Abstract
Farnesoid X receptor (FXR) plays important regulatory roles in bile acid, lipoprotein, and glucose homeostasis. Here, we have utilized Fxr−/− mice and mice deficient in scavenger receptor class B type I (SR-BI), together with an FXR-specific agonist and adenovirus expressing hepatocyte nuclear factor 4α or constitutively active FXR, to identify the mechanisms by which activation of FXR results in hypocholesterolemia. We identify a novel pathway linking FXR to changes in hepatic p-JNK, hepatocyte nuclear factor 4α, and finally SR-BI. Importantly, we demonstrate that the FXR-dependent increase in SR-BI results in both hypocholesterolemia and an increase in reverse cholesterol transport, a process involving the transport of cholesterol from peripheral macrophages to the liver for excretion into the feces. In addition, we demonstrate that FXR activation also induces an SR-BI-independent increase in reverse cholesterol transport and reduces intestinal cholesterol absorption. Together, these data indicate that FXR is a promising therapeutic target for treatment of hypercholesterolemia and coronary heart disease.
Keywords: Lipid/Cholesterol, Lipoprotein/Receptor, Receptors/Nuclear, Bile Acid, Cholesterol Metabolism, Cholesterol, FXR, HNF4α, Reverse Cholesterol Transport, SR-BI
Introduction
Farnesoid X receptor (FXR,2 NR1H4) is a member of the nuclear hormone receptor superfamily that plays important regulatory roles in reproduction, development, and metabolism (1). In 1999, specific bile acids were identified as endogenous ligands that potently activate FXR (2–4). However, bile acids can also stimulate other pathways in an FXR-independent manner (5). In contrast, synthetic FXR agonists, such as GW4064 (6), fexaramine (7), or WAY-362450 (8), specifically activate FXR. The expression of FXR is restricted to the liver, kidney, intestine, and adrenal glands (9–12). The single FXR gene encodes four isoforms, as a result of utilization of alternative promoters and alternative splicing between exons 5 and 6 (11, 12).
Fxr−/− mice have been shown to be hyperlipidemic and glucose-intolerant and exhibit insulin insensitivity (13–16). In contrast, oral administration of FXR agonists results in hypolipidemia, hypoglycemia, and increased insulin sensitivity (14–16). Interestingly, the liver appears to be particularly important in the hypolipidemic and hypoglycemic responses, as delivery of constitutively active FXR to the liver recapitulates the effects seen following oral gavage of the specific FXR agonist (14). Activation of hepatic FXR modulates the expression of many hepatic genes involved in lipid homeostasis, including SR-BI (14), CYP7A1, apoC-II, apoC-III, and human apoA-I (reviewed in Ref. 5). However, the underlying mechanism(s) resulting in hypocholesterolemia following FXR activation remains to be determined.
Hepatocyte nuclear factor 4α (HNF4α, NR2A1), another member of the nuclear receptor superfamily, is constitutively active (17) and highly expressed in the liver, with lower levels in the kidney, intestine, and pancreatic β cells (18, 19). Loss of HNF4α results in embryonic lethality (20), consistent with a critical role in development. More recently, generation of tissue-specific Hnf4α−/− mice has revealed that hepatic HNF4α, like hepatic FXR, is crucial for maintaining bile acid, lipid, and glucose homeostasis (21–23).
In this study, we identify a previously unrecognized cross-talk between FXR and HNF4α that results in increased hepatic expression of SR-BI and a reduction in plasma HDL. In addition, we show that activation of FXR increases reverse cholesterol transport via both SR-BI-dependent and -independent pathways and impairs intestinal cholesterol absorption. Together, the current studies have identified novel pathways that regulate FXR-mediated cholesterol homeostasis.
EXPERIMENTAL PROCEDURES
Mice, Diets, and Treatments
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Both Fxr−/− mice, backcrossed to C57BL/6 mice for 7 generations, and SR-BI−/− mice (on a mixed C57BL/6/129 background) have been described previously (13, 25). Studies involved male mice that were fed a standard chow diet (NIH31 modified mouse/rat diet, catalogue no. 7013, Harlan Teklad). Where indicated, 10–12-week-old mice were gavaged with either vehicle (2-hydroxypropyl-β-cyclodextrin, Sigma) or vehicle containing GW4064 (30 mg/kg, twice a day) for 6–8 days. Liver-specific Fxr−/− (l-Fxr−/−) mice and control littermates were gavaged with either vehicle or GW4064 (100 mg/kg) every 12 h for 24 h, as described previously (26). GW4064 was kindly supplied by Dr. Patrick R. Maloney (GlaxoSmithKline) (6). All experiments were approved by the Animal Care and Research Advisory Committee at UCLA and Northeastern Ohio Universities College of Medicine.
Primary Hepatocytes
Mouse primary hepatocytes were isolated and cultured as described previously (27). Three days after isolation, hepatocytes were infected with adenovirus for 48 h prior to RNA extraction.
AML12 Cells
AML12 cells were cultured as described previously (28) and treated with either vehicle (DMSO) or SP600125 (25 μm) for the indicated times. SP600125 was purchased from Calbiochem. Whole cell lysates were analyzed using Western blot assays.
Chromatin Immunoprecipitation Assay
Wild-type mice were treated with either vehicle or GW4064 (30 mg/kg, twice a day) for 7 days. Livers were homogenized in cold phosphate-buffered saline containing protease inhibitor mixture (Roche Applied Science), 2 μg/ml phenylmethylsulfonyl fluoride, 1 mm EDTA, and 1 mm EGTA. Chromatin immunoprecipitation was carried out using a chromatin immunoprecipitation assay kit (Millipore, MA) according to the manufacturer's protocol with minor modifications. Briefly, the cell lysates were fixed with formaldehyde at a final concentration of 1%, sonicated, and precleared with protein A beads. Aliquots of the precleared, sheared chromatin were then immunoprecipitated using mouse IgG or anti-HNF4α antibody (Santa Cruz Biotechnology). After elution, the resulting DNA was used for qRT-PCR analysis. PCR primers for quantification of DR-1A-, DR-1B-, or DR-1C-containing DNA fragments of the SR-BI gene, a known DR-1 element in the Cyp8b1 gene, or a fragment that does not contain any DR-1 element at −9.4 kb of SR-BI gene are listed in supplemental Table 1.
Electrophoretic Mobility Shift Assay (EMSA)
Three putative DR-1 response elements were identified in the mouse SR-BI gene using the NUBIScan computer algorithm. Oligonucleotides containing these putative DR-1s are shown in supplemental Table 2. EMSAs and competition studies were performed as described previously (29). For supershift assays, in vitro transcribed HNF4α proteins were preincubated with HNF4α antibody for 30 min, prior to addition of radiolabeled, double-stranded oligonucleotide probes.
Transient Transfection
HEK293 cells were plated in a 48-well plate and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Transient transfections were performed as described previously (11). Briefly, TK-luciferase reporter plasmids containing three copies of either DR-1A, DR-1B, or DR-1C were generated and co-transfected into HEK293 cells with plasmids expressing HNF4α (provided by Dr. Juro Sakai (30)) and β-galactosidase. After 36 h, cells were lysed and luciferase activities determined. Relative luciferase activities were obtained after normalization to β-galactosidase activity.
Plasma Lipid and Lipoprotein Analysis
Plasma cholesterol and high density lipoprotein cholesterol (HDL-C) were determined as described previously (27). For FPLC analysis, plasma from 7 to 8 mice was combined and 100–200 μl was used to determine plasma cholesterol lipoprotein profile (31).
Adenovirus Construction and Infection
The generation of Ad-VP16 and Ad-Fxrα2-VP16 has been described previously (14). A similar approach was used to generate Ad-Fxrα1-VP16, Ad-Fxrα3-VP16, Ad-Fxrα4-VP16, Ad-Hnf1α, and Ad-Hnf4α using corresponding mouse cDNAs and adenoviral vector pShuttle-IRES-hrGFP (Stratagene). All the adenoviruses were grown in Ad-293 cells (Stratagene) and purified using an Adeno-X virus purification kit (BD Biosciences). To infect primary hepatocytes, adenovirus was added at a multiplicity of infection of 10, and cells were harvested after 48 h. To overexpress genes in mice, 108–109 plaque formation units of adenovirus was transfused into each mouse via tail vein injection. In general, 6–7 days post-infection, mice were fasted for 6 h and then euthanized.
Real Time PCR
RNA was isolated using TRIzol Reagent (Invitrogen), and mRNA levels were determined by quantitative reverse transcription (qRT)-PCR using SYBR Green Supermix and a real time PCR machine from Bio-Rad or Applied Biosystems. The primer sequences for qRT-PCR are provided in supplemental Table 3. Results were normalized to 36B4 mRNA.
Western Blot Assay
Whole liver lysates (14) and cell membrane proteins (32) were prepared, and Western blot assays were performed as described previously (14). SR-BI and β-actin antibodies were from Novus Biologicals. HNF4α, p-JNK, JNK, and calnexin antibodies were from Santa Cruz Biotechnology. Lecithin:cholesterol acyltransferase antibody was kindly provided by Dr. John S. Parks (Wake Forest University, Winston-Salem, NC).
In Vivo Injection of SP600125
C57BL/6 mice were injected intraperitoneally with either vehicle (5% DMSO, 20% Cremophor EL, 75% saline) or SP600125 (30 mg/kg) (Calbiochem). After 3 h, livers were collected and total protein lysates isolated for Western blot assay.
In Vivo Reverse Cholesterol Transport (RCT)
The in vivo RCT was performed essentially as described previously (33). Briefly, J774 macrophages (ATCC, Manassas, VA), grown in suspension in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and penicillin/streptomycin, were cultured in uncoated cell culture dishes. To load cells with [3H]cholesterol and acetylated LDL (acLDL), J774 cells were incubated in Dulbecco's modified Eagle's medium, 10% fetal bovine serum containing 5 μCi/ml [3H]cholesterol, and 25 μg/ml acLDL for 48 h, followed by washing in phosphate-buffered saline and equilibration for 4 h in Dulbecco's modified Eagle's medium supplemented with 0.2% bovine serum albumin and penicillin/streptomycin. Before injection, cells were pelleted and resuspended in Eagle's minimum essential medium (ATCC, Manassas, VA). All the mice were caged individually with free access to water and food. To investigate the role of FXR activation in RCT, C57BL/6 mice were treated with either vehicle or GW4064 for a total of 7 days or infected with adenovirus expressing VP16 alone or FXRα2-VP16 for a total of 7 days. On day 5 of these experiments, mice were injected intraperitoneally with 0.5 ml of J774 cells (typically 5 × 106 cells) in Eagle's minimum essential medium containing 6.5–10 × 106 cpm. Blood was collected at 24 and 48 h, and plasma 3H-labeled tracers were assayed using a liquid scintillation counter (LSC). Feces were collected continuously from 0 to 48 h. At the end of the experiment, mice were perfused with cold phosphate-buffered saline. A portion of the liver was removed for lipid and RNA extraction.
Hepatic Lipid Extraction
Approximately 100 mg of liver was homogenized in methanol, and lipids were extracted in chloroform/methanol (2:1 v/v) as described previously (34). The lipids were collected, dried, and resuspended in toluene and counted in an LSC.
Fecal Cholesterol and Bile Acid Extraction
Fecal cholesterol and bile acids (BAs) were extracted as described previously (33). In brief, the collected feces were weighed and soaked in distilled water (100 mg of feces per 1 ml of water) at 4 °C overnight. The next day, an equal volume of ethanol was added, and feces were homogenized. A 200-μl aliquot was counted in an LSC to establish 3H-labeled total sterols. To extract fecal cholesterol and BAs, 2 ml of homogenized samples were mixed with 2 ml of ethanol, a known amount of [14C]cholic acid (internal control), and 400 μl of 10 n sodium hydroxide (NaOH). The samples were saponified at 100 °C for 1 h, cooled to room temperature, and extracted three times with 9 ml of hexane. The extracts were combined and solvent evaporated, and extracts were resuspended in toluene. Fecal [3H]cholesterol was then determined using an LSC. The remaining aqueous portion of feces was acidified to pH 1–2 with HCl and then extracted three times with 9 ml of ethyl acetate. The extracts were combined and solvent-evaporated, and extracts were resuspended in ethyl acetate. Fecal 3H-labeled BAs were then determined using an LSC.
Intestinal Cholesterol Absorption
Intestinal cholesterol absorption was performed as described previously (35). Briefly, C57BL/6J mice were gavaged with either vehicle or GW4064 (30 mg/kg, twice a day) for a total of 8 days. On day 5, these mice were injected with 2.5 μCi of [3H]cholesterol in Intralipid (Sigma) via tail vein injection, followed by immediate gavage with 1 μCi of [14C]cholesterol in median-chain triglycerides (MCT oil, Mead Johnson, Evansville, IN). After 72 h, blood and tissue were collected. The percent of the intestinal cholesterol absorption was calculated using the following formula: (percent of intragastric dose ([14C]cholesterol) per ml plasma) ÷ (percent of intravenous dose ([3H]cholesterol) per ml plasma) × 100.
Statistical Analysis
Statistical significance was analyzed using unpaired Student's t test or one- or two-way analysis of variance (GraphPad Prism software). Two-way analysis of variance was used to analyze the effect of GW4064 treatment in wild-type mice versus SR-BI−/− mice (Fig. 2, A and B, and Fig. 7, A and B); the data indicate that GW4064 treatment has significantly different effects in wild-type mice versus SR-BI−/− mice (p < 0.05). All the values are expressed as means ± S.E. Differences were considered statistically significant at p < 0.05.
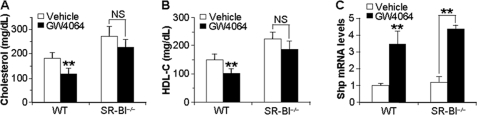
FIGURE 2.
SR-BI is required for GW4064 to lower plasma total cholesterol and HDL-C. A–C, SR-BI−/− mice and their wild-type (WT) littermates were gavaged with either vehicle or GW4064 for 6 days. After a 6-h fast, plasma total cholesterol (A) and HDL-C (B) levels were determined. Hepatic mRNA levels of Shp were determined by qRT-PCR (C) (n = 6–7 mice per group). **, p < 0.01 versus vehicle treatment. NS, not significant.
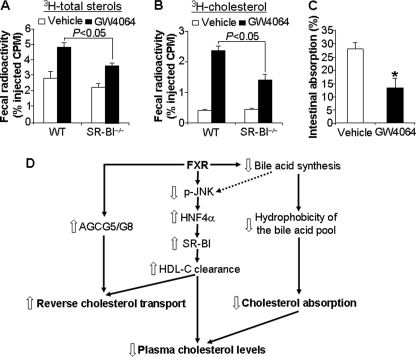
FIGURE 7.
Activation of FXR induces reverse cholesterol transport via SR-BI- dependent and SR-BI-independent pathways and reduces intestinal cholesterol absorption. A and B, SR-BI−/− mice and their wild-type (WT) littermates were gavaged with either vehicle or GW4064 for 7 days (n = 5 mice per group). On day 5, these mice were injected intraperitoneally with J774 macrophages preloaded with [3H]cholesterol and acLDL. Feces were collected for 48 h. Fecal 3H-labeled total sterols (A) and [3H]cholesterol (B) were extracted and quantified. C, C57BL/6J mice were gavaged with either vehicle or GW4064 for a total of 8 days (n = 5–6 mice per group). On day 5, these mice were injected intravenously with 2.5 μCi of [3H]cholesterol, followed by immediate gavage with 1 μCi of [14C]cholesterol. After 72 h, the radioactivity in the blood was determined using an LSC. *, p < 0.05 versus vehicle treatment. D, model showing how activated FXR regulates blood HDL-C levels and RCT. Activation of FXR lowers plasma cholesterol levels by increasing plasma cholesterol clearance via the FXR-pJNK-HNF4α-SR-BI-HDL pathway and by reducing intestinal cholesterol absorption. Activation of FXR inhibits Cyp7a1 and Cyp8b1 and hepatic bile acid synthesis, which may in turn cause the following: (i) reduced hepatic JNK activity (p-JNK) and subsequent induction of hepatic HNF4α expression, and (ii) reduced hydrophobicity index of the bile acid pool and subsequent reduction in intestinal cholesterol absorption. In addition, activation of FXR increases RCT via SR-BI-dependent and -independent (possibly ABCG5/ABCG8) pathways.
RESULTS
Activation of Hepatic FXR Specifically Lowers Plasma HDL-C
In agreement with earlier studies (14, 36, 37), we show that GW4064 treatment of wild-type but not Fxr−/− mice significantly lowered plasma total cholesterol and HDL-C (supplemental Fig. 1A). Analysis of the plasma by FPLC demonstrated that GW4064 treatment had no effect on HDL particle size, but specifically lowered the HDL-C levels of wild-type mice with little or no effect on plasma low density lipoprotein (Fig. 1A). Importantly, a decrease in HDL levels was also seen when non-human primates were treated with GW4064.3
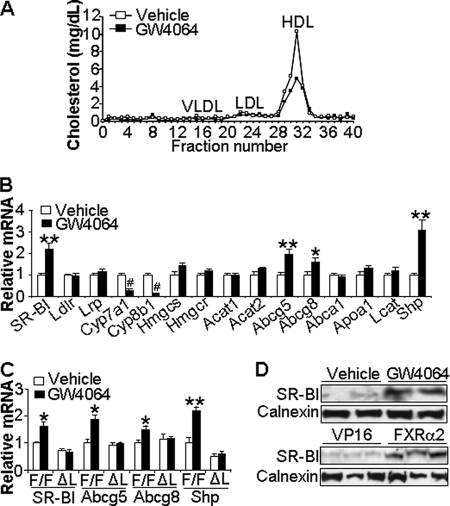
FIGURE 1.
Activation of hepatic FXR increases SR-BI expression and specifically lowers plasma HDL-C. A and B, wild-type (WT) mice were gavaged with either vehicle or GW4064 for 7 days (n = 5 mice per group). After a 6-h fast, blood was collected. Plasma lipoprotein/cholesterol profile was determined by FPLC (A) and hepatic mRNA levels of genes involved in lipid metabolism determined by qRT-PCR (B). C, liver-specific Fxr−/− (ΔL) mice and their wild-type littermates (F/F) were gavaged with either vehicle or GW4064 as described under “Experimental Procedures.” Hepatic mRNA levels were quantified by qRT-PCR (n = 4–5 mice per group). D, Western blot assays were performed to determine hepatic protein levels of wild-type mice treated with either vehicle or GW4064 for 7 days (top panel) or infected with Ad-VP16 (VP16) or Ad-Fxrα2-VP16 (Fxrα2) for 7 days (lower panel). LDLR, low density lipoprotein receptor; LRP, LDL receptor-related protein; CYP8B1, sterol 12-α-hydroxylase; HMGCS, HMG-CoA synthase; HMGCR, HMG-CoA reductase; ACAT, acyl-coenzyme A:cholesterol acyltransferase; VLDL, very low density lipoprotein; LDL, low density lipoprotein; LCAT, lecithin:cholesterol acyltransferase; ABCG5, ATP-binding cassette subfamily G member 5. *, p < 0.05; **, p < 0.01; #, p < 0.001 versus vehicle treatment.
A similar reduction in plasma total cholesterol and HDL-C was also observed in mice that had been infected 7 days earlier with adenovirus expressing constitutively active isoforms of FXR (fusion proteins of Fxrα1, -α2, -α3, or -α4 with VP16) (supplemental Fig. 1, B and C). Because adenovirus infection is essentially limited to the liver, these studies identify a crucial role for hepatic FXR in reducing plasma HDL levels.
Consequently, we profiled a panel of hepatic genes involved in cholesterol metabolism following a 7-day treatment of wild-type mice with GW4064. Induced genes included SR-BI, Abcg5, Abcg8 and Shp, a known FXR target gene (Fig. 1B). GW4064 treatment of Fxr−/− (supplemental Fig. 1D) or liver-specific Fxr knock-out (ΔL) mice (Fig. 1C) failed to induce hepatic SR-B1, Abcg5, Abcg8, or Shp mRNAs. In contrast, these same genes were induced following hepatic expression of FXR-VP16 (supplemental Fig. 1E and data not shown). As expected (5), Cyp7a1 and Cyp8b1 mRNA levels declined significantly, demonstrating the effectiveness of GW4064 or Ad-FXR-VP16 treatment (Fig. 1B and data not shown).
The specificity of these effects of FXR activation was further supported by the finding that mRNAs encoding the nuclear receptors liver receptor X receptor (Lxr), or peroxisome proliferator-activated receptor (Ppar) α, -β, -γ, or the SR-BI interacting protein Pdzk1 and the plasma protein levels of lecithin:cholesterol acyltransferase, all known to modulate lipid metabolism, were all unaffected by GW4064 treatment (supplemental Fig. 2, A and B). Based on these data, we conclude that induction of hepatic SR-BI, Abcg5, and Abcg8 is dependent upon activation of hepatic FXR and that one or all of these genes may be responsible for the observed hypocholesterolemia. Importantly, Western blot analysis of liver homogenates demonstrated that SR-BI protein levels were significantly increased (2-fold; p < 0.05) after treatment of mice with either GW4064 or adenovirus expressing FXR-VP16 (Fig. 1D).
FXR-dependent Hypolipidemia Involves SR-BI
Given the known role of SR-BI in regulating plasma HDL levels (38), we next investigate whether SR-BI is required for the FXR-dependent reduction in plasma cholesterol levels. SR-BI−/− mice and their wild-type littermates (all on a C57BL/6/129 background) were treated with either vehicle or GW4064. As expected (25), vehicle-treated SR-BI−/− mice have increased levels of plasma total and HDL cholesterol compared with vehicle-treated wild-type mice (Fig. 2, A and B). Consistent with the studies of Fig. 1 and supplemental Fig. 1 that utilized mice on a C57BL/6 background, there was a significant decrease in plasma total cholesterol and HDL-C levels following GW4064 treatment of wild-type littermates of the SR-B1−/− mice (Fig. 2, A and B; p < 0.01). The data of Fig. 2, A and B, show that treatment of SR-BI−/− mice with GW4064 resulted in a small but nonsignificant decrease in plasma total cholesterol (p = 0.11) and HDL-C (p = 0.21). Because hepatic Shp mRNA levels were induced 3–4-fold following GW4064 treatment of either SR-BI−/− or wild-type mice (Fig. 2C), we conclude that loss of SR-BI does not affect FXR function. Collectively, these data demonstrate that SR-BI plays a critical role in the FXR-dependent hypolipidemia.
Increased Hepatic Expression of HNF4α Induces SR-BI and Lowers Plasma HDL
The finding that SR-B1 was not induced following treatment of isolated primary hepatocytes with GW4064 suggests that SR-BI is not a direct target gene of FXR (supplemental Fig. 3 and also see supplemental Results). As discussed below, HNF4α plays a critical role in the induction of SR-B1 by FXR. Hence, the >94% decrease in Hnf4α levels in primary mouse hepatocytes, as compared with an intact liver (supplemental Fig. 3), likely accounts for the inability of GW4064 to induce SR-B1 in these cells.
To better understand the mechanism by which FXR activation results in increased hepatic expression of SR-BI and decreased plasma HDL levels, we initially focused on hepatocyte nuclear factor 1α (HNF1α) and HNF4α because previous studies have shown that deficiency of either factor was associated with defective HDL metabolism (21, 39). Consequently, we generated adenovirus expressing these two transcription factors and then infected either wild-type mice or primary hepatocyte cultures.
Infection of primary hepatocytes with the Hnf1α-expressing adenovirus significantly induced Fxr, a known HNF1α target gene (39), but had no effect on SR-BI expression (supplemental Fig. 4). In contrast, HNF4α overexpression significantly induced SR-BI and, as reported previously (30), Abcg5 and Abcg8 mRNAs (Fig. 3A). Srebp-2 mRNA levels were unchanged, consistent with specific gene activation by HNF4α (Fig. 3A).
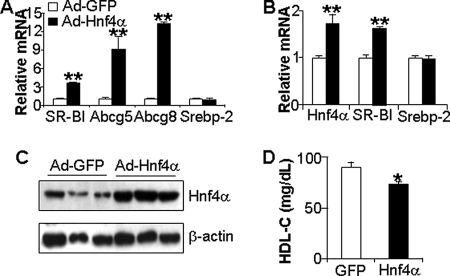
FIGURE 3.
Overexpression of HNF4α in the liver increases SR-BI expression and lowers plasma HDL-C levels. A, primary hepatocytes were isolated from wild-type mice and infected in triplicate dishes with adenovirus expressing GFP (Ad-GFP) or HNF4α. After 48 h, mRNA levels were quantified by qRT-PCR. B–D, C57BL/6J mice were transfused with Ad-GFP or Ad-Hnf4α (n = 5 mice per group). After 7 days and a 6-h fast, hepatic mRNA levels were quantified by qRT-PCR (B); hepatic protein levels were quantified by Western blot assays (C), and plasma HDL-C levels were determined (D). *, p < 0.05; **, p < 0.01 versus Ad-GFP infection.
In parallel studies, we infected wild-type mice with adenovirus expressing HNF4α or GFP and after 7 days analyzed hepatic gene expression; the data show that hepatic overexpression of Hnf4α resulted in increased SR-BI mRNA levels but no change in Srebp-2 (Fig. 3B). Thus, the in vitro and in vivo studies demonstrate that overexpression of HNF4α resulted in increased expression of SR-BI. Importantly, compared with wild-type mice overexpressing GFP, mice infected with Ad-Hnf4α both overexpressed HNF4α protein in the liver (Fig. 3C) and had a small but significant reduction in plasma HDL (Fig. 3D).
Activated FXR Induces HNF4α Protein Expression
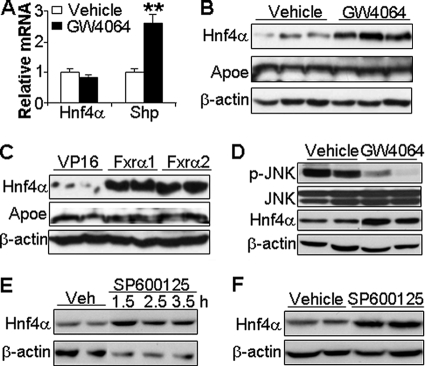
The induction of hepatic SR-BI by HNF4α led us to investigate whether Hnf4α is a downstream target of FXR. Treatment of wild-type mice with either GW4064 (Fig. 4A) or adenovirus expressing either FXRα1-VP16 or FXRα2-VP16 (supplemental Fig. 5) had no effect on hepatic Hnf4α mRNA levels, although Shp mRNA levels were highly induced, consistent with FXR being activated (Fig. 4A and supplemental Fig. 5). Interestingly, Western blot assays showed that hepatic HNF4α protein levels were highly induced following treatment of mice with either GW4064 (Fig. 4B) or Ad-FXR-VP16 (Fig. 4C). This increase was specific as hepatic ApoE protein levels were unchanged by either treatment (Fig. 4, B and C). We conclude that activation of hepatic FXR increases HNF4α protein levels by a post-transcriptional mechanism.
FIGURE 4.
Activation of FXR increases hepatic HNF4α protein but not mRNA levels. Wild-type mice were gavaged with either vehicle or GW4064 for 7 days (A, B, and D). Hepatic mRNA levels were determined by qRT-PCR (A), and hepatic protein levels were determined by Western blot assays (B and D). C, wild-type mice were infected with Ad-VP16, Ad-Fxrα1, or Ad-Fxrα2. After 7 days,. hepatic protein levels were determined by Western blot assays. E, AML12 cells were treated with SP600125 (25 μm) for the indicated times. Protein levels were determined by Western blot assay. F, C57BL/6J mice were injected intraperitoneally with either vehicle or SP600125 (30 mg/kg). After 3 h, mice were euthanized and hepatic protein levels determined by Western blot assays. **, p < 0.01 versus vehicle.
Previous studies have shown that bile acids result in phosphorylation and activation of JNK (40) and that activation of the JNK pathway in primary hepatocytes by exogenously added bile acids reduces HNF4α protein levels (41). Based on these earlier studies, we hypothesized that activation of FXR, and the subsequent repression of Cyp7a1 and Cyp8b1 (Fig. 1B) and bile acid synthesis, would result in decreased bile acid levels in a regulatory pool in the liver. We further hypothesized that such a decrease might result in decreased levels of p-JNK and increased levels of HNF4α protein.
Support for this latter hypothesis came from the demonstration that GW4064 treatment of wild-type mice markedly reduced hepatic levels of p-JNK and increased hepatic HNF4α protein levels (Fig. 4D). Total cellular JNK levels were unchanged by the GW4064 treatment (Fig. 4D).
SP600125 specifically inhibits JNK phosphorylation (42). Treatment of the murine liver cell line AML12 (Fig. 4E) or wild-type mice (Fig. 4F) with SP600125 resulted in a significant increase in HNF4α protein. Based on the data of Fig. 4, we propose that activation of hepatic FXR decreases hepatic p-JNK with a resultant increase in HNF4α protein expression by a process independent of changes in Hnf4α mRNA.
SR-BI Is a Direct Target Gene of HNF4α
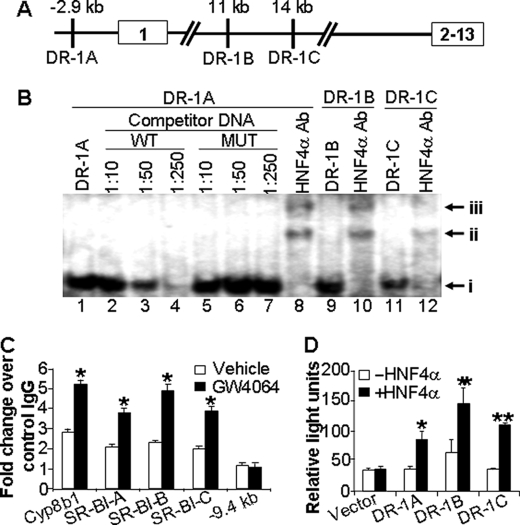
HNF4α is known to bind to direct repeat sequences (AGGTCA) separated by 1 bp (DR-1) to regulate gene transcription. To determine the mechanism by which HNF4α regulates SR-BI expression, we screened genomic sequences corresponding to 30 kb upstream and 30 kb downstream of the SR-BI transcriptional start site and identified three candidate HNF4α response elements as follows: one in the SR-BI promoter (DR-1A) and two in intron 1 (DR-1B and DR-1C) (Fig. 5A). EMSAs indicated that HNF4α protein bound to all three DR-1 elements (Fig. 5B, band i in lanes 1, 9, and 11). Wild-type (Fig. 5B, lanes 2–4) but not mutant (lanes 5–7) DR-1A oligonucleotides were able to compete away the binding of HNF4α protein from the radioactive probe (Fig. 5B). Similar results were obtained with DR-1B or DR-1C; binding of HNF4α protein to these sites was also competed away with wild-type but not mutant oligonucleotides (data not shown). Finally, the DNA-protein complexes were supershifted in the presence of HNF4α antibody in the EMSAs (Fig. 5B, bands ii and iii; compare lanes 1, 9, and 11 with lanes 8, 10, and 12, respectively). Collectively, the EMSA data demonstrate that HNF4α binds to the three candidate DR-1 elements in the SR-BI gene in vitro.
FIGURE 5.
SR-BI is a direct target of HNF4α. A, diagram showing the three putative DR-1-binding sites in the promoter or intron 1 of the mouse SR-BI gene. Exons are boxed. Distances are given from the transcriptional start site. B, EMSAs were performed. Band i represents the complex of HNF4α protein with DR-1A, DR-1B, or DR-1C (lanes 1, 9, and 11). The DNA-protein complexes were supershifted in the presence of HNF4α antibody (lanes 8, 10, and 12; bands ii and iii). MUT, mutant; WT, wild type. C, wild-type mice were treated with vehicle or GW4064 for 7 days. Liver lysates (n = 3 mice per group) were used for chromatin immunoprecipitation assays to determine the binding of HNF4α protein to DR-1A, DR-1B, DR-1C, or a sequence that does not contain any DR-1 element at −9.4 kb of SR-BI gene or a known DR-1 element in the Cyp8b1 gene (positive control). The results are shown in fold changes relative to the basal levels obtained using mouse IgG (control). D, triplicate dishes of HEK293 cells were transiently transfected with plasmids expressing HNF4α and β-galactosidase, together with a TK-luciferase reporter construct containing either SR-BI DR-1A-, DR-1B-, or DR-1C-binding sites or the empty vector. After 48 h, cells were harvested, and luciferase activities were determined and normalized to β-galactosidase activities. *, p < 0.05; **, p < 0.01 versus the absence of HNF4α.
To determine whether HNF4α binds to these same three DR-1 elements in vivo, chromatin immunoprecipitation assays were performed using liver lysates isolated from vehicle- and GW4064-treated mice. As shown in Fig. 5C, in the absence of the FXR agonist, hepatic HNF4α protein bound to the DR-1A, DR-1B, or DR-1C elements in the SR-BI gene ∼2-fold higher than the control that utilized a nonspecific IgG. Interestingly, GW4064 treatment increased the HNF4α binding to the DR-1 elements in the SR-B1 gene by ∼2-fold (Fig. 5C). Similar results were observed when we analyzed the DR-1 in the Cyp8b1 gene, a known HNF4α target gene (22). Increased binding of HNF4α to these response elements after GW4064 treatment is consistent with our finding that activation of FXR increases hepatic HNF4α protein levels. Taken together, the data demonstrate that HNF4α binds to the three DR-1 elements in vivo and that this interaction is increased following administration of GW4064 to mice.
To determine whether all three DR-1 elements are functional, they were each linked to a minimal thymidine kinase promoter-luciferase reporter. Transient transfection studies showed that co-transfection of these reporter genes with an HNF4α-expression plasmid resulted in a 2–3-fold increase in luciferase activity (Fig. 5D). In contrast, HNF4α had no effect on the minimal TK-luciferase vector (Fig. 5D). Interestingly, co-transfection of an HNF4α-expression plasmid did not affect the activity of a luciferase reporter gene controlled by the SR-BI promoter (−3.0 to +0.07 kb) containing the DR-1A element (data not shown). The latter observation suggests that at least two of the three HNF4α response elements (DR-1A, -B, and -C) may be required for HNF4α to stimulate transcription of the SR-BI gene.
Activation of FXR Increases Reverse Cholesterol Transport
Overexpression of hepatic SR-BI has been shown to increase RCT (43, 44). Furthermore, activation of liver X receptor (LXR) also increases RCT by a process involving induction of the sterol transporters ABCG5 and ABCG8 (45). The finding that activation of FXR increases hepatic expression of SR-BI, Abcg5, and Abcg8 (Fig. 1 and supplemental Fig. 1) suggested that FXR may regulate RCT.
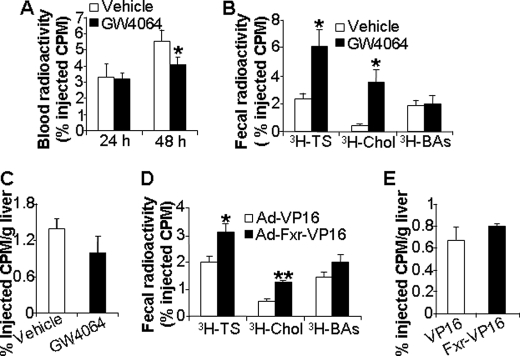
To test this latter hypothesis, wild-type mice were gavaged with either vehicle or the synthetic FXR agonist GW4064 for 5 days, followed by intraperitoneal injection of J774 macrophages that had been preincubated in vitro with [3H]cholesterol and acLDL. Blood was collected at 24 and 48 h, and feces were collected for 48 h. As shown in Fig. 6A, GW4064 treatment had no effect on plasma 3H-labeled tracers at 24 h but significantly reduced plasma 3H-labeled tracers after 48 h. These data suggest that GW4064 treatment leads to increased plasma cholesterol clearance. Analyses of fecal lipid extracts indicated that GW4064 treatment significantly increased the levels of fecal [3H]cholesterol ([3H]chol) and total 3H-labeled total sterols but had no effect on the levels of fecal 3H-labeled bile acids (3H-labeled BAs) (Fig. 6B). The finding that the 3H-labeled tracer did not accumulate in the liver (Fig. 6C) is consistent with increased flux of radiolabeled cholesterol from the blood into the bile and feces. Taken together, the data of Fig. 6, A–C, demonstrate that treatment of mice with the FXR agonist GW4064 increases cholesterol transport from extra-hepatic tissues to feces.
FIGURE 6.
Activation of FXR increases reverse cholesterol transport. A–C, wild-type mice were gavaged with either vehicle or GW4064 for 7 days. On day 5, these mice were injected intraperitoneally with J774 macrophages that had been preloaded with [3H]cholesterol and acLDL. Blood was taken at 24 and 48 h after injection of macrophages. Plasma 3H-labeled tracers were determined using LSC (A). Fecal total 3H-labeled sterol (3H-TS), [3H]cholesterol (3H-Chol), and 3H-labeled bile acids (3H-BAs) (B) or hepatic 3H-labeled tracers (C) were quantified (n = 7–8 mice per group). D and E, wild-type mice were transfused with Ad-VP16 or Ad-Fxrα2-VP16 by tail vein injection. On day 5 post-infection, these mice were injected intraperitoneally with J774 macrophages preloaded with [3H]cholesterol and acLDL. After 48 h, the mice were euthanized, and fecal (D) and hepatic (E) 3H-labeled tracers were determined (n = 5 mice per group). *, p < 0.05; **, p < 0.01 versus vehicle or Ad-VP16 treatment.
Administration of GW4064 by oral gavage is expected to activate FXR in the intestine, liver, and kidney (29). To determine whether activation of hepatic FXR alone is sufficient to increase RCT, wild-type mice were transfused with adenovirus expressing VP16 alone (control) or constitutively active FXR (FXRα2-VP16). After 5 days, we initiated RCT assays by intraperitoneal injection of J774 macrophages loaded with [3H]cholesterol and acLDL. Analysis of the data showed that hepatic expression of constitutively active FXR also significantly increased the levels of fecal [3H]cholesterol and total 3H-labeled sterols, but it had no effect on the levels of fecal 3H-labeled bile acids (Fig. 6D) or hepatic 3H-labeled sterols (Fig. 6E). These data demonstrate that activation of FXR, either following oral administration of a synthetic FXR agonist or following hepatic expression of constitutively active FXR, significantly increases RCT.
Activation of FXR Increases RCT via SR-BI-dependent and -independent Pathways
To better understand the importance of SR-BI in FXR-dependent RCT, we repeated the RCT assay comparing SR-BI−/− mice and their control littermates. As expected, GW4064 treatment of wild-type mice increased fecal levels of 3H-labeled total sterols (Fig. 7A) and [3H]cholesterol (Fig. 7B) consistent with increased RCT. Importantly, compared with the controls, the GW4064-dependent increase in fecal sterols and cholesterol was significantly attenuated in SR-BI−/− mice (Fig. 7, A and B; p < 0.05). Nonetheless, because treatment of SR-BI−/− mice with GW4064 did increase RCT, albeit to lower levels as compared with wild-type mice (Fig. 7, A and B), we conclude that FXR activation increases RCT via both SR-BI-dependent and -independent pathways.
Activation of FXR Reduces Intestinal Cholesterol Absorption
GW4064 treatment has been shown to reduce the BA pool size and decrease the hydrophobicity index (HI) of the BA pool (46). In addition, a reduction in the HI of the BA pool has been associated with a reduction in intestinal cholesterol absorption (47). These two studies suggest that GW4064 treatment might also decrease intestinal cholesterol absorption. We tested this hypothesis by administering dual radiolabeled isotopes to chow-fed mice pretreated with vehicle or GW4064. As shown in Fig. 7C, GW4064 treatment decreased intestinal cholesterol absorption by ∼50%.
DISCUSSION
In this study, we used loss-of-function and gain-of-function approaches to investigate the mechanism by which activation of FXR lowers plasma cholesterol levels. We demonstrate that activation of FXR lowers plasma HDL cholesterol levels by increasing RCT and reducing intestinal cholesterol absorption (Figs. 6 and 7). We also demonstrate that FXR increases RCT via SR-BI-dependent and -independent pathways (Fig. 7).
Although FXR has been shown to increase SR-BI expression (14, 48), both the underlying mechanism and the role of SR-BI in FXR-mediated hypolipidemia have not been elucidated. Previous studies have shown that bile acids increase JNK phosphorylation/activation (40). Here, we demonstrate that there is a profound decrease in hepatic p-JNK following activation of FXR in vivo. Such a finding is consistent with a decrease in bile acid pool size in GW4064-treated mice, presumably as a result of inhibition of Cyp7A1 (46). Importantly, we also show that activation of FXR results in increased levels of HNF4α protein (Fig. 3) by a process that is mimicked following specific inhibition of JNK phosphorylation with SP600125 (Fig. 4). Furthermore, we also demonstrate that the SR-BI gene is activated by HNF4α protein (Fig. 5). Thus, activation of FXR increases hepatic SR-BI expression through an FXR-JNK-HNF4α-SR-BI pathway. Finally, the importance of SR-BI in mediating the physiological changes resulting from FXR activation in vivo is apparent in studies with SR-BI−/− mice; we show that both FXR-dependent reduction in plasma total cholesterol and HDL-C and FXR-dependent increase in RCT are significantly attenuated in SR-BI−/− mice (Figs. 2 and 7). Thus, our data suggest that SR-BI plays an important role in FXR-mediated RCT and hypolipidemia.
High blood HDL levels are usually linked to increased RCT. Interestingly, overexpression of hepatic SR-BI has been shown to lower plasma HDL-C levels and increase RCT (43). Previous studies have also shown that increased hepatic expression of SR-BI reduces the size of atherosclerotic lesions (49, 50). This decrease may in part be due to increased RCT. In this study, although the steady levels of plasma HDL are reduced, activation of FXR results in enhanced HDL cholesterol transport to the feces. Consistent with our observations, more recent studies demonstrated that treatment of hyperlipidemic Ldlr−/− or Apoe−/− mice with FXR agonists resulted in a decrease in atherosclerotic lesions (8, 36, 37). These latter changes may result, at least in part, from increased RCT by SR-BI-dependent and -independent pathways, as well as a decrease in intestinal cholesterol absorption.
The end stage of RCT is removal of hepatic cholesterol from plasma HDL and the subsequent elimination of this sterol into bile. Activation of hepatic FXR is known to both repress bile acid synthesis and to induce expression of proteins, such as SR-BI, BSEP, MDR2, and ABCG5/ABCG8 (Fig. 1B), that efflux bile salts, phospholipids, and cholesterol into bile. Consistent with these latter observations, we show that activation of FXR increases RCT via both SR-BI-dependent and SR-BI-independent pathways (Fig. 7, A and B). The SR-BI-independent pathway may result from induction of hepatic Abcg5 and Abcg8 in response to FXR activation (Fig. 1B). Indeed, ABCG5/ABCG8 are known to be involved in biliary cholesterol secretion (51) and to be required for the liver X receptor-dependent increase in RCT (45). Consequently, we propose that ABCG5 and ABCG8 may also play a role in FXR-dependent increase in RCT. Future work will be directed to test whether biliary cholesterol levels and fecal actual mass of neutral sterols are also increased following FXR activation.
Surprisingly, although activation of FXR is known to repress bile acid synthesis, fecal bile acid levels remain unchanged (Fig. 6). The underlying mechanism may include the induction of bile acid transporters in the liver (BSEP and MRP2) and the repression of bile acid transporter ASBT in the intestine.
In addition to decreasing plasma HDL levels and increasing RCT, we show that activated FXR also reduces intestinal cholesterol absorption (Fig. 7C). The reduced intestinal cholesterol absorption identified in this study may contribute to the hypocholesterolemia that results from FXR activation. Jung et al. (46) have shown that GW4064 treatment significantly increased the ratio of muricholic acid to cholate in the bile acid pool thus reducing the HI. A reduction in the HI of the BA pool is known to reduce intestinal cholesterol absorption (47). Thus, the reduced intestinal cholesterol absorption following FXR activation (Fig. 7C) likely results from a reduction in the HI of the BA pool. We will test such a hypothesis in the future.
FXR is known to reduce plasma triglyceride levels. Previous data have shown that activation of FXR lowers plasma triglyceride levels via inhibition of hepatic lipogenesis and induction of hepatic Apoc-II (24). Thus, activation of FXR lowers plasma triglyceride and cholesterol levels via distinct mechanisms.
In summary, we have demonstrated that activation of FXR increases reverse cholesterol transport via SR-BI-dependent and SR-BI-independent pathways. The increase in hepatic SR-BI expression following FXR activation is through, at least in part, a novel pathway whereby activation of hepatic FXR results in decreased levels of p-JNK and a subsequent increase in protein levels of both HNF4α and SR-BI. We also demonstrate that activation of FXR reduces intestinal cholesterol absorption. These novel findings (Fig. 7D), together with the recent finding that FXR agonists protect against atherosclerosis (8, 36, 37), suggest that FXR is a therapeutic target for treatment of hypercholesterolemia and coronary heart disease.
Supplementary Material
Acknowledgments
We thank Drs. Monty Krieger and Junmei Yao at Massachusetts Institute of Technology for providing the SR-BI−/− mice and Dr. Krieger for comments, Dr. Karl H. Weisgraber at University of California, San Francisco, for apoE antibody, and Dr. Mohamad Navab and Greg Hough at UCLA for providing assistance with the FPLC analysis.
This work was supported, in part, by National Institutes of Health Grants HL30568 and HL68445 (to P. A. E.). This work was also supported by American Heart Association Beginning Grant-in-aid 0565173Y (to Y. Z.), Scientist Development Grant 0830255N (to Y. Z.), and a grant from the Laubisch Fund (to Y. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Results,” Figs. 1–7, Tables 1–3, and additional references.
T. Willson, personal communication.
- FXR
- farnesoid X receptor
- SR-BI
- scavenger receptor class B type I
- HNF4α
- hepatocyte nuclear factor 4α
- RCT
- reverse cholesterol transport
- FPLC
- fast protein liquid chromatography
- HDL
- high density lipoprotein
- HCL-C
- HDL cholesterol
- LSC
- liquid scintillation counter
- EMSA
- electrophoretic mobility shift assay
- qRT
- quantitative reverse-transcription
- GFP
- green fluorescent protein
- Ad
- adenovirus
- JNK
- c-Jun N-terminal kinase
- p-JNK
- phosphorylated JNK
- acLDL
- acetylated LDL
- BA
- bile acid
- TK
- thymidine kinase
- HI
- hydrophobicity index.
REFERENCES
- 1.Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. (2001) Science 294, 1866–1870 [DOI] [PubMed] [Google Scholar]
- 2.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. (1999) Science 284, 1362–1365 [DOI] [PubMed] [Google Scholar]
- 3.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. (1999) Science 284, 1365–1368 [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999) Mol. Cell 3, 543–553 [DOI] [PubMed] [Google Scholar]
- 5.Lee F. Y., Lee H., Hubbert M. L., Edwards P. A., Zhang Y. (2006) Trends Biochem. Sci. 31, 572–580 [DOI] [PubMed] [Google Scholar]
- 6.Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., Jones S. A., Willson T. M. (2000) J. Med. Chem. 43, 2971–2974 [DOI] [PubMed] [Google Scholar]
- 7.Downes M., Verdecia M. A., Roecker A. J., Hughes R., Hogenesch J. B., Kast-Woelbern H. R., Bowman M. E., Ferrer J. L., Anisfeld A. M., Edwards P. A., Rosenfeld J. M., Alvarez J. G., Noel J. P., Nicolaou K. C., Evans R. M. (2003) Mol. Cell 11, 1079–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flatt B., Martin R., Wang T. L., Mahaney P., Murphy B., Gu X. H., Foster P., Li J., Pircher P., Petrowski M., Schulman I., Westin S., Wrobel J., Yan G., Bischoff E., Daige C., Mohan R. (2009) J. Med. Chem. 52, 904–907 [DOI] [PubMed] [Google Scholar]
- 9.Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., Evans R. M., Weinberger C. (1995) Cell 81, 687–693 [DOI] [PubMed] [Google Scholar]
- 10.Seol W., Choi H. S., Moore D. D. (1995) Mol. Endocrinol. 9, 72–85 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Kast-Woelbern H. R., Edwards P. A. (2003) J. Biol. Chem. 278, 104–110 [DOI] [PubMed] [Google Scholar]
- 12.Huber R. M., Murphy K., Miao B., Link J. R., Cunningham M. R., Rupar M. J., Gunyuzlu P. L., Haws T. F., Kassam A., Powell F., Hollis G. F., Young P. R., Mukherjee R., Burn T. C. (2002) Gene 290, 35–43 [DOI] [PubMed] [Google Scholar]
- 13.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000) Cell 102, 731–744 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma K., Saha P. K., Chan L., Moore D. D. (2006) J. Clin. Invest. 116, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J. C., Gonzalez F. J., Kuipers F., Staels B. (2006) J. Biol. Chem. 281, 11039–11049 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez F. J. (2008) Drug Metab. Pharmacokinet. 23, 2–7 [DOI] [PubMed] [Google Scholar]
- 18.Drewes T., Senkel S., Holewa B., Ryffel G. U. (1996) Mol. Cell. Biol. 16, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang S., Tanaka T., Iwanari H., Hotta H., Yamashita H., Kumakura J., Watanabe Y., Uchiyama Y., Aburatani H., Hamakubo T., Kodama T., Naito M. (2003) Nucl. Recept. 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W. S., Manova K., Weinstein D. C., Duncan S. A., Plump A. S., Prezioso V. R., Bachvarova R. F., Darnell J. E., Jr. (1994) Genes Dev. 8, 2466–2477 [DOI] [PubMed] [Google Scholar]
- 21.Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. (2001) Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue Y., Yu A. M., Yim S. H., Ma X., Krausz K. W., Inoue J., Xiang C. C., Brownstein M. J., Eggertsen G., Björkhem I., Gonzalez F. J. (2006) J. Lipid Res. 47, 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura A., Yamagata K., Kakei M., Hatakeyama H., Takahashi N., Fukui K., Nammo T., Yoneda K., Inoue Y., Sladek F. M., Magnuson M. A., Kasai H., Miyagawa J., Gonzalez F. J., Shimomura I. (2006) J. Biol. Chem. 281, 5246–5257 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Edwards P. A. (2008) FEBS Lett. 582, 10–18 [DOI] [PubMed] [Google Scholar]
- 25.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 12610–12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I., Ahn S. H., Inagaki T., Choi M., Ito S., Guo G. L., Kliewer S. A., Gonzalez F. J. (2007) J. Lipid Res. 48, 2664–2672 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Castellani L. W., Sinal C. J., Gonzalez F. J., Edwards P. A. (2004) Genes Dev. 18, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbert M. L., Zhang Y., Lee F. Y., Edwards P. A. (2007) Mol. Endocrinol. 21, 1359–1369 [DOI] [PubMed] [Google Scholar]
- 29.Lee H., Zhang Y., Lee F. Y., Nelson S. F., Gonzalez F. J., Edwards P. A. (2006) J. Lipid Res. 47, 201–214 [DOI] [PubMed] [Google Scholar]
- 30.Sumi K., Tanaka T., Uchida A., Magoori K., Urashima Y., Ohashi R., Ohguchi H., Okamura M., Kudo H., Daigo K., Maejima T., Kojima N., Sakakibara I., Jiang S., Hasegawa G., Kim I., Osborne T. F., Naito M., Gonzalez F. J., Hamakubo T., Kodama T., Sakai J. (2007) Mol. Cell. Biol. 27, 4248–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., Fogelman A. M. (2004) Circulation 109, 3215–3220 [DOI] [PubMed] [Google Scholar]
- 32.Engelking L. J., Kuriyama H., Hammer R. E., Horton J. D., Brown M. S., Goldstein J. L., Liang G. (2004) J. Clin. Invest. 113, 1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naik S. U., Wang X., Da Silva J. S., Jaye M., Macphee C. H., Reilly M. P., Billheimer J. T., Rothblat G. H., Rader D. J. (2006) Circulation 113, 90–97 [DOI] [PubMed] [Google Scholar]
- 34.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 35.Turley S. D., Herndon M. W., Dietschy J. M. (1994) J. Lipid Res. 35, 328–339 [PubMed] [Google Scholar]
- 36.Hartman H. B., Gardell S. J., Petucci C. J., Wang S., Krueger J. A., Evans M. J. (2009) J. Lipid Res. 50, 1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mencarelli A., Renga B., Distrutti E., Fiorucci S. (2009) Am. J. Physiol. Heart Circ. Physiol. 296, H272–H281 [DOI] [PubMed] [Google Scholar]
- 38.Trigatti B., Rigotti A., Krieger M. (2000) Curr. Opin. Lipidol. 11, 123–131 [DOI] [PubMed] [Google Scholar]
- 39.Shih D. Q., Bussen M., Sehayek E., Ananthanarayanan M., Shneider B. L., Suchy F. J., Shefer S., Bollileni J. S., Gonzalez F. J., Breslow J. L., Stoffel M. (2001) Nat. Genet. 27, 375–382 [DOI] [PubMed] [Google Scholar]
- 40.Gupta S., Stravitz R. T., Dent P., Hylemon P. B. (2001) J. Biol. Chem. 276, 15816–15822 [DOI] [PubMed] [Google Scholar]
- 41.Li T., Jahan A., Chiang J. Y. (2006) Hepatology 43, 1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett B. L., Sasaki D. T., Murray B. W., O'Leary E. C., Sakata S. T., Xu W., Leisten J. C., Motiwala A., Pierce S., Satoh Y., Bhagwat S. S., Manning A. M., Anderson D. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Da Silva J. R., Reilly M., Billheimer J. T., Rothblat G. H., Rader D. J. (2005) J. Clin. Invest. 115, 2870–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., Krieger M. (1997) Nature 387, 414–417 [DOI] [PubMed] [Google Scholar]
- 45.Calpe-Berdiel L., Rotllan N., Fiévet C., Roig R., Blanco-Vaca F., Escolà-Gil J. C. (2008) J. Lipid Res. 49, 1904–1911 [DOI] [PubMed] [Google Scholar]
- 46.Jung D., Inagaki T., Gerard R. D., Dawson P. A., Kliewer S. A., Mangelsdorf D. J., Moschetta A. (2007) J. Lipid Res. 48, 2693–2700 [DOI] [PubMed] [Google Scholar]
- 47.Wang D. Q., Tazuma S., Cohen D. E., Carey M. C. (2003) Am. J. Physiol. Gastrointest. Liver Physiol. 285, G494–G502 [DOI] [PubMed] [Google Scholar]
- 48.Lambert G., Amar M. J., Guo G., Brewer H. B., Jr., Gonzalez F. J., Sinal C. J. (2003) J. Biol. Chem. 278, 2563–2570 [DOI] [PubMed] [Google Scholar]
- 49.Kozarsky K. F., Donahee M. H., Glick J. M., Krieger M., Rader D. J. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 721–727 [DOI] [PubMed] [Google Scholar]
- 50.Arai T., Wang N., Bezouevski M., Welch C., Tall A. R. (1999) J. Biol. Chem. 274, 2366–2371 [DOI] [PubMed] [Google Scholar]
- 51.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. (2002) J. Clin. Invest. 110, 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.