Abstract
The use of nonselective pharmacological inhibitors has resulted in controversy regarding the mechanism and consequences of p38 activation during myocardial infarction. Classic p38 inhibitors such as SB203580 rely on a critical “gatekeeper” threonine residue for binding. We addressed these controversies by using mice in which the p38α alleles were targeted to cause substitution of the gatekeeper residue and resistance to inhibition. In homozygous drug-resistant compared with wild-type hearts, SB203580 failed to inhibit the activating phosphorylation of p38 or to reduce the infarction caused by myocardial ischemia. However, BIRB796, a p38 inhibitor not reliant on the gatekeeper for binding, similarly reduced p38-activating phosphorylation and infarction in both wild-type and knock-in mice, thereby excluding a nonspecific inhibitor-dependent phenotype resulting from the targeting strategy. Furthermore, the activation during myocardial ischemia involved phosphorylation of both the threonine and tyrosine residues in the activation loop of p38 despite the phosphorylation of the threonine alone being sufficient to create the epitope for dual phosphospecific antibody binding. Finally, SB203580 failed to reduce infarction in heterozygous drug-resistant hearts, suggesting that near complete inhibition of p38α kinase activity is necessary to elicit protection. These results indicate that, during myocardial ischemia, p38α (i) is the dominant-active p38 isoform, (ii) contributes to infarction, (iii) is responsible for the cardioprotective effect of SB203580, and (iv) is activated by a mechanism consistent with autodiphosphorylation despite this necessitating the phosphorylation of a tyrosine residue by an archetypal serine/threonine kinase.
Keywords: Diseases/Atherosclerosis, Enzymes/Inhibitors, Organisms/Mammal, Phosphorylation, Phosphorylation/Kinases/Serine-Threonine, Phosphorylation/MAPs, Signal Transduction/Protein Kinases/MAP, Tissue/Organ Systems/Muscle/Heart
Introduction
Myocardial ischemia activates a number of kinases, including members of the p38 MAPK2 family (p38s). Activation of p38s is classically achieved by trans- and diphosphorylation of a threonine-glycine-tyrosine (TGY) motif by upstream kinases (1). However, an alternative mechanism involving autophosphorylation has been proposed under specific circumstances, including myocardial ischemia (2–5). Although many studies have used SB203580 and related p38 inhibitors during lethal myocardial ischemia to infer that p38 activation is detrimental (6), these inhibitors act on at least two of the four p38 isoforms. They also inhibit a variety of other kinases with a tertiary structure that has a hydrophobic groove adjacent to the ATP-binding pocket that resembles that formed around the threonine at position 106 in p38α and p38β (7). Thus, it is still unclear whether the attenuation of myocardial ischemic injury seen with SB203580 is due to the inhibition of p38s and, if so, whether it is the β or α isoform that is responsible (7, 8).
Another uncertainty is whether the reduction in p38 dual phosphorylation seen during myocardial ischemia in the presence of SB203580 is indicative of autophosphorylation or the inhibition of an upstream kinase involved in transphosphorylation (9). Furthermore, recent analysis of constitutively active p38 mutants and p38 phosphatases has revealed that the commonly used reagents to examine p38-activating phosphorylation cannot reliably differentiate between mono- or diphosphorylation of the TGY motif (10, 11). Because p38 is a serine/threonine kinase, it is possible that autophosphorylation results in the phosphorylation of the threonine residue alone and that such monophosphorylation may confer signaling specificity over diphosphorylated p38. Differentiating between these possibilities to elucidate mechanistic details may allow circumstance-specific inhibition of p38 or the targeting of an ischemia-selective substrate, which would circumvent the toxicity seen to date in early clinical trials of direct p38 inhibition (6, 9).
Another possibility is that the toxicity seen in clinical trials to date is the result of an off-target non-p38-dependent effect. However, inhibitors with different structures and mechanisms of binding seem to share similar side effect profiles, perhaps suggesting that it is a result of their on-target action (6, 9). If this is the case, then it is possible that toxicity could be avoided by dose titration to cause partial inhibition of p38. However, it is uncertain at present whether acute partial inhibition of p38 still results in cardioprotection.
Recently, a chemical genetic approach first described in vitro (12, 13) has been employed in mice in which Thr106 in p38α was substituted with a more bulky methionine, disrupting the hydrophobic groove on which many inhibitors depend for binding (8). We have made use of these mice to address the uncertainties highlighted above.
EXPERIMENTAL PROCEDURES
The knock-in mice harboring a T106M mutation in p38α on a C57BL/6 background have been described previously (8).
Retrograde Perfusion of Murine Hearts
After intraperitoneal pentobarbital (300 mg/kg) and heparin (150 units) administration, hearts were rapidly isolated from male p38α knock-in (drug-resistant (DR)) and colony isogenic wild-type (WT) mice and placed in ice-cold modified Krebs-Henseleit buffer (18.5 mmol of NaCl, 25.0 mmol of NaHCO3, 4.75 mmol of KCl, 1.18 mmol of KH2PO4, 1.19 mmol of MgSO4, 11.0 mmol of d-glucose, and 1.4 mmol of CaCl2). The excised hearts were mounted on a Langendorff apparatus and retrograde-perfused at a constant pressure of 80 mm Hg with Krebs-Henseleit buffer equilibrated with 95% O2 and 5% CO2 at 37 °C. A fluid-filled balloon inserted into the left ventricle monitored contractile function. The balloon was gradually inflated until the end-diastolic pressure was between 2 and 8 mm Hg. Atrial pacing was performed at 580 beats/min. Coronary flow was measured by timed collection of perfusate. More detailed methods and exclusion and inclusion criteria were as described previously (3, 9, 14, 15).
Analysis of Infarction Volume
The hearts were randomized to 10 μmol/liter SB203580 for 10 min or to 1 μmol/liter BIRB796 for 30 min prior to ischemia with blinding to the corresponding vehicle. Infarction was caused by 30 min of global ischemia followed by 2 h of reperfusion and delineated by 1% triphenyltetrazolium chloride. Triphenyltetrazolium chloride-negative infarction volume was expressed as a percentage of heart volume. All analyses of infarct size were done by an investigator who was blinded with regard to the group assignments. A more detailed description of the methods is provided in previous publications (3, 9, 15).
Immunoblot Analysis
Heart proteins were extracted after 10 min of global ischemia; separated on 10 or 12% SDS-polyacrylamide gels; transferred to polyvinylidene difluoride membranes, which were blocked for 2 h with 5% nonfat milk + 1% bovine serum albumin in Tris-buffered saline (pH 7.4) containing 0.1% Triton X-100; and probed overnight at 4 °C with the appropriate primary antibody as follows: total p38 (catalog no. 9212; T-p38), diphospho-p38 (catalog no. 9211; polyclonal; P-p38), or phospho-HSP27 (catalog no. 2401) from Cell Signaling; diphospho-p38 (catalog no. M8177; monoclonal; mP-p38) from Sigma; monophospho-Tyr182 of p38 (catalog no. 7975-R) from Santa Cruz Biotechnology; p38γ (MAB1347) from R&D systems; or phospho-TAB1 (Ser423) from Sir Philip Cohen (Protein Phosphorylation Unit, University of Dundee, Dundee, Scotland, United Kingdom). Glutathione S-transferase-tagged recombinant p38γ (catalog no. 7480) and p38α (catalog no. 7474) were from Cell Signaling. After washing and exposure for 2 h at room temperature to horseradish peroxidase-conjugated secondary antibody, antibody-antigen complexes were visualized by enhanced chemiluminescence as described previously (3, 14).
p38 Kinase Assay
This assay was based on ATF2 phosphorylation (catalog no. 9820, Cell Signaling). Hearts were homogenized in ice-cold 1× cell lysis buffer plus 1 mm phenylmethylsulfonyl fluoride and centrifuged at 14,000 rpm at 4 °C. The tissue lysate was collected, and 400 μl of supernatant was incubated with 40 μl of immobilized phospho-p38 (Thr180/Tyr182)-specific monoclonal antibody overnight at 4 °C. The tissue lysate/immobilized p38 was centrifuged at 14,000 rpm for 30 s at 4 °C. The pellet was washed twice with 500 μl of ice-cold 1× lysis buffer and finally washed with 500 μl of ice-cold 1× kinase buffer. The pellet was resuspended with 50 μl of kinase buffer supplemented with 200 μm ATP and 1 μg of ATF2 fusion protein in the presence and absence of 10 μm SB203580. The reactions were performed at 30 °C for 30 min with occasionally mixing. The reaction was terminated by the addition of 50 μl of 2× SDS sample buffer and boiled for 5 min before SDS-PAGE and Western blot analysis.
Characterization of Anti-phospho-p38 Antibodies Using Transfected HEK293 Cells
HEK293 cells were transfected at 70% confluency in Opti-MEM I using Lipofectin (both from Invitrogen) with peptide 6 (16) and the relevant plasmid DNAs. Wild-type p38α MAPK (TGY motif intact)- and TAB1-expressing plasmids were obtained from Jiahuai Han (The Scripps Research Institute, La Jolla, CA) (2). p38α MAPK constructs with either Thr180 or Tyr182 mutated to Ala180 (AGY) or Phe182 (TGF), respectively, were obtained from Oded Livnah (The Hebrew University of Jerusalem, Jerusalem, Israel) (10). MKK3 was from Par Gerwins (University of Uppsala, Uppsala, Sweden). All p38 constructs were hemagglutinin-tagged and cloned into the mammalian expression plasmid pcDNA3 (Invitrogen).
Statistics
Data sets were analyzed by two-way analysis of variance, and groups were compared using Tukey's test. A p value <0.05 was considered significant.
RESULTS
p38 MAPK Activation during Myocardial Ischemia in Hearts Expressing the WT or DR Form of p38α
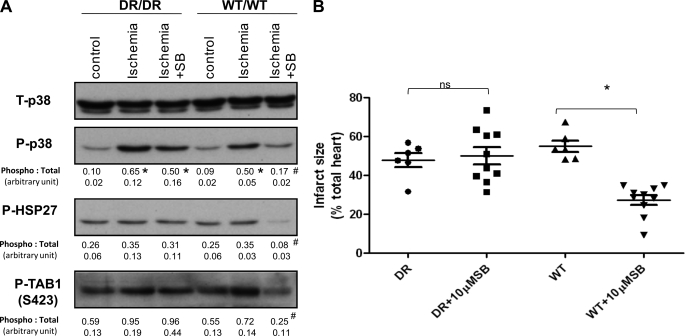
A variety of studies have demonstrated that the dual phosphorylation of p38 MAPK occurring during myocardial ischemia is reduced in the presence of SB203580 (3, 5, 6, 9). This observation could be the result of SB203580 acting upstream of p38 MAPK to inhibit an ischemia-responsive kinase (transphosphorylation) or acting directly on p38 MAPK to prevent autophosphorylation. To determine whether auto- or transphosphorylation is the dominant mechanism, we examined the phosphorylation of p38 MAPK and downstream substrates at 10 min of global ischemia with or without SB203580 pretreatment. p38 MAPK was similarly dual-phosphorylated during ischemia in both WT and DR hearts (Fig. 1A). This dual phosphorylation was inhibited by pretreatment with SB203580 in WT but not DR hearts. Ischemia also tended to enhance the already high basal phosphorylation of the downstream substrates HSP27 and TAB1 in both WT and DR myocardia, but this was inhibited only by SB203580 in WT hearts. These patterns of phosphorylation, which were quantified in at least three separate experiments, suggest that p38α is the dominant isoform activated during myocardial ischemia by a mechanism consistent with autophosphorylation.
FIGURE 1.
p38 MAPK activation during myocardial ischemia and sensitivity to myocardial infarction in hearts expressing the WT or DR form of p38α. A, DR and WT hearts were subjected to 10 min of ischemia in the presence and absence of SB203580 (SB). The values below each band represent quantification of band density from three separate experiments expressed as mean ± S.E. B, shown is the normalized infarction volume of DR and WT hearts in the presence of intraischemic SB203580 or vehicle. p38 in the DR/DR hearts was resistant to inhibition by SB203580 based on intact TAB1 and HSP27 phosphorylation, and this was accompanied by a failure of SB203580 to reduce infarct size. ns, not significant; *, p < 0.05 compared with the control; #, p < 0.05 compared with ischemia without SB203580.
Sensitivity to Myocardial Infarction in Hearts Expressing the WT or DR Form of p38α
The pharmacological inhibition of p38 MAPK by SB203580 reduces ischemic injury (6). However, SB203580 is not selective and is known to inhibit a number of other kinases (7, 8). To determine whether these “off-target” actions are relevant, we examined the sensitivity to infarction of WT and DR hearts in response to 30 min of global ischemia in the presence and absence of SB203580 (Fig. 1B). In the absence of SB203580, the sensitivity to infarction was similar in WT and DR hearts. Exposure to SB203580 significantly reduced infarct size in WT but not DR hearts (27.3 ± 8.1% versus 50.1 ± 13.8%, respectively). Although the hemodynamic data tended to follow infarction, there were no significant differences between the groups (Table 1). These results suggest that the cardioprotective action of SB203580 is dependent on the inhibition of p38α MAPK and not on another SB203580-sensitive target.
TABLE 1.
Morphometric and hemodynamic data in homozygous p38α (T106M) knock-in (DR) and WT mice
LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; Rep, reperfusion.
| Parameter | DR |
WT |
DR |
WT |
||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 6) | +10 mm SB203580 (n = 10) | Control (n = 6) | +10 mm SB203580 (n = 10) | Control (n = 6) | +1 mm BIRB796 (n = 6) | Control (n = 6) | +1 mm BIRB796 (n = 6) | |
| Body weight (g) | 30.7 ± 1.6 | 30.3 ± 0.7 | 32.9 ± 2.5 | 30.1 ± 0.5 | 23.88 ± 0.33 | 23.80 ± 0.80 | 27.12 ± 1.15 | 27.00 ± 0.88 |
| Heart weight (mg) | 0.136 ± 0.01 | 0.131 ± 0.01 | 0.155 ± 0.02 | 0.128 ± 0.0 | 0.096 ± 0.01 | 0.093 ± 0.05 | 0.110 ± 0.01 | 0.109 ± 0.05 |
| LVDP (mm Hg) | ||||||||
| Base line | 93.0 ± 11.4 | 87.0 ± 6.0 | 91.7 ± 9.4 | 84.6 ± 4.4 | 87.2 ± 7.5 | 86.7 ± 5.4 | 82.0 ± 4.4 | 84.3 ± 5.9 |
| Ischemic | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Rep 30 | 14.7 ± 5.9 | 24.6 ± 14.3 | 7.8 ± 4.0 | 42.2 ± 16.2 | 36.0 ± 8.5 | 46.5 ± 8.6a | 11.7 ± 4.2 | 47.2 ± 7.6a |
| Rep 60 | 17.3 ± 5.9 | 25.5 ± 10.5 | 13.0 ± 5.0 | 32.7 ± 9.3a | 27.3 ± 5.6 | 41.0 ± 3.2a | 16.2 ± 2.9 | 44.3 ± 5.8a |
| Rep 90 | 17.0 ± 4.6 | 24.1 ± 8.5 | 13.5 ± 4.0 | 29.1 ± 9.4a | 25.0 ± 5.5 | 37.0 ± 2.8a | 17.3 ± 3.1 | 39.5 ± 3.9a |
| Rep 120 | 15.7 ± 4.1 | 21.9 ± 8.2 | 13.0 ± 1.8 | 26.3 ± 7.2a | 20.7 ± 2.7 | 34.8 ± 2.3a | 16.2 ± 3.8 | 35.2 ± 4.6a |
| LVEDP (mm Hg) | ||||||||
| Base line | 4.8 ± 0.6 | 4.0 ± 1.3 | 3.7 ± 1.4 | 4.8 ± 0.9 | 3.3 ± 1.0 | 3.3 ± 0.8 | 4.8 ± 1.2 | 3.0 ± 0.9 |
| Ischemic | 50.2 ± 7.6 | 39.1 ± 8.8 | 48.8 ± 3.1 | 44.1 ± 10.3 | 30.3 ± 6.2 | 35.5 ± 8.0 | 33.3 ± 3.9 | 38.2 ± 5.6 |
| Rep 30 | 56.3 ± 11.2 | 40.5 ± 9.6a | 55.2 ± 8.4 | 30.6 ± 12.0a | 38.2 ± 9.4 | 19.2 ± 6.2a | 59.0 ± 2.4 | 18.8 ± 11.0a |
| Rep 60 | 45.7 ± 11.9 | 29.2 ± 8.2a | 47.0 ± 7.9 | 24.8 ± 9.4a | 32.3 ± 10.0 | 10.2 ± 4.0a | 47.7 ± 8.7 | 10.7 ± 6.7a |
| Rep 90 | 39.3 ± 12.0 | 24.0 ± 9.8a | 41.3 ± 9.7 | 20.1 ± 7.7a | 27.7 ± 12.4 | 7.0 ± 2.7a | 40.8 ± 11.2 | 8.2 ± 5.0a |
| Rep 120 | 36.2 ± 12.9 | 21.0 ± 9.7a | 36.3 ± 9.0 | 17.9 ± 6.4a | 25.2 ± 13.1 | 5.8 ± 2.9a | 37.2 ± 13.0 | 7.2 ± 4.3a |
| Coronary flow (ml/min) | ||||||||
| Base line | 3.1 ± 0.2 | 2.4 ± 0.4 | 3.3 ± 0.2 | 2.8 ± 0.5 | 3.1 ± 0.4 | 2.7 ± 0.3 | 2.9 ± 0.7 | 2.9 ± 0.6 |
| Ischemic | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Rep 30 | 2.0 ± 0.4 | 2.0 ± 0.3 | 2.3 ± 0.5 | 2.2 ± 0.4 | 2.6 ± 0.4 | 1.9 ± 0.4 | 2.0 ± 0.4 | 2.1 ± 0.6 |
| Rep 60 | 1.5 ± 0.1 | 1.8 ± 0.4 | 2.1 ± 0.6 | 1.9 ± 0.6 | 2.4 ± 0.5 | 1.8 ± 0.5 | 1.9 ± 0.5 | 1.9 ± 0.6 |
| Rep 90 | 1.4 ± 0.2 | 1.7 ± 0.3 | 2.0 ± 0.7 | 1.7 ± 0.5 | 2.3 ± 0.5 | 1.7 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.6 |
| Rep 120 | 1.3 ± 0.2 | 1.6 ± 0.3 | 1.9 ± 0.6 | 1.7 ± 0.4 | 2.2 ± 0.5 | 1.7 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.6 |
ap < 0.05 versus the corresponding control.
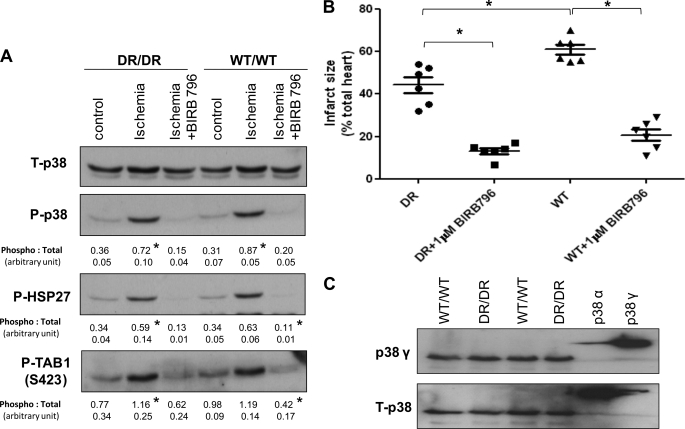
Effect of BIRB796 on p38 Dual Phosphorylation during Myocardial Ischemia
Diaryl urea-type p38 inhibitors make use of other unique structural motifs within p38 and thus, unlike SB203580, are able to inhibit the γ and δ isoforms, which in their native forms contain methionine at a position corresponding to Thr106 (6). BIRB796 is a diaryl urea with a slow onset of action that is thought to be the result of its binding to and subsequent stabilization of a relatively rare conformer that cannot be dual-phosphorylated (17). In both WT and DR hearts exposed to BIRB796 or vehicle for 30 min before ischemia, p38 dual phosphorylation and the phosphorylation of the downstream substrates HSP27 and TAB1 were similarly diminished, suggesting that the resistance to pharmacological inhibition in the DR hearts is dependent on the mode of inhibitor binding and reinforcing the selectivity of the chemical genetic approach we have adopted (Fig. 2A).
FIGURE 2.
Effect of BIRB796 on p38 diphosphorylation and sensitivity to myocardial infarction. A and B, the format was as described in the legend to Fig. 1, but DR and WT hearts were exposed to BIRB796 or vehicle for 30 min prior to ischemia. *, p < 0.05 compared with the control and ischemia + BIRB796. C, to ensure that the targeting of p38α in the DR hearts did not increased the expression of the SB203580-resistant p38γ isoform, heart homogenates were probed with the pan-isoform (T-p38)-selective and p38γ-selective primary antibodies. 16 ng of glutathione S-transferase-tagged recombinant p38α and p38γ was used to index antibody selectivity. The T-p38 antibody preferentially indicated p38α, but both isoforms seemed equally expressed in WT and DR hearts.
Effect of BIRB796 on Sensitivity to Myocardial Infarction
A theoretical concern is that the knock-in strategy nonspecifically alters the DR hearts in such a manner as to render them resistant to cardioprotection. We therefore exposed DR hearts to BIRB796 or vehicle. Compared with vehicle, BIRB796 significantly reduced infarction (13.2 ± 3.6% versus 44.3 ± 9.2%) (Fig. 2B) and improved post-ischemic hemodynamic recovery (Table 2) in both WT and DR hearts, suggesting that the knock-in strategy does not render the DR hearts resistant to cardioprotection by this agent.
TABLE 2.
Morphometric and hemodynamic data in heterozygous p38α (T106M) knock-in (DR/WT) and WT mice
Rep, reperfusion.
| Parameter | DR/WT |
WT/WT |
||
|---|---|---|---|---|
| Control (n = 6) | +10 mm SB203580 (n = 10) | Control (n = 6) | +10 mm SB203580 (n = 10) | |
| Body weight (g) | 24.95 ± 1.0 | 24.73 ± 0.1 | 24.99 ± 0.1 | 25.31 ± 0.1 |
| Heart weight (mg) | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.0 |
| LVDP (mm Hg) | ||||
| Base line | 78.7 ± 8.3 | 85.5 ± 11.1 | 77.3 ± 9.1 | 75 ± 7.9 |
| Ischemic | 0.0 ± 00 | 0.0 ± 00 | 0.0 ± 00 | 0.0 ± 00 |
| Rep 30 | 6.2 ± 2.3 | 4.5 ± 2.6 | 3.2 ± 2.2 | 13.7 ± 15.5a |
| Rep 60 | 12.3 ± 5.6 | 8 ± 2.8 | 7 ± 3.3 | 18.2 ± 9.2a |
| Rep 90 | 12.5 ± 3.2 | 10.3 ± 3.2 | 9.8 ± 4.1 | 22.5 ± 6.3a |
| Rep 120 | 12.7 ± 3.1 | 10.8 ± 3.3 | 10.5 ± 4.5 | 24 ± 4.3a |
| LVEDP (mm Hg) | ||||
| Base line | 4.7 ± 1.6 | 5.0 ± 1.8 | 5.3 ± 1.5 | 4.0 ± 1.7 |
| Ischemic | 61.2 ± 9.5 | 61.2 ± 4.0 | 69.3 ± 5.5 | 60.2 ± 11.7 |
| Rep 30 | 65.5 ± 8.3 | 70.3 ± 8.7 | 76.3 ± 7.1 | 66.7 ± 17.6 |
| Rep 60 | 60.2 ± 10.2 | 64.8 ± 11.9 | 66.8 ± 6.9 | 55.2 ± 13.4 |
| Rep 90 | 52.5 ± 11.6 | 60.3 ± 12.0 | 62 ± 7.0 | 48.7 ± 12.4 |
| Rep 120 | 50.2 ± 11.5 | 57.5 ± 12.8 | 58.3 ± 7.0 | 45.0 ± 11.4 |
| Coronary flow (ml/min) | ||||
| Base line | 2.4 ± 0.5 | 2.7 ± 0.5 | 2.2 ± 0.4 | 3.2 ± 0.4 |
| Ischemic | 0.0 ± 00 | 0.0 ± 00 | 0.0 ± 00 | 0.0 ± 00 |
| Rep 30 | 1.9 ± 0.2 | 2.0 ± 0.6 | 1.3 ± 0.5 | 2.0 ± 0.0a |
| Rep 60 | 1.4 ± 0.5 | 1.3 ± 0.8 | 1.0 ± 0.0 | 1.8 ± 0.4a |
| Rep 90 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.0 ± 0.0 | 1.5 ± 0.5 |
| Rep 120 | 1.0 ± 0.1 | 1.2 ± 0.4 | 1.0 ± 0.0 | 1.5 ± 0.5 |
ap < 0.05 versus the corresponding control.
One possibility that could explain the ability of BIRB796 to reduce infarction while SB203580 remains ineffective in the DR hearts is an increase in the expression of p38γ. If this were to be accompanied by a corresponding reduction in p38α, total p38 levels would appear similar in the DR and WT hearts, masking the shift in expression from an SB203580-sensitive to SB203580-resistant isoform. To exclude this possibility, we examined the expression of p38α and p38β using recombinant glutathione S-transferase-tagged isoforms as controls (Fig. 2C). As shown, the pan-isoform T-p38 antibody preferentially labeled p38α, and myocardial content appeared equal between genotypes. The anti-p38γ monoclonal antibody is relatively isoform-selective and demonstrates that p38γ is equally abundantly expressed in WT and DR hearts. Thus, the targeting strategy has not perturbed the expression of p38α or p38γ.
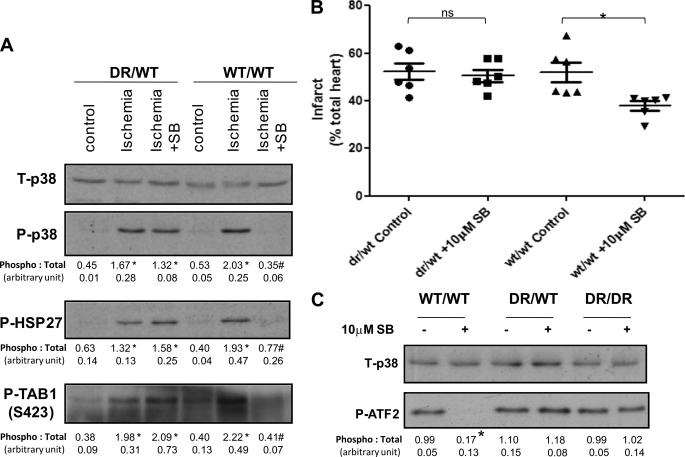
Is Partial Inhibition of p38α Sufficient to Reduce Infarction?
The experiments described above were performed in hearts harvested from mice homozygous for the alleles encoding either WT or DR p38α. To determine whether hearts from heterozygous mice behave similarly, the experiments depicted in Fig. 1 were repeated. Fig. 3A shows that the myocardial total p38 content was similar between heterozygous and wild-type mice, but despite the presence of a wild-type allele, p38 activity during ischemia remained resistant to inhibition by SB203580, which failed to reduce infarction (Fig. 3B). Because the WT and DR alleles caused equal p38α protein abundance within the myocardium (Fig. 1A), it is likely that partial inhibition is not sufficient to prevent downstream signaling or development of infarction. This was confirmed by the inability of SB203580 to significantly inhibit the in vitro phosphorylation of the p38 substrate ATF2 by endogenous p38 precipitated from crude heart homogenate (Fig. 3C).
FIGURE 3.
Effect of SB203580 on hearts from mice heterozygous for the p38α DR allele. A, hearts from mice harboring one (DR/WT) or no (WT/WT) p38α DR allele were subjected to 10 min of ischemia in the presence and absence of SB203580 (SB). The values below each band represent quantification of band density from three separate experiments expressed as mean ± S.E. B, shown is the normalized infarction volume of DR/WT and WT/WT hearts in the presence of intraischemic SB203580 or vehicle. *, p < 0.05 compared with the control. ns, not significant. C, shown are the results of an in vitro kinase assay with p38 precipitated from crude heart homogenate using ATF2 as substrate. The DR/WT hearts behaved like DR/DR hearts in that SB203580 did not prevent p38 activation or the phosphorylation of the downstream endogenous substrates HSP27 and TAB1 or the exogenous substrate ATF2 in an in vitro kinase assay.
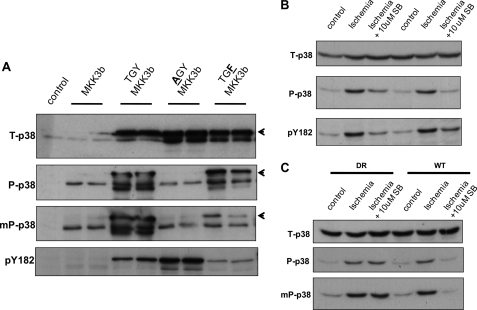
Di- Versus Monophosphorylation of the TGY Activation Motif
The results indicated that the dual phosphorylation of p38 was prevented only when SB203580 was able to bind p38α (WT versus DR lanes in Fig. 1A). This suggests that dual phosphorylation can proceed only when ATP binding is unhindered by SB203580 and therefore is likely dependent on kinase activity and, by inference, the result of autophosphorylation. p38 is an archetypal serine/threonine kinase, and it is uncertain how it would be capable of mediating phosphotransfer to Tyr182 required for dual phosphorylation. Recently, studies of constitutively active mutants of p38 (10) and p38 phosphatases (11) have highlighted that p38 can be catalytically active when phosphorylated at Thr180 alone. Moreover, the commonly used dual phosphospecific polyclonal antibodies may recognize this monophosphothreonine form. Thus, the supposedly dual phosphospecific p38 signal that diminishes in the presence of ATP-competitive inhibitors in Figs. 1–3, as well as in the previous literature (3, 6, 9), may represent monophosphorylation masquerading as dual phosphorylation. To examine this possibility, we characterized a range of antibodies using HEK293 cells expressing p38α with the TGY activation motif intact or with either the threonine or tyrosine residue substituted with alanine (AGY) or phenylalanine (TGF), respectively. The activation motifs were phosphorylated by coexpression of the upstream dual specificity kinase MKK3.
In Fig. 4A, ectopically expressed p38α ran above endogenous p38 as a result of the hemagglutinin tag (see arrowhead). The dual phosphospecific polyclonal antibody (P-p38) recognized endogenous and ectopic wild-type (TGY) p38 and ectopic p38 phosphorylated at threonine alone (TGF) but not at tyrosine alone (AGY). In contrast, the anti-phospho-Tyr182 antibody (pY182) recognized this form (AGY) as well as diphosphorylated p38 (TGY). Furthermore, a dual phosphospecific monoclonal antibody (mP-p38) was relatively selective and insensitive to phosphorylation of Thr180 alone (compare mP-p38 and P-p38). These antibodies were then used to examine p38 dual phosphorylation during ischemia. In outbred C57BL/6 mouse hearts subjected to ischemia in the presence and absence of SB203580, the dual phosphospecific signal seen with the polyclonal antibody (P-p38) closely resembled that seen with the anti-phosphotyrosine antibody (pY182) (Fig. 4B). Furthermore, in DR knock-in and corresponding WT hearts, the sensitivity and resistance to SB203580 involved phosphorylation of both the threonine and tyrosine residues of the TGY motif based on the similarity of immunodetection with the mP-p38 and P-p38 antibodies (Fig. 4C).
FIGURE 4.
Di- or monophosphorylation of p38 during myocardial ischemia. A, HEK293 cells were transfected as indicated. The three-letter codes denote the amino acid sequence in the activation motifs of ectopically expressed p38α with a tag that slowed migration (arrowheads). The anti-diphospho-p38 polyclonal antibody (P-p38) recognized phosphorylation of threonine alone (TGF = TGY), but the anti-diphospho-p38 monoclonal antibody was more selective for the true diphospho form (TGY ≫ TGF). The anti-phospho-Tyr182 antibody (pY182), unlike P-p38, was insensitive to threonine phosphorylation (TGY = AGY ≫ TGF). B, 10 min of ischemia resulted in phosphorylation of Tyr182, which was sensitive to SB203580 (SB). C, shown is the confirmation of TGY phosphorylation by the similarity in the patterns of immunoreactivity between anti-diphospho-p38 monoclonal and polyclonal antibodies.
DISCUSSION
Using a novel chemical genetic approach, we have shown that inhibition of the α isoform of p38 is responsible for the cardioprotection seen with SB203580 and that this isoform is likely activated by autophosphorylation of both the threonine and tyrosine residues in the activation loop. This information better defines the targets for the development of isoform- and circumstance-specific inhibitors of p38 for treatment of acute myocardial infarction.
There is already overwhelming evidence that pharmacological inhibition of p38 during myocardial ischemia is protective (6). However, there are a variety of explanations for this observation. One possibility is that protection is the result of the off-target inhibition of a non-p38 kinase. Another possibility is that it is the result of the “on-target” inhibition of either the α or β isoform of p38 or requires the simultaneous inhibition of both the α and β isoforms. The data provided in this work differentiate between these possibilities to clearly show that the on-target inhibition of the α isoform of p38 alone is the mechanism of protection. The chemical genetic approach we adopted reinforces and builds upon arguably less definitive findings in cell-based models of simulated ischemia with ectopic expression of mutant p38 isotypes (3, 18, 19) and in hearts of mice with p38α haploinsufficiency (20) or transgenic overexpression of dominant-negative upstream kinases or p38 isoforms (18).
Although the finding that it is myocardial p38α activation during ischemia that aggravates injury was expected, the findings regarding the sensitivity of p38 dual phosphorylation to pharmacological inhibition were not. The classic mechanism of p38 activation is the phosphorylation of Thr180 and Tyr182 in the activation loop by the upstream dual specificity kinases MKK3 and MKK6. These phosphorylations in turn result in a rotation of the two lobes of p38, enabling Asp168 in the C-terminal lobe and Lys53 in the N-terminal lobe to reorient, thereby enabling their coordination in the binding and catalysis of ATP (21). Thus, in the absence of Thr180 and Tyr182 phosphorylation, it is unclear how the kinase can bind ATP and autophosphorylate. Recent insights into how this may be achieved have come from a detailed screen of mutations of a yeast homolog of p38 that rescue growth in the absence of an upstream MKK. These mutations lie predominantly in a C-terminal extension of p38 that forms a bridge between the two lobes (10). The mutations disrupt the interactions with the N-terminal lobe to cause a reorientation that remodels the ATP-binding site. It is likely that a similar mechanism accounts for a physiological form of p38 autophosphorylation seen in T-cells that follows phosphorylation of Tyr323 within this extension (4). However, from a very recent study, it is clear that this form of autophosphorylation is confined to Thr180, excluding Tyr182 (4). Similar exclusive autophosphorylation of Thr180 occurs in the constitutively active mutants of p38α (10). The finding in our study that Tyr182 phosphorylation is also directly dependent on the ability of SB203580 to bind p38α is therefore surprising.
Similarly, in mice heterozygous for the p38α DR allele, we predict but cannot prove that there is equal expression of WT and DR forms of p38 within the heart, yet these hearts seem fully resistant to the effects of SB203580. It is possible that this is the result of a relative excess of p38α kinase activity that results in non-linearity and efficient phosphorylation of downstream substrates. More difficult to explain is the intact diphosphorylation of p38 shown in Fig. 3A. The cause for this is unknown but maybe related to the suggestion that p38, like ERK (extracellular signal-regulated kinase), dimerizes and that autophosphorylation is in fact autotransphosphorylation of one p38α monomer by another. Thus, the uninhibited DR form would be capable of transactivating the SB203580-bound and SB203580-inhibited WT form.
Based on the studies summarized above, it is entirely feasible that Thr180 is autophosphorylated. However, it is more difficult to envisage how a Ser/Thr kinase such as p38 can phosphorylate Tyr182. Nonetheless, this situation is exactly analogous to GSK3α and GSK3β, Ser/Thr kinases that are also activated by autophosphorylation of Tyr279 and Tyr216, respectively (22). A similar situation occurs with the activating Tyr autophosphorylation of the Ser/Thr DYRKs (23). Moreover, the positions of the autophosphorylated Tyr residues in GSKs and DYRKs correspond to Tyr180 in p38α. However, unlike p38, the autophosphorylation of GSKs and DYRKs does not seem dynamic and occurs while the proteins are immature (23, 24).
Given the differences between p38α and the GSKs and DYRKs, could there be alternative explanations for the phosphorylation of Tyr182 that are still consistent with our data? One possibility is that the phosphorylation is mediated by an extrinsic tyrosine kinase but that this event requires the priming and rate-limiting autophosphorylation of Thr180. Another possibility is that ATP binding may help enable the intramolecular rearrangements that promote diphosphorylation, and thus, the binding of SB203580, by excluding ATP, prevents this event. Such a scenario is possible because ATP and SB203580 have different contact points within the nucleotide-binding pocket (13), and the prevention of ATP binding, rather than of catalytic activity, has recently been shown to underlie the illusion of autophosphorylation in kinase-dead mutants of protein kinase C (25).
In conclusion, we have adopted a chemical genetic approach to demonstrate that the short-term intraischemic inhibition of p38α in the intact heart reduces infarction. Furthermore, the mechanism of activation of p38α during myocardial ischemia is entirely consistent with autoactivation. This information may allow a refinement of the current strategies to target p38 in ischemic heart disease.
Acknowledgment
We thank Sir Philip Cohen for providing anti-phospho-TAB1 (Ser423) antibody and BIRB796.
This work was supported by a Royal Thai Government scholarship (to S. K.) and by Medical Research Council Project Grant G0802033 and British Heart Foundation Project Grant 07/073/23432.
- MAPK
- mitogen-activated protein kinase
- DR
- drug-resistant
- WT
- wild-type
- MKK
- MAPK kinase
- GSK
- glycogen synthase kinase
- DYRK
- dual specificity tyrosine phosphorylation-regulated kinase.
REFERENCES
- 1.Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. (1996) Mol. Cell. Biol. 16, 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R. J., Luo Y., Han J. (2002) Science 295, 1291–1294 [DOI] [PubMed] [Google Scholar]
- 3.Tanno M., Bassi R., Gorog D. A., Saurin A. T., Jiang J., Heads R. J., Martin J. L., Davis R. J., Flavell R. A., Marber M. S. (2003) Circ. Res. 93, 254–261 [DOI] [PubMed] [Google Scholar]
- 4.Mittelstadt P. R., Yamaguchi H., Appella E., Ashwell J. D. (2009) J. Biol. Chem. 284, 15469–15474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiedler B., Feil R., Hofmann F., Willenbockel C., Drexler H., Smolenski A., Lohmann S. M., Wollert K. C. (2006) J. Biol. Chem. 281, 32831–32840 [DOI] [PubMed] [Google Scholar]
- 6.Clark J. E., Sarafraz N., Marber M. S. (2007) Pharmacol. Ther. 116, 192–206 [DOI] [PubMed] [Google Scholar]
- 7.Godl K., Wissing J., Kurtenbach A., Habenberger P., Blencke S., Gutbrod H., Salassidis K., Stein-Gerlach M., Missio A., Cotten M., Daub H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15434–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Keefe S. J., Mudgett J. S., Cupo S., Parsons J. N., Chartrain N. A., Fitzgerald C., Chen S. L., Lowitz K., Rasa C., Visco D., Luell S., Carballo-Jane E., Owens K., Zaller D. M. (2007) J. Biol. Chem. 282, 34663–34671 [DOI] [PubMed] [Google Scholar]
- 9.Jacquet S., Nishino Y., Kumphune S., Sicard P., Clark J. E., Kobayashi K. S., Flavell R. A., Eickhoff J., Cotten M., Marber M. S. (2008) J. Biol. Chem. 283, 11964–11971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diskin R., Lebendiker M., Engelberg D., Livnah O. (2007) J. Mol. Biol. 365, 66–76 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y. Y., Mei Z. Q., Wu J. W., Wang Z. X. (2008) J. Biol. Chem. 283, 26591–26601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyers P. A., van den Ijssel P., Quinlan R. A., Goedert M., Cohen P. (1999) FEBS Lett. 451, 191–196 [DOI] [PubMed] [Google Scholar]
- 13.Gum R. J., McLaughlin M. M., Kumar S., Wang Z., Bower M. J., Lee J. C., Adams J. L., Livi G. P., Goldsmith E. J., Young P. R. (1998) J. Biol. Chem. 273, 15605–15610 [DOI] [PubMed] [Google Scholar]
- 14.Bellahcene M., Jacquet S., Cao X. B., Tanno M., Haworth R. S., Layland J., Kabir A. M., Gaestel M., Davis R. J., Flavell R. A., Shah A. M., Avkiran M., Marber M. S. (2006) J. Am. Coll. Cardiol. 48, 545–555 [DOI] [PubMed] [Google Scholar]
- 15.Nishino Y., Webb I. G., Davidson S. M., Ahmed A. I., Clark J. E., Jacquet S., Shah A. M., Miura T., Yellon D. M., Avkiran M., Marber M. S. (2008) Circ. Res. 103, 307–314 [DOI] [PubMed] [Google Scholar]
- 16.Hart S. L., Collins L., Gustafsson K., Fabre J. W. (1997) Gene Ther. 4, 1225–1230 [DOI] [PubMed] [Google Scholar]
- 17.Kuma Y., Sabio G., Bain J., Shpiro N., Márquez R., Cuenda A. (2005) J. Biol. Chem. 280, 19472–19479 [DOI] [PubMed] [Google Scholar]
- 18.Kaiser R. A., Bueno O. F., Lips D. J., Doevendans P. A., Jones F., Kimball T. F., Molkentin J. D. (2004) J. Biol. Chem. 279, 15524–15530 [DOI] [PubMed] [Google Scholar]
- 19.Saurin A. T., Martin J. L., Heads R. J., Foley C., Mockridge J. W., Wright M. J., Wang Y., Marber M. S. (2000) FASEB J. 14, 2237–2246 [DOI] [PubMed] [Google Scholar]
- 20.Otsu K., Yamashita N., Nishida K., Hirotani S., Yamaguchi O., Watanabe T., Hikoso S., Higuchi Y., Matsumura Y., Maruyama M., Sudo T., Osada H., Hori M. (2003) Biochem. Biophys. Res. Commun. 302, 56–60 [DOI] [PubMed] [Google Scholar]
- 21.Wilson K. P., Fitzgibbon M. J., Caron P. R., Griffith J. P., Chen W., McCaffrey P. G., Chambers S. P., Su M. S. (1996) J. Biol. Chem. 271, 27696–27700 [DOI] [PubMed] [Google Scholar]
- 22.Cole A., Frame S., Cohen P. (2004) Biochem. J. 377, 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochhead P. A., Sibbet G., Morrice N., Cleghon V. (2005) Cell 121, 925–936 [DOI] [PubMed] [Google Scholar]
- 24.Lochhead P. A., Kinstrie R., Sibbet G., Rawjee T., Morrice N., Cleghon V. (2006) Mol. Cell 24, 627–633 [DOI] [PubMed] [Google Scholar]
- 25.Cameron A. J., Escribano C., Saurin A. T., Kostelecky B., Parker P. J. (2009) Nat. Struct. Mol. Biol. 16, 624–630 [DOI] [PubMed] [Google Scholar]