Abstract
We screened a protoberberine backbone derivative library for compounds with anti-proliferative effects on p53-defective cancer cells. A compound identified from this small molecule library, cadein1 (cancer-selective death inducer 1), an isoquinolinium derivative, effectively leads to a G2/M delay and caspase-dependent apoptosis in various carcinoma cells with non- functional p53. The ability of cadein1 to induce apoptosis in p53-defective colon cancer cells was tightly linked to the presence of a functional DNA mismatch repair (MMR) system, which is an important determinant in chemosensitivity. Cadein1 was very effective in MMR+/p53− cells, whereas it was not effective in p53+ cells regardless of the MMR status. Consistently, when the function of MMR was blocked with short hairpin RNA in SW620 (MMR+/p53−) cells, cadein1 was no longer effective in inducing apoptosis. Besides, the inhibition of p53 increased the pro-apoptotic effect of cadein1 in HEK293 (MMR+/p53+) cells, whereas it did not affect the response to cadein1 in RKO (MMR−/p53+) cells. The apoptotic effects of cadein1 depended on the activation of p38 but not on the activation of Chk2 or other stress-activated kinases in p53-defective cells. Taken together, our results show that cadein1 may have a potential to be an anti-cancer chemotherapeutic agent that is preferentially effective on p53-mutant colon cancer cells with functional MMR.
Keywords: Apoptosis, Cancer/Colon, Cell/Apoptosis, Diseases/Cancer/Therapy, Colon cancer, DNA mismatch repair, cadein1, p38, p53
Introduction
The p53 tumor suppressor is essential for maintaining genomic stability in mammals. When cells are subjected to stress signals such as hypoxia, radiation, or chemotherapeutic drugs, p53 is activated, and its ubiquitin-dependent degradation is blocked leading to an accumulation of active p53 transcription factor (1). Activated p53 regulates most downstream signals for cell cycle arrest and apoptosis by affecting the expression of its target genes. Transcriptional targets of p53 include the cyclin-dependent kinase inhibitor, p21/WAF1, and genes involved in cell death, including BAX, PUMA, NOXA, and Fas (2, 3). Because p53 activation inhibits cell proliferation, mutation of the p53 gene or disruption of pathways that lead to p53 activation has been frequently observed in most types of human cancer (4).
The p53-dependent induction of apoptosis in response to genotoxic damages is an important aspect of tumor suppression. Thus, the loss of p53 in human cancers not only contributes to aggressive tumor behavior but often leads to resistance to radiation and chemotherapeutic drugs. For example, treatment of p53+/+ mouse thymocytes with radiation results in apoptosis, whereas p53−/− thymocytes are resistant. p53+/+ mouse embryonic fibroblasts transformed by adenoviral E1A protein and Ha-ras oncogene undergo apoptosis in response to γ-irradiation or chemotherapeutic agents, whereas p53−/− fibroblasts are resistant to these anti-cancer therapies (5). In addition, some p53 mutations in cancers suppress the function of p73, which induces apoptosis through a p53-independent mechanism (6). Thus, the common loss of p53 function in cancer cells presents a major limitation for anti-cancer therapies.
DNA mismatch repair (MMR)3 is a post-replicative DNA repair process that corrects single-base mismatches and small mismatched loops in the daughter strand of newly replicated DNA. Loss of MMR by mutation of MSH2 or MLH1 is responsible for the majority of cases of hereditary nonpolyposis colon cancer and is also common in a variety of sporadic cancers including endometrial, ovarian, breast, prostate, lung, and pancreatic cancer (7–9). In addition to an increased rate of mutation throughout the genome, the loss of MMR often alters the sensitivity to some therapeutic DNA damaging agents. MMR deficiency results in strong resistance to the base analog, antimetabolite 6-thioguanine, moderate resistance to methylating agents, such as N-methyl-N′-nitro-N-nitrosoguanidine and temozolomide, and weak resistance to cisplatin and carboplatin (10–13). In the case of cisplatin, the loss of both MMR and p53 leads to a cooperative increase in resistance and limits the therapeutic potential of cisplatin for colon cancer (14). Although the mechanism is not clear, the decreased sensitivity to DNA damaging agents caused by loss of MMR is likely to be due to decreased levels of apoptosis resulting from impaired detection of DNA adducts or a failure to initiate repair (11).
In this study we prepared a small library based on the backbone of protoberberine and screened for compounds with effective anti-proliferative activity. In particular, we searched for an anti-proliferative compound selective to cells without wild type p53 function, as loss of p53 function is a common feature of most cancer cells and often accompanies resistance to anti-cancer therapeutics. A new isoquinolinium derivative, cadein1 (cancer-selective death inducer 1), was identified in the screen, which induces apoptosis most effectively in p53-defective carcinoma cells with functional MMR. The apoptotic effect of cadein1 in cancer cells depends on the activation of p38. Given that decreased sensitivity to chemotherapies in human cancer cells without functional p53 is a major drawback of cancer treatment, cadein1 may be a potent anti-cancer agent against human cancers, specifically p53-deficient cancers with functional MMR.
EXPERIMENTAL PROCEDURES
Synthesis of the Modified Isoquinolinium Derivative Cadein1
Cadein1 (C33H48ClF3NO2, Mr 564.20), an isoquinolinium salt, was synthesized as follows. Briefly, 3,4-dimethoxyphenylethylamine (Aldrich) was acylated with long chain acid chloride (CH3(CH2)14COCl, triethylamine/ethylene dichloride, room temperature, 95%) to yield amide. Amide was subsequently treated with phosphorus oxychloride (POCl3, CH3CN, reflux, 82%) to give isoquinoline, which was further reacted with 2, 6-difluorobenzyllbenzyl chloride (2,6-F2C6H4CH2Cl, CH3CN, reflux, 62%) to yield the desired isoquinolinium salt as a yellow solid. The isoquinolinium salt compound was verified by spectroscopic analysis (1H NMR, 13C NMR, high resolution mass spectrometry).
Cell Culture and Screening of Isoquinolinium Derivatives
WI-38 human normal lung fibroblast cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Eagle's minimum essential medium with 10% fetal bovine serum (all from HyClone, Logan, UT). Human normal lung fibroblast IMR90, human cervical carcinoma HeLa, and human embryonic kidney HEK293 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. SW620 and RKO human colon cancer cells were grown in Leibovitz's L-15 medium and minimum Eagle's medium with 10% fetal bovine serum, respectively. The four sublines of HCT116 human colorectal adenocarcinoma, HCT116, HCT116-Ch3, HCT116-E6, and HCT116-Ch3/E6, human colorectal cell lines (HT29, DLD-1, and SW480), and H1299 human lung cancer cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 with 10% fetal bovine serum. The chromosome-complemented lines (HCT116-Ch3 and HCT116-Ch3/E6) were maintained in medium containing 400 μg/ml G418, and the cell lines expressing Papillomavirus E6 (HCT116-E6 and HCT116-Ch3/E6) were cultured in medium supplemented with 80 μg/ml hygromycin B. All cells were cultured in 5% CO2 in a medium with penicillin and streptomycin at 37 °C. For screening of 80 isoquinolinium derivatives, cells were grown for 1 day to 60–70% confluence and treated with various concentrations of derivatives. To determine the anti-proliferative effects of isoquinolinium derivatives including cadein1, the morphology of the cells was observed with a phase contrast microscope (Fig. 1B, Model CK2, Olympus Optical Co.).
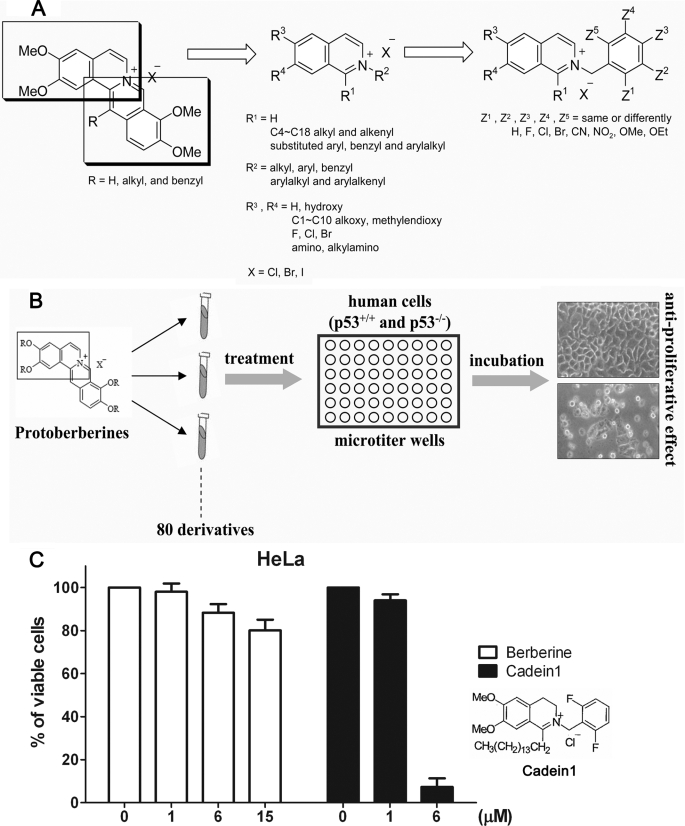
FIGURE 1.
Cadein1, a novel isoquinolinium derivative, was identified by its potent cytotoxic effects on p53-mutant cancer cells. A, shown is a scheme for the synthesis of isoquinolinium derivatives including cadein1. B, shown is a scheme for screening the anti-proliferative effects of isoquinolinium derivatives in p53-negative HeLa and HCT116-Ch3/E6 cancer cells and in p53-positive HEK293 and IMR90 cells. The cells were treated with a range of different concentrations of derivatives for 24 h. Cell morphology was assessed using a phase contrast microscope. C, the anti-proliferative effect of cadein1 was measured by MTT assays and compared across a range of different concentrations of the lead compound berberine for 24 h in HeLa cells. Three independent experiments were performed at each point, and the mean value is plotted with S.D.
Chemical Treatment and Cell Cycle Assay
A stock solution of cadein1 (1 mg/ml) was prepared in 70% (v/v) ethanol. Stock solutions of berberine (1 mg/ml) and SB203580 (10 mm, AG Scientific) were prepared by dissolving these compounds in dimethyl sulfoxide (DMSO) and diluted to the final concentration in media. Unless indicated otherwise, cells were grown for 1 day to 60–70% confluency and treated with various concentrations of cadein1. Cells were synchronized at G1/S by a modified double thymidine block (15). For fluorescence-activated cell sorter analysis, cells were trypsinized, fixed with cold 70% ethanol (−20 °C), treated with 100 μg/ml RNase A (Sigma), and stained with 100 μg/ml propidium iodide (Sigma). The DNA content of cells was measured by flow cytometry (BD Biosciences) and analyzed using Cell Quest software.
Cell Viability Assay
Cell proliferation was evaluated by spectrophotometric measurement of the mitochondrial dehydrogenase activity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (16). Cells (5 × 103 cells/ml) were plated in triplicate in 96-well plates for 1 day and incubated with varying concentrations of cadein1 (1, 3, 4, 5, 6, or 9 μm) for 24 h. After incubation with MTT (5 mg/ml), the dissolved formazan product was measured on a microplate reader (SOFTmax PRO 4.0).
Immunoblotting
Cells were lysed in a buffer containing 17 mm Tris, pH 8.0, 50 mm NaCl, 0.3% Triton X-100, and protease inhibitors. Specific proteins were detected using the following primary antibodies: phospho-p38, p38, p53 (Santa Cruz Biotechnology, Santa Cruz, CA); cleaved caspase-3, cleaved PARP, phospho-Chk2 (Thr-68), phospho-p38 (Thr-180/Tyr-182), p38, PARP, phospho-stress-activated protein kinase/JNK, p-ERK1/2 (Cell Signaling Technology, Inc., Beverly, MA); cyclin A, cyclin B1, cyclin E (NeoMarkers, Fremonts, CA); MLH1, p53 (BD Pharmingen); γ-H2AX(Ser-139) (Upstate biotechnology, NY). Immunoreactive bands were normalized to those of control actin (Santa Cruz Biotechnology) or α-tubulin (Calbiochem).
Transfection
Wild type p53 in pcDNA3.1 and pSuper-Neo-p53 were kindly provided by Dr. H. W. Lee (Yonsei University, Seoul, Korea) and Dr. J. Shin (Sungkyunkwan University, Suwon, Korea), respectively. shp53-pLKO.1 puro vector was purchased from Addgene (Cambridge, MA). pSuperRetro-shMLH1 was kindly provided by Dr. A. Zhitkovich (Brown University). The cells were transfected with those constructs using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's protocol. Transient transfection of wild type p38 and dominant-negative p38 in HeLa cells was performed with the Effectene kit (Qiagen, Valencia, CA), according to the manufacturer's instructions.
RESULTS
A Novel Isoquinolinium Derivative Cadein1 Efficiently Blocks Proliferation of p53-deficient Carcinoma Cells
The use of phytochemicals as anti-cancer chemotherapeutics has attracted considerable interest. Among 121 prescription drugs in use for cancer therapy, 90 are derived from natural plant sources (17, 18). Protoberberine-derived alkaloids, such as berberine, have a wide range of pharmacological activities, including cytotoxicity against several carcinomas in vitro (19, 20). Thus, we generated a privileged small scale chemical library by modifying one of the two isoquinoline rings of protoberberine (Fig. 1A and Ref. 21) and screened the anti-proliferative effects of 80 isoquinolinium derivatives in several carcinoma cell lines and non-cancerous cells (Fig. 1B).
Given that decreased sensitivity to anti-cancer agents in p53-deficient human cancer cells is the major limitation to cancer therapeutics, our search was focused on identifying an anti-proliferative compound selective to cells without wild type p53 function (Fig. 1B). Thus, in the screen we compared the anti-proliferative effect of each compound in p53-negative cancer cells, such as HeLa and HCT116-Ch3/E6, and in p53-positive HEK293 cells and IMR90 primary cells. Among these compounds, one new isoquinolinium derivative efficiently blocked the proliferation of p53-negative carcinoma cells but not of normal cells (data not shown). We designated this isoquinolinium derivative, cadein1 (cancer-specific death inducer 1).
The anti-proliferative effect of cadein1 in p53-negative carcinoma cells was very strong compared with the lead compound berberine (Fig. 1C). As shown in Fig. 1C, cadein1 significantly inhibited the proliferation of HeLa cells at 6 μm, whereas the lead compound, berberine, did not affect HeLa cells at low concentrations (15 μm or less).
Cadein1 Efficiently Induces Apoptosis in p53-defective Cancer Cells via the Caspase Pathway and Transient G2/M Delay
To determine the optimal conditions for cadein1 treatment, we first performed MTT assays to assess the effective concentration range for inhibition of cell growth in HeLa, IMR90, and WI-38 cells. As observed in the screen, HeLa cells with non-functional p53 were more sensitive to cadein1 than were p53-positive IMR90 and WI-38 primary cells (Fig. 2A). The viability of HeLa cells was 18.6% at 6 μm cadein1, whereas IMR90 and WI-38 cells were 61.55 and 74.13% viable, respectively (Fig. 2A). Additionally, in low concentrations (9 μm or less) of cadein1, a strong cytotoxic effect was observed in other p53-defective carcinoma cells including human colorectal HT29, SW480, SW620, and HCT116-Ch3/E6 cells and human lung cancer H1229 cells (data not shown). These observations strongly suggest that cadein1 is able to inhibit the proliferation of p53-deficient cancer cells.
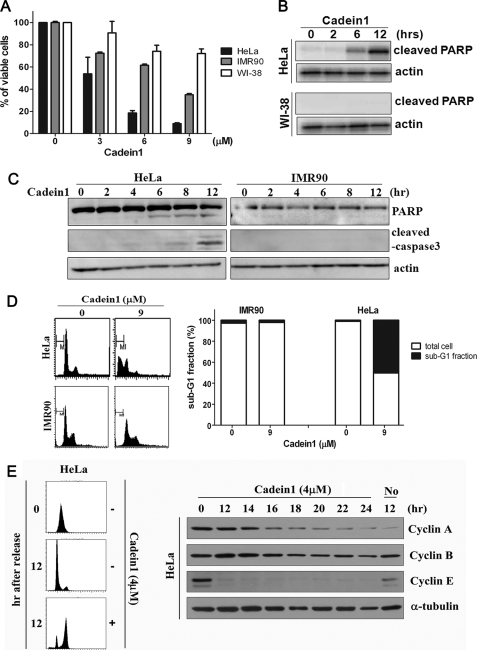
FIGURE 2.
Cadein1 efficiently induces apoptosis and G2/M delay in p53-defective HeLa cells. A, the viability of HeLa, WI-38, and IMR90 cells was evaluated for a range of different concentrations of cadein1 for 24 h by MTT assay. Three independent experiments were performed for each point, and the mean value is plotted with S.D. B, HeLa and WI-38 cells were treated with 6 μm cadein1 for up to 12 h. Cells were analyzed by Western blot with antibodies against cleaved-PARP. C, HeLa and IMR90 cells were treated with 6 μm cadein1 for up to 12 h. Cells were collected every 2 h and analyzed by Western blot with antibodies against PARP and cleaved caspase-3. Actin blots are shown as loading controls. D, DNA contents of HeLa and IMR90 cells were analyzed by flow cytometry, as described under “Experimental Procedures,” and the number of cells in the sub-G1 phase over the total cells was calculated. The number of cells in sub-G1 phase (M1) over the total number of cells is plotted as percentages. E, 12 h after release from a double thymidine block, HeLa cells were incubated in the media containing either 4 μm cadein1 or no cadein1 for up to 24 h. DNA contents in HeLa cells were analyzed by flow cytometry at each time point. Cyclin A, cyclin B, and cyclin E are markers of cell cycle progression. Levels of each cyclin at each time point were measured by immunoblots. α-Tubulin was used as a loading control.
To examine whether cadein1-induced cell death is the result of apoptosis, we evaluated the expression of proteins in the apoptotic pathway after cadein1 treatment. Because the cleavage of PARP (substrate of caspase-3, poly(ADP-ribose)polymerase) is a hallmark of apoptotic cell death, whole cell lysates from HeLa and WI-38 were subjected to Western blotting using the antibody against cleaved-PARP. When incubated with 6 μm cadein1 for 12 h, activated PARP was significantly detected in HeLa cells, but no cleaved PARP was observed in WI-38 cells (Fig. 2B).
We analyzed the cadein1-induced apoptotic cell death by examining the expression of caspase-3 (effecter caspase) and PARP in HeLa and IMR90 cells after treatment with cadein1. When cells were treated with 6 μm cadein1, as expected, cleaved caspase-3 and PARP were detected in a time-dependent manner in HeLa cells but not in IMR90 cells (Fig. 2C). These results confirm that cadein1-induced apoptosis in p53-defective cancer cells is mediated by activation of caspase-3.
To better assess the hypersensitivity of p53-negative cells to cadein1, the DNA content of cells was analyzed after incubation with 9 μm cadein1 for 24 h. As shown in Fig. 2D, the sub-G1 fraction, which presumably contains dying cells, was highly increased in HeLa cells (9 μm, 50.27%), whereas a negligible sub-G1 fraction was observed in IMR90 cells (9 μm, 2.11%). These observations showed that, at low concentrations, cadein1 efficiently induces cell death in HeLa cells but not in IMR90 cells, supporting that p53-defective cancer cells are more sensitive to cadein1 than p53-positive normal cells.
To investigate the effect of cadein1 on cell cycle progression, we analyzed the cell cycle profiles of cadein1-treated HeLa cells. After synchronization at G1 via a double thymidine block, HeLa cells were released from the G1 block in the presence of 4 μm cadein1. Cadein1 induced a transient G2/M delay in HeLa cells as compared with non-treated cells (Fig. 2E). In addition, cyclin A degradation was delayed, and cyclin B persisted in the cadein1-treated cells over 24 h (Fig. 2E), suggesting that a cell cycle delay at G2/M occurs in response to cadein1 treatment. However, cyclin E was not detectable in these cells due to the G2/M delay (Fig. 2E).
Cadein1 Sensitively Inhibits Proliferation of p53-deficient Colon Cancer Cells with Functional MMR
Because p53 cooperates with the MMR to induce cell death in response to some chemotherapeutic compounds in colon cancer cells (14), the effect of an MMR deficiency on cadein1-induced cell death of p53-negative cells was examined. We assessed the cytotoxic effect of cadein1 across a panel of sublines of the MMR-deficient HCT116 human colorectal adenocarcinoma cells because each HCT116 subline contains different combinations of p53 and MMR functions in the same genetic background. Four sublines of HCT116 colon cancer cells were used: HCT116 (MMR−/p53+), HCT116-Ch3 (MMR+/p53+), HCT116-E6 (MMR−/p53−), and HCT116-Ch3/E6 (MMR+/p53−). HCT116 contains a hemizygous mutation in hMLH1 resulting in a truncated, nonfunctional protein (22). The chromosome 3-complemented cells (identified here as HCT116-Ch3 and HCT116-Ch3/E6) are competent in DNA mismatch repair, as MMR function has been restored by transfer of a copy of MLH1 on chromosome 3 (14, 23). In HCT116-E6 and HCT116-Ch3/E6 cell lines, constitutive high level expression of the human Papillomavirus type-16 E6 gene disrupts p53 function, as it promotes the ubiquitin-dependent degradation of p53 (14). The HCT116 sublines were exposed to a range of cadein1 (0–4 μm) for 24 h, and the anti-proliferative effect of cadein1 on the HCT116 sublines was measured by an MTT assay. Exposure to cadein1 caused a dose-dependent loss of cell viability (Fig. 3A). The percentages of cell viability in HCT116-Ch3/E6 (MMR+/p53−), HCT116-E6 (MMR−/p53−), HCT116-Ch3 (MMR+/p53+), and HCT116 (MMR−/p53+) cells treated with 4 μm cadein1 were 17.7, 49, 55.4, and 68.6%, respectively (Fig. 3A). The HCT116-Ch3/E6 (MMR+/p53−) cells displayed the most significant sensitivity to cadein1 among the four HCT116 sublines. Although this line does not have functional p53, the HCT116-E6 (MMR−/p53−) cells had decreased sensitivity to cadein1 relative to HCT116-Ch3/E6 cells (Fig. 3A). Interestingly, the viability of HCT116-Ch3 (MMR+/p53+) cells and of HCT116 (MMR−/p53+) cells was not much different at low doses of cadein1 (4 μm or less) even though they have different MMR phenotypes. MMR-deficient cells were resistant to cadein1 even in a p53-negative background (E6-expressed cells; HCT116-Ch3/E6 and HCT116-E6 cells). However, the loss of MMR did not affect the cadein1 sensitivity of p53-proficient cells. These results demonstrate that p53-negative colon cancer cells are selectively sensitive to cadein1, and functional MMR leads to more profound cadein1-induced cell death in p53-deficient cells (Fig. 3A).
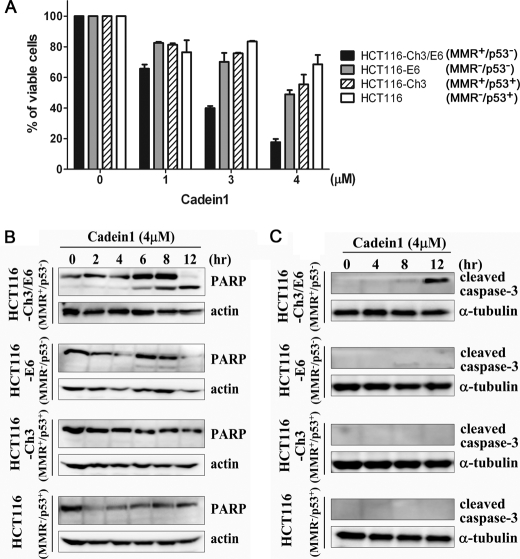
FIGURE 3.
Cadein1 sensitivity of a p53-mutant colon cancer cell line is increased with functional MMR. A, HCT116-Ch3/E6, HCT116-E6, HCT116-Ch3, and HCT116 cells were treated with various concentrations of cadein1 for 24 h. Cell viability was assessed by MTT assay. Three independent experiments were performed at each point, and the mean value was plotted with S.D. B and C, HCT116-Ch3/E6, HCT116-E6, HCT116-Ch3, and HCT116 cells were treated with 4 μm cadein1 for the indicated times. Cell extracts were immunoblotted with anti-PARP (B) and anti-cleaved capapse-3 antibodies (C). Actin (B) and α-tubulin (C) were used as loading controls.
To understand the selectivity and mechanism of cadein1-induced apoptotic cell death in HCT116 cell sublines, we evaluated the expression of proteins in the apoptotic pathway after cadein1 treatment. Cleaved PARP was most significantly detected in cadein1-treated HCT116-Ch3/E6 (MMR+/p53−) cells relative to other HCT116 sublines (Fig. 3B). We also confirmed apoptotic effect of cadein1 by evaluating the expression of caspase-3 in these cells. As expected, cleaved caspase-3 was detected only in HCT116-Ch3/E6 cells treated with 4 μm cadein1 (Fig. 3C), verifying that cadein1-induced apoptosis in p53-defective cancer cells is mediated by activation of caspase-3. Altogether these observations confirmed that p53-negative cells were more sensitive to cadein1, and the effect of p53 dependence was only observed in MMR-proficient cells. In short, the activity of p53 and MMR determines the sensitivity of colon cancer cells to the cytotoxic effect of cadein1.
Cadein1-induced Apoptosis Requires Both p53 Deficiency and MMR Proficiency in Colon Carcinoma Cell Lines
To confirm the function of MMR and p53 for cadein1-induced apoptosis in colon cancer cells, we tested other colon cancer cells, HT29 (MMR+/p53−) and DLD-1 (MMR−/p53−), for sensitivity to cadein1. As expected, the viability of HT29 cells that have nonfunctional p53 and functional MMR (MMR+/p53−) was severely decreased by cadein1 relative to DLD-1 (MMR−/p53−) cells (Fig. 4A). Noticeably, the viability in HT29 cells treated with cadein1 was 10.6% at 4 μm, whereas the viability of DLD-1 cells was 51% at 4 μm (Fig. 4A). Consistent with the results obtained from HCT116 sublines (Fig. 3, B and C), activated caspase-3 and PARP were significantly detected in HT29 (MMR+/p53−) cells treated with at least 5 μm cadein1 for 12 h but were not in cadein1-treated DLD-1 (MMR−/p53−) cells (Fig. 4B). These results confirm that only MMR-proficient cells are sensitive to cadein1 when p53 is nonfunctional. In HT29 cells, ∼90% inhibition of growth was observed in Fig. 4A, whereas a 25% cleavage of PARP was detected in Fig. 4B. This discrepancy between the MTT assay and the apoptotic markers is likely due to the difference in incubation time with cadein1. HT29 cells were incubated with cadein1 for 24 h in the MTT assay of Fig. 4A and for 12 h in Fig. 4B. In fact, the % of viable cells was 47.11% for the 12 h incubation but 9.75% for the 24-h incubation in HT29 cells treated with 5 μm cadein1 (supplemental Fig. 1).
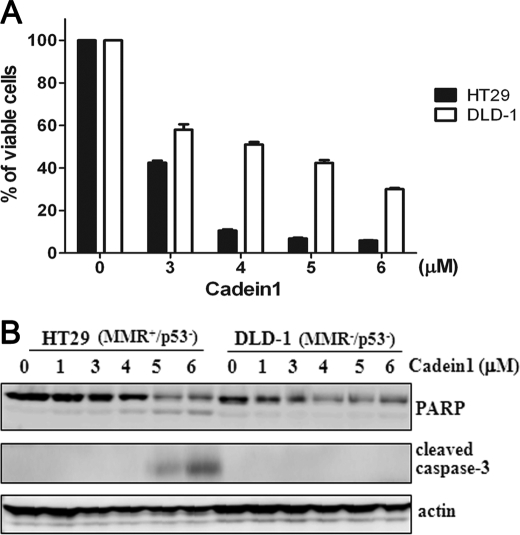
FIGURE 4.
Cadein1 induces apoptosis in MMR-positive HT29 but not in MMR-negative DLD-1 colon cancer cells. A and B, HT29 and DLD-1 cells were treated with different concentrations of cadein1 for 24 h (A) or 12 h (B). A, cell viability was measured by MTT assay. Three independent experiments were performed at each point, and the mean value was plotted with S.D. B, the expressions of cleaved caspase-3 and PARP were analyzed by Western blots. Actin was used as a loading control.
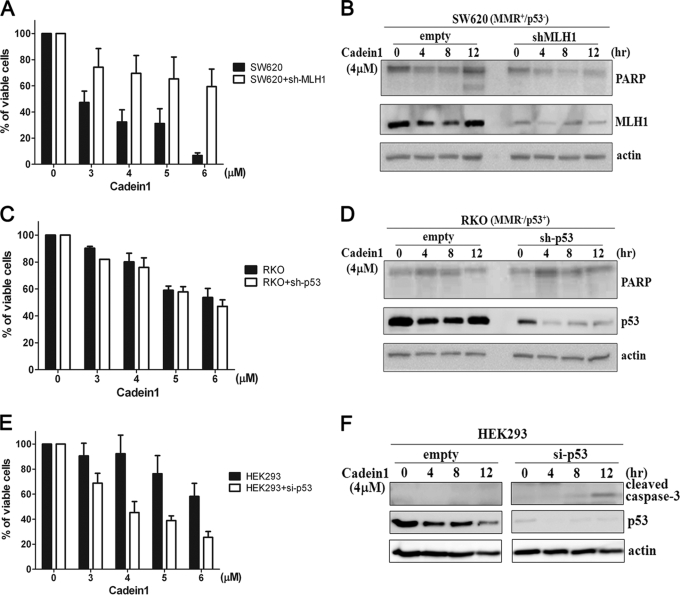
The function of MMR and p53 for cadein1-induced apoptosis is further studied in MMR-positive SW620 (MMR+/p53−) and p53-positive RKO (MMR−/p53+) colon cancer cells. Consistent with the results obtained from the HCT116 sublines, SW620 cells (Fig. 5, A and B) were more sensitive to cadein1 than RKO cells (Fig. 5, C and D). To validate the role of MMR and p53 in cadein1-induced apoptosis, MMR or p53 functions were knocked down with short hairpin RNAs in SW620 (Fig. 5, A and B) and RKO (Fig. 5, C and D) cells. The viability of SW620 cells was 32% at 4 μm cadein1, whereas MMR-depleted SW620 (shMLH1-transfected SW620) cells were 70% viable by 4 μm cadein1 treatment (Fig. 5A). When these cells were treated with 4 μm cadein1, activated PARP was not detected in MMR- depleted SW620 cells (shMLH1-transfected SW620 cells), although these cells are p53-negative (Fig. 5B). In contrast to MMR-depleted SW620 cells, MMR-deficient RKO cells that were knocked down with p53 did not change the sensitivity to cadein1 (Fig. 5C), and the PARP cleavage was not observed (Fig. 5D). These observations also support the notion that cadein1-induced apoptosis in p53-deficient colon cancer cells depends on the function of MMR.
FIGURE 5.
Cadein1-induced apoptosis requires both p53 deficiency and MMR proficiency. A and B, SW620 cells were transfected with short hairpin RNA-MLH1 or vector only (empty) and treated with different concentrations of cadein1 for 24 h (A) or with 4 μm cadein1 for indicated times (B). A, cell viability was measured by MTT assay. Three independent experiments were performed at each point, and the mean value was plotted with S.D. B, the expression of PARP and MLH1 was analyzed by Western blots. C and D, RKO cells were transfected with short hairpin RNA-p53 or vector only (empty) and treated with different concentrations of cadein1 for 24 h (C) or with 4 μm cadein1 for indicated times (D). C, cell viability was measured by MTT assay. Three independent experiments were performed at each point, and the mean value was plotted with S.D. D, cell extracts were immunoblotted with PARP and p53 antibodies. E and F, HEK293 cells were transfected with pSuper-Neo-p53-GFP and treated with different concentrations of cadein1 for 24 h (E) or with 4 μm cadein1 for the indicated times (F). E, cell viability was measured by MTT assay. Three independent experiments were performed at each point, and the mean value was plotted with S.D. F, cell extracts were immunoblotted with cleaved caspase-3 and p53 antibodies. Actin was used as a loading control. si-, small interfering.
To confirm the role of p53 in cadein1-induced apoptosis, p53- and MMR-positive HEK293 cells were transfected with small interfering RNA p53 and then treated with cadein1. Although the HEK293 cells were not normal cells, they were MMR- and p53-positive cells and have reduced sensitivity to cadein1 relative to HeLa cells (supplemental Fig. 2). When cells were treated with 6 μm cadein1, the viability of HeLa cells was 18.6%, whereas HEK293 cells were 61.6% viable (supplemental Fig. 2A). In addition, with 6 μm cadein1 treatment, the cleaved caspase-3 and PARP were detected in HeLa cells in a time-dependent manner, whereas the activation of caspase-3 and PARP was not detected in HEK293 cells (supplemental Fig. 2B). As shown in supplemental Fig. 2C, the sub-G1 fraction was highly increased in HeLa cells (9 μm, 50.3%), whereas a negligible sub-G1 fraction was observed in HEK293 cells (9 μm, 2.93%). Thus, we evaluated the expression of apoptotic proteins in cadein1-treated HEK293 cells where p53 was depleted by small interfering RNA p53. As shown in Fig. 5E, 92% of HEK293 cells were viable at 4 μm cadein1, whereas the viability at 4 μm cadein1 was 45% in p53-depleted HEK293 cells. In addition, when treated with 4 μm cadein1 for 12 h, the cleaved caspase3 was detected only in p53-depleted HEK293 cells but not in HEK293 cells (Fig. 5F). These observations support that the loss of p53 is directly associated with cadein1-induced apoptosis when MMR is functional.
We further studied our results that cadein1 induces apoptosis in p53-deficient cells with functional MMR by examining whether the recovery of p53 abrogates cadein1 sensitivity in MMR-proficient cells. HT29 (MMR+/p53−) cells were transfected with a p53-expressing vector, and their proliferative capacity was tested by MTT assay after treatment with a range of cadein1 concentrations (supplemental Fig. 3A). Interestingly, in contrast to what was observed in HT29 cells, the cytotoxic effect of cadein1 was reduced in p53-overexpressed HT29 cells. These results were verified by the reduction of cleaved PARP and caspase3 in p53-overexpressing HT29 cells relative to p53-deficient HT29 cells (supplemental Fig. 3B). In contrast to p53-overexpressing HT29 cells, neither the activation nor the reduction of cleaved PARP and caspase3 was detected in p53-overexpressing DLD-1 (MMR−/p53−) cells relative to p53-deficient DLD-1 cells (data not shown). These observations, even though they were performed under ectopic overexpression of p53, show that the decreased cadein1 sensitivity of p53-transfected cells is due to the expression of functional p53 and that the effect of p53 depends on the presence of functional MMR. Taken together, these results confirm that MMR function facilitates the cadein1-induced apoptosis in p53-negative cancer cells and functional p53 enables cells to resist to cadein1 even in the presence of MMR.
We verified the role of p53 in cadein1-induced apoptosis in other p53-deficient cells. HeLa cells were examined after transfection with a p53-expression plasmid. Activated caspase3 was strongly detected in cadein1-treated HeLa cells, as previously described in supplemental Fig. 3B. However, consistent with the results obtained from HT29 cells, the level of cleaved caspase3 in response to cadein1 treatment was reduced in HeLa cells expressing p53 (supplemental Fig. 3C). Thus, the gain of p53 function is directly correlated with resistance to cadein1 in MMR-proficient cells.
Cadein1 Induces the Activation of p38 but Not of the DNA Damage Response Proteins or Stress-activated Kinases
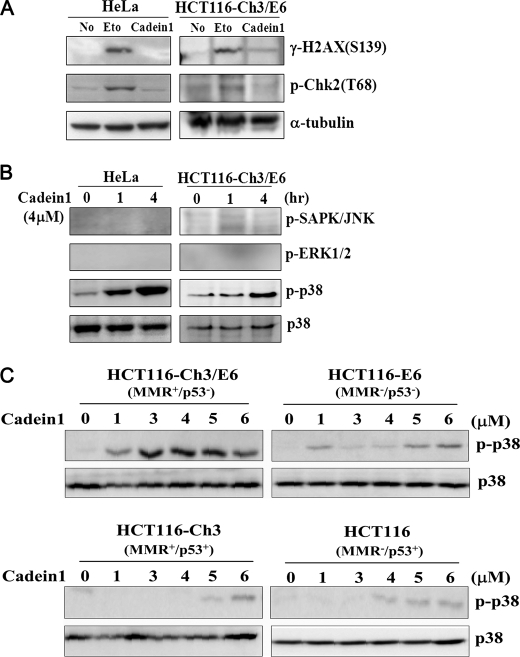
To understand the mechanism responsible for the G2/M delay and cell death caused by cadein1 treatment, we first investigated whether the ATM/Chk2 DNA damage checkpoint pathway impinges on the cadein1-induced G2/M delay. We measured γ-H2AX phosphorylation of Ser-139 by the ATM (ataxia-telangiectasia mutated) kinase (24) in cells treated with cadein1. Although etoposide produced γ-H2AX phosphorylation, treatment with 4 μm cadein1 did not induce γ-H2AX phosphorylation in any of the cell types tested (Fig. 6A). In addition, cadein1 failed to induce the phosphorylation and activation of Chk2, although Chk2 phosphorylation was induced by etoposide (Fig. 6A). These observations demonstrate that cadein1 induces a G2/M delay and cell death via an ATM-independent mechanism.
FIGURE 6.
Cadein1 activates p38 in p53-defective cancer cells with functional MMR to induce cell death. A, immunoblots for γ-H2AX and phosphorylated Chk2 were performed after treatment with 4 μm cadein1 or 20 μm etoposide (Eto) for 12 h in HeLa and HCT116-Ch3/E6 cells. Etoposide was used as a positive control for DNA damage. α-Tubulin is shown as a loading control. B, HeLa and HCT116-Ch3/E6 cells were treated with 4 μm cadein1 for 1 and 4 h. Cells extracts were immunoblotted with anti-phospho-p38, anti-p38, anti-phospho-stress-activated protein kinase (SAPK)/JNK, and anti-phospho-ERK1/2 antibody, respectively. C, HCT116-Ch3/E6, HCT116-E6, HCT116-Ch3, and HCT116 cells were treated with various concentrations of cadein1 for 12 h. The activation of p38 was analyzed by immunoblots with anti-phospho-p38 and anti-p38 antibody.
Because the p38 pathway mediates apoptotic signal transduction and is involved in the delayed G2/M transition induced by topoisomerase II and histone deacetylase inhibitors (24–26), we then tested whether cadein1 activates p38. Interestingly, 4 μm cadein1 induced p38 phosphorylation in a time-dependent manner in HeLa and HCT116-Ch3/E6 cells (Fig. 6B). However, cadein1 did not activate other stress-activated kinases such as ERKs and JNKs (Fig. 6B). These data suggest that the efficient apoptotic effects of cadein1 in cancer cells distinctively correlate with p38 activation. Accordingly, these results demonstrate that the cadein1-induced G2/M delay and cell death are dependent on p38 activation but not on the ATM-mediated DNA damage checkpoint pathway or other stress-activated kinases.
We then tested how cadein1 regulates the activation of p38 in HCT116 sublines. Consistent with the results shown in Fig. 3, HCT116-Ch3/E6 (MMR+/p53−) cells, which were most sensitive to cadein1, had detectable levels of phosphorylated p38 after lower doses (1–5 μm) of cadein1 treatment, compared with the other sublines (Fig. 6C). These results suggest that the cadein1 sensitivity and cadein1-induced apoptotic cell death directly correlate with p38 activation in HCT116-Ch3/E6 (MMR+/p53−) cells.
Cadein1-induced Apoptosis by p38 Activation Correlates with p53 Deficiency in Cancer Cells
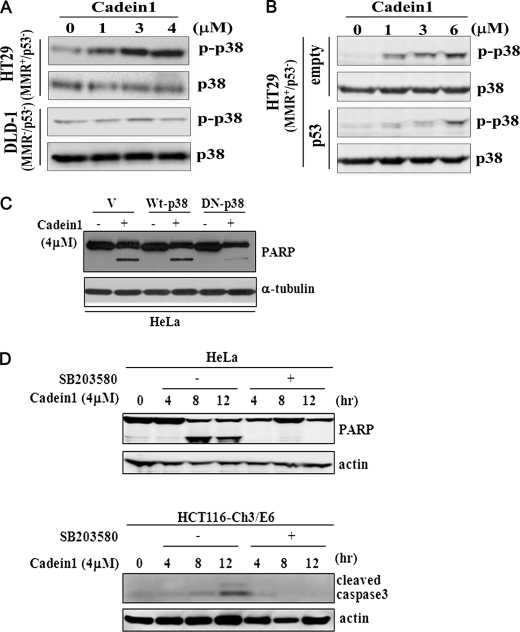
Consistent with the results obtained from the HCT116 sublines shown in Fig. 6C, after 4 μm cadein1 treatment, phosphorylation of p38 was also significantly activated in HT29 (MMR+/p53−) cells but not in MMR-deficient DLD-1 (MMR+/p53−) cells (Fig. 7A). As described in this study, the cytotoxicity of cadein1 was effective in HT29 cells but reduced in p53-overexpressed HT29 cells, whereas it did not change in DLD-1 cells and in p53-overexpressing DLD-1 (MMR−/p53−) cells (supplemental Fig. 3, A and B and data not shown). To confirm whether cadein1-induced p38 activation for cell death is linked to the presence or absence of functional p53, we measured the phosphorylation of p38 in p53-transfected HT29 cells. As shown in Fig. 7B, the restoration of p53 function reduced the phosphorylation of p38 in MMR-proficient HT29 cells. These results demonstrate that MMR facilitates cadein1-induced p38 activation in p53-negative cancer cells, but functional p53 reduces the phosphorylation of p38 even in the presence of functional MMR.
FIGURE 7.
p38 activation in cadein1-induced apoptosis depends on p53 deficiency in cancer cells with functional MMR. A, HT29 and DLD-1 cells were treated with different concentrations of cadein1 for 12 h. The activation of p38 was analyzed by immunoblots with anti-phospho-p38 and anti-p38 antibody. B, HT29 cells were transfected with pcDNA3.1-p53 expression plasmid or vector only (empty) and treated with different concentrations of cadein1 for 12 h. Cell extracts were analyzed by Western blots with anti-phospho-p38 and anti-p38 antibody. C, ectopic expression of dominant-negative p38 (DN-p38) alleviated the effect of cadein1. HeLa cells were transfected with pcDNA3.1 vector (V) and plasmids for wild type p38 (Wt-p38) and dominant-negative p38 (DN-p38). One day after transfection the cells were treated with 4 μm of cadein1 for 12 h. PARP was analyzed by an immunoblot. α-Tubulin protein is shown as a loading control. D, HeLa and HCT116-Ch3/E6 cells were pretreated with 10 μm SB203580 for 1 h, and then 4 μm cadein1 was added to cells for the indicated times. PARP and cleaved caspase3 were analyzed by immunoblots. Actin was used as a loading control.
To further verify the role of p38 in cadein1-induced cell death of cancer cells, HeLa cells were transfected with dominant-negative p38. When cells were treated with 4 μm cadein1, the level of cleaved PARP was clearly decreased in cells ectopically expressing dominant-negative p38 relative to cells expressing the wild type p38 (Fig. 7C). In addition, when cadein1-sensitive HeLa and HCT116-Ch3/E6 cells were pretreated with 10 μm concentrations of a p38-specific inhibitor, SB203580, for 1 h and followed with 4 μm cadein1, cadein1-induced cell death was prevented, as determined by the levels of caspase3 and PARP cleavage (Fig. 7D). These observations demonstrate that the apoptotic effects of cadein1 depend on p38 activation.
DISCUSSION
More than 50% of human tumors have a mutation leading to defects in the function of the tumor suppressor p53 (27). Inheriting a mutated allele of p53 increases the susceptibility to cancer and reduces sensitivity to anti-cancer therapies. Because cells with defective p53 are relatively resistant to apoptosis and, thus, to chemotherapeutic agents, it is appropriate to seek out cytotoxic agents that induce apoptosis in p53-defective cells. On the basis of this logic, we conducted a screen for anti-an proliferative agent(s) that selectively affects p53-deficient cancer cells.
In this report we describe cadein1, an isoquinolinium derivative that efficiently induces cell death in p53-defective cancer cell lines. Cadein1 has a strong potential as an anti-proliferative chemotherapeutic agent with a wide range of applicability, because it has a selective efficacy in p53-deficient cancer cells. At low concentrations, cadein1 preferentially blocks the proliferation of p53-defective cancer cells, whereas it has no effect on normal cells with intact p53 function (Fig. 2).
Just as cadein1 efficiently induces apoptosis in p53-defective cancer cells through activation of the caspase pathway, some clinically useful chemical agents are known to be more effective in tumor cells with mutant p53 (28). Taxol, which inhibits microtubule dynamics, is a good example. In contrast to DNA-damaging agents that lead to p53-dependent apoptosis, taxol induces apoptosis regardless of the status of p53, and loss of p53 appears to sensitize cells to cell death stimuli (29). In addition, the synthetic retinoid fenretinide induces p53-indepenent apoptosis of cancer cells and acts synergistically with chemotherapeutic drugs. The upstream signaling events induced by fenretinide include an increase in the intracellular levels of ceramide, which eventually lead to oxidative stress and apoptosis (30).
MMR deficiency results in strong resistance to a chemotherapeutic agent (10–13). In the case of cisplatin, a loss of both MMR and p53 causes a cooperative increase in resistance and limits the therapeutic potential of cisplatin in the colon cancer cells (14). We showed that the efficient cell death induced by cadein1 in p53-deficient cancer cells also depends on MMR proficiency (Figs. 3–5). The question, then, is how cadein1 efficiently induces apoptosis in p53-deficient and MMR-proficient cells. In the MMR-proficient cells, the G2/M arrest induced by DNA methylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) was accompanied by phosphorylation of p53, but the cell death by MNNG was p53-independent (31, 32). In addition, the sensitivity of cells to cancer chemotherapeutic agents such as temozolomide, 6-thioguanine, and cisplatin was affected by MMR (33–35). Therefore, MMR process is clearly linked to cell cycle checkpoint activation and cell death, although the mechanism is not clearly understood at present. Previous reports showed that the MMR-dependent signaling cascade is linked with the ATM and ataxia telangiectasia and Rad3-related (ATR) as well as the JNK/ stress-activated protein kinase and p38 (33–40). Temozolomide was shown to activate the mitogen-induced p38 kinase and the G2 checkpoint in MMR-proficient glioma and colon cancer (HCT116-ch3) cells, whereas no activation was observed in the glioma cells treated with small interfering RNA against hMLH1 (33). Recently, the p38 pathway was reported to be involved in checkpoint and surveillance in DNA damaging cellular states (24–26). Interestingly, cadein1 also induced G2/M arrest and activated the p38 pathway in MMR-proficient cells but not the ATM-Chk2 pathway (Figs. 6 and 7). The general mechanisms linking the MMR system, p53, and apoptosis should be further understood for a detailed mechanistic study of cadein1.
We showed that cadein1-induced apoptosis depends on p38 activation in p53-defective cancer cells (Fig. 7). There are some known chemotherapeutic agents that also activate p38 MAPK and trigger p53-independent apoptosis in tumor cells. Bortezomib induces apoptosis in esophageal squamous carcinoma cells through the activation of p38 mitogen-activated protein kinase pathway (41). This compound causes a G2-M phase cell cycle arrest and p53-independent apoptosis associated with caspase cleavage and Noxa (41). Asiatic acid effectively induces apoptosis and S-G2/M phase arrest through activation of the ERK1/2 and p38 MAPK pathway in human breast cancer cells (42).
Activated p38 MAP kinase has been reported to mediate apoptosis by Bid cleavage, mitochondrial dysfunction, and caspase-3, when reactive oxygen species are induced (43). Berberine, the lead compound of cadein1, is known to generate reactive oxygen species and thereby activate sustained phosphorylation of JNK and p38 MAPK, leading to cell death (44). Thus, one possible mechanism for cadein1-triggered apoptosis is through the generation of cellular stress, such as reactive oxygen species and the subsequent activation of the p38 kinase stress response pathway. Future studies will be necessary to understand the mechanism of how cadein1 induces cell death by activating p38 in p53-defective cells.
Importantly, cadein1 may have the potential to be effective in the treatment of the many human cancers that lose p53 but retain MMR proficiency. Although additional animal and preclinical studies are required to evaluate the clinical effectiveness of this agent, this compound may have important clinical applications. Further efforts to develop an efficient anticancer drug against p53-defective cancer cells by modifying cadein1 may be useful as well. As such, we suggest that this new compound be extensively explored.
Supplementary Material
Acknowledgments
Cadein1 was patented by K. Song and J. Park (Korea patent 10-2005-46749). We appreciate Drs. H.-W. Lee (Yonsei University, Seoul, Korea), J. Shin (Sungkyunkwan University, Suwon, Korea), and A. Zhitkovich (Brown University, RI) for pcDNA3-p53, pSuper-Neo-p53 p53, and shMLH1 plasmids.
This work was supported by National Research and Development Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea Grant NCCR-0920300 and in part by Korea Health Industry Development Institute (Grant B05-0016-AM0815-07A3-00030B).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- MMR
- DNA mismatch repair
- PARP
- poly(ADP-ribose)polymerase
- ATM
- ataxia-telangiectasia mutated
- MAPK
- mitogen-activated protein kinase
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- JNK
- c-Jun N-terminal kinase
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Fuster J. J., Sanz-González S. M., Moll U. M., Andrés V. (2007) Trends Mol. Med. 13, 192–199 [DOI] [PubMed] [Google Scholar]
- 2.Gudkov A. (2003) Cancer Biol. Ther. 2, 444–445 [DOI] [PubMed] [Google Scholar]
- 3.Wei C. L., Wu Q., Vega V. B., Chiu K. P., Ng P., Zhang T., Shahab A., Yong H. C., Fu Y., Weng Z., Liu J., Zhao X. D., Chew J. L., Lee Y. L., Kuznetsov V. A., Sung W. K., Miller L. D., Lim B., Liu E. T., Yu Q., Ng H. H., Ruan Y. (2006) Cell 124, 207–219 [DOI] [PubMed] [Google Scholar]
- 4.El-Deiry W. S. (2003) Oncogene 22, 7486–7495 [DOI] [PubMed] [Google Scholar]
- 5.Lowe S. W., Ruley H. E., Jacks T., Housman D.E. (1993) Cell 74, 957–967 [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Prives C. (2007) Oncogene 26, 2220–2225 [DOI] [PubMed] [Google Scholar]
- 7.Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. (1993) Cell 75, 1027–1038 [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. (1994) Science 263, 1625–1629 [DOI] [PubMed] [Google Scholar]
- 9.Fishel R., Kolodner R. D. (1995) Curr. Opin. Genet. Dev. 5, 382–395 [DOI] [PubMed] [Google Scholar]
- 10.Griffin S., Branch P., Xu Y. Z., Karran P. (1994) Biochemistry 33, 4787–4793 [DOI] [PubMed] [Google Scholar]
- 11.Kat A., Thilly W. G., Fang W. H., Longley M. J., Li G. M., Modrich P. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6424–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink D., Nebel S., Aebi S., Zheng H., Cenni B., Nehmé A., Christen R. D., Howell S. B. (1996) Cancer Res. 56, 4881–4886 [PubMed] [Google Scholar]
- 13.Liu L., Markowitz S., Gerson S. L. (1996) Cancer Res. 56, 5375–5379 [PubMed] [Google Scholar]
- 14.Lin X., Ramamurthi K., Mishima M., Kondo A., Christen R. D., Howell S. B. (2001) Cancer Res. 61, 1508–1516 [PubMed] [Google Scholar]
- 15.Stein G. S., Borun T. W. (1972) J. Cell Biol. 52, 292–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosmann T. (1983) J. Immunol. Methods 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 17.Craig W. J. (1997) J. Am. Diet Assoc. 97, S199–S204 [DOI] [PubMed] [Google Scholar]
- 18.Craig W. J. (1999) Am. J. Clin. Nutr. 70, 491S–499S [DOI] [PubMed] [Google Scholar]
- 19.Orfila L., Rodríguez M., Colman T., Hasegawa M., Merentes E., Arvelo F. (2000) J. Ethnopharmacol. 71, 449–456 [DOI] [PubMed] [Google Scholar]
- 20.Iizuka N., Oka M., Yamamoto K., Tangoku A., Miyamoto K., Miyamoto T., Uchimura S., Hamamoto Y., Okita K. (2003) Int. J. Cancer 107, 666–672 [DOI] [PubMed] [Google Scholar]
- 21.Kim H. J., Park J. E., Jin S., Kim J. H., Song K. (2006) Chem. Biol. 13, 881–889 [DOI] [PubMed] [Google Scholar]
- 22.Boyer J. C., Umar A., Risinger J. I., Lipford J. R., Kane M., Yin S., Barrett J. C., Kolodner R. D., Kunkel T. A. (1995) Cancer Res. 55, 6063–6070 [PubMed] [Google Scholar]
- 23.Koi M., Umar A., Chauhan D. P., Cherian S. P., Carethers J. M., Kunkel T. A., Boland C. R. (1994) Cancer Res. 54, 4308–4312 [PubMed] [Google Scholar]
- 24.Reinhardt H. C., Aslanian A. S., Lees J. A., Yaffe M. B. (2007) Cancer Cell 11, 175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikhailov A., Shinohara M., Rieder C. L. (2004) J. Cell Biol. 166, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saya H. (2007) 19th Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB) Meeting, Seoul, Korea, May 27–31, 2007, Abstr. S9-3, Korean Society for Biochemistry and Molecular Biology, Seoul, Korea [Google Scholar]
- 27.Levesque A. A., Eastman A. (2007) Carcinogenesis 28, 13–20 [DOI] [PubMed] [Google Scholar]
- 28.Weinstein J. N., Myers T. G., O'Connor P. M., Friend S. H., Fornace A. J., Jr., Kohn K. W., Fojo T., Bates S. E., Rubinstein L. V., Anderson N. L., Buolamwini J. K., van Osdol W. W., Monks A. P., Scudiero D. A., Sausville E. A., Zaharevitz D. W., Bunow B., Viswanadhan V. N., Johnson G. S., Wittes R. E., Paull K. D. (1997) Science 275, 343–349 [DOI] [PubMed] [Google Scholar]
- 29.Wahl A. F., Donaldson K. L., Fairchild C., Lee F. Y., Foster S. A., Demers G. W., Galloway D. A. (1996) Nat. Med. 2, 72–79 [DOI] [PubMed] [Google Scholar]
- 30.Corazzari M., Lovat P. E., Oliverio S., Di Sano F., Donnorso R. P., Redfern C. P., Piacentini M. (2005) Biochem. Biophys. Res. Commun. 331, 810–815 [DOI] [PubMed] [Google Scholar]
- 31.Cejka P., Stojic L., Mojas N., Russell A. M., Heinimann K., Cannavó E., di Pietro M., Marra G., Jiricny J. (2003) EMBO J. 22, 2245–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickman M. J., Samson L. D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10764–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirose Y., Katayama M., Stokoe D., Haas-Kogan D. A., Berger M. S., Pieper R. O. (2003) Mol. Cell. Biol. 23, 8306–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamane K., Taylor K., Kinsella T. J. (2004) Biochem. Biophys. Res. Commun. 318, 297–302 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Qin J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15387–15392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stojic L., Mojas N., Cejka P., Di Pietro M., Ferrari S., Marra G., Jiricny J. (2004) Genes Dev. 18, 1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nehmé A., Baskaran R., Aebi S., Fink D., Nebel S., Cenni B., Wang J. Y., Howell S. B., Christen R. D. (1997) Cancer Res. 57, 3253–3257 [PubMed] [Google Scholar]
- 38.Nehmé A., Baskaran R., Nebel S., Fink D., Howell S. B., Wang J. Y., Christen R. D. (1999) Br. J. Cancer 79, 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franchitto A., Pichierri P., Piergentili R., Crescenzi M., Bignami M., Palitti F. (2003) Oncogene 22, 2110–2120 [DOI] [PubMed] [Google Scholar]
- 40.Brown K. D., Rathi A., Kamath R., Beardsley D. I., Zhan Q., Mannino J. L., Baskaran R. (2003) Nat. Genet. 33, 80–84 [DOI] [PubMed] [Google Scholar]
- 41.Lioni M., Noma K., Snyder A., Klein-Szanto A., Diehl J. A., Rustgi A. K., Herlyn M., Smalley K. S. (2008) Mol. Cancer Ther. 7, 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu Y. L., Kuo P. L., Lin L. T., Lin C. C. (2005) J. Pharmocol. Exp. Ther. 313, 333–344 [DOI] [PubMed] [Google Scholar]
- 43.Zhuang S., Demirs J. T., Kochevar I. E. (2000) J. Biol. Chem. 275, 25939–25948 [DOI] [PubMed] [Google Scholar]
- 44.Hsu W. H., Hsieh Y. S., Kuo H. C., Teng C. Y., Huang H. I., Wang C. J., Yang S. F., Liou Y. S., Kuo W. H. (2007) Arch. Toxicol. 81, 719–728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.