Abstract
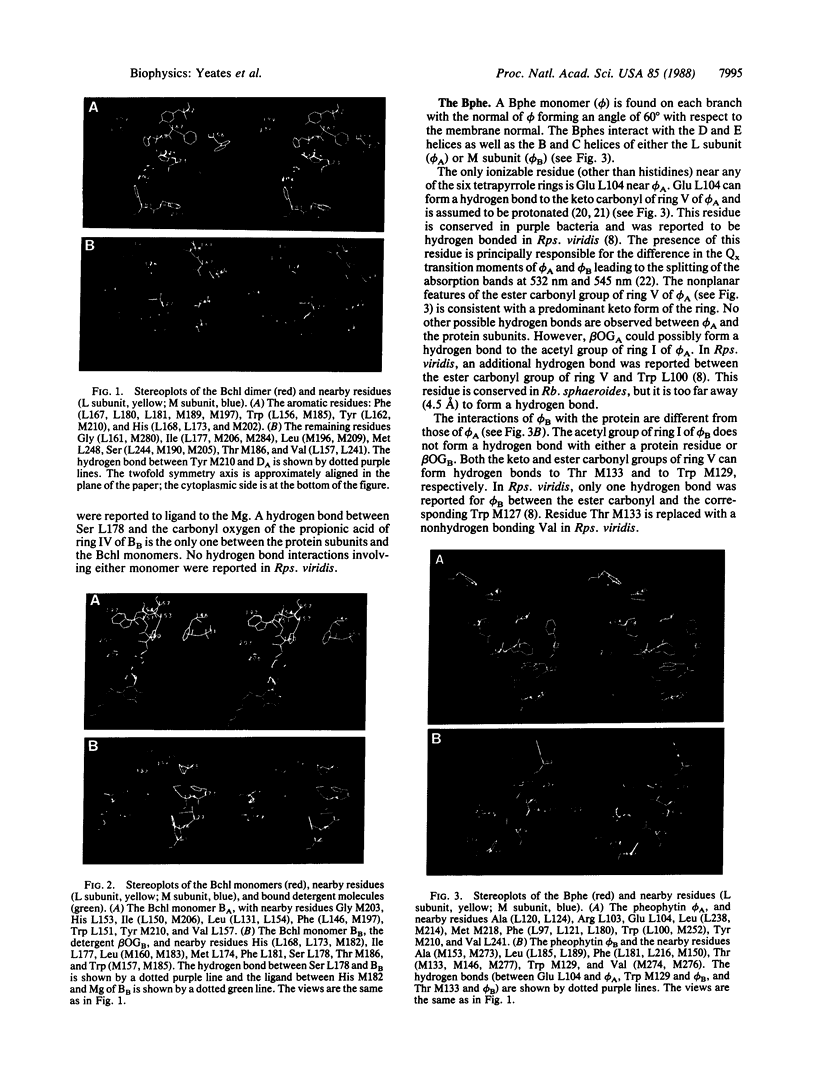
The three-dimensional structures of the cofactors and protein subunits of the reaction center (RC) from the carotenoidless mutant strain of Rhodobacter sphaeroides R-26 and the wild-type strain 2.4.1 have been determined by x-ray diffraction to resolutions of 2.8 A and 3.0 A with R values of 24% and 26%, respectively. The bacteriochlorophyll dimer (D), bacteriochlorophyll monomers (B), and bacteriopheophytin monomers (phi) form two branches, A and B, that are approximately related by a twofold symmetry axis. The cofactors are located in hydrophobic environments formed by the L and M subunits. Differences in the cofactor-protein interactions between the A and B cofactors, as well as between the corresponding cofactors of Rb, sphaeroides and Rhodopseudomonas viridis [Michel, H., Epp, O. & Deisenhofer, J. (1986) EMBO J. 3, 2445-2451], are delineated. The roles of several structural features in the preferential electron transfer along the A branch are discussed. Two bound detergent molecules of beta-octyl glucoside have been located near BA and BB. The environment of the carotenoid, C, that is present in RCs from Rb. sphaeroides 2.4.1 consists largely of aromatic residues of the M subunit. A role of BB in the triplet energy transfer from D to C and the reason for the preferential ease of removal of BB from the RC is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agalidis I., Lutz M., Reiss-Husson F. Binding of carotenoids on reaction centers from Rhodopseudomonas sphaeroides R 26. Biochim Biophys Acta. 1980 Feb 8;589(2):264–274. doi: 10.1016/0005-2728(80)90043-2. [DOI] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C. Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions. J Mol Biol. 1981 Nov 15;152(4):737–762. doi: 10.1016/0022-2836(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Hamlin R. Multiwire area X-ray diffractometers. Methods Enzymol. 1985;114:416–452. doi: 10.1016/0076-6879(85)14029-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Kakitani T., Honig B., Crofts A. R. Theoretical studies of the electrochromic response of carotenoids in photosynthetic membranes. Biophys J. 1982 Jul;39(1):57–63. doi: 10.1016/S0006-3495(82)84490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos T. L. Heme enzyme crystal structures. Adv Inorg Biochem. 1988;7:1–36. [PubMed] [Google Scholar]
- Tronrud D. E., Schmid M. F., Matthews B. W. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1.9 A resolution. J Mol Biol. 1986 Apr 5;188(3):443–454. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]










