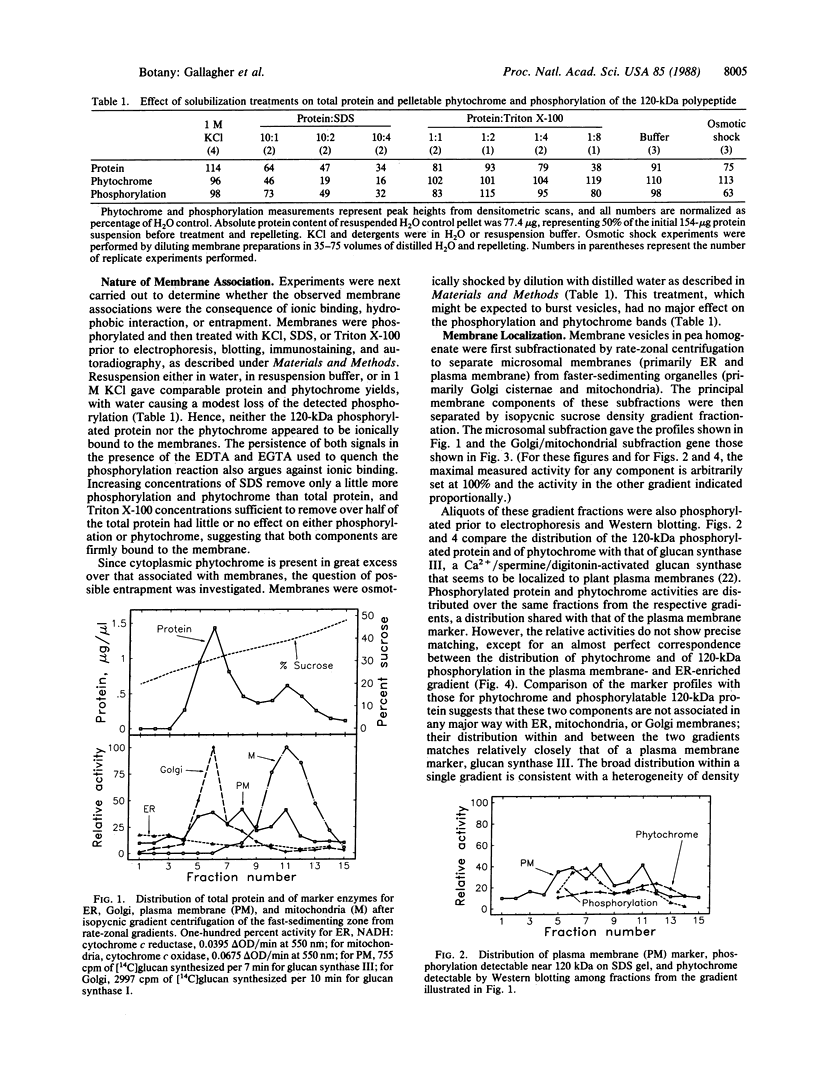
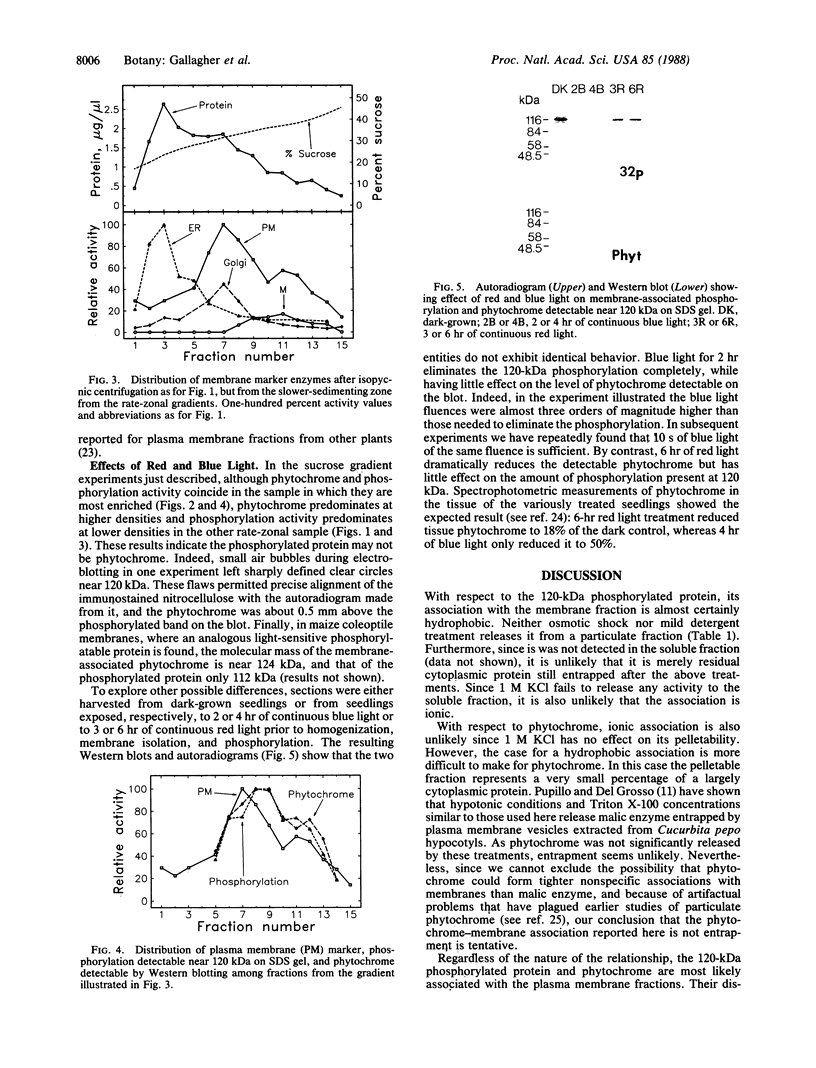
Abstract
Irradiation of etiolated pea (Pisum sativum L.) seedlings with white light affects two proteins, both of monomer molecular mass near 120 kDa. Both proteins have been detected in association with plasma membrane fractions. The first is identifiable in that it becomes heavily phosphorylated when the membranes are incubated with exogenous ATP. The second of these proteins is phytochrome, as determined by electrophoretic transfer (Western) blot analysis. Measurable phosphorylation and phytochrome (the latter detected by antigenicity) decline when the tissue is irradiated with white light prior to membrane isolation and in vitro phosphorylation. The phosphorylated protein is probably not phytochrome for three reasons. (i) It shows a slightly different distribution in sucrose gradients. (ii) Red light causes a gradual decline in the phytochrome that is associated with membrane fractions but has a negligible effect on the phosphorylatable protein; blue light, on the other hand, causes significantly slower loss of phytochrome than does red light but brings about a rapid decline in the phosphorylation signal. (iii) The molecular masses are not identical. The association of both proteins with membrane fractions is probably neither ionic nor, at least for the phosphorylatable protein, the consequence of entrapment of soluble proteins in vesicles formed during tissue extraction. Phytochrome is lost from the membrane fractions during irradiation, as judged by loss of antigenicity. Whether the phosphorylatable protein is lost, a specific kinase is lost, phosphatase activity increases, or phosphorylatable sites are blocked as a consequence of blue light treatment is not known.
Keywords: blue light, phytochrome, protein phosphorylation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordonnier M. M., Pratt L. H. Comparative Phytochrome Immunochemistry as Assayed by Antisera against Both Monocotyledonous and Dicotyledonous Phytochrome. Plant Physiol. 1982 Sep;70(3):912–916. doi: 10.1104/pp.70.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M. M., Pratt L. H. Immunopurification and initial characterization of dicotyledonous phytochrome. Plant Physiol. 1982 Feb;69(2):360–365. doi: 10.1104/pp.69.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Lissemore J. L., Quail P. H. Analysis of cloned cDNA and genomic sequences for phytochrome: complete amino acid sequences for two gene products expressed in etiolated Avena. Nucleic Acids Res. 1985 Dec 9;13(23):8543–8559. doi: 10.1093/nar/13.23.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Briggs W. R. Photochemical and Nonphotochemical Reactions of Phytochrome in vivo. Plant Physiol. 1966 Mar;41(3):467–474. doi: 10.1104/pp.41.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Fatty acid acylation of eukaryotic cell proteins. Methods Enzymol. 1983;96:795–801. doi: 10.1016/S0076-6879(83)96067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R. A., Lissemore J. L., Quail P. H. Nucleotide and amino acid sequence of a Cucurbita phytochrome cDNA clone: identification of conserved features by comparison with Avena phytochrome. Gene. 1986;47(2-3):287–295. doi: 10.1016/0378-1119(86)90072-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]