Abstract
Extracellular nucleotides transmit signals into the cells through the P2 family of cell surface receptors. These receptors are amply expressed in human blood vessels and participate in vascular tone control; however, their signaling mechanisms remain unknown. Here we show that in smooth muscle cells of isolated human chorionic arteries, the activation of the P2Y2 receptor (P2Y2R) induces not only its partition into membrane rafts but also its rapid internalization. Cholesterol depletion with methyl-β-cyclodextrin reduced the association of the agonist-activated receptor into membrane rafts but did not affect either the UTP-mediated vasoconstrictions or the vasomotor responses elicited by both serotonin and KCl. Ex vivo perfusion of human chorionic artery segments with 1–10 μm UTP, a selective P2Y2R agonist, displaced the P2Y2R localization into membrane rafts within 1 min, a process preceded by the activation of both RhoA and Rac1 GTPases. AG1478, a selective and potent inhibitor of the epidermal growth factor receptor tyrosine kinase activity, not only blocked the UTP-induced vasomotor activity but also abrogated both RhoA and Rac1 activation, the P2Y2R association with membrane rafts, and its internalization. Altogether, these results show for the first time that the plasma membrane distribution of the P2Y2R is transregulated by the epidermal growth factor receptor, revealing an unsuspected functional interplay that controls both the membrane distribution and the vasomotor activity of the P2Y2R in intact human blood vessels.
Keywords: G proteins/Low Molecular Weight, Lipid/Raft, Receptors/Endocytosis, Signal Transduction/G proteins, EGF Receptor, Purine Receptors, RTK Transactivation, Smooth Muscle Cell
Introduction
The plasma membrane (PM)4 nucleotide receptors of the P2Y (P2YR) family are all Gq or Gi protein-coupled receptors (GPCRs); this family is composed of eight members (P2YR1, P2YR2, P2YR4, P2YR6, and P2YR11–14) (1). The P2YRs are ubiquitous and amply expressed in the brain and other organs (2). They are activated by either purines, such as ADP/ATP, or pyrimidines, including UTP, UDP, CTP, or UDP-glucose (3). Moreover, P2YRs are pharmacological targets in processes like platelet aggregation and treatment of cystic fibrosis (4, 5).
Both ADP and UTP have been recently recognized as signals involved in the contractility of the human vascular wall through the activation of P2Y1/2Rs (6, 7). In particular, the activation of P2Y1/2Rs by ADP, ATP, or UTP stimulates smooth muscle contraction in human chorionic arteries (HCA), whereas these nucleotides relax microvessels in the placental cotyledon through the release of nitric oxide (7). Notwithstanding its contribution to vascular smooth muscle tone, little is known about its cellular mechanisms.
Microregionalization is a common feature of cell signaling. Clathrin-coated pits, caveolae, and membrane rafts contain high concentrations of signaling molecules. An array of GPCR has also been identified in caveolae or rafts (reviewed in Ref. 8). In many cases, the receptor microregionalization is sensitive to ligand stimulation, altering the receptor clustering in or out of membrane rafts. We recently showed that in HCA, the P2Y1R-mediated vasocontractile activity depends on its association with membrane rafts (9). Although membrane rafts have been involved in signal transduction mediated by UTP (10), neither the P2Y2R cell membrane distribution nor the molecular mechanisms involved have been clarified.
Membrane rafts are a dynamic assembly of cholesterol, glycosphingolipids, and proteins, such as caveolin, flotillins, Src family kinases, and glycosylphosphatidylinositol-linked proteins (11, 12). Cholesterol is a vital and major component of membrane rafts; it is known that its extraction with MβCD causes a loss in identifiable caveolae (13, 14). The actin cytoskeleton also appears to play a role. Actin network disruption by latrunculin B, a toxin isolated from the red sponge Negombata magnifica (formerly Latrunculia magnifica), results in loss of the caveolae-F actin association (15). Filamin, an actin cross-linking protein, is one of the proteins identified as ligand of caveolin-1 (16). Interestingly, activation of the P2Y2R induces its association with filamin and actin reorganization in aortic SMC (17). Because of its increasing role in signal transduction, the mechanisms that govern organization of these microdomains are a matter of intense research and debate. Interestingly, recent data show that epidermal growth factor (EGF) induces the coalescence of different membrane rafts (18), making it likely that the EGF·EGF receptor (EGFR) complex also transregulates signaling pathway(s) mediated by several other physiological inputs.
Connected to the role of P2Y1/2Rs in cell proliferation, cell migration, and vascular tone control (6, 7, 9, 19–23), EGF is also a key regulator in a variety of cellular systems. It binds to EGFR, triggering tyrosine kinase activity that results in the activation of several signaling proteins, such Ras, phosphatidylinositol 3-kinase, and phospholipase Cγ pathways, leading to mitogenic events (24, 25). The EGFR also plays a role in vascular physiology. EGF induces contractility of vascular and gastric smooth muscles (24, 26, 27). Moreover, activation of receptors for angiotensin and catecholamines results in transactivation of the EGFR, most probably by activation of metalloproteinases and release of EGFR ligands, such as HB-EGF (28), a process associated with smooth muscle cell growth and contraction, respectively (29, 30).
To date, the P2Y1R and P2Y2R are the only nucleotide receptors clearly demonstrated to transactivate the EGFR (19–23, 31). Thus, it becomes possible to hypothesize that EGFR acts downstream of the P2Y2R signaling pathway to regulate nucleotide-induced vasoconstriction. The transactivation of the EGFR activity by GPCRs is a complex molecular process that governs many aspects of the cell fate (32, 33), nevertheless, the question of whether EGFR in turn transregulates GPCR activity in intact human tissues is a challenging issue that remains to be addressed (34).
Here we report that in the SMC of HCA, the P2Y2R is not associated with membrane rafts; however, selective receptor activation with 1 μm UTP results in its rapid partition to raft domains. Stimulation with higher UTP concentrations further caused the internalization of the P2Y2R, a finding concomitant with the fading of the UTP-evoked contractions in these human vessels. Membrane raft disruption by tissue cholesterol depletion resulted in reduction of the receptor mass associated with these microdomains, a procedure that nonetheless did not modify the UTP-induced vasomotor responses. Remarkably, partition of the P2Y2R into rafts and its internalization were found to depend on the activation of the EGFR and the actin cytoskeleton, implying RhoA and Rac1 GTPase activity. The vasomotor action elicited by P2Y2R activation was also dependent on the EGFR tyrosine kinase activity. These results show for the first time that membrane distribution and the vasomotor activity of the P2Y2R are processes transregulated by the EGFR, revealing an unprecedented functional interplay between the P2Y2R and the EGFR in smooth muscle cells.
EXPERIMENTAL PROCEDURES
P2YR Ligands and Drug Providers
ATP trisodium salt, ADP disodium salt, UTP trisodium salt, UDP disodium salt, uridine, serotonin (5-HT) as the hydrochloride salt, MβCD, and antiproteases were purchased from Sigma. MRS 2365 as the trisodium salt was purchased from Tocris Bioscience (Ellisville, MO). AG1478, latrunculin B, and PD98059 were purchased from Calbiochem. Percoll was from Amersham Biosciences. For the preparation of buffers, we only used analytical grade reagents, which were obtained from Merck.
Obtainment of Human Placentas
Full-term placentas from normal pregnancies delivered vaginally or by caesarean section were derived from the maternity ward associated with the Department of Obstetrics and Gynecology of the School of Medicine at the P. Catholic University Clinical Hospital, Santiago. The ethics committees from the School of Medicine and the Faculty of Biology approved the experimental protocols using human tissues; the guidelines for the handling of human materials were strictly adhered to. Appropriate informed consent was obtained as requested by the School of Medicine Ethics Committee. Concerted actions with other investigators in our Department allowed a more complete study of this organ; whereas we dissected chorionic blood vessels, other colleagues used the cord or the body of the placentas for independent protocols. The corresponding ethical regulations were strictly adhered to.
At least 60 placentas were used in the experiments reported in this study. Full-term placentas were transported to the laboratory within 5–15 min of childbirth; immediately thereafter, segments of superficial HCA were carefully dissected from the main body to perform the protocols. For some experiments, segments conserved an intact endothelium, whereas in other experiments, the vessel segments were manually denuded of the endothelial cell layer by gently rubbing the internal vessel surface with a cotton swab; this procedure was described to eliminate the internal endothelial layer without damaging the adjacent smooth muscle layer (35). To perform other protocols, as will be specified separately, HCA vessel segments were perfused with one of the nucleotide receptor agonists for 1, 2, or 4 min; immediately thereafter, these vessel segments were placed in liquid nitrogen until further tissue processing. Functional assays were also performed to assess the biological activity of the P2Y2R upon challenge with selective nucleotide agonists or the removal of the tissue cholesterol. Each of these protocols will be detailed below.
Detergent-free purification of membrane rafts and plasma membrane-enriched fraction isolation from HCA were done according to the methods described by Song et al. (36) and Smart et al. (37), respectively, and modified as we described previously (9).
Immunoblotting
Antibodies for the human P2Y1R were generated and characterized previously (7). Antibodies against P2Y2R, P2X1R, RhoA, Cdc42, caveolin-3, flotillin-1, Gq, phospho-ERK, and Na+/K+ ATPase (β-subunit) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against Rac were from Cytoskeleton, Inc. (Denver, CO), and anti-ERK antibodies (pan-ERK) from BD Biosciences Pharmingen.
Proteins were separated by SDS-PAGE on 10% acrylamide gels and transferred to polyvinylidene difluoride membranes. These membranes were incubated with the specific antibodies to detect the indicated proteins and visualized using horseradish peroxidase-conjugated secondary antibody and the ECL detection system (SuperSignal® West Femto, Pierce). Immunoblots were digitized in a VISTA-T630 UMax scanner driven by Adobe Photoshop CS (Adobe Systems, Mountain View, CA); quantitative analysis was done with the ImageJ software (National Institutes of Health).
Ex Vivo Tissue Perfusion with P2Y2R Agonists
A 3–5-cm segment from the second bifurcation of the principal superficial chorionic artery was carefully dissected from the placental body. The tissue was immediately denuded of the endothelial cell layer as described previously (9). Immediately thereafter, one end of the vessel was cannulated with PE 190 tubing and perfused with 95% O2, 5% CO2 gassed Krebs-Ringer buffer maintained at 37 °C at a flow of 4 ml/min; the vessels were placed inside a 1.5-ml Eppendorf tube, keeping humid and warm the external surface of the tissue. In this way, the buffer bathed the inside and outside of the vessel during the ex vivo perfusion procedure. After an equilibrium period of 15–20 min, the tissues were perfused with 1 μm UTP for 4 min. Upon completion of the perfusion procedure, the tissues were rapidly dismounted from the perfusion set and immediately immersed in liquid nitrogen until tissue processing for fractionation and sucrose gradient application as detailed above. Control experiments performed without freezing the tissue showed that these procedures did not alter the membrane raft localization of the P2Y2R and control proteins.
To test the agonist specificity of the putative receptors, we next perfused separated artery segments for 4 min with one of the following nucleotides: 10 nm MRS 2365, 100 nm ADP, 1 μm UTP, or 1 μm uridine. Each of these studies was repeated in 3–4 separate vessels obtained from independent placentas.
To examine whether the receptor microregionalization was altered by agonist activation and to assess the timing required for the P2Y2R to incorporate into raft domains, additional experiments were performed by perfusing 1 μm UTP for either 1, 2, or 4 min; immediately thereafter, blood vessels were immersed into liquid nitrogen for sucrose gradient centrifugation. To test the participation of actin cytoskeleton and the involvement of the EGFR on P2Y2R raft translocation, we perfused 100 nm latrunculin B or 100 nm AG1478 during 30 min. Next, UTP was added to a final concentration of 1 μm and perfused for another 4 min. The vessels were dismounted and processed as described previously.
All these experiments were performed in Krebs-Ringer solutions gassed with 95% O2, 5% CO2 at 37 °C; protocols were repeated in at least 3–4 independent placentas. Parallel bioassays determined the course of the vasocontractile activity ensued by agonist application and the viability of the tissues; these protocols will be detailed below.
Quantification of Tissue Cholesterol Content following Methyl-β-cyclodextrin Treatment
Extraction and quantification of tissue cholesterol were done exactly as we described previously (9).
Vascular Reactivity Assays
Intact segments of HCA were dissected from surrounding tissues; 0.3–0.5-cm width rings were carefully prepared for bioassays as previously detailed (9). Muscular contractions were evoked with 1–2-min applications of increasing UTP concentrations; once the maximal contraction was reached, the agonists were rapidly rinsed, avoiding desensitization of the motor responses.
To assess whether removal of the membrane cholesterol influenced the vasomotor responses elicited by UTP, the next series of experiments examined the effect of tissue incubation with 10 mm MβCD. These experiments were performed exactly as we described previously (9). Once the rings reached the maximal contraction evoked by 70 mm KCl, 1 μm UTP was added to the tissue bath for less than 2 min to avoid tissue desensitization. Next, tissues were exposed to 10 mm MβCD for 90 min, after which the challenge with UTP was repeated. Results evaluated the contractile responses evoked by each vasomotor agonist before and after treatment with MβCD.
Receptor Internalization Assays
In analogy to the observation of the loss of the vasomotor response following 10–100 μm UTP applications, an additional set of experiments analyzed the PM distribution of the P2Y2R in HCA segments perfused ex vivo with either 1, 10, or 100 μm UTP. The procedure for the PM assay of the P2Y2R was described above. To test the effects of AG1478 on the receptor internalization, we perfused Krebs-Ringer solution gassed with 95% O2, 5% CO2 at 37 °C containing 100 nm AG1478 for 30 min. Then UTP was added to a final concentration of 10 μm and perfused for another 4 min.
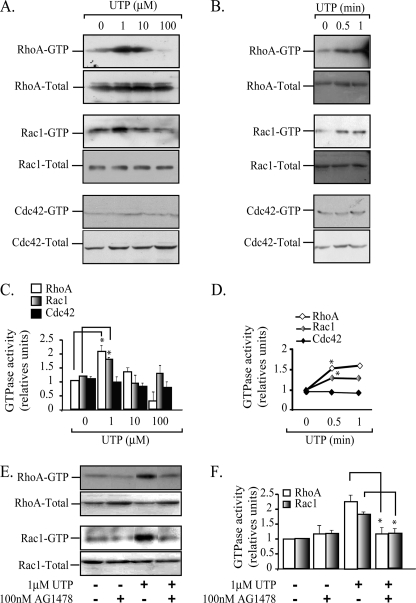
RhoA, Rac1, and Cdc42 Pull-down Activity Assays
RhoA activation was studied using as substrate the RhoA binding domain of rhoketin. Rac1 and Cdc42 were studied using as substrate the Pak1 binding domain. Both were contained in a glutathione S-transferase fusion proteins (GST-RhoA binding domain and GST-Pak1 binding domain) kindly provided by Dr. Keith Burridge (University of North Carolina) (38). Briefly, HCA segments were perfused with Krebs-Ringer solution gassed with 95% O2, 5% CO2 at 37 °C and a flow rate of 4 ml min−1 containing 1 μm UTP for 4 min. HCA segments were ground in a cold slab in the presence of pull-down buffer (50 mm Tris, pH 7.6, 0.5 mm MgCl2, 500 mm NaCl, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10 μg/ml each of aprotinin and leupeptin, and 1 mm phenylmethylsulfonyl fluoride). The extracts were centrifuged at 14,000 rpm for 10 min to eliminate debris. The supernatant was sequentially incubated for 1 h with 30 μg of either GST-RhoA binding domain or GST-Pak1 binding domain coupled to glutathione-Sepharose beads to precipitate RhoA-GTP or Rac1-GTP (38). Total RhoA, Rac1, and Cdc42 present in 30 μg of whole cell lysates used for loading controls and pulled down RhoA-GTP, Rac1-GTP, and Cdc42-GTP were detected by immunoblot using the respective monoclonal antibodies.
To analyze the time dependence of GTPase activation mediated by UTP, HCA segments were perfused with 1 μm UTP for 0.5 and 1 min. Agonist perfusion and pull-down assays were done as described above. Similarly, studies were performed to evaluate the UTP concentration dependence for the GTPases activation. For this purpose, HCA segments were perfused with 1, 10, and 100 μm UTP for 4 min. Finally, to test the effects of AG1478 on the GTPase activity, we perfused Krebs Ringer solution gassed with 95% O2, 5% CO2 at 37 °C containing 100 nm AG1478 for 30 min. Then UTP was added to a final concentration of 1 μm and perfused for another 4 min. The vessels were dismounted and processed as described above.
Quantification of Vasomotor Responses and Statistical Analysis
Nucleotide-induced isometric vasomotor responses were quantified as the tension developed by each agonist in intact vessel rings or vessels denuded of the endothelial cell layer. The motor responses were quantified as the tension force that was expressed in g of tension developed; results were generally normalized agonist the standard of 70 mm KCl used at the beginning of each bioassay. At least 4–6 separate rings were examined per agonist examined; the rings were derived each time from separate placentas. Statistical analysis used Student's t test when appropriate or Dunnett's tables for multiple comparisons with a single control. In all cases, a p value less than 0.05 was considered significant.
RESULTS
The P2Y2R Partitions into Membrane Rafts upon Agonist Stimulation in HCA-SMC
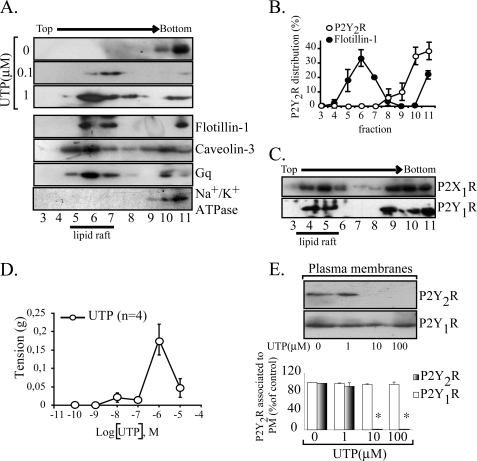
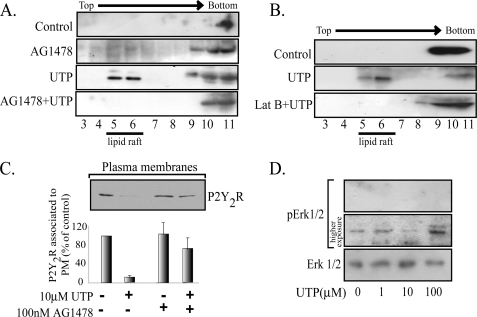
Membrane rafts enriched in glycosphingolipids and cholesterol are currently isolated by flotation in sucrose gradients as low density membranes. Analysis of the gradient's fractions by Western blot indicated that the inactive P2Y2R did not associate with membrane rafts, as evidenced by its co-migration with the β subunit of Na+/K+ ATPase (n = 10), a non-membrane raft protein. As membrane raft markers, we showed that either flotillin-1 or caveolin-3, the muscle-caveolin variant (Fig. 1A), were consistently found floating in low density fractions containing 15–25% sucrose. In addition, protein Gq, the signaling partner of the P2Y2R (39), was also partially localized in low density fractions (Fig. 1A). Densitometric analysis of the bands indicated that 100% (n = 10) of the total P2Y2R mass was excluded from the raft fractions (Fig. 1B). We also examined the distribution of two other nucleotide receptors. As much as 50 and 30% of the P2X1R and P2Y1R, respectively, were also found in low density fractions (Fig. 1C) (9). Similar distribution of the P2Y2R was observed in parallel experiments performed using the OptiPrep™ procedure (9). These results suggest that inactive P2Y2R does not have the properties for being targeted to membrane rafts.
FIGURE 1.
UTP changes the plasma membrane distribution of the P2Y2R. The association of the P2Y2R with raft domains was assessed in sodium carbonate extracts separated in sucrose gradients; membrane rafts generally correspond to fractions 4–7 that were enriched with the endogenous markers flotillin-1 and caveolin-3. In non-stimulated HCA, the P2Y2R localized exclusively in fractions 9–11 co-migrating with the non-membrane raft protein Na+/K+ ATPase subunit β (representative immunoblots of three independent experiments are shown). Furthermore, the P2Y2R signaling partner Gq was distributed in raft and non-raft fractions (A). The P2Y2R associates with membrane rafts upon agonist stimulation. HCA segments were perfused ex vivo with different concentrations of UTP for 4 min (n = 3 each). Immunoblots from sucrose density gradients show that 0.1–1 μm UTP caused a rapid and nearly complete P2Y2R enrichment in membrane rafts (A). For comparative purposes, densitometry of the sodium carbonate immunoblots for P2Y2R and flotillin-1 are shown in B. Symbols, average values; bars, S.E. (n = 3). As a control, we observed that the P2Y1R and the P2X1R from these vessels partially localized in raft fractions (C). The contractile response elicited by UTP was plotted as a concentration-response curve; maximal response was attained with 1 μm, and larger concentrations elicited less response (D). Symbols, average values; bars, S.E. (n = 4). E, P2Y2R internalization induced at 10–100 μm UTP. HCA segments were perfused with 1, 10, or 100 μm UTP for 4 min; as controls, separated vessels were perfused with agonist-free buffer. Cells were harvested, SMC-plasma membranes were isolated by centrifugation, and 15 μg of these fractions were subjected to Western blot analysis using the indicated antibodies. Representative immunoblots are shown in the upper part of E; columns, mean values; bars, S.E. *, p < 0.05 as compared with the controls.
Because GPCR activation may induce in and out translocations from membrane rafts (40–42), we assessed whether P2Y2R activation changed its membrane localization. Perfusion of HCA segments with 0.1 or 1 μm UTP for 4 min showed a concentration-dependent translocation of the P2Y2R into low density fractions (Fig. 1A). Lower UTP concentrations (0.1–1 nm) did not modify the receptor distribution in the sucrose gradient (data not shown). Increasing UTP concentration to 1 μm produced an apparent further increase in the receptor translocation because we consistently observed that nearly 50% of the receptor translocated to the raft domains (Fig. 1A). Furthermore, a 4-min tissue perfusion with an agonist-free buffer provided evidence that 100% of the P2Y2R was present in the non-raft fractions, therefore discarding nonspecific effects due to shear stress or other variables related to the perfusion procedure.
On the other hand, UTP elicited a slow rise in the vasoconstriction response that attained its maximum with 1 μm UTP (Fig. 1D). The contraction was not sustained, because shortly after reaching its maximum, the response faded rapidly to basal tension (data not shown). Further increasing the concentration to 10 μm did not evoke a larger vasomotor effect; in contrast, we repeatedly noted an evident reduction in the motor response (Fig. 1D).
P2Y2R Internalization Is a Rapid and Concentration-dependent Process
To further investigate the molecular mechanism accounting for the rapid decrease in the vasomotor response, we next examined whether desensitization might be related to P2Y2R internalization. Western blot analysis of the PM-enriched fractions showed that HCA perfused for 4 min with 1 μm UTP did not modify the P2Y2R present in the PM (Fig. 1E), a result that further reinforced our notion that the partition of the activated P2Y2R into the low density fractions is due to receptor association with membrane rafts from the cell surface. In contrast, increasing UTP to 100 μm induced the complete clearance of the PM-associated P2Y2R (Fig. 1E). As a control, it was shown that tissue perfusion with UTP did not change either the P2Y1R fraction associated with the PM (Fig. 1E) or its membrane raft association (9). These PM-enriched fractions showed only a minor or null contamination with other cell markers (9, 37).
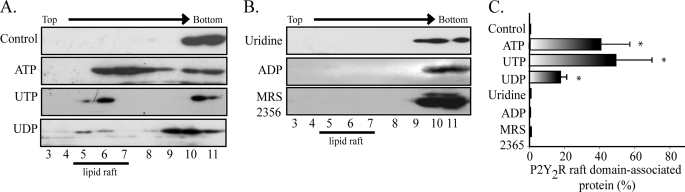
P2Y2R Occupation with UTP Analogues Induces Receptor Partitioning into Membrane Rafts
To further substantiate that the activation of the P2Y2R is necessary for its translocation into membrane rafts, we assessed whether structural analogues mimicked the effect of UTP. Perfusion of HCA segments with ATP or UDP also evidenced a displacement of the receptor toward the low density fractions (Fig. 2, A and C), although the latter redistributed the P2Y2R with a lesser potency (Fig. 2, A and C). Consonant with the notion that receptor partition into membrane rafts is dependent on P2Y2R activation, uridine did not elicit receptor translocation into low density fractions. Likewise, other nucleotides with preferential affinity for the P2Y1R, such as ADP (100 nm) or MRS 2365 (10 nm), did not mimic the effect of UTP (Fig. 2, B and C).
FIGURE 2.
The P2Y2R translocation in membrane rafts is ligand-dependent. To examine whether the P2Y2R association with membrane raft depends on the occupation of the P2Y2R, HCA were perfused for 4 min with either 10 μm ATP, 1 μm UTP, or 1 μm UDP. A, representative immunoblots showing that all of these nucleotides induced the association of the P2Y2R receptor to membrane rafts. Neither 1 μm uridine nor 100 nm ADP or 10 nm MRS 2365 evoked the receptor displacement into raft domains, as shown in representative immunoblots shown in B. Quantification of the immunoblots illustrates the fraction of the P2Y2R associated with membrane rafts evoked by these nucleotide and their analogs (C). Columns, mean values; bars, S.E. *, p < 0.05 as compared with the controls (n = 3–4 per assay).
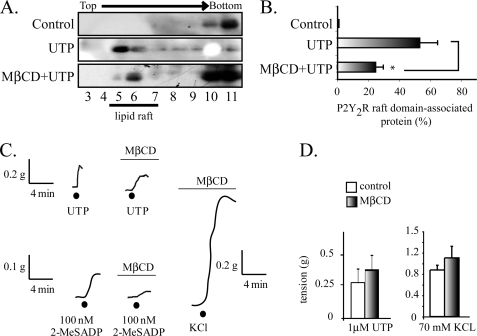
MβCD Reduces the UTP-induced P2Y2R Partitioning into Membrane Rafts without Modifying Its Vasomotor Activity
Tissue treatment with MβCD reduced by 60% the UTP-induced P2Y2R association with low density fractions (n = 3, p < 0.05; Fig. 3, A and B), an effect attributed to the 50% reduction of the tissue cholesterol content elicited by the dextrin treatment (9). MβCD did not modify either the UTP vasomotor responses or those elicited by KCl (Fig. 3, C and D), although the responses became slower, retarding the maximum response. In contrast, parallel experiments showed that cholesterol depletion abrogated the vasoconstrictions evoked by 2-MeSADP (Fig. 3C), confirming our previous report (9). Thus, these data strongly suggest that the vasocontractile action of UTP is independent of the P2Y2R association with raft domains, a finding that contrasts with the P2Y1R, and highlights novel aspects that govern the signaling by nucleotides in vascular SMC.
FIGURE 3.
The UTP-mediated vasoconstrictions are insensitive cholesterol depletion. Representative immunoblots from sucrose gradients and their quantification show that perfusion of HCA with Krebs Ringer buffer containing 10 mm MβCD for 1.5 h caused a 60% reduction in the P2Y2R recovered in raft fractions (A and B). C shows representative tracings of the vasomotor activity elicited by HCA rings prior to and following treatment with MβCD. Cholesterol depletion by MβCD did not modify either 1 μm UTP- or 70 mm KCl-evoked vasomotor responses but substantially decreased vasoconstrictions induced by 100 nm 2-MeSADP. D shows a quantification of these responses. Columns, mean values; bars, S.E. (n = 3–4 per assay). *, p < 0.05 when comparing the effect of UTP in tissues with and without MβCD treatment.
UTP Induces RhoA and Rac1 GTPase Activation in HCA-SMC, Implicating EGFR Signaling
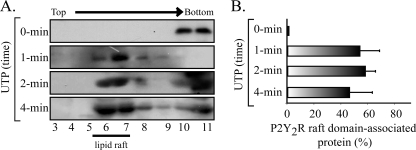
To gain further insight into the translocation mechanism, we next determined the kinetics of this process. HCA perfused with 1 μm UTP for 1, 2, or 4 min provided evidence that the displacement of the P2Y2R to low density fractions was completed within the first minute; prolonging agonist exposure for up to 4 min did not yield evidence of further receptor movements (Fig. 4, A and B), an indication that translocation is a rapid event in the P2Y2R signaling cascade.
FIGURE 4.
UTP induced a rapid association of the P2Y2R with membrane rafts. Kinetics of the P2Y2R association with membrane rafts. HCA segments were perfused ex vivo with 1 μm UTP for 1, 2, and 4 min (n = 3 each). A shows representative immunoblots from sucrose density gradients. UTP elicited a rapid enrichment of the P2Y2R into raft fractions after a 1-min perfusion with the ligand compared with a control protocol perfused without the agonist addition. Quantification of these experiments are depicted in B. Columns, mean values; bars, S.E. (n = 4). *, p < 0.05 when comparing control versus UTP-perfused tissues.
Because UTP is known to activate small GTPases of the Rho family in different cell types (43–46) and the EGFR activates Vav2, a guanine nucleotide exchange factor for RhoA and Rac1 (47), we next assessed whether the activated P2Y2R signals toward the EGFR and Rho family GTPases as part of its molecular mechanism. Pull-down assays using the RhoA and Rac1 substrate GST-rhoketin and GST-PAK, respectively (38, 48), showed a significant 2-fold activation of RhoA and Rac1 in HCA perfused ex vivo with 1 μm UTP. 10–100 μm UTP did not evoke activation of these GTPases (Fig. 5, A and C). Kinetic experiments showed that increased RhoA and Rac1 activities were detected within 30 s of 1 μm UTP perfusion with a time course compatible with the translocation of the P2Y2R to low density fractions (Fig. 5, B and D). Activation of Cdc42 was not detected under these conditions (Fig. 5, A–D). Thus, there is a correlation between the level of RhoA and Rac1 activation and the occupation of the P2Y2R in HCA smooth muscle cells. Moreover, this finding suggests that P2Y2R signaling involves EGFR activity and that the actin cytoskeleton actively participates in the pathways triggered by P2Y2R.
FIGURE 5.
The UTP-mediated activity of both RhoA and Rac1 is dependent on EGFR tyrosine kinase activity. The activation of both RhoA and Rac1, but not Cdc42, precedes the partition of the P2Y2R into membrane rafts. UTP-mediated activation of RhoA, Rac1, and Cdc42 was measured in HCA segments perfused with 1, 10, and 100 μm UTP; densitometric analysis showed a concentration-dependent activation of RhoA and Rac1, reaching a maximum at 1 μm UTP (A and C). Kinetic experiments using 1 μm UTP showed that the increase in the GTP loading of RhoA and Rac occurs within the first 30 s of UTP perfusion (B and D). Cdc42 was not activated under these conditions. Control experiments were performed in HCA segments perfused with agonist-free buffer. Data were normalized to the relative activation of the protein compared with the control and assigned an arbitrary value of 1; bars, S.E. (n = 3 per assay). *, p < 0.05 when comparing control versus UTP-perfused tissues. HCA segments were perfused sequentially with Krebs-Ringer buffer with or without 100 nm AG1478 for 30-min and the same buffer supplemented with 1 μm UTP for another 4 min. Activation of RhoA and Rac1 GTPases by UTP were completely blocked by the EGFR tyrosine kinase inhibitor (E and F). *, p < 0.05 as compared with the same protocol without AG 1478 (n = 3–4 per assay).
The P2Y2R Signaling and Its Membrane Distribution Are Dependent on the EGFR
We next assessed whether activation of the P2Y2R by UTP activates the EGFR signaling cascade. HCA segments were perfused ex vivo with 1 μm UTP in the absence and in the presence of 100 nm AG1478, a highly selective inhibitor of the EGFR tyrosine kinase activity. Tissue perfusion with AG1478 completely blocked the UTP-induced activation of RhoA and Rac1 (Fig. 5, E and F). Surprisingly, AG1478 also fully abrogated the UTP-mediated P2Y2R association with the low density fractions, an effect that was mimicked by the actin sequestering drug latrunculin B (Fig. 6, A and B). AG1478 also blocked the UTP-mediated internalization of the P2Y2R (Fig. 6C). Control experiments showed that latrunculin B did not modify the P2Y1R association with membrane rafts (data not shown). The P2Y2R activates the MAPK/ERK pathway in several cell lines, in many cases by transactivation of the EGFR (10, 20, 22, 46, 49, 50). Perfusion of HCA for 4 min with 0–100 μm UTP showed no changes in the phospho-content of ERK1/2 (Fig. 6D), strongly suggesting that the membrane distribution of the P2Y2R and its signaling in this fetal tissue are independent of the MAPK/ERK pathway. Thus, the EGFR is linked not only to the P2Y2R intracellular signaling mechanism but also transregulates the plasma membrane distribution of the P2Y2R.
FIGURE 6.
The P2Y2R plasma membrane distribution is dependent on EGFR tyrosine kinase activity and actin cytoskeleton. The UTP-mediated partitioning of the P2Y2R into membrane rafts was also blocked by either AG1478 or 100 nm latrunculin B (A and B). HCA segments were perfused sequentially with Krebs-Ringer buffer with or without 100 nm AG1478 for 30 min and the same buffer supplemented with 10 μm UTP for another 4 min. As controls, separated vessels were perfused with agonist-free buffer. C shows that the P2Y2R internalization induced by 100 μm UTP is dependent on the EGFR tyrosine kinase activity. HCA segments were perfused with 100 μm UTP for 4 min; as controls, separated vessels were perfused with agonist-free buffer. Cells were harvested; SMC-plasma membranes were isolated by centrifugation; 15 μg of these fractions were subjected to Western blot analysis using the indicated antibody. Representative immunoblots are shown in the upper part of C. Columns, mean values; bars, S.E. HCA segments were perfused ex vivo with 0–100 μm UTP for 4 min. Cell-equivalent amounts of lysate were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were probed with monoclonal anti-phospho-ERK antibodies and developed with the ECL enhanced chemiluminescence detection system (SuperSignalR West Femto, Pierce). As a loading control, membranes were stripped and probed for total ERK. A representative blot of three independent experiments is shown in D. No increases in the phospho-content of ERK were detected after UTP perfusions.
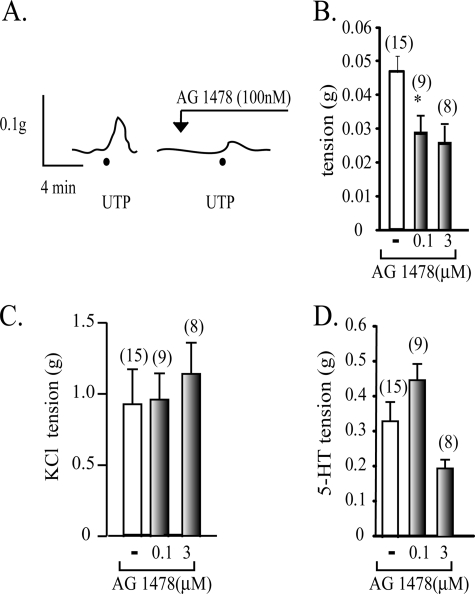
P2Y2R-mediated Vasomotor Activity Is Dependent on the EGFR
To directly assess whether the vasomotor response elicited by P2Y2R activation involves EGFR signaling, we examined the contractile activity of UTP in HCA rings preapplied with AG1478. HCA rings pretreated with 100 nm AG1478 evoked only 40% of the vasoconstriction induced by 1 μm UTP. Increasing AG1478 up to 3 μm was unable to completely block the UTP-mediated vasoconstrictions (Fig. 7, A and B). The blockade of the UTP-mediated contractions was specific, as demonstrated by the insensitivity of KCl-evoked contractions to the EGFR blocker (Fig. 7C). AG1478 showed a dual action on the magnitude of the 5-HT-induced contractions; at 100 nm, this inhibitor increased by 40% the 5-HT-mediated activity, whereas at 3 μm, we detected a ∼50% reduction (Fig. 7D).
FIGURE 7.
The UTP-mediated vasoconstrictions are dependent on EGFR signaling. A representative tracing of a vascular reactivity assay of a HCA ring shows that the contractile response of 1 μm UTP was halved by 0.1 or 3 μm AG1478 (A and B). As a control, AG1478 did not modify the KCl-induced vasoconstrictions (C). As an additional control, we also evaluated whether the vasomotor responses evoked by 300 nm 5-HT were modified by AG1478. Quantification of the polygraphic recordings showed that 0.1 μm AG1478 increased by 40% the 5-HT-induced contractions, whereas at 3 μm, these were halved (D). Columns, mean average contraction (n = number of independent experiments); bars, S.E.; *, p < 0.05.
Collectively, these results strongly suggest that the vasomotor activity of the P2Y2R involves transactivation of the EGFR which, in turn, retrotransregulates the PM distribution of the P2Y2R by mechanisms involving Rho GTPases.
DISCUSSION
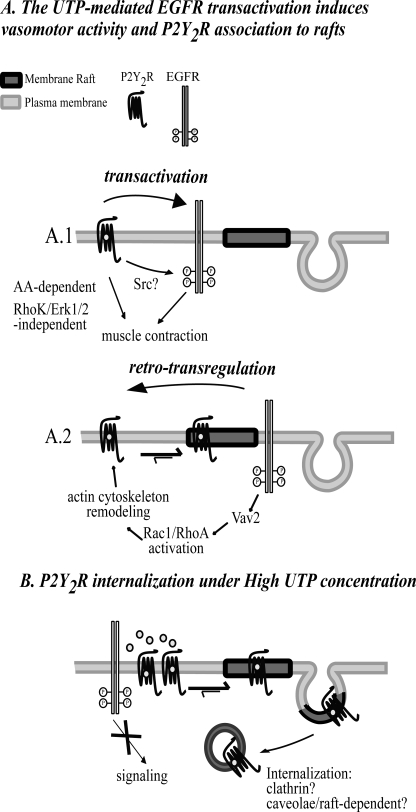
Three major findings highlight novel aspects of the P2Y2R cell biology. First, the inactive P2Y2R is excluded from membrane rafts of HCA-SMC; however, upon selective activation, nearly 50% of the receptor mass rapidly associates to these microdomains. Second, the P2Y2R membrane distribution requires EGFR activity and actin cytoskeleton remodeling, highlighting the role of RhoA and Rac1 GTPases in P2Y2R signaling. In addition, a rapid and concentration-dependent P2Y2R internalization is functionally reflected in UTP concentration-response curves. Finally, bioassays concur in showing that the P2Y2R-evoked vasoconstrictions are of slow onset and involve indirectly the EGFR pathway cascade, highlighting the importance of complementing cell biology studies with physiological bioassays (see Fig. 8).
FIGURE 8.
Model of P2Y2R membrane distribution and its regulation by agonists. UTP-mediated activation of the P2Y2R induces transactivation of the EGFR, leading to vessel contraction (A.1) in turn, the EGFR retrotransregulates the membrane distribution of the P2Y2R through a mechanism involving RhoA and Rac1 GTPases, and actin cytoskeleton remodeling (A.2). Under high UTP concentration, the P2Y2R is rapidly internalized, avoiding excessive P2Y2R signaling (B).
Membrane rafts, which include caveolae and non-caveolae microdomains, have been amply described (51, 52). In non-stimulated HCA-SMC, the P2Y2R was located outside membrane rafts, and these corresponded to the receptors competent for smooth muscle contraction. Although several GPCRs have previously been shown to reside in rafts and caveolae, we have discovered that the activated P2Y2R shared this location. This is the first report showing raft localization for active P2Y2R. Although our biochemical methods of preparing membrane rafts amply support the conclusions (9), the ultimate proof will require high resolution microscopy. Unfortunately, the P2Y2R antibody has limitations in the usage of these methodologies.
The finding that tissue exposure to UTP or ATP and to a minor extent UDP but not to other structurally related nucleotides reveals that selective P2Y2R occupation is required for the receptor association with rafts. In the same tissue, the fraction of the P2Y2R found in membrane rafts exceeds the proportion of the P2Y1R, which never was over 30–35%. Myristylation and/or palmitylation of the Src family tyrosine kinases and Ras proteins have been shown to be critical for its association with membrane rafts (53, 54). Consistently, the P2Y2R, which lacks potential S-alkylation cysteines (55), was not detected in raft domains. We now show evidence indicating that the actin cytoskeleton plays a role in this process; latrunculin B, a known disrupter of the actin cytoskeleton remodeling, abrogated the UTP-evoked receptor microregionalization.
Because the UTP-evoked vasomotor activity was blocked by AG1478, we infer that EGFR transactivation is a major signaling event involved in P2Y2R-mediated contractile activity. Transactivation of the EGFR by GPCR agonists was first reported by Daub et al. (56) in rat fibroblasts and subsequently observed in additional cell types and for other GPCR ligands (32). This process may involve the activation of the tyrosine kinases Pyk2 and Src (57, 58) or the proteolytic processing of the pro-heparin-binding EGF (32). Both mechanisms result in rapid tyrosine phosphorylation and activation of the EGFR. UTP-mediated EGFR transactivation was previously reported in 1321N1 astrocytoma cells (19). The mechanism of the EGFR transactivation in HCA remains unknown, but the recent report describing the activation of the Pyk2/Src pathway by UTP suggests that it may likewise be involved in HCA vasoconstriction (19). The EGFR expression levels in HCA seem to be very low, given that we were unable to detect it by Western blots even after immunoprecipitation (31). Notwithstanding and surprisingly, AG1478 not only reduced the vasomotor activity mediated by UTP but also completely blocked the UTP-mediated partition of the P2Y2R into membrane rafts and its internalization. These observations strongly suggest that EGFR is indeed a downstream element in the P2Y2R signaling; thus, we propose that the transactivated EGFR in turn retrotransregulates the plasma membrane distribution the P2Y2R, which is also a novel functional role for the EGFR (see Fig. 8, A.2). A recent report from Ma and co-workers (34) described how EGF induces internalization of the δ- and μ-opioid receptors through GRK2 activation in HEK293 cells. To our knowledge, our present data represent the first example of a tyrosine kinase receptor that transregulates the membrane distribution of a GPCR in a human tissue with a functional correlate.
It was essential to address whether the receptor signaling leading to vasomotor activity occurred in rafts. Disruption of these microdomains with MβCD did not essentially perturb the UTP-evoked vasomotor responses or the contractions elicited by KCl or 5-HT, whereas it abrogated the P2Y1R-induced vasocontractile responses (7) (this study). We conclude that P2Y2R signaling occurs at membrane sites other than raft domains and that the receptor translocation into rafts must occur after signaling. Taken together, these observations strongly suggest that the environment of the PM microdomain in which the P2Y2R operates dictates signal output. In fact, the UTP-mediated activation of p38 in HeLa cells was reported to be blocked by cholesterol depletion or knocking down of flotillin-2; in contrast, the ERK1/2 pathway was not affected (10).
The PM is a highly dynamic structure exposed to constant internalization and recycling of endocytic vesicles (59). Endocytosis occurs through clathrin-dependent and -independent mechanisms, although the precise nature of the clathrin-independent pathways remains unclear. Tissue exposure to UTP resulted in P2Y2R internalization, a process that required UTP concentrations larger than necessary to elicit maximal vasomotor responses. Although 1 μm UTP, which caused the maximal contraction, did not evidence a detectable P2Y2R internalization even after 60 min of ligand perfusion (data not shown), increasing 10-fold the concentration of UTP evoked a rapid and nearly complete clearance of the receptor from the PM-enriched fractions, a likely indication that the P2Y2R was internalized. This finding is related in all likelihood to the vasomotor concentration-response curve (Fig. 1D). Other tissues and/or cell lines require larger concentration and a longer exposure to UTP to elicit significant receptor internalization (60–62). Most likely, low agonist concentration results in poor β-arrestin recruitment needed for efficient internalization (62). On the other hand, rapid internalization could result in uncoupling of the receptor from its signaling machinery, avoiding overstimulation triggered by sustained receptor activation. The finding that RhoA and Rac1 activity also follow an activation peak supports this interpretation.
Whether the P2Y2R follows a clathrin-dependent or -independent pathway has been controversial (62, 63). For the EGFR. it is known that “selection” of a specific internalization route depends on the EGF concentration (i.e. low ligand concentration induces receptor sequestration through a clathrin-dependent pathway, whereas high ligand concentration targets the EGFR to a clathrin-independent/raft-dependent pathway, which finally transports cargoes to the degradative pathway) (64). Although the present results are in line with this latter hypothesis, we were unable to detect reduction in its expression by Western blots even after 3 h of UTP perfusion (data not shown). Future work using selective inhibition/knockdowns of proteins involved in these internalization processes will be necessary to definitively address these issues.
Low UTP concentrations increased both RhoA and Rac1 activities 2-fold without affecting Cdc42 activity. Although we did not explore which guanine nucleotide exchange factors are involved, the most plausible candidate is Vav2, which has RhoA and Rac1 guanine nucleotide exchange factor activity and also is phosphorylated and activated by EGFR (47). In this way, the blocking action of AG1478 demonstrated that RhoA and Rac1 were both activated downstream from the P2Y2R/EGFR axis. Most likely, this process commands the translocation of the P2Y2R into membrane rafts and the receptor's internalization kinetics (see Fig. 8).
The P2Y2R is known to activate a variety of signaling pathways, including the mitogen-activated protein kinase ERK1/2 and the small GTPases of the Rho family (43, 45, 65). However, until now, it was unclear whether these pathways were involved in HCA vasoconstrictions induced by nucleotides. The P2Y2R has been shown to mediate proliferative signaling through UTP, a process most probably involving transactivation of the EGFR/ERK pathway (10, 20, 22, 46, 49). However, we could not detect any increase in phospho-ERK1/2 after perfusion of HCA with 1–100 μm UTP. Furthermore, HCA perfusion with PD98059, a well known mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor, was unable to block the vasomotor activity mediated by UTP (data not shown). Most likely, the P2Y2R/EGFR axis in this human fetal tissue is coupled to signaling pathways related to short term responses, such as those described here.
RhoA-dependent signaling controls smooth muscle cell functions, such as contraction and proliferation (66–68). The classical hypothesis establishes that the vasocontractile action of RhoA results from the activation of Rho-dependent kinase, which phosphorylates the myosin light chain phosphatase, allowing a phosphorylation increase of the myosin light chain and contraction at a constant [Ca2+], a phenomenon called Ca2+ sensitization (69, 70). We now describe that 10 μm Y27632, a Rho-dependent kinase inhibitor, did not block the UTP-evoked translocation of the P2Y2R into rafts or its vasocontractile action; however, Y27632 significantly halved the 5-HT-mediated vasomotor activity (supplemental Fig. 1). Thus, the UTP-mediated contractile activity partially follows after EGFR activation (discussed below) rather than direct release of intracellular calcium reservoirs elicited by IP3, explaining its relatively delayed onset as compared with the faster motor responses elicited by other vasoconstrictors, such as 5-HT, noradrenaline, or angiotensin II, known to depend directly on intracellular calcium release. Therefore, the vasomotor action of UTP is indirect in nature (see Fig. 8), since it requires EGFR activation, a finding that could account for the rather modest component attributed to nucleotides as compared with other agents known to play a key role in regulating the vascular wall. Likewise, the P2Y1R-evoked vasoconstrictions account for only 20% of those induced by KCl; thus, the purinergic component governing vascular tone is but one of a multiple set of endogenous compounds that concur in vascular wall tone regulation (71).
We demonstrated years ago that arachidonic acid (AA) and thromboxane τ receptors are involved in HCA vasoconstrictions mediated by the P2Y2R (7). The role of the AA cascade in the generation of potent and efficacious vasoactive metabolites is of paramount importance for the maintenance of the vasomotor tone of human placental vessels (72–74). We demonstrated that prostaglandin E2 or F2α (35) and even adenosine contracts main conductance placental vessel rings, probably by the release of thromboxane (74), reiterating the importance of AA metabolites in the regulation of placental vasomotor tone. AA has been shown to activate protein kinase C, which in turn phosphorylates the MLCP regulator CIP17 (75). AA also can be produced by EGFR activation; therefore, it is possible that in HCA-SMC the UTP-mediated EGFR transactivation generates AA responsible in part for vasoconstrictions.
In summary, Fig. 8 illustrates our model linking P2Y2R membrane distribution with vasomotor responses. P2Y2R signaling starts out of raft domains. As a result, the EGFR is transactivated by an as yet non-defined mechanism, which results in the activation of a pathway that probably includes Vav2 and RhoA/Rac1 GTPases. Actin remodeling follows, allowing the translocation of the P2Y2R into the raft domains. The physiological consequences of this P2Y2R-EGFR interplay remain to be examined at the light of recent findings relating nucleotide receptor oligomerization and signaling (76, 77). Finally, these findings should be extended not only to SMC from other human vascular beds but also to other organs and cell types where the P2Y2R and the EGFR are co-expressed. Because the EGFR is a target for anticancer drugs (78) and plays a fundamental role in vascular physiology (28–30, 79, 80), the present findings highlight novel clinically relevant opportunities with therapeutic potential.
Supplementary Material
Acknowledgments
We are greatly indebted to the personnel of the maternity ward from the Clinical Hospital of the P. Catholic University for collaboration in obtaining human placentas and other vascular biopsies. We thank Dr. J. F. Miquel for help in cholesterol measurements. We thank Dr. Antonia Silva and Rebecca Katchen for their assistance with manuscript corrections. We especially thank A. Silva and A. Aldana for continuous support.
This work was supported by Fondo Nacional de Areas Prioritarias Grant 13980001, Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia Grant PFB12/2007, and the Millenium Institute for Fundamental and Applied Biology, which is financed in part by the Ministerio de Planificación y Cooperación de Chile.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- PM
- plasma membrane
- P2YR
- P2Y receptor
- P2Y2R
- P2Y2 receptor
- P2Y1R
- P2Y1 receptor
- MβCD
- methyl-β-cyclodextrin
- GPCR
- G protein-coupled receptor
- HCA
- human chorionic artery
- SMC
- smooth muscle cell(s)
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- 5-HT
- serotonin
- AA
- arachidonic acid
- ERK
- extracellular signal-regulated kinase
- GST
- glutathione S-transferase
- MAPK
- mitogen-activated protein kinase
- MRS 2365
- [[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl] 2,3dihydroxybicyclo[3.1.0]hex-1-yl]methyl]diphosphoric acid monoester trisodium salt.
REFERENCES
- 1.North R. A. (2002) Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. (2007) Physiol. Rev. 87, 659–797 [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. (2006) Trends Pharmacol. Sci. 27, 166–176 [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. (2006) Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 5.Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A., Weisman G. A. (2006) Pharmacol. Rev. 58, 281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buvinic S., Briones R., Huidobro-Toro J. P. (2002) Br. J. Pharmacol. 136, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buvinic S., Poblete M. I., Donoso M. V., Delpiano A. M., Briones R., Miranda R., Huidobro-Toro J. P. (2006) J. Physiol. 573, 427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. (2007) Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 9.Norambuena A., Poblete M. I., Donoso M. V., Espinoza C. S., González A., Huidobro-Toro J. P. (2008) Mol. Pharmacol. 74, 1666–1677 [DOI] [PubMed] [Google Scholar]
- 10.Sugawara Y., Nishii H., Takahashi T., Yamauchi J., Mizuno N., Tago K., Itoh H. (2007) Cell. Signal. 19, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 11.Simons K., Toomre D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 12.Brown D. A., London E. (2000) J. Biol. Chem. 275, 17221–17224 [DOI] [PubMed] [Google Scholar]
- 13.Hailstones D., Sleer L. S., Parton R. G., Stanley K. K. (1998) J. Lipid Res. 39, 369–379 [PubMed] [Google Scholar]
- 14.Westermann M., Steiniger F., Richter W. (2005) Histochem. Cell Biol. 123, 613–620 [DOI] [PubMed] [Google Scholar]
- 15.Mundy D. I., Machleidt T., Ying Y. S., Anderson R. G., Bloom G. S. (2002) J. Cell Sci. 115, 4327–4339 [DOI] [PubMed] [Google Scholar]
- 16.Stahlhut M., van Deurs B. (2000) Mol. Biol. Cell 11, 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu N., Erb L., Shivaji R., Weisman G. A., Seye C. I. (2008) Circ. Res. 102, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofman E. G., Ruonala M. O., Bader A. N., van den Heuvel D., Voortman J., Roovers R. C., Verkleij A. J., Gerritsen H. C., van Bergen En Henegouwen P. M. (2008) J. Cell Sci. 121, 2519–2528 [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Liao Z., Camden J., Griffin K. D., Garrad R. C., Santiago-Pérez L. I., González F. A., Seye C. I., Weisman G. A., Erb L. (2004) J. Biol. Chem. 279, 8212–8218 [DOI] [PubMed] [Google Scholar]
- 20.Morris J. B., Pham T. M., Kenney B., Sheppard K. E., Woodcock E. A. (2004) J. Biol. Chem. 279, 8740–8746 [DOI] [PubMed] [Google Scholar]
- 21.Schafer R., Sedehizade F., Welte T., Reiser G. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 285, L376–L385 [DOI] [PubMed] [Google Scholar]
- 22.Soltoff S. P., Avraham H., Avraham S., Cantley L. C. (1998) J. Biol. Chem. 273, 2653–2660 [DOI] [PubMed] [Google Scholar]
- 23.Wagstaff S. C., Bowler W. B., Gallagher J. A., Hipskind R. A. (2000) Carcinogenesis 21, 2175–2181 [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg M. D. (1995) Mol. Cell Biochem. 149, 77–85 [DOI] [PubMed] [Google Scholar]
- 25.Jorissen R. N., Walker F., Pouliot N., Garrett T. P., Ward C. W., Burgess A. W. (2003) Exp. Cell Res. 284, 31–53 [DOI] [PubMed] [Google Scholar]
- 26.Berk B. C., Brock T. A., Webb R. C., Taubman M. B., Atkinson W. J., Gimbrone M. A., Jr., Alexander R. W. (1985) J. Clin. Invest. 75, 1083–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muramatsu I., Hollenberg M. D., Lederis K. (1985) Can J. Physiol. Pharmacol. 63, 994–999 [DOI] [PubMed] [Google Scholar]
- 28.Hao L., Du M., Lopez-Campistrous A., Fernandez-Patron C. (2004) Circ. Res. 94, 68–76 [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Chalothorn D., Jackson L. F., Lee D. C., Faber J. E. (2004) Circ. Res. 95, 989–997 [DOI] [PubMed] [Google Scholar]
- 30.Thomas W. G., Brandenburger Y., Autelitano D. J., Pham T., Qian H., Hannan R. D. (2002) Circ. Res. 90, 135–142 [DOI] [PubMed] [Google Scholar]
- 31.Buvinic S., Bravo-Zehnder M., Boyer J. L., Huidobro-Toro J. P., González A. (2007) J. Cell Sci. 120, 4289–4301 [DOI] [PubMed] [Google Scholar]
- 32.Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. (1999) Nature 402, 884–888 [DOI] [PubMed] [Google Scholar]
- 33.Hackel P. O., Zwick E., Prenzel N., Ullrich A. (1999) Curr. Opin. Cell Biol. 11, 184–189 [DOI] [PubMed] [Google Scholar]
- 34.Chen Y., Long H., Wu Z., Jiang X., Ma L. (2008) Mol. Biol. Cell 19, 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdecantos P., Briones R., Moya P., Germain A., Huidobro-Toro J. P. (2003) Placenta 24, 17–26 [DOI] [PubMed] [Google Scholar]
- 36.Song K. S., Li S., Okamoto T., Quilliam L. A., Sargiacomo M., Lisanti M. P. (1996) J. Biol. Chem. 271, 9690–9697 [DOI] [PubMed] [Google Scholar]
- 37.Smart E. J., Ying Y. S., Mineo C., Anderson R. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterman-Storer C. M., Worthylake R. A., Liu B. P., Burridge K., Salmon E. D. (1999) Nat. Cell Biol. 1, 45–50 [DOI] [PubMed] [Google Scholar]
- 39.Erb L., Liao Z., Seye C. I., Weisman G. A. (2006) Pflugers Arch. 452, 552–562 [DOI] [PubMed] [Google Scholar]
- 40.Chini B., Parenti M. (2004) J. Mol. Endocrinol. 32, 325–338 [DOI] [PubMed] [Google Scholar]
- 41.Ostrom R. S., Insel P. A. (2004) Br. J. Pharmacol. 143, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike L. J. (2003) J. Lipid Res. 44, 655–667 [DOI] [PubMed] [Google Scholar]
- 43.Sauzeau V., Le Jeune H., Cario-Toumaniantz C., Vaillant N., Gadeau A. P., Desgranges C., Scalbert E., Chardin P., Pacaud P., Loirand G. (2000) Am. J. Physiol. Heart Circ. Physiol. 278, H1751–H1761 [DOI] [PubMed] [Google Scholar]
- 44.Seye C. I., Yu N., González F. A., Erb L., Weisman G. A. (2004) J. Biol. Chem. 279, 35679–35686 [DOI] [PubMed] [Google Scholar]
- 45.Bagchi S., Liao Z., Gonzalez F. A., Chorna N. E., Seye C. I., Weisman G. A., Erb L. (2005) J. Biol. Chem. 280, 39050–39057 [DOI] [PubMed] [Google Scholar]
- 46.Liao Z., Seye C. I., Weisman G. A., Erb L. (2007) J. Cell Sci. 120, 1654–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B. P., Burridge K. (2000) Mol. Cell Biol. 20, 7160–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benard V., Bokoch G. M. (2002) Methods Enzymol. 345, 349–359 [DOI] [PubMed] [Google Scholar]
- 49.Elia M. G., Muscella A., Romano S., Greco S., Di Jeso B., Verri T., Storelli C., Marsigliante S. (2005) Cell. Signal. 17, 739–749 [DOI] [PubMed] [Google Scholar]
- 50.Chang S. J., Tzeng C. R., Lee Y. H., Tai C. J. (2008) Cell. Signal. 20, 1248–1255 [DOI] [PubMed] [Google Scholar]
- 51.Ikonen E. (2001) Curr. Opin. Cell Biol. 13, 470–477 [DOI] [PubMed] [Google Scholar]
- 52.Pike L. J. (2004) Biochem. J. 378, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resh M. D. (1994) Cell 76, 411–413 [DOI] [PubMed] [Google Scholar]
- 54.Hancock J. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 373–384 [DOI] [PubMed] [Google Scholar]
- 55.Costanzi S., Mamedova L., Gao Z. G., Jacobson K. A. (2004) J. Med. Chem. 47, 5393–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daub H., Weiss F. U., Wallasch C., Ullrich A. (1996) Nature 379, 557–560 [DOI] [PubMed] [Google Scholar]
- 57.Murasawa S., Mori Y., Nozawa Y., Masaki H., Maruyama K., Tsutsumi Y., Moriguchi Y., Shibasaki Y., Tanaka Y., Iwasaka T., Inada M., Matsubara H. (1998) Hypertension 32, 668–675 [DOI] [PubMed] [Google Scholar]
- 58.Saito Y., Berk B. C. (2001) J. Mol. Cell Cardiol. 33, 3–7 [DOI] [PubMed] [Google Scholar]
- 59.Maxfield F. R., McGraw T. E. (2004) Nat. Rev. Mol. Cell Biol. 5, 121–132 [DOI] [PubMed] [Google Scholar]
- 60.Sromek S. M., Harden T. K. (1998) Mol. Pharmacol. 54, 485–494 [DOI] [PubMed] [Google Scholar]
- 61.Tulapurkar M. E., Schäfer R., Hanck T., Flores R. V., Weisman G. A., González F. A., Reiser G. (2005) Cell Mol. Life Sci. 62, 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann C., Ziegler N., Reiner S., Krasel C., Lohse M. J. (2008) J. Biol. Chem. 283, 30933–30941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tulapurkar M. E., Zündorf G., Reiser G. (2006) J. Neurochem. 96, 624–634 [DOI] [PubMed] [Google Scholar]
- 64.Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seye C. I., Yu N., Jain R., Kong Q., Minor T., Newton J., Erb L., González F. A., Weisman G. A. (2003) J. Biol. Chem. 278, 24960–24965 [DOI] [PubMed] [Google Scholar]
- 66.Gong M. C., Iizuka K., Nixon G., Browne J. P., Hall A., Eccleston J. F., Sugai M., Kobayashi S., Somlyo A. V., Somlyo A. P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 1340–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loirand G., Cario-Toumaniantz C., Chardin P., Pacaud P. (1999) J. Physiol. 516, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seasholtz T. M., Majumdar M., Kaplan D. D., Brown J. H. (1999) Circ. Res. 84, 1186–1193 [DOI] [PubMed] [Google Scholar]
- 69.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. (1996) Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- 70.Kureishi Y., Kobayashi S., Amano M., Kimura K., Kanaide H., Nakano T., Kaibuchi K., Ito M. (1997) J. Biol. Chem. 272, 12257–12260 [DOI] [PubMed] [Google Scholar]
- 71.Maguire J. J., Davenport A. P. (2005) Trends Pharmacol. Sci. 26, 448–454 [DOI] [PubMed] [Google Scholar]
- 72.Ylikorkala O., Mäkilä U. M. (1985) Am. J. Obstet. Gynecol. 152, 318–329 [DOI] [PubMed] [Google Scholar]
- 73.Walters W. A., Boura A. L. (1991) Reprod. Fertil. Dev. 3, 475–481 [DOI] [PubMed] [Google Scholar]
- 74.Donoso M. V., López R., Miranda R., Briones R., Huidobro-Toro J. P. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H2439–H2449 [DOI] [PubMed] [Google Scholar]
- 75.Somlyo A. P., Somlyo A. V. (2003) Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
- 76.Choi R. C., Simon J., Tsim K. W., Barnard E. A. (2008) J. Biol. Chem. 283, 11050–11063 [DOI] [PubMed] [Google Scholar]
- 77.Ecke D., Hanck T., Tulapurkar M. E., Schäfer R., Kassack M., Stricker R., Reiser G. (2008) Biochem. J. 409, 107–116 [DOI] [PubMed] [Google Scholar]
- 78.Gschwind A., Fischer O. M., Ullrich A. (2004) Nat. Rev. Cancer 4, 361–370 [DOI] [PubMed] [Google Scholar]
- 79.Lemarié C. A., Tharaux P. L., Esposito B., Tedgui A., Lehoux S. (2006) Circ. Res. 99, 434–441 [DOI] [PubMed] [Google Scholar]
- 80.Ushio-Fukai M., Zuo L., Ikeda S., Tojo T., Patrushev N. A., Alexander R. W. (2005) Circ. Res. 97, 829–836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.