodal iontophoresis followed by cathodal electroporation and that of cathodal iontophoresis followed by anodal electroporation (data not shown). This is likely a result of the dominant effect of iontophoresis on the delivery of charged macromolecules like siRNA masking the effect of electroporation in the present study.
Figs. 3J–3L presents the cross-section cryostat images of corneas immediately and at 6 and 24 hr after the treatment of Cy3-labeled siRNA with the combined protocol of cathodal iontophoresis and anodal electroporation (n = 3). The red fluorescence of Cy3 observed in the corneal epithelium after the treatment suggests the delivery and retention of siRNA in the corneal epithelium (Figs. 3J and 3K). The high level of Cy3 fluorescence in the upper corneal epithelial cell layers shown in the images was believed to be both extracellular and intracellular as indicated by whole mount confocal microscopy in a separate study (data not shown). The siRNA then depleted to below the detection limit 24 hr post-treatment as shown in the cross-section images (Fig. 3L).
Passive delivery of the siRNA was also examined without iontophoresis or electroporation treatments (passive control). Very weak to no fluorescence (n = 3) was observed in the eye after passive delivery of the siRNA in vivo (Fig. 3M), and there was no detectable fluorescence in the cornea after cryostat sectioning (Fig. 3N). In these passive transport experiments, siRNA was delivered only on the cornea surface. This result also indicates no significant corneal delivery of the siRNA due to other factors such as osmolality and ionic strength effects of the siRNA solution.
3.3. Assessment of cornea after iontophoresis and electroporation treatment
The cornea appearance was checked for possible damages under the stereomicroscope after each treatment. The 0.5 mA iontophoresis, 20 V electroporation, and their combinations generally did not cause any cornea damages by visual examination—the appearance of the cornea was normal post-treatments (data not shown). This observation is consistent with the previous report suggesting that the application of 20–25 mA/cm2 iontophoresis for 5 min was relatively safe (Hughes and Maurice, 1984). However, cloudiness on the cornea was seen in our preliminary delivery study of siRNA using 2 mA cathodal iontophoresis, indicating corneal tissue damages. Interestingly, no corneal opacity was observed when 2 mA iontophoresis was applied in the dextran experiments in which the donor solution was dextran in PBS. Therefore, the 2 mA iontophoresis protocol was used only in the dextran experiments.
4. Discussion
4.1. Electrically assisted delivery of macromolecules
The cornea is primarily composed of epithelial, stromal, and endothelial layers. Tight junctions are present in the epithelium forming a major barrier to the delivery of molecules into the cornea (Hamalainen et al., 1997). For the delivery of hydrophilic molecules, the paracellular route of the epithelial layer is the predominant pathway, and the permeability of the epithelium is a function of their molecular size. The results of the passive delivery study (Fig. 2F, 2G, Fig. 3M, and 3N) indicate that hydrophilic macromolecules dextrans and siRNA were not effectively delivered to the mouse corneal epithelium without the assistance of iontophoresis or electroporation.
With the present iontophoresis and electroporation protocols, the feasibility to deliver macromolecules to the corneal epithelium was demonstrated. Ocular iontophoresis is noninvasive compared to other techniques such as intrastromal injection. Iontophoresis was effective in delivering macromolecules such as siRNA and dextran up to 70 kDa into the cornea. Electroporation was less effective than iontophoresis. While iontophoresis alone could deliver the macromolecules into the cornea, the retention (or extent of cell penetration) of the macromolecules in the cornea was shorter after iontophoresis than in the combined iontophoresis and electroporation protocol. The combination of iontophoresis and electroporation was believed to be critical to effectively deliver these macromolecules into the corneal epithelium with significantly improved retention.
The delivery of genes into corneal cells in vivo has been previously reported (Oshima et al., 1998; Sakamoto et al., 1999; Blair-Parks et al., 2002; Oshima et al., 2002). However, intracameral injection to the anterior chamber (Oshima et al., 1998; Sakamoto et al., 1999), subconjunctival injection (Blair-Parks et al., 2002), or intrastromal injection (Oshima et al., 2002; Zhou and Dean, 2007) was required before electric pulses were applied to deliver plasmid DNA into the corneal cells. The single-stranded antisense oligonucleotide (~7 kDa) was also reported to be effectively delivered into the cornea with iontophoresis (Berdugo et al., 2003). The results in the present study show that the double-stranded siRNA with molecular weight around 13 kDa can be successfully delivered into the cornea in vivo by using iontophoresis and electroporation, which is needle-free and noninvasive. To our knowledge, this is the first study of electrically assisted noninvasive corneal delivery of siRNA. The successful delivery of the model siRNA to the corneal epithelium in the present study suggests that iontophoresis and electroporation can be a useful technique to deliver therapeutic siRNAs for the treatment of cornea diseases although further optimization may be required for the technique in clinical therapy. For example, fast clearance and depletion of siRNA from the corneal epithelium imply the necessity of multiple dosing in clinical therapy. This noninvasive method can also be a useful research tool in ocular research. An ongoing study is to determine the activity of a targeting siRNA after the siRNA is delivered into the corneal epithelium using the combined iontophoresis and electroporation protocol.
4.2. Mechanisms of electrically assisted transport of macromolecules
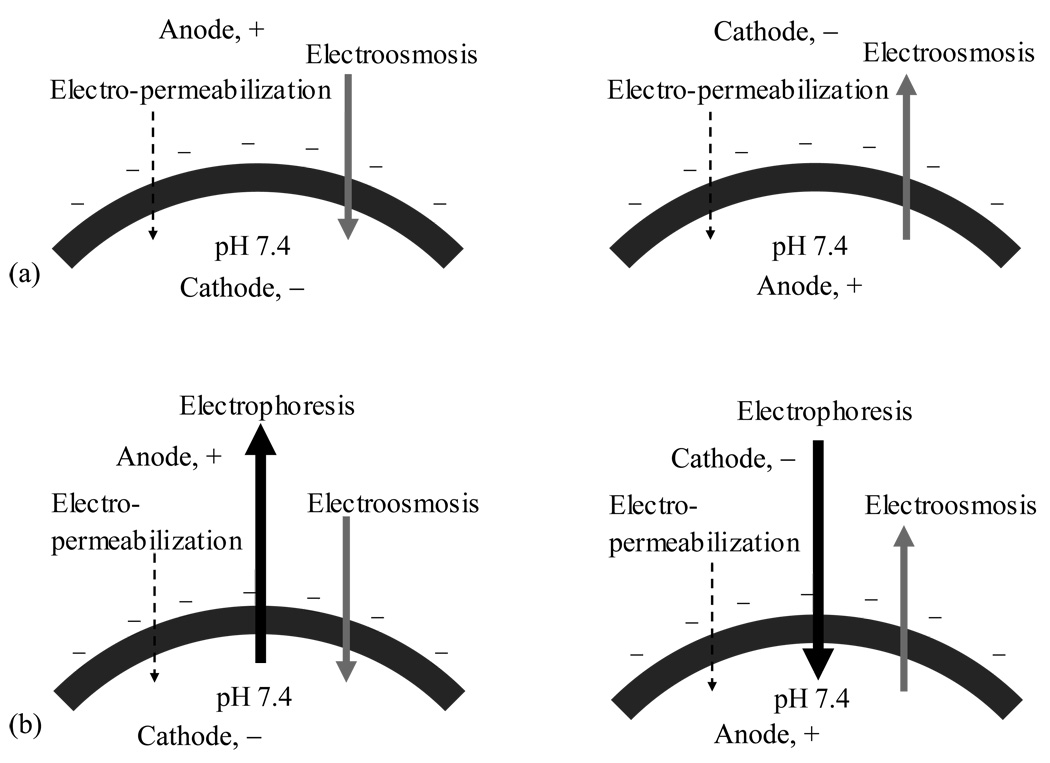
The present findings on the mechanisms of electrically assisted transport of dextrans and siRNA into the corneal epithelium are summarized in a schematic diagram in Fig. 4. Anodal iontophoresis enhanced the delivery of dextrans into the cornea, and cathodal iontophoresis was found not to be effective. The significant enhancement of the delivery of the neutral permeants with anodal iontophoresis suggests that electroosmosis was a flux enhancing mechanism in corneal iontophoretic delivery of macromolecules. In addition, the lack of enhancement in cathodal iontophoresis for dextrans suggests that iontophoresis-induced permeabilization was less effective than electroosmosis in the delivery of these macromolecules; if the effect of electro-permeabilization was significantly greater than that of electroosmosis, enhancement should be observed during cathodal iontophoresis (compared to that of anodal iontophoresis). It should be noted that the concentrations of dextrans applied were the same in their respective anodal and cathodal iontophoresis experiments, and this allowed the direct comparison of the extent of dextran delivery in these experiments. For siRNA, electrophoresis was found to be an important flux enhancing mechanism. Significant enhancement was observed in the delivery of siRNA into the cornea with cathodal iontophoresis. Because electroosmosis was from the anode to cathode in the cornea (i.e., electroosmosis was working against electrophoresis during cathodal iontophoresis), this also suggests that the effect of electrophoresis was stronger than those of electroosmosis and electro-permeabilization for the delivery of siRNA into the cornea; if the effects of electroosmosis and electro-permeabilization are greater than that of electrophoresis, greater siRNA delivery enhancement would be observed during anodal iontophoresis. Although the amounts of siRNA delivered into the corneal epithelium were not quantified, the comparison of the fluorescent intensity of siRNA in the cornea delivered from the same siRNA solution in the experiments allowed the comparison of the effects of the different flux enhancing mechanisms in electrically facilitated transport of siRNA. The siRNA results suggest that electrophoresis is the major flux enhancing mechanism for polyelectrolytes in corneal iontophoretic delivery.
Fig. 4.
Illustration of the mechanisms of anodal (left panel) and cathodal (right panel) iontophoretic transport of (a) neutral dextrans and (b) negatively charged siRNA across the negatively charged cornea under the normal physiological condition. From the results of dextrans, the rank order of the effects is: electroosmosis > electro-permeabilization. From the results of siRNA, the rank order of the effects is: electrophoresis > electroosmosis > electro-permeabilization.
Electroporation assists the transport of permeants by creating transient pores in a cell membrane (Glaser et al., 1988). The permeant is transported through these pores mainly by diffusion with some contributions from electrophoresis and electroosmosis. The pores would then reseal over time after the application of the electric field (Benz and Zimmermann, 1981). The present study suggests that the combination of cathodal iontophoresis and electroporation is effective in delivering and retaining siRNA in the corneal epithelium. siRNA was delivered into the corneal epithelium and was retained longer in the epithelium under the combined cathodal iontophoresis and electroporation protocol than that of iontophoresis alone. One possible explanation is that cathodal iontophoresis drove the negatively charged siRNA into the epithelium and electroporation further assisted the delivery by permeabilizing the cell membrane. While greater delivery of the siRNA with cathodal electroporation than with anodal electroporation suggested the contribution of electrophoresis during cathodal electroporation delivery, this effect was masked by the dominant effect of iontophoresis in the combination protocols. This may account for the observed indifference between these two different combination protocols (the combination protocol of cathodal iontophoresis and anodal electroporation vs. the combination of cathodal iontophoresis and cathodal electroporation).
5. Conclusions
A model siRNA was successfully delivered into the corneal epithelium by cathodal iontophoresis alone. Combining cathodal iontophoresis and electroporation enhanced siRNA delivery and prolonged its retention in the cornea. The successful delivery of dextrans up to 70 kDa by anodal iontophoresis suggests the feasibility of electrically facilitated corneal delivery of macromolecules with molecular sizes larger than siRNA. The present study also examined the interplay of electrophoresis, electroosmosis, and electro-permeabilization in macromolecule delivery to the cornea. The effect of electroosmosis was significant as demonstrated in the study with dextrans, but for polyelectrolytes such as siRNA, electrophoresis was the dominant flux-enhancing mechanism over electroosmosis and electro-permeabilization.
Acknowledgements
This research was supported in part by NIH grants EY015181, EY010556, and EY011845, Research to Prevent Blindness, and Ohio Lions Eye Research Foundation. The authors thank Dr. Johanna T.A. Meij, Dr. Yujin Zhang, Dr. Hongshan Liu, Dr. Mindy K. Call, Dr. T-H. Young, and Ms. Poonam Chopra for helpful discussion and their help in the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asahara T, Shinomiya K, Naito T, Shiota H. Induction of gene into the rabbit eye by iontophoresis: preliminary report. Jpn J Ophthalmol. 2001;45:31–39. doi: 10.1016/s0021-5155(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–1197. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- Bejjani RA, Andrieu C, Bloquel C, Berdugo M, BenEzra D, Behar-Cohen F. Electrically assisted ocular gene therapy. Surv Ophthalmol. 2007;52:196–208. doi: 10.1016/j.survophthal.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Benz R, Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim Biophys Acta. 1981;640:169–178. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- Berdugo M, Valamanesh F, Andrieu C, Klein C, Benezra D, Courtois Y, Behar-Cohen F. Delivery of antisense oligonucleotide to the cornea by iontophoresis. Antisense Nucleic Acid Drug Dev. 2003;13:107–114. doi: 10.1089/108729003321629647. [DOI] [PubMed] [Google Scholar]
- Blair-Parks K, Weston BC, Dean DA. High-level gene transfer to the cornea using electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi TB, Lee DA. Transscleral and transcorneal iontophoresis of vancomycin in rabbit eyes. J Ocul Pharmacol. 1988;4:153–164. doi: 10.1089/jop.1988.4.153. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Orucov F, Aldouby Y, Frucht-Pery J, Domb AJ. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J Control Release. 2008;126:156–161. doi: 10.1016/j.jconrel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106:386–390. doi: 10.1016/j.jconrel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Fattal E, Bochot A. Ocular delivery of nucleic acids: antisense oligonucleotides, aptamers and siRNA. Adv Drug Deliv Rev. 2006;58:1203–1223. doi: 10.1016/j.addr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Gilmore IR, Fox SP, Hollins AJ, Akhtar S. Delivery strategies for siRNA-mediated gene silencing. Curr Drug Deliv. 2006;3:147–145. doi: 10.2174/156720106776359159. [DOI] [PubMed] [Google Scholar]
- Glaser RW, Leikin SL, Chernomordik LV, Pastushenko VF, Sokirko AI. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Halhal M, Renard G, Courtois Y, BenEzra D, Behar-Cohen F. Iontophoresis: from the lab to the bed side. Exp Eye Res. 2004;78:751–757. doi: 10.1016/j.exer.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- Hughes L, Maurice DM. A fresh look at iontophoresis. Arch Ophthalmol. 1984;102:1825–1829. doi: 10.1001/archopht.1984.01040031483028. [DOI] [PubMed] [Google Scholar]
- Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, Kompella UB, Ambati BK. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- Jun AS, Larkin DF. Prospects for gene therapy in corneal disease. Eye. 2003;17:906–911. doi: 10.1038/sj.eye.6700565. [DOI] [PubMed] [Google Scholar]
- Kao WW. Particle-mediated gene transfer to ocular surface epithelium. Adv Exp Med Biol. 2002;506:1297–1308. doi: 10.1007/978-1-4615-0717-8_189. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Kim SW. Efficient siRNA Delivery with Non-viral Polymeric Vehicles. Pharm Res. 2009;26:657–666. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]
- Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J Control Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Maurice D. Iontophoresis and transcorneal penetration of tobramycin. Invest Ophthalmol Vis Sci. 1989;30:1181–1182. [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF. Effect of iontophoresis on transcorneal permeation 'in vitro' of two beta-blocking agents, and on corneal hydration. Int J Pharm. 2003;250:423–429. doi: 10.1016/s0378-5173(02)00557-4. [DOI] [PubMed] [Google Scholar]
- Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–2079. doi: 10.1016/j.addr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sakamoto T, Hisatomi T, Tsutsumi C, Sassa Y, Ishibashi T, Inomata H. Targeted gene transfer to corneal stroma in vivo by electric pulses. Exp Eye Res. 2002;74:191–198. doi: 10.1006/exer.2001.1117. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sakamoto T, Yamanaka I, Nishi T, Ishibashi T, Inomata H. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 1998;5:1347–1354. doi: 10.1038/sj.gt.3300725. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Oshima Y, Nakagawa K, Ishibashi T, Inomata H, Sueishi K. Target gene transfer of tissue plasminogen activator to cornea by electric pulse inhibits intracameral fibrin formation and corneal cloudiness. Hum Gene Ther. 1999;10:2551–2557. doi: 10.1089/10430349950016889. [DOI] [PubMed] [Google Scholar]
- Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci. 1998;39:2554–2561. [PubMed] [Google Scholar]
- Singh N, Higgins E, Amin S, Jani P, Richter E, Patel A, Kaur R, Wang J, Ambati J, Dong Z, Ambati BK. Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in human corneal cells and inhibits and regresses murine corneal neovascularization. Cornea. 2007;26:65–72. doi: 10.1097/ICO.0b013e31802b4201. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- Tanelian DL, Barry MA, Johnston SA, Le T, Smith G. Controlled gene gun delivery and expression of DNA within the cornea. Biotechniques. 1997;23:484–488. doi: 10.2144/97233st06. [DOI] [PubMed] [Google Scholar]
- Vaka SR, Sammeta SM, Day LB, Murthy SN. Transcorneal iontophoresis for delivery of ciprofloxacin hydrochloride. Curr Eye Res. 2008;33:661–667. doi: 10.1080/02713680802270945. [DOI] [PubMed] [Google Scholar]
- Voigt M, de Kozak Y, Halhal M, Courtois Y, Behar-Cohen F. Down-regulation of NOSII gene expression by iontophoresis of anti-sense oligonucleotide in endotoxin-induced uveitis. Biochem Biophys Res Commun. 2002;295:336–341. doi: 10.1016/s0006-291x(02)00656-3. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Carlson EC, Liu CY, Kao CW, Hu FR, Kao WW. Cis-regulatory elements of the mouse Krt1.12 gene. Mol Vis. 2002;8:94–101. [PubMed] [Google Scholar]
- Yamashita T, Sonoda S, Suzuki R, Arimura N, Tachibana K, Maruyama K, Sakamoto T. A novel bubble liposome and ultrasound-mediated gene transfer to ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp Eye Res. 2007;85:741–748. doi: 10.1016/j.exer.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Dursun D, Dubovy S, Miller D, Alfonso E, Forster RK, Behar-Cohen F, Parel JM. Lontophoresis for the treatment of paecilomyces keratitis. Cornea. 2002;21:131–132. doi: 10.1097/00003226-200201000-00029. [DOI] [PubMed] [Google Scholar]
- Zhou R, Dean DA. Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp Biol Med. 2007;232:362–369. [PMC free article] [PubMed] [Google Scholar]