Abstract
Sepsis is a severe medical condition causing a large number of deaths worldwide. Recent studies indicate that the septic susceptibility is attributable to the vascular ATP-sensitive K+ (KATP) channel. However, the mechanisms underlying the channel modulation in sepsis are still unclear. Here we show evidence for the modulation of vascular KATP channel by septic pathogen lipopolysaccharides (LPS). In isolated mesenteric arterial rings, phenylephrine (PE) produced concentration-dependent vasoconstriction that was relaxed by pinacidil, a selective KATP channel opener. The PE response was disrupted with a LPS treatment. In acutely dissociated aortic smooth myocytes the LPS treatment augmented KATP channel activity, and hyperpolarized the cells. Quantitative PCR analysis showed that LPS raised Kir6.1 and SUR2B transcripts in a concentration-dependent manner, which was suppressed by transcriptional inhibition. Consistently, the same LPS treatment did not affect Kir6.1/SUR2B channels in a heterologous expression system. The LPS effect on Kir6.1 and SUR2B expression was abolished in the presence of NF-κB inhibitors. Several other Toll-like receptor ligands also stimulated Kir6.1 and SUR2B expression to a similar degree as LPS. Thus, the effect of LPS on vasodilation involves up-regulation of KATP channel expression, in which the NF-κB-dependent signaling plays an important role.
Keywords: Channels/Potassium, Receptors/Toll-like, Tissue/Organ Systems/Muscle/Smooth, Signal transduction, Transcription Regulation, Kir6.1, Lipopolysaccharide, SUR2B, Sepsis, Transcription
Introduction
Septic shock caused by several septic pathogens including bacterial lipopolysaccharides (LPS)3 is a severe medical condition characterized by lethal cardiovascular dysfunction and hypotension (1, 2). Despite the widespread antibiotic usage, the incidence of sepsis continued to rise over the past two decades from 164,000 in 1979 to 660,000 in 2000, and the related cases of in-hospital deaths rose from 44,000 in 1979 to 120,000 in 2000 (3). Dysfunction of the cardiovascular system plays a major role in the septic mortality. Accumulating evidence indicates that cardiovascular responses to septic pathogens are rather diverse with very different prognosis among individuals, although the underlying mechanisms for the sepsis susceptibility are unclear (4). Recent studies indicate that functional integrity of vascular KATP channels is a crucial factor for the sepsis susceptibility (5, 6).
The vascular KATP channels are members of the inward rectifier K+ channel family. These channels consist of 4 pore-forming Kir6.x subunits and 4 sulfonylurea receptor (SUR) subunits. The Kir6.1/SUR2B is the major isoform in vascular smooth muscles (VSM). The VSM KATP channel is modulated by several vasoactive hormones and neurotransmitters, such as α and β adrenergic receptor agonists, angiotension II, arginine vasopressin, adenosine, calcitonin gene-related peptide, vasoactive intestinal polypeptide, etc. (7–11). In addition, several metabolites including ATP, ADP, pH, epoxyeicosatrienenoic acids, H2S are important KATP channel regulators (12–15). Genetic knock-out of either subunit of the vascular KATP channel leads to spontaneous coronary vasospasm and sudden death, consistent with their function in vascular tone regulations (16, 17).
The VSM KATP channel has recently been shown to play a critical role in septic susceptibility. Studies with chemical mutations genome-wide have led to an identification of four strains of mice that are highly vulnerable to various septic pathogens (6). All of these mice carry a null mutation of the Kir6.1 gene (Kcnj8). Consistently, mice with Kir6.1-knock-out exhibit cardiovascular abnormalities with a high mortality when exposed to a sublethal dose of LPS (5). Although these studies indicate that the VSM KATP channel is an important player in systemic responses to sepsis, how the channel is affected by LPS remains unclear, and several questions are open as to how the LPS exposure affects whole cell KATP currents, whether the increase in KATP currents is a result of the up-regulation of channel protein expression or a direct effect on channel activity, what the intracellular signaling pathways underscore the changes. To address these questions, we performed this study.
MATERIALS AND METHODS
Chemicals and cDNAs
Chemicals used in our studies were purchased from Sigma unless otherwise stated. LPS (Escherichia coli 0127:B8) was purchased from Sigma. Lipoteichoic acid (LTA), bacterial CpG DNA (CpG), flagellin, and polyinosinic:polycytidylic acid (Poly I:C) were obtained from InvivoGen (San Diego, CA). All chemicals were prepared as high concentration stocks in double-distilled H2O (ddH2O) or dimethyl sulfoxide (DMSO), and were diluted to experimental concentrations immediately before usage. The final concentration of DMSO was <0.1%.
Rat Kir6.1 (GenBankTM accession D42145) and mouse SUR2B (GenBankTM accession D86038) were cloned in a eukaryotic expression vector, pcNDA3.1, and used for mammalian cell expression. Human TLR4 (GenBankTM accession NM_138554) and CD14 were cloned in pcDNA3 (GenBankTM accession NM_000591) by Dr. Golenbock at the University of Massachusetts. Human MD-2 (GenBankTM accession NM_015364) was cloned in a mammalian expression vector pEFBOS by Dr. Sachiko Akashi-Takamura at the University of Tokyo.
Cell Culture
All types of cells were grown at 37 °C in a humidified atmosphere of 95% air and 5%CO2, and were routinely split when the cell density reached 90–100% confluence. Rat aortic smooth muscle cells (A10, CRL-1476, ATCC) were cultured as a monolayer in the DMEM with 10% fetal bovine serum (FBS). Human embryonic kidney cells (HEK293, CRL-1573, ATCC) were grown in the DMEM-F12 medium supplemented with 10% FBS and penicillin/streptomycin.
Transfection
The HEK293 cells were used to express the KATP channels. Transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in which 0.7 μg of Kir6.1, 2.1 μg of SUR2B, 1.0 μg of TLR4, 0.2 μg of MD2, and 0.2 μg of CD14 were added to a 35-mm Petri dish. To facilitate the identification of positively transfected cells, 0.4 μg of green fluorescent protein (GFP) cDNA (pEGFP-N2; Clontech, Palo Alto, CA) was added to the cDNA mixture. Cells were dissociated from the monolayer using 0.25% trypsin ∼24 h after transfection. A few drops of the cell suspension were added onto 5× 5-mm coverslips in a 35-mm Petri dish. Cells were then incubated in DMEM-F12 for 24–48 h before experiments.
Acute Dissociation of Vascular Smooth Myocytes
All animal experiments complied with the Institutional Animal Care and Use Committee approval of the Georgia State University. Mice (15–20 g) were anesthetized by inhalation of saturated halothane vapor followed by cervical dislocation. The aorta was dissected free, cut into small segments (1 mm), and placed in 5-ml solution containing (in mm): 140 NaCl, 5.4 KCl, 1 MgCl2, 0.1 CaCl2, 10 HEPES, and 10 d-glucose at room temperature for 10 min. The tissues were then placed in 1-ml solution with 20 units of papain (Worthington) and 1.25 mg dithiothreitol. After digestion for 15 min at 35 °C, the tissue was washed once and then transferred to a 1 ml solution containing 440 units of collagenase (CLS II, Worthington) and 1.25 mg of trypsin inhibitor (Sigma) for 5–10 min. After being thoroughly washed, the tissue was moved to a 1 ml solution containing 20% fetal bovine serum and triturated with a fire-polished Pasteur pipette to obtain single smooth muscle cells. Cells were stored on ice and used within 8 h. A drop of cells was put in a 35-mm tissue culture dish, and cells were allowed to attach to the surface in 15 min. Cells that had clear smooth muscle morphology and did not show evident swelling or shrinkage were used for patch studies.
Mesenteric Arterial Rings
Mesenteric arterial rings were obtained from Sprague-Dawley rats (250–350 g) in accordance with the guidelines for the care and use of laboratory animals by Georgia State University and Harbin Medical University. The rats were anesthetized by inhaling saturated halothane vapor followed by cervical dislocation. The mesenteric arteries were dissected free and transferred to ice-cold Krebs solution containing (in mm): 118.0 NaCl, 25.0 NaHCO3, 3.6 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11.0 glucose, and 2.5 CaCl2. The arteries were cut into 6–8 endothelium-intact rings of 2 mm in length and stored in Krebs solution. Endothelium-denuded rings were also used in which the endothelium was removed by a rough plastic tube and tested by the loss of response to acetylcholine. During the experiment, a ring was mounted on a force-electricity transducer (Model FT-302, iWorx/CBSciences, Dover, NH) in a tissue bath. With a 0.5-g preload, the ring was allowed to equilibrate in the tissue bath for 30 min when the tension was reduced to ∼0.3 g. The tissue bath was filled with Krebs solution and perfused with 5% CO2 at 36 °C. The arterial tone was measured as changes in isometric force. Only rings that showed a clear vasoconstriction response to 1.0 μm phenylephrine were used in the study.
Electrophysiology
Patch clamp experiments were performed at room temperature as described previously. In brief, fire-polished patch pipettes with resistance of 40–50 MΩ were made with 1.2-mm borosilicate glass capillaries. Whole cell recording was performed in single-cell voltage clamp. Current records were low-pass filtered (2 kHz, Bessel 4-pole filter, −3 dB), digitized (20 kHz, 16-bit resolution), and stored on a computer hard drive for later analysis using the Clampfit 9 software (Axon Instruments). The bath solution contained (in mm): 10.0 KCl, 135.0 potassium gluconate, 5.0 EGTA, 5.0 glucose, and 10.0 HEPES (pH 7.4). The pipette solution contained (in mm): 10.0 KCl, 133.0 potassium gluconate, 5.0 EGTA, 5.0 glucose, 1 K2ATP, 0.5 NaADP, and 10.0 HEPES (pH 7.4), in which the free Mg2+ concentration was adjusted to 1 mm using a [Ca2+]/[Mg2+] calculation software. For membrane potential measurement from aortic smooth muscle cells, bath solution contained (in mm): 3.0 KCl, 140.0 NaCl, 1.0 CaCl2, 1.0 MgCl2, 10.0 glucose, and 10.0 HEPES (pH 7.4 with NaOH). Pipette solution is the same as that used in whole cell patch clamping.
Reverse Transcription PCR (RT-PCR)
Total RNA was extracted from mouse aorta with an RNeasy Mini kit (Qiagen) according to the manufacturer's protocol. cDNA was reverse-transcribed from 0.5 μg of total RNA in a 20-μl reaction containing 200 units of Superscript II reverse transcriptase (Invitrogen), 300 ng of random hexamers, 0.5 mm dNTPs, 40 units of RNaseOut, and 10 mm dithiothreitol. The RT product was treated with 5 units of RNase H for 20 min. For PCR analysis of KATP channel subunits, we designed primers targeting the mRNA sequence of mouse KATP channel subunits (Table 1). PCR was performed in a Perkin Elmer GeneAmp 2400 in a final volume of 50 μl including 1 μl of the RT product, 1.25 units of GoTaq DNA polymerase (Promega, Madison, WI), 250 μm dNTP, 2.5 μl of DMSO, and 0.5 μm primers. The cycling conditions were 95 °C for 5 min followed by 30 cycles of 45 s at 95 °C, 45 s at 52 °C, 75 s at 72 °C, and a final elongation for 10 min at 72 °C. 5 μl of PCR products were separated by electrophoresis on a 1% agarose gel and visualized with ethidium bromide under UV fluorescence.
TABLE 1.
RT-PCR primers
| Target gene | Primers | Accession no. | Size |
|---|---|---|---|
| bp | |||
| Kir6.1 | Fw: TGGCTGCTCTTCGCTATC | NM_008428 | 578 |
| Re: GGGCTACGCTTGTCAATC | |||
| Kir6.2 | Fw: AGGGCATTATCCCTGAGG | NM_010602 | 569 |
| Re: GCGTTGATCATCAGCCC | |||
| SUR2B | Fw: GAAGTCCTCCTTATCCCTGG | NM_011511 | 592 |
| Re: ACGGACAAACGAGGCAAAC | |||
| GAPDH | Fw: TGCTGAGTATGTCGTGGAG | NM_008084 | 668 |
| Re: ACCAGGAAATGAGCTTGAC |
Real-time Quantitative RT-PCR (qPCR)
qPCR was performed with an Applied Biosystems 7500 Fast Real-Time PCR system. Primers were specifically designed using Applied Biosystems Primer Express 3.0 and are listed in Table 2. The specificity of the primers was confirmed with a BLAST program. Each 20-μl reaction contained 1× Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen), 0.2 μm forward and reverse primers, 0.04 μl ROX reference dye, and 0.4 μl of cDNA. Thermal cycling conditions included an initial UNG incubation at 50 °C for 2 min, Platinum TaqDNA polymerase activation at 95 °C for 2 min, 40 cycles of denaturing at 95 °C for 3 s, and annealing and extension at 60 °C for 30 s, followed by routine melting curve analysis. Relative quantitation (RQ) of target gene expression was calculated by the 2−ΔΔCt method (18). The first step in the RQ analysis is to normalize target gene expression level to GAPDH (ΔCt). The second step is to compare the difference between normalized target gene expression in LPS-treated and untreated samples (ΔΔCt). Each experiment was repeated 2–3 times in 3–4 samples.
TABLE 2.
Real time PCR primers
| Target gene | Primers | Accession no. | Size |
|---|---|---|---|
| bp | |||
| Kir6.1 | Fw: CGCAAACCCGAGTCTTCTAGGA | NM_008428 | 101 |
| Re: CCTGGCCAACATCTTCCTTTCAC | |||
| Kir6.2 | Fw: GCCCTGCGTCACAAGCA | NM_010602 | 39 |
| Re: GGACCTCGATGGAGAAAAGGA | |||
| SUR2B | Fw: CCATAGCTCATCGGGTTCACA | NM_011511 | 133 |
| Re: CGGACAAACGAGGCAAACAC | |||
| GAPDH | Fw: CCAGCCTCGTCCCGTAGA | NM_008084 | 179 |
| Re: TGCCGTGAGTGGAGTCATACTG |
Nuclear Protein Extraction and Western Blotting
A10 cells in 90–100% confluence were rendered quiescent in Dulbecco's modified Eagle's medium with 0.5% fetal bovine serum for 6 h before experiments. The cell line provided large quantity of samples for protein study. Nuclear proteins were extracted using Nuclear Extract kit (Active Motif, Carlsbad, CA). Protein concentration was determined by BCA assay (Pierce). The nuclear proteins in Laemmli sample buffer were boiled in 95–100 °C for 5 min. The samples (50 μg) were loaded onto each well, separated on 10% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Bio-Rad). Nonspecific binding sites were blocked by a 1-h incubation of the membranes in TBST/5% nonfat milk, followed by blotting with primary antibodies diluted in TBST/5% bovine serum albumin overnight. Most primary antibodies were purchased from Cell Signaling Technology (Boston, MA). Anti-p65 IgG was provided by Santa Cruz Biotechnology (Santa Cruz, CA). The membrane was then incubated in horseradish peroxidase-conjugated secondary antibody (1:104, Jackson Immunoresearch), and detected by SuperSignal ECL substrate (Thermo Scientific, Rockford, IL) according to the manufacturer's instruction.
Data Analysis
Data are presented as the mean ± S.E. of each group. Differences in means were tested with a Student's t test and were accepted as significant if p ≤ 0.05.
RESULTS
KATP Channels in the LPS-induced Vascular Hyporeactivity to Vasoconstrictor
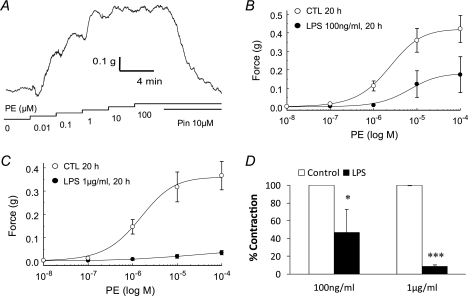
Vascular responses to LPS exposure were studied in isolated and perfused rings from mesenteric arteries. The mesenteric artery was adopted, because it is a favorable resistant artery model. Endothelium was mechanically removed immediately before mounting. The endothelium elimination was confirmed as the rings failed to respond to 1 μm carbachol (11). The rings were mounted on force electricity transducers with a 0.3 g of preload and allowed to equilibrate for 30 min before experiments. Isometric contraction was produced with the adrenergic α-receptor agonist PE that produced concentration-dependent constrictions with the maximum effect reached at ∼1 μm (Fig. 1A). At the maximum constriction, pinacidil, a KATP channel opener, relaxed the vasoconstriction almost completely, suggesting that KATP channels are involved in the vascular tone regulation (Fig. 1A). Previous studies have shown that the concentration of LPS can reach 100 ng/ml (19, 20). The concentration can be elevated even up to 200-fold when bacteria were lysed during antibiotic therapy (21). A pretreatment of the rings with LPS for 20 h impaired the PE reactivity (Fig. 1B). The LPS effect had clear concentration dependence. The vascular reactivity to 1 μm PE was almost completely lost with an exposure to 1 μg/ml LPS, and reduced by >60% with 0.1 μg/ml LPS (Fig. 1, B–D). Hence, we adopted 1 μg/ml LPS in the further studies. Such a concentration was used in dissecting signaling pathways rather than mimicking the in vivo condition in sepsis. Therefore, the signaling pathways under study may not be immediately related to sepsis.
FIGURE 1.
KATP channels play a role in vascular responses to LPS treatment. A, in an isolated rat mesenteric arterial ring, PE produced a concentration-dependent vasoconstriction. At the peak contraction, the KATP channel opener pinacidil relaxed the ring almost completely in the presence of 100 μm PE. B, relationship of the contractile force with PE concentrations was studied in endothelium-denuded rings. The relationship was described using the Hill equation with EC50 2.2 μm in the control group (CTL). The contractility was markedly diminished after a pretreatment with LPS (100 ng/ml) for 20 h. The EC50 of PE became 6.3 μm. Data are presented as means ± S.E. (n = 9 rings for control; n = 7 for LPS). C, similar experiments were done with 1 μg/ml LPS. A greater impair in contractility occurred with the LPS exposure (n = 6 for control; n = 5 for LPS). D, in comparison to their experiments controls, the ED rings lost about 42% contractility with 100 ng/ml LPS (*, p < 0.05, n = 15), and >90% with 1 μg/ml LPS (***, p < 0.001, n = 9).
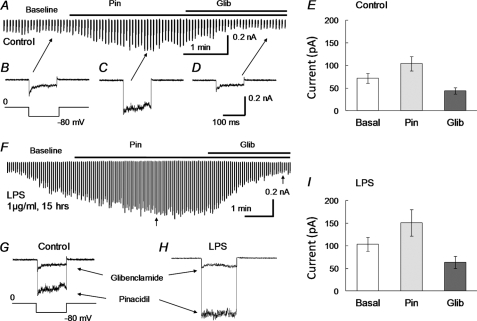
Increase in Whole Cell KATP Currents with LPS Exposure
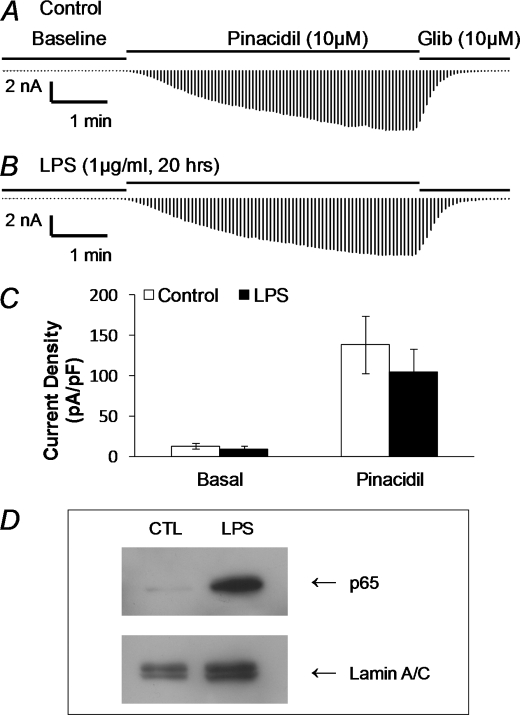
K+ currents were studied in whole cell voltage clamp. High concentration of K+ (145 mm) was applied to the bath and pipette solutions, and membrane potentials of the cell were held at 0 mV with step hyperpolarizing pulses to −80 mV applied to the cell (9). Under this condition, the aortic SMCs (n = 21) exhibited small basal currents upon formation of the whole cell configuration (72.1 ± 10.5 pA, Fig. 2, A, B, E). Pinacidil (10 μm) augmented the currents by 44% (104.2 ± 16.1 pA, Fig. 2, A, C, E). The pinacidil-activated currents were strongly inhibited by 10 μm glibenclamide (43.9 ± 7.1 pA, Fig. 2, A, D, E), consistent with the expression of functional KATP channels in the SMCs. In another group of SMCs (n = 17), the basal currents increased by 43% (103.6 ± 15.2 pA, Fig. 2, F, I) after a treatment with 1 μg/ml LPS overnight. The amplitude of the pinacidil-activated currents was further augmented by 45% (150.9 ± 29.5 pA, Fig. 2, F, H, I). The glibenclamide-sensitive currents were 63.7 ± 13.6 pA (Fig. 2, F, H, I).
FIGURE 2.
Augmentation of KATP currents with LPS incubation. Whole cell voltage clamp was performed in freshly dissociated aortic SMCs. The bath solutions contained 145 mm K+. The same solution was used in the recording pipette with addition of 1 mm ATP, 0.5 mm ADP, and 1 mm free Mg2+. A, in a control experiment, small inward currents were seen upon the formation of the whole cell configuration. The currents were increased by pinacidil (10 μm). The maximal activation was reached in 2 min, whereas glibenclamide (10 μm) reduced currents to a level even below the baseline. B–D, individual records of inward currents at baseline (B) and with pinacidil (Pin, C) or glibenclamide (Glib, D). E, summary of the current amplitude under these conditions (n = 21 cells). F, the pinacidil- and glibenclamide-sensitive currents were studied in another SMC that had been treated with LPS (1 μg/ml) overnight. The current amplitude increased significantly after the whole cell patch formation, presumably produced by intracellular dialysis of ADP and Mg2+. The currents were further augmented by pinacidil, reaching a peak that doubled that without LPS treatment in A. The pinacidil-activated currents were completely suppressed by glibenclamide. G, superimposed are individual currents obtained from C and D. H, current traces were similarly taken from F at the position indicated by arrows. I, effect of LPS on whole cell currents of dissociated SMCs (n = 17 cells).
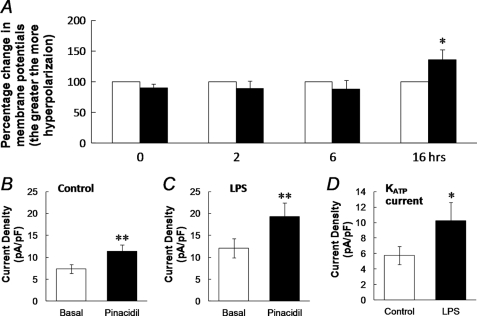
Membrane potentials of SMCs freshly dissociated from the mouse aorta were examined in whole cell current clamp. With physiological concentration of K+ in the bath and pipette solutions, the SMCs had a resting membrane potential of −56.4 ± 3.8 mV (n = 10). The effect of LPS on membrane potentials was studied in two groups of cells with one group treated with 1 μg/ml LPS and the other with the solvent vehicle. Although no significant changes in membrane potentials were seen between these groups at 0, 2, and 6 h of exposure (p > 0.05, n = 9–11 cells for each time point), at 15–18 h the membrane potentials were significantly more hyperpolarizing in the LPS-treated group than in the vehicle-treated (−60.1 ± 3.5 mV, n = 6) in comparison to the control group (−46.5 ± 4.9 mV, n = 6; p < 0.05; Fig. 3A, supplemental Fig. S1). The augmentation of KATP currents may result from an up-regulation of the channel expression, post-translational modulation of channel activity (e.g. channel protein phosphorylation) or both. We therefore carried out studies to test these possibilities.
FIGURE 3.
A, effect of LPS on membrane potentials (Vm) was studied in vascular SMCs freshly dissociated from the mouse aorta by parallel comparison of the Vm recorded from cells treated with and without LPS. Although no obvious changes in Vm were seen with an LPS treatment for 0, 2, and 6 h, significant hyperpolarization occurred with a 16 h exposure (*, p < 0.05; n = 10). B–D, enhancement of current density with LPS exposure. The current density was calculated by dividing the current amplitude by the whole cell capacitance in each cell. Without LPS, the pinacidil-activated current (I) density was ∼54% greater than the basal currents (B, n = 21, **, p < 0.01). After an overnight treatment with LPS (1 μg/ml), the density of basal currents increased by 50%, whereas the pinacidil-activated current density was further elevated by 59% (C, n = 17). KATP currents were isolated by subtraction of the glibenclamide-sensitive currents from the pinacidil-activated currents (Fig. 2, G and H). The KATP current density was significantly enhanced after LPS exposure (*, p < 0.05, D).
Surface Expression
To show the effect of LPS exposure on the surface expression of KATP channels, we analyzed the current density in dissociated aortic SMCs by dividing the whole cell current amplitude by membrane capacitance. The density of the pinacidil-activated currents was 11.4 ± 1.4 pA/pF (n = 21, Fig. 3B) without LPS treatment. After a treatment of the cells with LPS (1 μg/ml) overnight, the current density increased to 19.3 ± 3.1 pA/pF (n = 17), which was 69% greater than that before LPS exposure (p < 0.01 and Fig. 3C). Despite the large increase in the current density, the net effect of pinacidil remained similar (60.1% over the basal currents after LPS treatment versus 54.2% before).
The KATP currents were isolated by subtracting the currents with the glibenclamide (10 μm) treatment from the currents with the pinacidil (10 μm) treatment as shown in Fig. 2, G and H. The isolated currents were then divided by whole cell capacitance to get the current density. The KATP current density was 5.8 ± 1.2 pA/pF (n = 21 cells) without LPS treatment and increased significantly to 10.3 ± 2.3 pA/pF (n = 17 cells, p < 0.05) after an overnight exposure to LPS (1 μg/ml, Fig. 3D), suggesting that the LPS exposure augments the surface expression of functional KATP channels.
Concentration-dependent Stimulation of Kir6.1/SUR2B Transcription
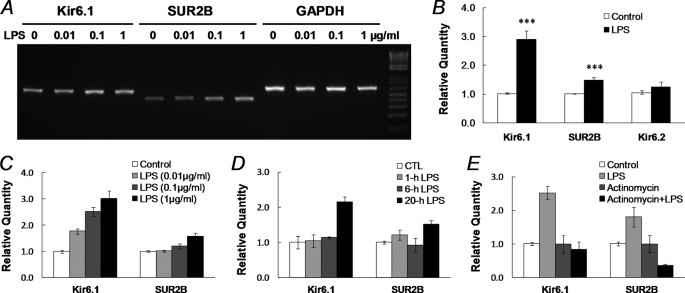
At the mRNA level, the expression of Kir6.1, Kir6.2, and SUR2B was studied in smooth muscle tissues of the mouse aorta. We use mouse tissues because the information related to alternative splicing in mouse ABCC9 were more detailed in GenBankTM®. RT-PCR with the mRNAs extracted from cultured endothelium-denuded aortic rings showed that the levels of Kir6.1 and SUR2B transcripts were significantly higher in the LPS-treated groups than in the control group (Fig. 4A). In contrast, the Kir6.2 mRNA expression did not show any evident change (Fig. 4A), whereas SUR2A transcript level was too low to form a significant KATP isoform (supplemental Fig. S2). These results thus indicate that the Kir6.1/SUR2B isoform of KATP channels is targeted.
FIGURE 4.
Augmentation of KATP mRNA expression after LPS exposure. A, total RNAs were extracted from dissociated SMCs from the mouse aorta after 20 h of incubation with LPS in 0, 0.01, 0.1, and 1 μg/ml. In RT-PCR analysis, the LPS treatment led to up-regulation of both Kir6.1 and SUR2B transcripts in a concentration-dependent fashion. B, quantitative real-time PCR was performed to quantify KATP channel expression. Expression levels of target genes were normalized to the GAPDH mRNA level using the 2−ΔΔCt method (18). LPS (1 μg/ml, 20 h) increased Kir6.1 transcripts by ∼1.9-fold, and SU2B by ∼0.5-fold (***, p < 0.001, n = 48 and 50 samples from 14 mince, respectively), whereas Kir6.2 did not show significant increase (p > 0.05, n = 33). Note that the expression of the KATP channel in LPS-treated groups was measured and normalized to the vehicle control. C, concentration-dependent Kir6.1 and SUR2B expression following 20 h of LPS treatment. Data were collected from three independent experiments with 3–4 samples in each. D, time dependence. A clear up-regulation of Kir6.1 and SUR2B mRNA expression was observed with LPS (1 μg/ml) exposure at 20 h but not at 1 and 6 h. E, after a 20-h treatment with actinomycin D (2 μg/ml, added 1 h before 1 μg/ml LPS), the enhancement of Kir6.1 and SUR2B expression was totally eliminated. Data were obtained from three independent experiments with 3–4 samples in each.
Quantitative PCR analysis showed that LPS (1 μg/ml, 20 h) enhanced Kir6.1 transcripts by ∼1.9-fold, and SUR2B transcripts by 0.5-fold in the dissociated aortic SMCs (p < 0.001, n = 47 and 49, respectively; Fig. 4B), whereas Kir6.2 transcripts did not show significant increase (p > 0.05, n = 32). The effect of LPS on Kir6.1 and SUR2B expression relied on LPS concentrations (Fig. 4, A and C). In the concentration as low as 0.01 μg/ml, LPS raised Kir6.1 mRNA expression by 0.8-fold. LPS further stimulated the Kir6.1 expression by 1.5- and 2.0-fold in 0.1 μg/ml and 1 μg/ml, respectively. In the concentration 0.01 μg/ml, LPS did not exhibit stimulatory effect on SUR2B. A small effect was seen with 0.1 μg/ml LPS, whereas LPS in 1 μg/ml increased SUR2B mRNA by 0.6-fold (Fig. 4C). The up-regulation of Kir6.1 and SUR2B expression occurred at ∼20 h of LPS (1 μg/ml) exposure, whereas no significant increase in Kir6.1 and SUR2B expression was found at 1 h and 6 h (Fig. 4D). Therefore, a 20-h treatment with 1 μg/ml LPS was adopted in further studies.
Actinomycin D (2 μg/ml), an RNA polymerase II inhibitor that binds DNA at the transcription initiation complex and blocks RNA elongation (22), totally eliminated the LPS-induced Kir6.1 and SUR2B expression (Fig. 4E). These suggest that transcriptional mechanisms are required for the LPS effects.
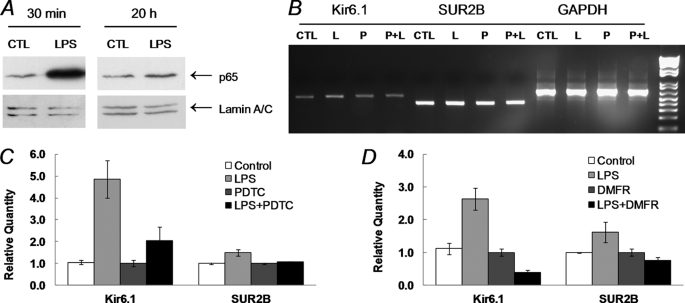
Necessity of NF-κB
Because nuclear factor κB (NF-κB) is a critical player in the Toll-like receptor 4 (TLR4) signaling pathways activated by LPS, it is possible that LPS enhances the KATP channel expression via the NF-κB pathway. Indeed, we found that p65, a subunit of NF-κB, displayed a strong nuclear accumulation after a 30-min LPS treatment. The nuclear accumulation returned to nearly the basal level after 20 h (Fig. 5A). The NF-κB pathway was further studied using NF-κB inhibitors. We tested pyrrolidine dithiocarbamate (PDTC, 100 μm) that prevents phosphorylation of IκB (23), and dimethyl fumarate (DMFR, 100 μm), a NF-κB inhibitor that blocked the nuclear entry of p65 after its release from IκB (24). One of the NF-κB inhibitors was added to aortic tissues 1 h before LPS administration. LPS stimulated Kir6.1 mRNA expression by 3.9-fold, and SUR2B by 1.5-fold in the control group (Fig. 5, B and C). After overnight incubation with PDTC, the LPS-induced Kir6.1 and SUR2B expression was strongly suppressed (Fig. 5, B and C). Similarly, LPS-elevated Kir6.1 and SUR2B mRNA expression were also inhibited by DMFR (Fig. 5D). These data indicated that the NF-κB signaling is necessary for the KATP channel up-regulation.
FIGURE 5.
Role of NF-κB in the LPS-induced KATP channel expression. A, A10 aortic smooth muscle cell line was cultured in the presence or absence of LPS (1 μg/ml). Nuclear extracts were obtained from A10 cells, separated in 10% SDS-PAGE, and then transferred to polyvinylidene difluoride membrane. Western blot analysis was performed using anti-p65 antibody with Lamin A/C as the loading control. The p65 was accumulated in nucleus after 30-min LPS stimulation. The p65 nuclear accumulation returned to basal level with 20 h of treatment. B, RT-PCR evidence for the suppression of LPS effects by the NF-κB inhibitor PDTC. Total RNAs extracted from dissociated mouse aortic SMCs were subject to RT-PCR after a 20-h incubation with or without LPS. PDTC was applied 1 h before LPS treatment. The up-regulation of Kir6.1 and SUR2B following an exposure to 1 μg/ml LPS (L) was both blocked by PDTC (P, 0.1 mm). C and D, qPCR analysis of the effect LPS (1 μg/ml, 20 h) in the presence of PDTC (0.1 mm, C) and another NF-κB inhibitor DMFR (0.1 mm, D). Both were applied to the tissue 1 h before LPS administration. The expression of Kir6.1 and SUR2B in the LPS-treated groups was strongly suppressed with either NF-κB inhibitor. The data were collected from 3–4 independent experiments with three samples in each.
KATP Channels Derived from Expression Vectors
If transcriptional regulation is critical, LPS may have little effect on the KATP channels derived from expression vectors. To address this issue, we performed experiments in HEK293 cells transfected transiently with Kir6.1/SUR2B. The HEK293 cell line was chosen for several reasons: 1) The expression of Kir6.1 and SUR2B from plasmids is most likely independent of transcriptional regulations; 2) we have previously shown that post-translational regulations of the Kir6.1/SUR2B channel such as Kir6.1 and SUR2B phosphorylations can take place in HEK293 cells (9, 11); and 3) the intracellular signaling pathway for LPS is intact in cells, although membrane expression of TLR4, MD2, and CD14 is absent (25). Thereby, TLR4, MD2, and CD14 were also cotransfected to cells. Under this condition, the basal current density of LPS-treated cells did not increase at all in comparison to the vehicle-treated cells (9.8 ± 2.7 pA/pF, n = 13, versus 12.8 ± 3.3 pA/pF, n = 15, respectively, p > 0.05). The pinacidil-induced currents were not different between LPS- and vehicle-treated cells either (104.6 ± 29.0 pA/pF, n = 13, versus 138.7 ± 35.8 pA/pF, n = 15, respectively, p > 0.05) (Fig. 6, A–C). These were not due to a failure of activation of intracellular signaling systems by LPS, as our results indicated that LPS affected HEK cells, resulting in a clear nuclear accumulation of p65 subunit after a 30-min treatment (Fig. 6D). The different responses between native SMCs and HEK293 cells to LPS are thus consistent with the transcriptional regulation of KATP channel expression in endotoxemia.
FIGURE 6.
LPS failed to change Kir6.1/SUR2B channel activity in a heterologous expression system. A, Kir6.1/SUR2B were co-expressed with TLR4/MD2/CD14 in HEK293 cells, and whole cell currents were studied as shown in Fig. 2. The current amplitude increased markedly in response to pinacidil (10 μm), and was inhibited by glibenclamide (Glib, 10 μm). B, in another cell treated with LPS (1 μg/ml) overnight, the currents showed similar responses to pinacidil and Glib. C, comparison of the current density between the control (n = 15) and LPS-treated cells (n = 13). Both basal current density and pinacidil-induced current density were not significant changed after overnight LPS incubation (1 μg/ml, p > 0.05). D, stimulation of NF-κB signaling with LPS exposure in HEK293 cells. HEK293 cells were transfected with human TLR4/MD2/CD14 cDNAs. Two days after transfection, Western blot analysis was performed on the nuclear extracts from cells. The p65 accumulation was clearly seen 30 min after LPS stimulation.
Effects of Other TLR Ligands on Kir6.1 mRNA Expression
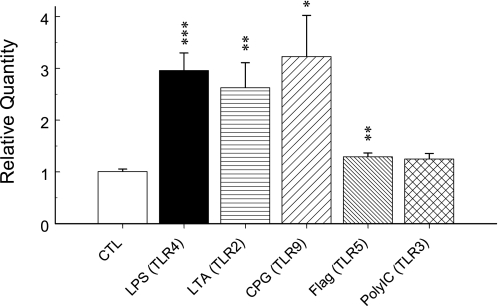
Besides LPS, other bacterial pathogens also contribute to pathogenesis of sepsis (6). LTA, a substantial immunostimulatory component of Gram-positive bacteria and a ligand of TLR2, and bacterial CpG DNA (CpG), a ligand of TLR9, displayed a stimulatory effect on Kir6.1 mRNA expression (Fig. 7). The stimulatory effect of flagellin, a ligand of TLR5 that forms the filament in bacterial flagellum, is weak but significant. Polyinosinic:polycytidylic acid (poly(I:C)), a synthetic double-stranded RNA that is a ligand of TLR3 and used to mimic viral infections, did not change Kir6.1 mRNA expression significantly.
FIGURE 7.
The effect of other TLR ligands on Kir6.1 mRNA expression. Total RNAs were extracted from dissociated SMCs from the mouse aorta after 20 h of incubation with different TLR ligands. Real-time PCR was performed to quantify Kir6.1 mRNA expression. LPS (1 μg/ml) increased Kir6.1 transcripts by ∼2.0-fold (***, p < 0.001), LTA (1 μg/ml) by ∼1.6-fold (**, p < 0.01), CpG (10 μg/ml) ∼2.2-fold (*, p < 0.05), and flagellin (1 μg/ml) ∼0.3-fold (**, p < 0.01), whereas poly(I:C) (25 μg/ml) did not show an obvious effect on Kir6.1 expression (∼0.3-fold, p > 0.05). Data were obtained from two independent experiments with 3–4 samples in each.
DISCUSSION
The outcome of sepsis is determined by not only pathogens but also cardiovascular response (1). Indeed, the major cause of death in sepsis is hypotension and hypoperfusion of several vital organs. Accounting for these are excessive vasodilation and hyporeactivity to vasoconstrictors, in which the vascular KATP channel has been recently shown to play a critical role (5). The vascular KATP channels regulate resting membrane potentials, controlling voltage-gated Ca2+ channels, cytosolic Ca2+ levels, and the excitation-contraction coupling in smooth muscles (26).
A previous study shows that administration of glibenclamide can lead to recovery of blood pressure in dogs with endotoxemia (27). In contrast, glibenclamide does not show any effect in control group, suggesting channel activity is enhanced in the LPS-treated animals. Another study indicates that the KATP channel pore blocker PNU-37883A rather than SUR subunit inhibitor glibenclamide or tolbutamide attenuates the LPS-induced vascular hyporeactivity to PE, which may be due to the functional change of SUR subunit during exposure of LPS, leading to insensitivity to sulfonylurea (28). In our current study, a significant hyperpolarization is revealed in LPS-treated aortic SMCs. Consistent with these findings, another group finds that mesenteric SMCs dissociated from the LPS-injected rats display hyperpolarization that can be blocked by glibenclamide (29). Direct evidence for the LPS effect on the KATP currents has been shown in our whole cell patch clamp, in which KATP currents in SMCs treated with LPS were significantly increased over the control group.
The augmentation of whole cell KATP currents is due to an increase in surface expression as the channel density increases significantly. It has been reported that rat diaphragm with a 24–48 h LPS treatment showed elevated Kir6.1 mRNA by 3-fold and Kir6.1 protein by 8-fold (30). In experimental colitis, the Kir6.1 mRNA expression in colonic smooth muscle is enhanced markedly, whereas SUR2B mRNA decreases slightly (31). The increase in the KATP expression, especially Kir6.1 subunit, is believed to contribute to the dysfunction of visceral smooth muscle contraction during inflammation (30, 31). In vascular SMCs, we have shown, for the first time, that both Kir6.1 and SUR2B mRNA are up-regulated after LPS treatment. The effect is very likely to be due to the newly synthesized mRNAs. 1) The augmentation of KATP channel activity in endotoxemia was associated with increased Kir6.1/SUR2B mRNA levels. 2) In the presence of actinomycin, LPS failed to stimulate Kir6.1 and SUR2B mRNA expression. Indeed, the SUR2B mRNA level was even lower than that without LPS exposure, indicating that the increased Kir6.1 and SUR2B expression with LPS exposure occur at the transcriptional level. 3) In a heterologous expression system, a similar LPS exposure did not stimulate the Kir6.1/SUR2B channel activity, as the expression of these channels derived from the expression vector via the CMV promoter is not subject to transcriptional regulation.
The up-regulation of the vascular KATP channel expression is not only produced by LPS. Several other TLR ligands including the Gram-positive bacterial TLR2 ligand and bacterial TLR9 ligand also display a strong stimulatory effect on Kir6.1 mRNA expression. These observations are preliminary, and further studies are still needed to understand mechanisms underlying KATP channel regulation.
Several intracellular signaling systems may be involved in the Kir6.1 and SUR2B up-regulation during endotoxemia. NF-κB is a key player. Activation of NF-κB increases the expression of the gene encoding the proinflammatory cytokine TNFα (32). Interestingly, previous studies have shown that the coronary vasodilation induced by TNFα is alleviated in Kir6.1-null mice, suggesting KATP channel is necessary for the TNFα-induced vasodilation (5). In the present study, we have examined the causality between KATP and NF-κB. Our results have shown that p65 is accumulated in the nucleus as soon as 30 min after LPS stimulation. A pretreatment with the NF-κB inhibitor PDTC or DMFR attenuates significantly the LPS-induced KATP channel expression, indicating that NF-κB signaling is necessary for the up-regulation of the vascular KATP channel during endotoxemia. It is noteworthy that both of the NF-κB inhibitors have certain nonspecific effects beyond inhibiting NF-κB, for example, PDTC could activate Akt (33), whereas DMFR also inhibits mitogen- and stress-activated protein kinase 1,2 (34). Because the nonspecific effects do not overlap with each other, the consistency of their effects on the LPS-induced KATP channel expression supports that NF-κB signaling plays a role.
As an important player in vascular tone regulation, the KATP channels are subject to multiple levels of control. At the post-translational level, it is targeted by numerous hormones and neurotransmitters by PKA and PKC phosphorylations (9, 10). At the transcriptional level, it is regulated by NF-κB-dependent intracellular signaling following the exposure of LPS. As a result of the up-regulation, the KATP channels contribute to vasodilation in resistant blood vessels leading to an altered distribution of blood supply (27, 35). Persistent low perfusion is lethal, and can cause multiple organ failure. The relaxation of coronary arterial SMCs and the increase in coronary circulation may, to a large degree, compensate the metabolic needs of myocardium and secure sufficient cardiac output in the early stage of sepsis (1, 36).
In conclusion, our results indicate the decrease in the contractility of vascular smooth muscle in endotoxemia is attributable to the increased activity of the KATP channel. Such an effect is not limited to LPS but is also shared by several other TLR ligands. The effect of LPS appears to be mediated by enhancing the expression of Kir6.1 and SUR2B in which the NF-κB signaling system plays a role. The demonstration of a molecular target of septic pathogens and the likely intracellular signaling pathway may help the design of therapeutic modalities for the control of hypotension and hyporeactivity to vasoconstrictors in septic shock.
Supplementary Material
Acknowledgments
We thank Timothy Tower for proofreading the manuscript. Kir6.1 and SUR2B cDNAs were gifts from Dr. Susumu Seino at Kobe University, Japan, and Yoshihisa Kurachi at Osaka University, Japan, respectively. Human TLR4 and CD14 cDNAs were kindly provided by Dr. Golenbock at the University of Massachusetts, Worcester, MA. Human MD2 cDNA was a gift from Dr. Sachiko Akashi-Takamura at the University of Tokyo, Japan.
This work was supported, in whole or in part, by the National Institutes of Health Grant HD060959 (to C. J.). This work was also supported by the National Natural Science Foundation of China (Grant number 30470752, to D. L. Z.) and by the American Heart Association (Grant 09GRNT2010037, to C. J.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- LPS
- lipopolysaccharide
- RT
- reverse transcription
- SMC
- smooth muscle cell
- LTA
- lipoteichoic acid
- PE
- phenylephrine
- SUR
- sulfonylurea receptor
- RQ
- relative quantitation
- HEK
- human embryonic kidney
- DMEM
- Dulbecco's modified Eagle's medium
- pF
- picofarads.
REFERENCES
- 1.Hotchkiss R. S., Karl I. E. (2003) N. Engl. J. Med. 348, 138–150 [DOI] [PubMed] [Google Scholar]
- 2.Merx M. W., Weber C. (2007) Circulation 116, 793–802 [DOI] [PubMed] [Google Scholar]
- 3.Martin G. S., Mannino D. M., Eaton S., Moss M. (2003) N. Engl. J. Med. 348, 1546–1554 [DOI] [PubMed] [Google Scholar]
- 4.Watanabe E., Zehnbauer B., Deutschman C. S. (2009) Crit. Care Med. 37, 357–360 [DOI] [PubMed] [Google Scholar]
- 5.Kane G. C., Lam C. F., O'Cochlain F., Hodgson D. M., Reyes S., Liu X. K., Miki T., Seino S., Katusic Z. S., Terzic A. (2006) FASEB J. 20, 2271–2280 [DOI] [PubMed] [Google Scholar]
- 6.Croker B., Crozat K., Berger M., Xia Y., Sovath S., Schaffer L., Eleftherianos I., Imler J. L., Beutler B. (2007) Nat. Genet. 39, 1453–1460 [DOI] [PubMed] [Google Scholar]
- 7.Bonev A. D., Nelson M. T. (1996) J. Gen. Physiol. 108, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. (1990) Nature 344, 770–773 [DOI] [PubMed] [Google Scholar]
- 9.Shi W., Cui N., Shi Y., Zhang X., Yang Y., Jiang C. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R191–R199 [DOI] [PubMed] [Google Scholar]
- 10.Shi Y., Wu Z., Cui N., Shi W., Yang Y., Zhang X., Rojas A., Ha B. T., Jiang C. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1205–R1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Shi Y., Guo S., Zhang S., Cui N., Shi W., Zhu D., Jiang C. (2008) Biochem. Biophys. Acta 1778, 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Wu J., Li L., Chen F., Wang R., Jiang C. (2003) Circ. Res. 92, 1225–1232 [DOI] [PubMed] [Google Scholar]
- 13.Kamouchi M., Kitamura K. (1994) Am. J. Physiol. Heart Circ. Physiol. 266, H1687–H1698 [Google Scholar]
- 14.Ye D., Zhou W., Lee H. C. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H358–H364 [DOI] [PubMed] [Google Scholar]
- 15.Tang G., Wu L., Liang W., Wang R. (2005) Mol. Pharmacol. 68, 1757–1764 [DOI] [PubMed] [Google Scholar]
- 16.Chutkow W. A., Pu J., Wheeler M. T., Wada T., Makielski J. C., Burant C. F., McNally E. M. (2002) J. Clin. Invest. 110, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miki T., Suzuki M., Shibasaki T., Uemura H., Sato T., Yamaguchi K., Koseki H., Iwanaga T., Nakaya H., Seino S. (2002) Nat. Med. 8, 466–472 [DOI] [PubMed] [Google Scholar]
- 18.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. (1989) J. Infect. Dis. 159, 195–204 [DOI] [PubMed] [Google Scholar]
- 20.Scheifele D. W., Olsen E. M., Pendray M. R. (1985) Am. J. Clin. Pathol. 83, 227–229 [DOI] [PubMed] [Google Scholar]
- 21.Gong P., Angelini D. J., Yang S., Xia G., Cross A. S., Mann D., Bannerman D. D., Vogel S. N., Goldblum S. E. (2008) J. Biol. Chem. 283, 13437–13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobell H. M. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 5328–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong H., SuYang H., Erdjument-Bromage H., Tempst P., Ghosh S. (1997) Cell 89, 413–424 [DOI] [PubMed] [Google Scholar]
- 24.Loewe R., Holnthoner W., Gröger M., Pillinger M., Gruber F., Mechtcheriakova D., Hofer E., Wolff K., Petzelbauer P. (2002) J. Immunol. 168, 4781–4787 [DOI] [PubMed] [Google Scholar]
- 25.Divanovic S., Trompette A., Atabani S. F., Madan R., Golenbock D. T., Visintin A., Finberg R. W., Tarakhovsky A., Vogel S. N., Belkaid Y., Kurt-Jones E. A., Karp C. L. (2005) Nat. Immunol. 6, 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quayle J. M., Nelson M. T., Standen N. B. (1997) Physiol. Rev. 77, 1165–1232 [DOI] [PubMed] [Google Scholar]
- 27.Landry D. W., Oliver J. A. (1992) J. Clin. Invest. 89, 2071–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien A. J., Thakur G., Buckley J. F., Singer M., Clapp L. H. (2005) Br. J. Pharmacol. 144, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C. C., Chen S. J., Garland C. J. (2004) Br. J. Pharmacol. 142, 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czaika G., Gingras Y., Zhu E., Comtois A. S. (2000) Muscle Nerve 23, 967–969 [DOI] [PubMed] [Google Scholar]
- 31.Jin X., Malykhina A. P., Lupu F., Akbarali H. I. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287, G274–G285 [DOI] [PubMed] [Google Scholar]
- 32.Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. (1986) Science 234, 470–474 [DOI] [PubMed] [Google Scholar]
- 33.Malm T. M., Iivonen H., Goldsteins G., Keksa-Goldsteine V., Ahtoniemi T., Kanninen K., Salminen A., Auriola S., Van Groen T., Tanila H., Koistinaho J. (2007) J. Neurosci. 27, 3712–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesser B., Johansen C., Rasmussen M. K., Funding A. T., Otkjaer K., Kjellerup R. B., Kragballe K., Iversen L. (2007) J. Invest. Dermatol. 127, 2129–2137 [DOI] [PubMed] [Google Scholar]
- 35.Gardiner S. M., Kemp P. A., March J. E., Bennett T. (1999) Br. J. Pharmacol. 128, 1772–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon M. A., Correa R., Alexander H. R., Koev L. A., Cobb J. P., Kim D. K., Roberts W. C., Quezado Z. M., Scholz T. D., Cunnion R. E. (1994) Am. J. Physiol. 266, H757–H768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.