Abstract
The identity of the source of the biological reductant needed to convert cobalamin to its biologically active form adenosylcobalamin has remained elusive. Here we show that free or protein-bound dihydroflavins can serve as the reductant of Co2+Cbl bound in the active site of PduO-type ATP-dependent corrinoid adenosyltransferase enzymes. Free dihydroflavins (dihydroriboflavin, FMNH2, and FADH2) effectively drove the adenosylation of Co2+Cbl by the human and bacterial PduO-type enzymes at very low concentrations (1 μm). These data show that adenosyltransferase enzymes lower the thermodynamic barrier of the Co2+ → Co+ reduction needed for the formation of the unique organometalic Co–C bond of adenosylcobalamin. Collectively, our in vivo and in vitro data suggest that cobalamin reductases identified thus far are most likely electron transfer proteins, not enzymes.
Keywords: Enzymes/Catalysis, Enzymes/Flavin, Enzymes/Reductase, Metabolism, Vitamins and Cofactors/Adenosylcobalamin, Electron Transfer Proteins, Redox Potential
Introduction
Cobalamin (Cbl)3 is an essential nutrient for animals, lower eukaryotes, and prokaryotes (1). Only some archaea and bacteria synthesize AdoCbl de novo, a process that involves at least 25 proteins (1). Enzymes that use AdoCbl (AdoCbl, coenzyme B12) catalyze intramolecular rearrangements (2, 3), deaminations (4), dehydrations (5), reductions (6, 7), or reductive dehalogenations (8). In humans, AdoCbl is required for the metabolism of branched chain amino acids, short chain fatty acids, and cholesterol (9).
The cobalt-carbon bond of AdoCbl lies at the heart of the reactivity of this coenzyme. This is a unique organometallic bond that serves as a radical initiator (10). ATP:cob(I)alamin adenosyltransferase (ACA) enzymes catalyze the transfer of the adenosyl moiety from ATP to cob(I)alamin, resulting in the formation of this unique cobalt-carbon bond. Three types of ACA enzymes have been described thus far, CobA, PduO, and EutT (11–13). Although ACA enzymes carry out the same reaction, they do not share sequence homology at the nucleotide or amino acid level.
For adenosylation to occur, the cobalt ion of corrinoids must be reduced in two consecutive one-electron reductions from Co3+ → Co2+ → Co+ (14). We previously reported that the reducing environment of the cell drives the Co3+ → Co2+ reduction (14, 15). The Co2+ → Co+ reduction, however, is more challenging because the reduction potential of Co2+/+ couple in solution is −610 mV (16), which is lower than any known physiological reductant in the cell.
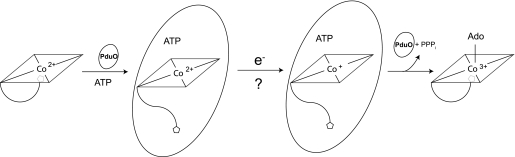
To solve this problem, the cell uses ACA enzymes to facilitate the reduction of cob(II)alamin before adenosylation can occur (Fig. 1). In this process, ACA enzymes bind 5-coordinate cob(II)alamin and displace the lower ligand 5,6-dimethylbenzimidazole, resulting in a 4-coordinate cob(II)alamin intermediate that lacks axial ligands (17–22). In this 4-coordinate cob(II)alamin intermediate, the 3dz2 orbital of the cobalt ion is stabilized, raising the reduction potential ∼250 mV and bringing the reduction potential of Co2+/+Cbl to within physiological range (20). Recent structural and kinetic analyses revealed that a conserved phenylalanine in the active site of the PduO-type ACA enzyme is critical to the formation of the 4-coordinate cob(II)alamin intermediate (17, 21).
FIGURE 1.
Proposed mechanism for the reduction and adenosylation of cobalamin. PduO-type ACA enzymes (oval) bind 5-coordinate cob(II)alamin and facilitate its reduction to cob(I)alamin by displacing the lower ligand. The latter event generates a 4-coordinate cob(II)alamin intermediate whose redox potential is raised enough so it can accept one electron from dihydroflavin. The resulting cob(I)alamin nucleophile attacks ATP to generate coenzyme B12 (AdoCbl) and triphosphate. In these studies, we investigated the source of the electron for the reduction of cob(II)alamin to cob(I)alamin.
Even though we now have a better understanding of the mechanism of this unfavorable reduction, we still do not know the identity of the physiological reductant for the Co2+ → Co+ reduction, and how the electron is delivered. This has been a point of interest for several decades. Because the reduction potential of the Co2+ → Co+ reduction in solution is outside the physiological range, all scenarios investigated thus far invoke the participation of a reductase and/or an electron transfer protein.
In the case of the CobA ACA enzyme of Salmonella enterica (SeCobA), in vitro evidence for the involvement of a reduction system was reported (23). In this case, flavodoxin A (FldA) is reduced by the NADPH-dependent ferredoxin (flavodoxin) protein reductase (Fpr), and reduced FldA transfers one electron to cob(II)alamin present in the active site of SeCobA yielding cob(I)alamin. The latter is the nucleophile that attacks the 5′C of ATP yielding AdoCbl and triphosphate (23). The putative role of the Fpr/FldA system in cob(II)alamin reduction was supported by the results from studies of FldA/CobA interactions (24).
There are several reports of putative cobalamin reductases in the literature. For example, in Brucella melitensis, the flavoprotein CobR was reported to reduce the cobalt ion from Co3+ → Co2+ → Co+ in several corrinoids, including intermediates of the corrin ring biosynthetic pathway and cobalamin (25).
In the case of the PduO-type human adenosyltransferase (hATR), the source of the electron involved in the reduction of cob(II)alamin is unknown, and initial searches for the source of this electron putatively identified methionine synthase reductase (26) as a candidate reductant driving the reaction. This hypothesis was based on results of in vitro studies, which showed that, in the presence of hATR, methionine synthase reductase reduced cob(II)alamin to cob(I)alamin (27). An important caveat to the idea that methionine synthase reductase is the physiological reductant of hATR is that methionine synthase reductase is found in the cytosol, where methionine is synthesized (28), and not in the mitochondrion where AdoCbl synthesis occurs, leaving unanswered the questions about the origin of the electron involved in the hATR reaction.
The reductant needed for the synthesis of AdoCbl in bacteria that use PduO-type ACA enzymes is also unknown. Sampson et al. (29) reported results of in vitro experiments, which suggested that, in S. enterica, the PduS protein catalyzes two consecutive one-electron reductions needed to convert cob(III)alamin to cob(I)alamin. To our knowledge, there is no in vivo evidence to support any of the abovementioned results.
In this study, we report the dihydroflavin-dependent reduction of cob(II)alamin to cob(I)alamin, but only when cob(II)alamin is bound to the active site of PduO-type ACA enzymes. We show that flavoproteins, whose functions are unrelated to AdoCbl biosynthesis, can drive PduO-bound cob(II)alamin reduction.
EXPERIMENTAL PROCEDURES
Cloning of Genes into Overexpression Vectors
The MMAB human gene (encodes the PduO-type corrinoid adenosyltransferase; hATR) was synthesized by GenScript with Escherichia coli codon usage and NheI and NotI restriction sites at the 5′ and 3′ ends, respectively. To generate a recombinant protein of hATR with an rTEV protease-cleavable N-terminal hexahistidine (His6) tag, the GenScript construct was cut with NheI and NotI enzymes, and the fragment was ligated into vector pTEV3 (30) cut with the same enzymes; the resulting plasmid was named PDU46.
The S. enterica pduS gene was PCR-amplified using strain TR6583 genomic DNA as template and primers that included NdeI and BamHI restriction sites at the 5′ and 3′ ends, respectively. The amplified fragment was ligated into plasmid pET15b (EMB Biosciences) yielding plasmid pPDU47, which directed the synthesis of His6 N-terminally tagged PduS (H6-PduS). DNA sequences were confirmed at the DNA sequencing facility of the University of Wisconsin, Madison.
Production and Purification of Proteins
hATR protein was produced by transforming the plasmid PDU46 into E. coli strain BL21(DE3) for overexpression. Strains were grown at 37 °C with shaking in 1.5 liter of lysogenic broth (LB) medium (31, 32) supplemented with ampicillin (100 μg/ml). After the culture reached an absorbance at 650 nm (A650) of 0.6–0.7, synthesis of phage T7 RNA polymerase enzyme was induced by the addition of isopropyl β-d-thiogalactopyranoside to a final concentration of 0.5 mm. Cells were grown for an additional 5 h at 37 °C with shaking. Cells were harvested by centrifugation at 12,000 × g with a Beckman/Coulter Avanti J-25I centrifuge equipped with a JLA-16.250 rotor. The cell pellet was frozen at −80 °C until used. His6-hATR protein was purified from cell pellets that were thawed and resuspended in 30 ml of Tris-HCl buffer (0.1 m, pH 8.0 at 4 °C) containing the protease inhibitor phenylmethanesulfonyl fluoride (0.8 mm), imidazole (20 mm), and NaCl (0.5 m). Cells were broken using a French pressure cell (1.03 × 107 kilopascals). Cell-free extracts were cleared by centrifugation at 4 °C for 1 h at 45,000 × g. The resulting supernatant was filtered (0.45 mm; Nalgene), and proteins were resolved on a 5-ml HisTrap FF column (Amersham Biosciences). Proteins were desorbed from the column with a linear gradient of Tris-HCl buffer (0.1 m, pH 8.0 at 4 °C) containing imidazole (0.5 m) and NaCl (0.5 m). Fractions containing hATR protein were pooled and incubated for 2 h at room temperature with rTEV protease (33–35) in a 2:50 rTEV:hATR molar ratio. The hATR, rTEV protein mixture was sequentially dialyzed at 4 °C against buffer A (0.1 m Tris-HCl, pH 8 at 4 °C) containing 0.5 m NaCl, 2 mm EDTA, buffer B (buffer A lacking EDTA), and buffer C (0.1 m Tris-HCl, pH 8 at 4 °C) containing 10 mm imidazole and 0.5 m NaCl. The rTEV, hATR protein mixture was loaded onto a 5-ml HisTrap FF column. The flow-through was collected and dialyzed against Tris-HCl buffer (0.1 m, pH 8.0 at 4 °C) containing 0.5 m NaCl and 10% glycerol (v/v). Tag-less hATR used for kinetic analysis was stored at −80 °C until used.
The plasmid encoding the SeH6-PduS protein was transformed into the E. coli strain BL21 (DE3) and was used for overexpression. Overproduction strains were grown at 28 °C with shaking in super broth medium supplemented with ampicillin (100 μg/ml). When the culture reached an A650 = 0.6, isopropyl β-d-thiogalactopyranoside (0.5 mm) was added, and the culture was shifted to 15 °C and grown overnight at that temperature. Cells were harvested by centrifugation and were broken by French pressing (as above), and the SeH6-PduS protein was isolated as described for hATR.
The LrPduO wild-type protein and the variant LrPduOF112A contained an rTEV protease-cleavable N-terminal His6 tag. Tagged SeH6-PduS protein was isolated using Ni2+ affinity chromatography as described elsewhere (36). The tag was from homogeneous SeH6-PduS with rTEV protease prepared as described (37). FMN reductase, FldA, ferredoxin (flavodoxin):NAD(P)+ reductase (Fpr), and SeCobA were produced and purified as described, without modifications (23, 38).
In Vitro Activity Assays
Adenosylation activity assays of Cbl were performed using the continuous spectrophotometric methods described elsewhere (21, 36). Cob(III)alamin was chemically reduced to cob(I)alamin with Ti(III)citrate (36). In assays that employed cob(II)alamin, a reduced electron transfer protein (FldA, Fpr, PduS, or YdjA) or free dihydroflavins were used to reduce cob(II)alamin to cob(I)alamin. Free dihydroflavins were generated inside an anoxic chamber by incubating FAD/FMN with NADH (1 mm) for 3 h. The flavin cofactor of Fpr was reduced to dihydroflavin by incubating the protein with NADH (1 mm). This sample of reduced Fpr was used to reduce the cofactor of FldA. The concentration of the electron transfer proteins was either one-to-one molar ratio or ≥10-fold in excess to the concentration of the adenosyltransferase.
Cob(II)alamin reduction was assessed using a continuous spectrophotometric assay that monitored changes in the absorbance at 374 nm (36). The reaction mixture contained FMN/FAD (0.1–10 μm), NADH (1 mm), ATP (1 mm), PduO-type enzyme (7–10 μg/ml), FMN reductase (10 μg/ml), KCl2 (0.1 m), MgCl2 (1.5 mm), Tris-Cl buffer (0.2 m, pH 8 at 37 °C). Because the reduction of cob(II)alamin was coupled to the adenosylation of cob(I)alamin, the reaction was started by the addition of the PduO-type adenosyltransferase. FMN reductase was used to accelerate the reduction of flavins and was not required for the reduction of cob(II)alamin when FMNH2 or FADH2 was added to reaction.
Adenosylation activity assays of Cbi were performed using an end point assay inside an anoxic chamber. The reactions were incubated in the dark at room temperature for 4 h. The reaction mixture contained the same reagents as in the reduction of cob(II)alamin assay (see above) except Co(II) cobinamide was the substrate. The presence of AdoCbi was assessed spectophotometrically and by bioassay using an AdoCbl/AdoCbi auxotrophic strain of S. enterica (JE7180). A 2.5-μl sample of the reaction products was spotted on non-carbon essential minimal medium (39) seeded with strain JE7180. The non-carbon essential medium was supplemented with 90 mm ethanolamine, 150 μm 5,6-dimethylbenzimidazole, 0.5 mm methionine, 0.5 mm glycerol, 1 mm MgSO4, 30 mm NH4Cl, and trace minerals.
RESULTS AND DISCUSSION
Dihydroflavins Drive the Conversion of Cob(II)alamin to AdoCbl by PduO-type ACA Enzymes
The search for the physiological reductant needed for the conversion of vitamin B12 into coenzyme B12 has been going on for decades, with claims of the existence of different reducing systems being reported (14, 40). In the last few years, however, ACA enzymes have been shown to critically contribute to this process by increasing the reduction potential of cob(II)alamin to within physiological range (18–20, 22). It remains unclear, however, whether the Co(II) → Co(I) reduction is driven by an enzyme or by an electron transfer protein.
We found that the Lactobacillus reuteri PduO (LrPduO) enzyme can adenosylate cob(II)alamin in the presence of free dihydroflavins (FMNH2 or FADH2). This was surprising, because the idea that a reductase enzyme is required for the reduction of cob(II)alamin has been generally accepted. To determine whether free dihydroflavin-driven cob(II)alamin reduction worked with other PduO-type ACA enzymes, we substituted the human PduO-type adenosyltransferase for the bacterial enzyme in the reaction mixture. Our results showed that indeed dihydroflavins quantitatively drove the adenosylation of cob(II)alamin by the human adenosyltransferase enzyme (Fig. 2A).
FIGURE 2.
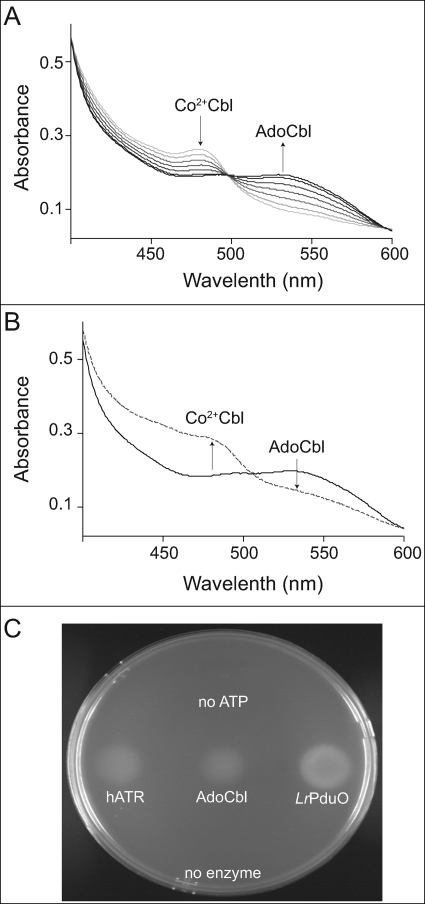
Free dihydroflavins drive the conversion of 4-coordinate cob(II)alamin bound to PduO-type ACA enzymes to AdoCbl. A, spectral changes associated with the enzymatic conversion of cob(II)alamin by hATR. Arrows and increasing darkness in the spectra represent successive time points after the reaction was initiated by addition of hATR (light gray, 1 min; black, 10 min). B, spectral changes associated with photolysis of AdoCbl. The presence of AdoCbl was confirmed by exposing the product of reaction mixture from A (black) under a tungsten lamp for 10 min in an anoxic cuvette (dotted line). C, presence of AdoCbl in reaction mixture A was confirmed by bioassay using an AdoCbl auxotroph strain of S. enterica (JE7180).
The formation of AdoCbl was confirmed based on two parameters. First, spectral changes of the corrinoid in the reaction mixture (i.e. an increase in A525 nm (AdoCbl), with a concomitant decrease at A473 nm), revealed the conversion of cob(II)alamin to AdoCbl. Second, exposure of the latter to light under anoxic conditions resulted in homolytic cleavage of the Co–C bond yielding cob(II)alamin (Fig. 2B). Together, these data supported the conclusion that AdoCbl was present in the mixture. AdoCbl was not detected in control experiments performed in the absence of dihydroflavins or the PduO-type enzymes.
Further evidence that AdoCbl was present in the reaction mixture was obtained by bioassay. For this purpose, we used an AdoCbl auxotrophic strain of S. enterica (JE7180, cobA366::Tn10d(cat+) eut1141(ΔeutT)), which cannot grow on ethanolamine, unless AdoCbl is provided in the medium (41). Reaction products were spotted on a no-carbon essential/ethanolamine medium agar plate seeded with strain JE7180. Cell growth was assessed after overnight incubation in the dark at 37 °C. Only reaction mixtures that included PduO-type enzymes, ATP, and cob(II)alamin supported growth of JE7180; control reactions lacking ATP or PudO-type enzymes did not (Fig. 2C).
Formation of the 4-Coordinate Cob(II)alamin Intermediate Is Required for Dihydroflavin-driven AdoCbl Synthesis
PduO-type ACA enzymes facilitate the unfavorable reduction of cob(II)alamin by generating a 4-coordinate intermediate in the active site of the enzyme (17, 19, 21, 22). The conserved residue Phe-112, located in the active site of PduO, is critical for the displacement of the lower ligand base of cob(II)alamin (17, 21). The LrPduOF112A variant enzyme cannot displace the lower ligand and is therefore inactive (36).
Control experiments showed that free dihydroflavins did not reduce cob(II)alamin in the absence of the bacterial or human PduO-type ACA enzyme, nor did they drive the reduction of cob(II)alamin bound to the active site of the LrPduOF112A variant (data not shown). The above results led us to conclude that dihydroflavins can reduce 4-coordinate but not 5-coordinate cob(II)alamin.
Reduction of Enzyme-bound 4-Coordinate Cob(II)alamin Occurs at Low Concentrations of Dihydroflavins
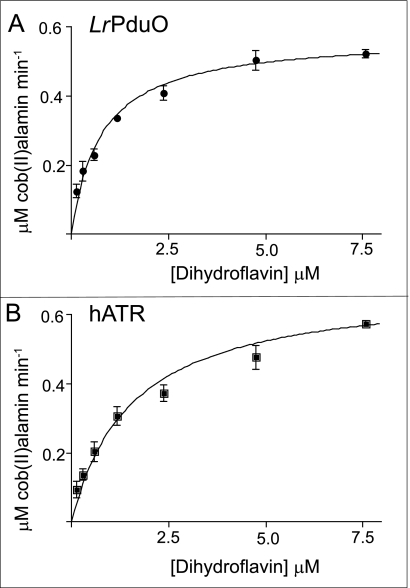
To address how much dihydroflavin was required to reduce 4-coordinate cob(II)alamin, we analyzed the rate of reduction by varying the concentration of dihydroflavin in the presence of LrPduO or hATR (Fig. 3, A and B, respectively). Remarkably, the dihydroflavin concentrations resulting in half the maximal velocity of the reaction catalyzed by LrPduO or hATR were 0.8 ± .1 and 1.6 ± 0.2 μm, respectively. The velocity of cob(II)alamin reduction was the same when dihydroriboflavin, reduced flavin mononucleotide (FMNH2), or reduced flavin adenine dinucleotide (FADH2) was used as the reducing agent of cob(II)alamin. Dihydroflavins reduced cob(II)alamin twice as fast in the presence of hATR compared with LrPduO (kcat, 4.1 × 10−2 s−1 versus 2.2 × 10−2 s−1). Together, these results strongly suggest that once the PduO-type adenosyltransferase binds cob(II)alamin and the 4-coordinate intermediate is formed, the reduction potential of the cobalt ion is raised to levels where the Co(II) → Co(I) reduction can occur without the participation of an accessory protein.
FIGURE 3.
Rate of cob(II)alamin reduction driven by FMNH2. Kinetics of the initial rate of reduction of cob(II)alamin using free dihydroflavins as the source of the electron in a coupled assay with LrPduO (A) and hATR (B). The concentration of FMNH2 in the reaction mixture needed to drive the reaction at half the maximal velocity was 0.8 ± 0.1 μm for LrPduO and 1.6 ± 0.2 μm for hATR.
Rate Comparisons between Dihydroflavin- and Reduced Flavoprotein-driven AdoCbl Synthesis
Although it was clear that free dihydroflavins (riboside, mononucleotide, and dinucleotide forms) drove the conversion of 4-coordinate cob(II)alamin to AdoCbl, it was unclear whether free dihydroflavins were the physiological reductant needed for this reaction. An alternative source of reducing power would be flavoproteins. To analyze how well dihydroflavins reduced 4-coordinate cob(II)alamin, we compared the rate of adenosylation by bacterial and human PduO-type ACA enzymes when free dihydroflavins or reduced flavoproteins were used as reductant.
To obtain a point of reference, we determined the catalytic competence of the PduO-type ACA enzyme tested. To do this, we used Ti(III)citrate to reduce cob(II)alamin to cob(I)alamin. The activity obtained with chemically reduced cob(II)alamin indicated the limit of the rate of formation of AdoCbl occurred once the Co(I) nucleophile was formed; this rate was set as 100% (Fig. 4).
FIGURE 4.
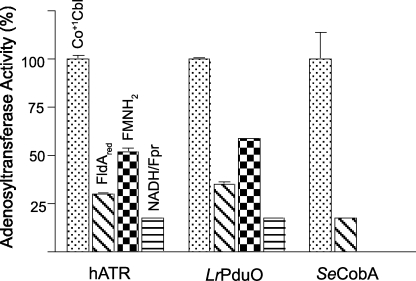
Rate of adenosylation by dihydroflavins. Cob(I)alamin was generated using Ti(III)citrate as reducing agent. The rate of adenosylation by the ACA enzyme with cob(I)alamin (dotted) was considered to be 100%. Adenosylation by PduO-type ACA enzymes using free dihydroflavins (black and white squares) was at least half the rate compared with the adenosylation rate measured with cob(I)alamin. Reduced FldA (FldAred, diagonal lines) and Fpr (Fprred, horizontal lines) also drove the reduction of cob(II)alamin, although not as fast as free FMNH2 or FADH2. SeCobA protein was used as control to confirm that CobA-type ACA enzymes can only use FldAred as reductant but not Fpr. The concentration of flavoproteins was at least 10-fold in excess compared with concentrations of ACA enzymes.
Surprisingly, the rate of AdoCbl production with chemically reduced cob(I)alamin was only 2-fold faster than the rate obtained when free FADH2 or FMNH2 was used to reduce 4-coordinate cob(II)alamin bound to hATR or LrPduO (Fig. 4). When an excess of Fpr or FldA was used in lieu of FMNH2 or FADH2, the rates of AdoCbl formation were half as fast as those observed with free dihydroflavins (Fig. 4). Most likely, the slower rate of AdoCbl formation when flavoproteins were used as the reductant was due to the less frequent collision rate between the dihydroflavin in the reductase and the 4-coordinate cob(II)alamin in the active site of PduO-type ACA enzymes.
What Is the Physiological Reductant for the Reduction of 4-Coordinate Cob(II)alamin in the Active Site of PduO-type ACA Enzymes?
Based on the above data, and the fact that the concentration of free dihydroflavins inside the cell is not known, we hypothesized that reduced flavoproteins whose flavin cofactor was close to the surface of the protein would reduce the 4-coordinate cob(II)alamin in the active site of PduO-type ACA enzymes.
To test this hypothesis, we analyzed three unrelated flavoproteins. We note that in S. enterica none of the chosen flavoproteins interact with the PduO enzyme, because the latter localizes to the lumen of the compartment known as the PduO metabolosome (42). Hence, adenosylation of cob(II)alamin resulting from the use of reduced flavoproteins could not be explained by expected interactions between PduO and the flavoprotein. The first two proteins we used were flavodoxin (FldA, FMN) and ferredoxin (flavodoxin):NADPH reductase (Fpr, FAD), which were previously shown to function together as reductants in the adenosylation of cob(II)alamin by the S. enterica CobA ACA enzyme (23). Reduced flavodoxin (FldAred) served as the electron donor for the adenosylation of cob(II)alamin by PduO- and CobA-type ACA enzymes (Fig. 4). In contrast, Fpr alone reduced cob(II)alamin bound to PduO-type ACA enzymes but did not reduce cob(II)alamin bound to SeCobA (Fig. 4). The rate of adenosylation, when Fpr served as the cob(II)alamin reductant (in one-to-one molar ratio with hATR), was 4-fold slower than when free dihydroflavins served as the reductant (Table 1). The rate of Fpr-dependent adenosylation of cob(II)alamin was very similar to the reported adenosylation rate using methionine synthase reductase as the reductant (0.07 versus 0.09 nmol min−1 μg−1 of hATR), even though the rates for methionine synthase reductase were determined using methionine synthase reductase concentrations 10-fold higher than the concentrations of hATR (27).
TABLE 1.
Specific activities of hATR and LrPduO using different electron sources for the adenosylation of cob(II)alamin
The concentrations of Fpr and PduS were at a one-to-one molar ratio to the concentrations of PduO-type ACA enzymes.
| Reductant | Specific activity |
|
|---|---|---|
| hATR | LrPduO | |
| nmol min−1 μg−1of enzyme | ||
| Ti(III)citrate | (4.9 ± 0.2) × 10−1 | (2.5 ± 0.1) × 10−1 |
| Dihydroflavins | (2.5 ± 0.1) × 10−1 | (1.4 ± 0.1) × 10−1 |
| Fpr | (7.2 ± 0.9) × 10−2 | (4.5 ± 0.8) × 10−2 |
| PduS | (2.6 ± 0.2) × 10−2 | (2.3 ± 0.3) × 10−2 |
We tested a third flavoprotein, YdjA, whose function is unknown and unrelated to AdoCbl biosynthesis. YdjA is a putative FMN-dependent nitroreductase enzyme (43).
Even though the rate of reduction of cob(II)alamin bound to PduO-type ACA enzymes by YdjA was too slow to be accurately measured, reduced YdjA did drive the adenosylation of cob(II)alamin (data not shown). The presence of AdoCbl in reaction mixtures containing YdjA was confirmed using a bioassay and in vitro by homolytic cleavage of the Co–C bond (as in Fig. 2). Control reaction mixtures lacking YdjA did not yield AdoCbl (data not shown).
As with Fpr and FldA, it is unlikely that YdjA is the physiological partner for the PduO in S. enterica. A more likely explanation for these results is that dihydroflavin bound to YdjA nonspecifically transferred one electron to the 4-coordinate cob(II)alamin species bound to the active site of LrPduO.
It appears that, for efficient transfer of the electron, the location of the dihydroflavin cofactor is important. The crystal structure of YdjA shows that FMN is close to the surface (RCSB Protein Data Bank codes 3bm1 and 3bm2) (43). Hence, it is possible that any flavoprotein with a surface-exposed cofactor can drive the adenosylation of 4-coordinate cob(II)alamin. The fact that dihydroflavins (free or bound to protein) can drive the adenosylation of cob(II)alamin would explain why human or bacterial cob(II)alamin reductases involved in the adenosylation of Cbl have not been identified.
Putative Bifunctional Cobalamin Reductase of S. enterica PduS Protein Is a Flavoprotein
Sampson et al. (29) proposed that, in S. enterica, the PduS (SePduS) protein is the source of electrons for the cob(III)alamin → cob(II)alamin and the cob(II)alamin → cob(I)alamin reductions. It is important to point out that these results were obtained in vitro using cell-free extracts and that supporting in vivo evidence was not reported.
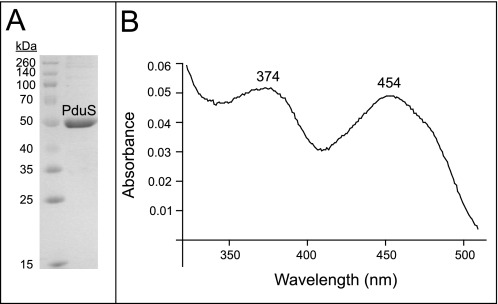
To gain insights into the function of SePduS, we isolated the protein under oxic and anoxic conditions (Fig. 5A). Notably, the fractions containing highly purified SePduS were yellow, suggesting the presence of a flavin cofactor (Fig. 5B). Based on these results, we hypothesized that SePduS reduced 4-coordinate cob(II)alamin bound to LrPduO or hATR not necessarily because SePduS had cob(II)alamin reductase activity but because it contained a flavin that could be reduced, and the resulting dihydroflavin could transfer one electron to 4-coordinate cob(II)alamin. SePduS was a good reductant of 4-coordinate cob(II)alamin, regardless of whether it was purified in the presence or absence of oxygen. The rates of adenosylation of cob(II)alamin in the presence of SePduS were within the same range as compared with Fpr or the previously reported methionine synthase reductase (Table 1).
FIGURE 5.
UV-visible spectra of SePduS and its flavin cofactor. A, purified SePduS separated by SDS-PAGE. Protein mass markers are shown on the left. B, spectral characteristics of flavin after heating SePduS to isolate cofactor.
Sampson et al. (29) also reported that the SePduO enzyme was required to observe the proposed cob(II)alamin reductase activity of SePduS. The explanation from the authors (29) for this requirement was that SePduO served as the means to sequester and protect the reactive cob(I)alamin nucleophile from rapid, nonspecific quenching.
Based on our results, we suggest that the lack of the proposed cob(II)alamin reductase activity of SePduS observed in the absence of SePduO was due to the inability of dihydroflavins to reduce 5-coordinate cob(II)alamin species in solution, which would be the species present in a reaction mixture lacking SePduO. This alternative interpretation of the data obtained by Sampson et al. (29) is supported by results reported here and by crystallographic data presented elsewhere (21), which indicate that the 4-coordinate cob(II)alamin is formed only upon binding to the active site of PduO-type ACA enzymes and that when 5-coordinate cob(II)alamin is bound to the active site, the enzyme is inactive.
Although we think it is plausible that SePduS might be involved in the transfer of an electron to the Co(II) ion, we are unconvinced that SePduS has cobalamin reductase activity. It is more likely that PduS is an electron transfer protein and not an enzyme.
Explanation of Previous Results
During the course of the structural analysis of LrPduO in complex with cob(II)alamin and ATP, we observed the formation of AdoCbl in the absence of any electron transfer protein but in the presence of the FMN reductase enzyme, NADH, and FMN. The simplest explanation for this observation would be consistent with the data reported here, i.e. during crystallization, FMNH2 drove the reduction of the 4-coordinate cob(II)alamin intermediate in the active site of LrPduO. The availability of cob(I)alamin in the presence of ATP led to the formation of AdoCbl.
Adenosylation of Incomplete Corrinoids
In S. enterica, CobA is involved in the de novo biosynthesis of the corrin ring (44) and the synthesis of the nucleotide loop that tethers the lower ligand to the ring (45). Spectroscopic data showed that SeCobA generated the 4-coordinate corrinoid intermediate more readily with cobinamide (Cbi, Cbl precursor lacking the lower ligand) than with Cbl (18, 20).
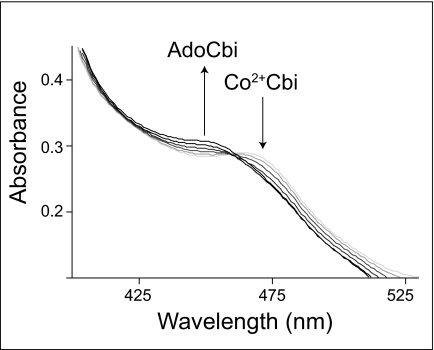
In Fig. 6, we show that CobA can adenosylate cob(II)inamide (evident by the increase of AdoCbi at A455 nm with the concomitant decrease of Co2+Cbi at A465 nm) using free dihydroflavins as the reductant. The presence of AdoCbi was confirmed using bioassay conditions that demanded growth of strain JE7180 on ethanolamine (data not shown).
FIGURE 6.
Adenosylation of cobinamide. Spectral changes are associated with the conversion of cob(II)inamide to AdoCbi by SeCobA using free dihydroflavins as reductants. Arrows and increasing darkness in the spectra represent successive time points after the reaction was initiated by addition of SeCobA.
Previously, we showed that free dihydroflavins did not drive the reduction of cob(II)alamin bound to SeCobA (23). These results were intriguing, because spectroscopic data showed that the 4-coordinate cob(II)alamin species forms in the active site of SeCobA, albeit not as readily as the 4-coordinate of cob(II)inamide (20). Insufficient reaction time (30 min) was one plausible explanation for why dihydroflavins did not drive the reduction of cob(II)alamin in the active site of SeCobA (15). Thus, we extended the reaction time to 12 h inside an anoxic chamber in the dark. Under extended incubation conditions, SeCobA generated AdoCbl when free dihydroflavins were present. However, in contrast with PduO-type enzymes, FldAred was the only flavoprotein that drove the adenosylation of cob(II)alamin bound to SeCobA.
The LrPduOF112A variant, which cannot generate the 4-coordinate species of cob(II)alamin, did adenosylate cob(II)inamide when dihydroflavins were used as reductant. These results further support the importance of the role of the ACA enzyme in the generation of the 4-coordinate intermediate, which is needed to raise the redox potential so the reduction of Co(II) can occur. The hATR enzyme also used dihydroflavins to drive the adenosylation of cob(II)inamide. These results indicate that PduO-type ACA enzymes, regardless of their origin, have similar catalytic capabilities.
Criteria for Cobalamin Reductases
We suggest that the assignment of “cobalamin reductase” activity to any protein must be based on two criteria as follows: (i) evidence of binding of the corrinoid substrate to the protein; and (ii) for Co(II) corrinoid reductases, evidence of the formation of a 4-coordinate Co(II) corrinoid species in the site of the enzyme. To date, magnetic circular dichroism spectroscopy has proven to be a powerful means to establish the existence of 4-coordinate Co(II) corrinoid species. In the absence of this information, and in light of the data reported herein and structural data published elsewhere (21), we suggest that the proteins identified thus far as cobalamin reductases are not enzymes but are electron transfer proteins.
Conclusions
We draw two conclusions from the studies reported here. First, the redox potential of 4-coordinate Co(II) corrinoid species generated in the active site of ACA enzymes is within the physiological range but that of 5-coordinate cob(II)alamin is not. This is based on our results where PduO-type ACA enzymes can adenosylate cob(II)alamin in the presence of free dihydroflavins. Second, cobalamin reductases described thus far are most likely electron transfer proteins and not enzymes, and we propose the following criteria for the assignment of cobalamin reductase function to a protein. Because the Co3+ → Co2+ reduction can be driven in solution by dihydroflavins (15), evidence of binding of Co(III) corrinoids to the active site of the protein is central to the assignment of cob(III)alamin reductase function. As for cob(II)alamin reductases, the formation of a 4-coordinate cob(II)alamin species in the active site of the protein is proposed as the diagnostic feature of a bona fide cob(II)alamin reductase. In the absence of such information, putative cobalamin reductases should be considered as electron transfer proteins and not enzymes.
Acknowledgments
We thank Michael Gray for the gift of homogeneous YdjA protein.
This work was supported, in whole or in part, by National Institutes of Health Grant GM40313 from USPHS (to J. C. E.-S.).
- Cbl
- cobalamin
- AdoCbl
- adenosylcobalamin
- cob(III)alamin
- Co3+Cbl
- cob(II)alamin
- Co2+Cbl
- cob(I)alamin
- Co+Cbl
- hATR
- human adenosyltransferase
- ACA
- ATP:corrinoid adenosyltransferase
- rTEV
- recombinant tobacco etch virus
- Fpr
- ferredoxin (flavodoxin) protein reductase
- FldA
- flavodoxin A
- FMNH2
- dihydroflavin mononucleotide
- FADH2
- dihydroflavin adenine dinucleotide
- Cbi
- cobinamide.
REFERENCES
- 1.Warren M. J., Raux E., Schubert H. L., Escalante-Semerena J. C. (2002) Nat. Prod. Rep. 19, 390–412 [DOI] [PubMed] [Google Scholar]
- 2.Halpern J. (1985) Science 227, 869–875 [DOI] [PubMed] [Google Scholar]
- 3.Buckel W., Bröker G., Bothe H., Pierik A. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 757–782, John Wiley & Sons, Inc., New York [Google Scholar]
- 4.Bandarian V., Reed G. H. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 811–833, John Wiley & Sons, Inc., New York [Google Scholar]
- 5.Toraya T. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 783–809, John Wiley & Sons, Inc., New York [Google Scholar]
- 6.Fontecave M. (1998) Cell. Mol. Life Sci. 54, 684–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontecave M., Mulliez E. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 731–756, John Wiley & Sons, Inc., New York [Google Scholar]
- 8.Wohlfarth G., Diekert G. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 871–893, John Wiley & Sons, Inc., New York [Google Scholar]
- 9.Banerjee R., Chowdhury S. (1999) in Chemistry and Biochemistry of B12 ( Banerjee R. ed) pp. 707–729, John Wiley & Sons, Inc., New York [Google Scholar]
- 10.Frey P. A., Hegeman A. D. (2007) Enzymatic Reaction Mechanisms, pp. 189–252, Oxford University Press, Oxford [Google Scholar]
- 11.Suh S., Escalante-Semerena J. C. (1995) J. Bacteriol. 177, 921–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson C. L., Buszko M. L., Bobik T. A. (2004) J. Bacteriol. 186, 7881–7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buan N. R., Escalante-Semerena J. C. (2006) J. Biol. Chem. 281, 16971–16977 [DOI] [PubMed] [Google Scholar]
- 14.Walker G. A., Murphy S., Huennekens F. M. (1969) Arch. Biochem. Biophys. 134, 95–102 [DOI] [PubMed] [Google Scholar]
- 15.Fonseca M. V., Escalante-Semerena J. C. (2000) J. Bacteriol. 182, 4304–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lexa D., Saveant J. M. (1983) Acc. Chem. Res. 16, 235–243 [Google Scholar]
- 17.St Maurice M., Mera P., Park K., Brunold T. C., Escalante-Semerena J. C., Rayment I. (2008) Biochemistry 47, 5755–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stich T. A., Buan N. R., Brunold T. C. (2004) J. Am. Chem. Soc. 126, 9735–9749 [DOI] [PubMed] [Google Scholar]
- 19.Stich T. A., Yamanishi M., Banerjee R., Brunold T. C. (2005) J. Am. Chem. Soc. 127, 7660–7661 [DOI] [PubMed] [Google Scholar]
- 20.Stich T. A., Buan N. R., Escalante-Semerena J. C., Brunold T. C. (2005) J. Am. Chem. Soc. 127, 8710–8719 [DOI] [PubMed] [Google Scholar]
- 21.Mera P. E., St Maurice M., Rayment I., Escalante-Semerena J. C. (2009) Biochemistry 48, 3138–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park K., Mera P. E., Escalante-Semerena J. C., Brunold T. C. (2008) Biochemistry 47, 9007–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca M. V., Escalante-Semerena J. C. (2001) J. Biol. Chem. 276, 32101–32108 [DOI] [PubMed] [Google Scholar]
- 24.Buan N. R., Escalante-Semerena J. C. (2005) J. Biol. Chem. 280, 40948–40956 [DOI] [PubMed] [Google Scholar]
- 25.Lawrence A. D., Deery E., McLean K. J., Munro A. W., Pickersgill R. W., Rigby S. E., Warren M. J. (2008) J. Biol. Chem. 283, 10813–10821 [DOI] [PubMed] [Google Scholar]
- 26.Leclerc D., Odièvre M., Wu Q., Wilson A., Huizenga J. J., Rozen R., Scherer S. W., Gravel R. A. (1999) Gene 240, 75–88 [DOI] [PubMed] [Google Scholar]
- 27.Leal N. A., Olteanu H., Banerjee R., Bobik T. A. (2004) J. Biol. Chem. 279, 47536–47542 [DOI] [PubMed] [Google Scholar]
- 28.Froese D. S., Wu X., Zhang J., Dumas R., Schoel W. M., Amrein M., Gravel R. A. (2008) Mol. Genet. Metab. 94, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson E. M., Johnson C. L., Bobik T. A. (2005) Microbiology 151, 1169–1177 [DOI] [PubMed] [Google Scholar]
- 30.Rocco C. J., Dennison K. L., Klenchin V. A., Rayment I., Escalante-Semerena J. C. (2008) Plasmid 59, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertani G. (1951) J. Bacteriol. 62, 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertani G. (2004) J. Bacteriol. 186, 595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haspel J., Blanco C., Jacob J., Grumet M. (2001) BioTechniques 30, 60–61 [DOI] [PubMed] [Google Scholar]
- 34.Kapust R. B., Waugh D. S. (2000) Protein Expr. Purif. 19, 312–318 [DOI] [PubMed] [Google Scholar]
- 35.Shih Y. P., Wu H. C., Hu S. M., Wang T. F., Wang A. H. (2005) Protein Sci. 14, 936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St Maurice M., Mera P. E., Taranto M. P., Sesma F., Escalante-Semerena J. C., Rayment I. (2007) J. Biol. Chem. 282, 2596–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blommel P. G., Fox B. G. (2007) Protein Expr. Purif. 55, 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall D. A., Jordan-Starck T. C., Loo R. O., Ludwig M. L., Matthews R. G. (2000) Biochemistry 39, 10711–10719 [DOI] [PubMed] [Google Scholar]
- 39.Berkowitz D., Hushon J. M., Whitfield H. J., Jr., Roth J., Ames B. N. (1968) J. Bacteriol. 96, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe F., Nakano Y. (1997) Methods Enzymol. 281, 289–295 [DOI] [PubMed] [Google Scholar]
- 41.Buan N. R., Suh S. J., Escalante-Semerena J. C. (2004) J. Bacteriol. 186, 5708–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havemann G. D., Bobik T. A. (2003) J. Bacteriol. 185, 5086–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi J. W., Lee J., Nishi K., Kim Y. S., Jung C. H., Kim J. S. (2008) J. Mol. Biol. 377, 258–267 [DOI] [PubMed] [Google Scholar]
- 44.Escalante-Semerena J. C., Suh S. J., Roth J. R. (1990) J. Bacteriol. 172, 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Toole G. A., Escalante-Semerena J. C. (1993) J. Bacteriol. 175, 6328–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]