Abstract
Previous studies have demonstrated that morphine, administered systemically or directly into the PAG, produces a significantly greater degree of antinociception in males in comparison to females. As the midbrain periaqueductal gray (PAG) and its descending projections to the rostral ventromedial medulla (RVM) constitute an essential neural circuit for opioid-based analgesia, the present studies were conducted to determine if sex differences in the anatomical organization of the PAG-RVM pathway, and its activation during persistent inflammatory pain, could account for sex-based differences in opioid analgesia. In the rat, retrograde tracing was combined with Fos immunocytochemistry to investigate sexual dimorphism in the organization of the PAG-RVM circuit and its activation by persistent inflammatory pain induced by intraplantar injection of complete Freund's adjuvant (CFA). The ability of morphine to suppress the activation of the PAG-RVM circuit was also examined. Sexually dimorphic retrograde labeling was observed within the dorsomedial and lateral/ventrolateral PAG at all rostrocaudal levels, with females having significantly more PAG-RVM output neurons in comparison to males. While no sex differences were noted in the activation of the PAG-RVM circuit by persistent inflammatory pain, significantly more double labeled cells were found in males in comparison to females. Systemic administration of morphine significantly suppressed CFA-induced Fos in the PAG in males only. The results of these studies demonstrate that both the anatomical organization, and functional activation, of the PAG-RVM circuit is sexually dimorphic, and may provide the anatomical substrate for sex-based differences in morphine analgesia.
Keywords: pain, antinociception, endogenous descending pathway, morphine analgesia
Introduction
It is becoming increasingly clear that morphine produces a differential degree of analgesia in males and females. In studies using either acute (Kepler et al., 1989; Islam et al., 1993; Cicero et al., 1996; Cicero et al., 1997) or persistent pain models (Cook and Nickerson, 2005; Wang and Murphy, 2005), morphine has been shown to produce a significantly greater degree of analgesia in males in comparison to females. Similar findings have also been reported in humans (Cepeda and Carr, 2003; Miller and Ernst, 2004). The midbrain periaqueductal gray (PAG), along with its descending projections to the rostral ventromedial medulla (RVM), is one of the primary anatomical substrates mediating opioid analgesia. Administration of mu opioid receptor agonists into the PAG produces potent analgesia that is blocked by central or systemic administration of the opioid antagonist naloxone (Satoh et al., 1983; Jensen and Yaksh, 1986; Bodnar et al., 1988). Similarly, lesions of the PAG or intra-PAG administration of mu opioid receptor (MOR) antagonists (Wilcox et al., 1979; Ma and Han, 1991; Zhang et al., 1998) attenuate the antinociceptive effects of systemic morphine. Together, these studies indicate that the PAG is an essential locus for opioid-mediated analgesia.
There are no direct projections from the PAG to the dorsal horn of the spinal cord. Rather, the PAG projects heavily onto reticulospinal neurons of the RVM (Beitz, 1982; Beitz et al., 1983; van Bockstaele et al., 1991; Cameron et al., 1995b), and this PAG-RVM-spinal cord circuit is essential for the analgesic actions of systemic or intra-PAG morphine. Lesions of the RVM or dorsolateral funiculus (DLF) completely abolish PAG opiate-produced analgesia (Proudfit and Anderson, 1975; Basbaum et al., 1977; Chance et al., 1978; Mohrland and Gebhart, 1980; Proudfit, 1980; Guo and Tang, 1990). In fact, lesions of the DLF attenuate all analgesic manipulations tested, including systemic, intraventricular and intrathecally administered morphine, indicating that the PAG-RVM-spinal cord pathway is the critical neural circuit for opioid-induced analgesia.
The anatomical and physiological organization of the PAG-RVM-spinal cord circuit has been well characterized in a variety of species, including the rat (Basbaum and Fields, 1978; Behbehani and Fields, 1979; Beitz, 1982; Jones and Light, 1992; Williams et al., 1995), cat (Basbaum et al., 1978; Shah and Dostrovsky, 1980; Abols and Basbaum, 1981; Sandkuhler et al., 1987a; Sandkuhler et al., 1987b; Holstege and Cowie, 1989), primate (Mantyh, 1983; Gerhart et al., 1984), and rabbit (Haselton et al., 1988). However to date, all of these studies have been conducted exclusively in male animals. Therefore, it is not known if the PAG-RVM-spinal cord circuit is organized anatomically in a similar manner in females. Sex differences in the organization of this circuit may provide the anatomical substrate for the observed sex differences in opioid analgesia.
The present studies were conducted to determine if the PAG-RVM circuit is organized differentially in males and females. Additional behavioral studies were performed to examine the activation of this circuit by persistent inflammatory pain. The results of these studies demonstrate for the first time that the PAG-RVM circuit is sexually dimorphic in both its anatomical organization and in its functional activation during persistent inflammatory pain. Collectively, these data suggest that the PAG, and its descending projections to the RVM, may provide the anatomical substrate for the observed sex differences in morphine analgesia. Portions of this paper have been previously published in abstract form (Loyd and Murphy, 2004b).
Methods
Subjects
Fifteen adult intact male Sprague-Dawley rats and fifteen adult cycling female Sprague-Dawley rats (Zivic-Miller) were used in these experiments. Animals were weight-matched (250-300g) and housed in same-sex pairs in separate rooms on a 12:12 hour light: dark cycle (lights on at 7:00A.M.). Access to food and water was ad libitum throughout the experiment except during surgery. These studies were performed in strict compliance with the Institutional Animal Care and Use Committee at Georgia State University. All efforts were made to minimize any possible suffering by the animal, and to reduce the number of animals used.
Iontophoresis Injections
Twelve male and twelve female rats were anesthetized with ketamine/xylazine (50 mg/kg / 10mg/kg; s.c.) and placed in a stereotaxic frame upon achieving a deep surgical plane of anesthesia. The skull was adjusted so that bregma and lambda were at the same dorsoventral coordinate. Glass micropipettes (10-20 μM) were filled with the retrograde tracer Fluorogold (FG; 2% soln. w/v in saline; Fluorochrome Inc.) and lowered into the NRM at the following coordinates (in mm): AP: −2.0 Lambda; ML: 0.0; DV: −8.5. FG was iontophoresed (50/50 duty cycle, 7.5 μA current) for 20 min. Pipettes remained in place for five minutes after injection to prevent backflow of tracer along the pipette tract and to facilitate neuronal uptake. Special care was taken to ensure that all injection protocols were comparable for males and females. Following surgery the animals were allowed to recover under a heat lamp in clean cages, and returned to their original housing facilities upon recovery from the anesthetic.
Inflammatory Hyperalgesia
Ten days following surgery, persistent inflammatory pain was induced by injection of complete Freund's adjuvant (CFA; Mycobacterium tuberculosis; Sigma; 200 μl), suspended in an oil/saline (1:1) emulsion, into the plantar surface of the right hindpaw. Animals were under brief Isoflo (isofluorane, USP) anesthesia during the plantar injections. We have previously used this procedure to induce persistent inflammatory pain and reported no sex differences in the degree of edema produced by intraplantar CFA, nor in the ensuing hyperalgesia, assayed using the paw thermal stimulator (Wang and Murphy, 2005).
Morphine Analgesia
Twenty-four hours following the induction of inflammation, all FG-injected animals were given a subcutaneous injection of either morphine sulfate (4.5 mg/kg, s.c.; NIDA) or the drug vehicle as a control. Morphine sulfate was prepared fresh in a saline vehicle on the day of the experiment.
Perfusion fixation
One hour after morphine administration, animals were given a euthanizing dose of Nembutal (160 mg/kg; i.p.). Upon achieving a deep surgical plane, the animals were perfused transcardially (descending aorta clamped) with 100-200 ml of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator, followed immediately by 250 ml of 4% paraformaldehyde in 0.1M phosphate buffer containing 2.5% acrolein (Polyscience). Following fixation, a final rinse with the sodium chloride/sodium nitrate solution was used to remove any residual acrolein from the animal. Immediately following perfusion, the brains were removed and a mark made along the base of the brain to demarcate left from right side. The tissue was stored at 4°C in 30% sucrose solution until sectioned. The dura and pia matter were removed and the brains were cut into 25μm coronal sections with a Leica 2000R freezing microtome and stored free-floating in cryoprotectant-antifreeze solution (Watson et al., 1986) at –20°C until immunocytochemical processing was initiated.
Immunocytochemistry
A 1:6 series through the rostrocaudal axis of each brain was processed for FG and/or Fos immunoreactivity as previously described (Murphy and Hoffman, 2001). Briefly, sections were removed from the cryoprotectant-antifreeze solution, rinsed extensively in potassium phosphate-buffered saline, and then reacted for 20 minutes in 1% sodium borohydride to remove excess aldehydes. Sections were then incubated in primary antibody solution directed against either Fos or FG in KPBS containing 0.1% Triton X for 1 hour at room temperature followed by 48 hours at 4°C. Cells containing Fos were identified using the polyclonal rabbit anti-Fos antibody (Oncogene, Cat. No. PC38; lot no. 4194) at a concentration of 1:50,000. This rabbit antiserum was prepared against the synthetic peptide (SGFNADYEASSSRC) corresponding to amino acids 4-17 of human c-Fos. In western blots, this antibody recognizes the ~55 kDa c-Fos and ~62 kDa v-Fos proteins, and does not cross-react with the ~39 kDa Jun protein (manufacturer's technical information). FG containing cells were recognized using the polyclonal rabbit anti-Fluorogold antibody (Chemicon, Cat. No. AB153; lot no. 25060005) at a concentration of 1:10,000. This antibody was raised against the chemical compound hydroxystildamidine. No cytoplasmic FG staining was present in animals in which the tracer failed to be ejected from the electrode. In addition, the retrograde labeling pattern in the PAG noted with this antibody is identical to published reports using alternative retrograde tracers (Rizvi et al., 1996; Beitz et al., 1983).
After rinsing in KPBS, the tissue was incubated for 1 hour in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, 1:200), rinsed in KPBS, followed by a 1 hour incubation in avidin-biotin peroxidase complex (1:10; ABC Elite Kit, Vector Labs). After rinsing in KPBS and sodium acetate (0.175 M; pH 6.5), Fos was always visualized as a black reaction product using nickel sulfate intensified 3,3’-diaminobenzidine solution containing 0.08% hydrogen peroxide in sodium acetate buffer. FG was always visualized as a brown reaction product using 3,3’-diaminobenzidine containing 0.08% hydrogen peroxide in Tris buffer (pH 7.2). The reaction product was terminated after 7-25 minutes by rinsing in sodium acetate buffer.
For dual labeling, sections were first processed for Fos immunocytochemistry as described above. Following visualization of Fos, sections were rinsed several times in KPBS and then incubated for 1 hour at room temperature in primary antibody directed against FG followed by 48 hours at 4°C. Secondary and ABC reactions are as stated above. FG was visualized as a brown reaction product using 3,3’-diaminobenzidine containing 0.08% hydrogen peroxide in Tris buffer (pH 7.2) for a brown cytoplasmic reaction product. Sections were mounted out of saline onto gelatin-subbed slides, air-dried overnight, dehydrated in a series of graded alcohols, cleared in Histoclear, and cover-slipped using Histomount.
Data Analysis and Presentation
Previous anatomical studies have demonstrated that the PAG is not a homogenous structure, but rather has distinct afferents, efferents and neurochemical composition that is dependent on the rostrocaudal level (van Bockstaele et al., 1991; Bandler and Shipley, 1994). Therefore, the number of FG+, Fos+, or Fos+FG neurons was determined for six rostrocaudal levels of PAG (Bregma −6.72, −7.04, −7.74, −8.00, −8.30, −8.80). Fos counts were made ipsilateral to the CFA injected paw. In our preliminary studies (Loyd and Murphy, 2004), no differences were noted in the number of FG+ cells for the left versus right side of PAG; similarly, no laterality was observed in CFA induced Fos expression in the PAG. Therefore, FG, Fos and Fos+FG labeled cells were only counted for the side ipsilateral to the CFA injected paw.
Results for each rostrocaudal level of the PAG are expressed as the mean ± standard error of the mean (SEM). Only animals with comparable injection sites limited to the RVM were used for analysis. Neurons were counted in two PAG subdivisions: dorsomedial and lateral/ventrolateral; these subdivisions are clearly distinguishable based on the distribution of retrograde labeling from the RVM (van Bockstaele et al., 1991; Bandler and Shipley, 1994). Sections (25 μm thick) were immunocytochemically processed in a 1:6 series; therefore, each section analyzed is separated by 125 μm. Cell counts were adjusted using the protocol outlined by (Guillery, 2002). PAG somal sizes range between 11-40 μm (Lovick and Stezhka, 1999), with a mean somal length of 14.8 μm (Beitz, 1985; Beitz and Shepard, 1985). As the different classes of cells examined (Fos+/FG−, Fos−/FG+, Fos+/FG+) may differ in cell size, only cells with a distinct nucleus were quantified. Nuclear diameters were determined for each cell population, and each cell population was corrected for its own mean nuclear diameter.
Analysis of variance (ANOVA) was used to test for significant main effects of sex (male, female), PAG subdivision (dorsomedial, lateral/ventrolateral), PAG level (Bregma –6.72 through −8.80); treatment (morphine, saline) was included as a fourth factor where relevant. P≤0.05 was considered significant for all analyses. To determine the PAG area, an image of the section was captured with a 4x objective on a Nikon Eclipse E800 microscope using a Sensys digital camera (Biovisions Technology, Exton, PA). With IP Spectrum software (Scanalytics, Fairfax, VA), the PAG area (in mm2) was determined.
For data presentation, a representative animal from each experimental group was selected and the distribution of FG and Fos within the PAG were plotted using a Nikon Drawing Tube attached to a Nikon Optiphot microscope. Plots were imported to the computer and finalized using Adobe Illustrator 10. Photomicrographs were generated using a Synsys digital camera attached to a Nikon Eclipse E800 microscope. Images were captured with IP Spectrum software and finalized using Adobe Photoshop 7.0. Alterations to the images were strictly limited to enhancement of brightness/contrast.
RESULTS
Sexually Dimorphic Anatomical Organization of the PAG-RVM Circuit
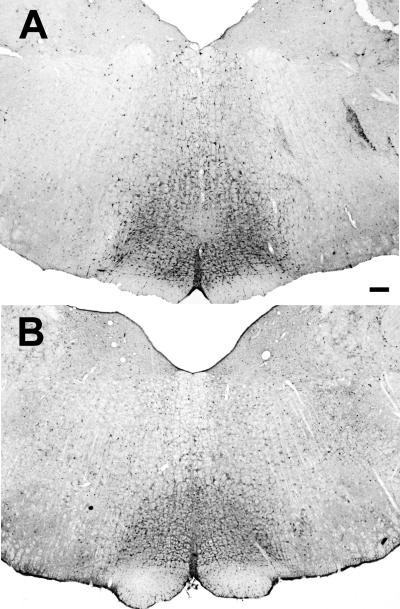
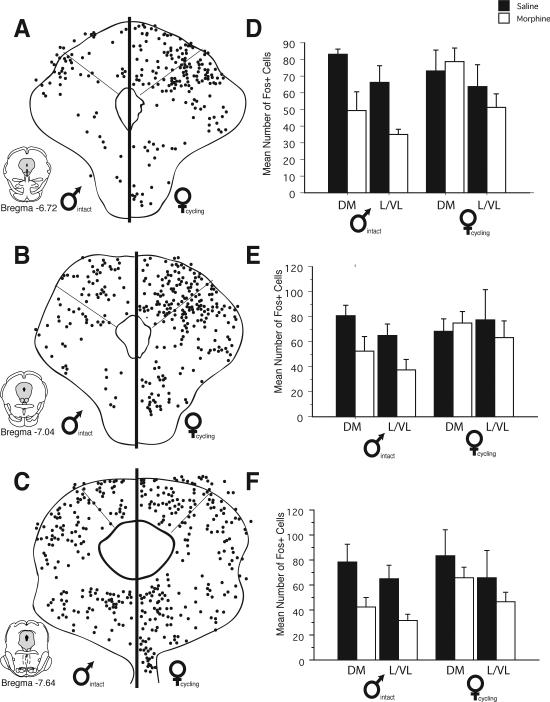
Figure 1 shows an example of a typical iontophoretic injection of the retrograde tracer Fluorogold (FG) into the RVM of a male (top; n=12) and female (bottom; n=12) rat. All injections were located on the midline and dorsal to the pyramidal tract, just caudal to the level of the facial nucleus (Lambda –2.0). Injections outside of the RVM were not included for analysis. Injection of FG into the RVM produced dense retrograde labeling throughout the rostral-caudal axis of the PAG in both males and females (Figure 2). Within the rostral half of PAG (Bregma –6.72 - –7.64), retrogradely labeled cells were concentrated within the dorsomedial and lateral regions of PAG. A few scattered cells were present within the ventrolateral PAG. More caudally (Bregma –8.0 - –8.30), retrogradely labeled cells were most prominent within the lateral and ventrolateral regions of PAG. At the caudal pole of PAG (Bregma –8.80), retrograde labeling was primarily localized within the dorsal half of PAG, with a few scattered cells present in the ventrolateral region.
Figure1.
Representative examples of FG injection into the RVM of a male (top) and female (bottom) rat. Scale bar: 200μm.
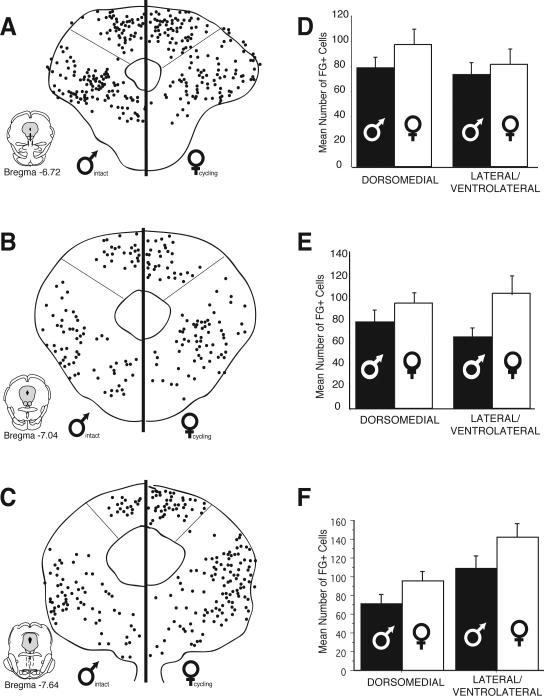
Figure 2.
Distribution of cells retrogradely labeled from the rostral ventromedial medulla in males and females at six rostrocaudal levels of the periaqueductal gray. Each black circle represents one FG-immunoreactive cell. Bar graphs show the mean number (± S.E.M.) of FG-immunoreactive cells for the dorsomedial and lateral/ventrolateral regions of PAG.
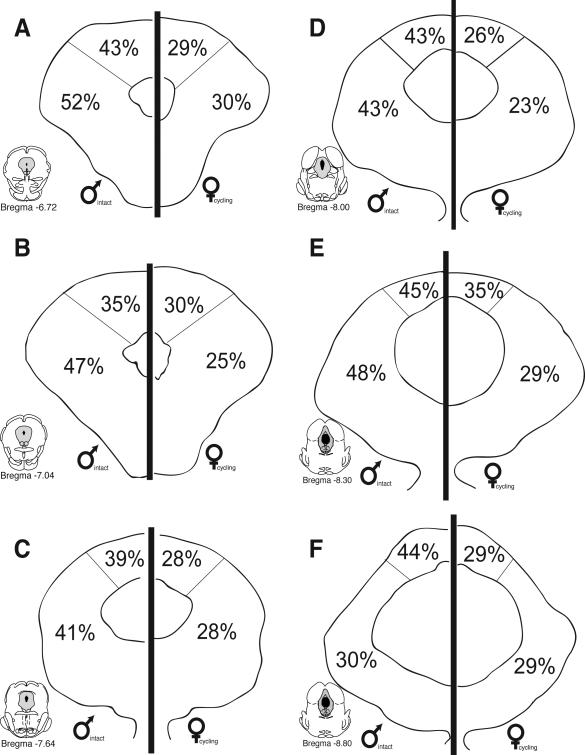
Although there were no differences in the overall distribution pattern of retrogradely labeled cells for males and females, sex differences in the actual number of FG positive cells were evident at all rostro-caudal levels of PAG. For example, at mid-levels of PAG (Bregma –7.64), the mean number of FG+ cells in the lateral/ventrolateral region was 109 ± 13 for males, in comparison to 142 ± 15 for females. More caudally (Bregma –8.30), the mean number of retrogradely labeled cells within the lateral/ventrolateral PAG was 32% higher in females (175 ± 19) in comparison to males (133 ± 14). An ANOVA on the number of FG labeled cells revealed significant main effects for sex (F(1,248)=26.10, p<0.01), PAG subdivisions (F(1, 248)=27.40, p<0.01) and a significant subdivisions by rostrocaudal level interaction (F(5,248)=12.10, p<0.01). A significant interaction between level and subdivisions was expected based on previous studies demonstrating shifts in the regional distribution of PAG-RVM output neurons that are dependent on the rostro-caudal level of PAG (Bandler and Shipley, 1994).
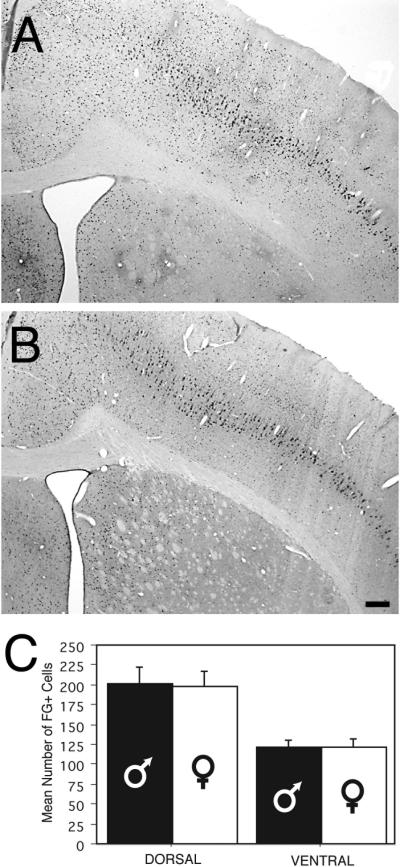
The observed sex differences in the number of PAG-RVM output neurons was not due to sex differences in FG transport rates; the number of FG+ cells within layer 5 of the motor/somatosensory cortex was comparable for males and females ((F(1,8) = .009, p>0.05); Figure 3). Sex differences in retrograde labeling are not due to differences in the area of the PAG for males and females; no significant differences were noted in the mean area (mm2) for males in comparison to females (F(1, 48)=1.70, p>0.05).
Figure 3.
Photomicrographs of cortical retrograde labeling in the male (A) and female (B) rat (Bregma –1.0). Scale bar: 200μm. Bar graph (C) shows the mean number (+ S.E.M.) of retrogradely labeled cells in the cortex of males and females. Labeling was localized to pyramidal cells in layer 5. Dorsal counts include primarily motor cortex, while ventral counts are primarily somatosensory cortex.
Sexually Dimorphic Activation of the PAG-RVM Circuit
The results from the above studies demonstrate that the PAG-RVM circuit is sexually dimorphic in its anatomical organization, with females having significantly more PAG-RVM output neurons than males. The next series of experiments were conducted to determine if the activation of the PAG-RVM circuit by persistent inflammatory pain was comparable for males and females. Intraplantar injection of CFA induced extensive Fos expression throughout the rostral-caudal axis of PAG (Figure 4). Fos+ cells were distributed throughout all regions of the PAG with no sex differences noted in the distribution; similarly, an ANOVA revealed no significant main effect of sex in the number of Fos+ cells induced by intraplantar CFA (F(1,107)=0.02, p>0.05).
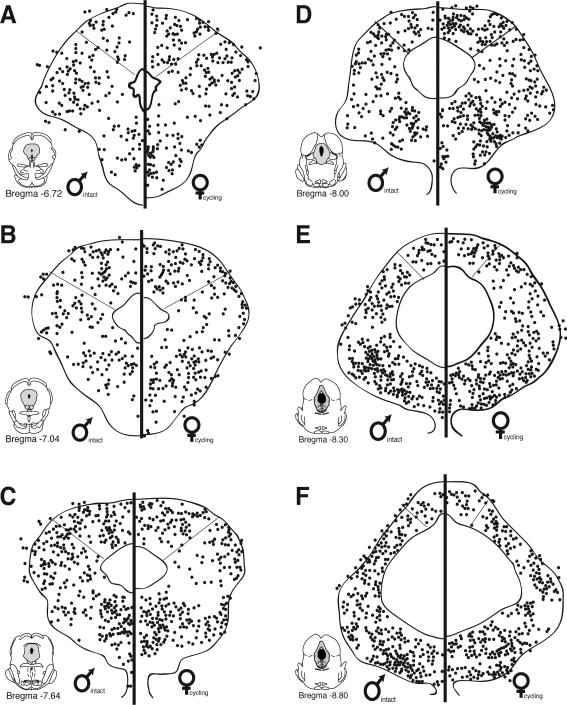
Figure 4.
Distribution of CFA induced Fos within the periaqueductal gray of males and females across six rostrocaudal levels. Each black circle represents one Fos+ cell. No sex differences were noted in either the distribution or number of Fos+ cells for males and females.
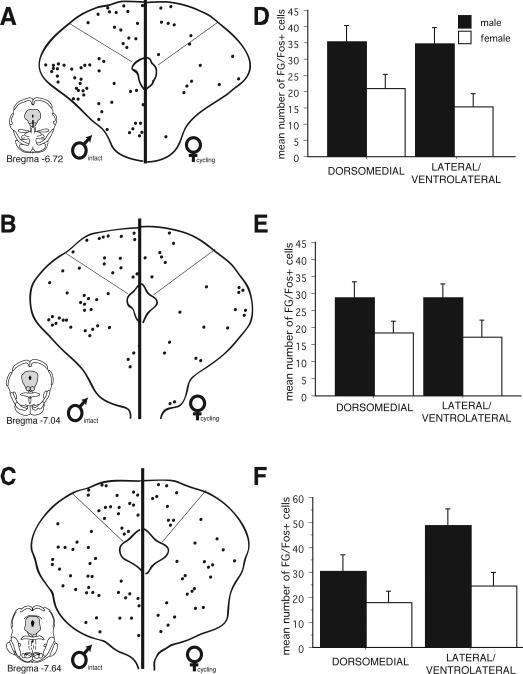
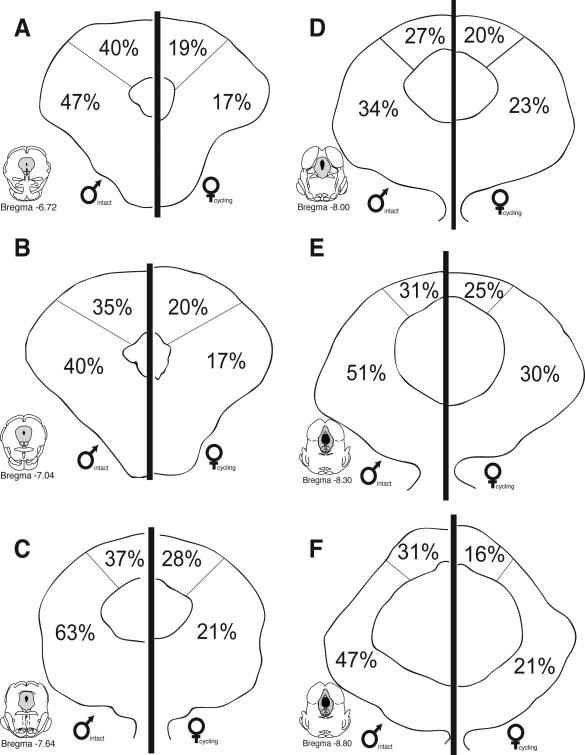
While no significant differences were noted in the overall number of Fos+ cells for males versus females, the number of PAG-RVM output neurons that expressed CFA-induced Fos was significantly greater in males in comparison to females. Figure 5 shows the distribution of PAG cells retrogradely labeled from the RVM that co-expressed CFA-induced Fos and an example of CFA-induced Fos and retrograde labeling from the RVM in the male is presented in Figure 6. At all rostrocaudal levels of PAG, and within all regions analyzed, dual labeled cells were more prevalent in males in comparison to females. An ANOVA on the number of dual labeled neurons revealed significant main effects for sex (F(1,108)=39.30, p<0.01), PAG subdivisions (F(1,108)=23.50, p<0.01) and level (F(5,108)=2.20, p<0.05). In addition, a significant levels by subdivision interaction was noted (F(5,108)=6.50, p<0.01); no other interactions reached significance (p>0.05).While FG/Fos+ cells were distributed within the dorsomedial and lateral/ventrolateral regions of PAG, sex differences in the activation of the PAG-RVM circuit were most evident within the lateral/ventrolateral PAG, where in males, approximately 50% of all PAG-RVM output neurons expressed Fos, in comparison to approximately 20% in females (Figure 7). An indication of the degree of variability in the percentile data is shown in Figure 10.
Figure 5.
Distribution of PAG-RVM output neurons that expressed CFA-induced Fos for males and females across six rostrocaudal levels of the PAG. Each black circle represents one double-labeled (FG+Fos) cell. Bar graphs display the mean number of FG/Fos cells (± S.E.M.) for each level and region.
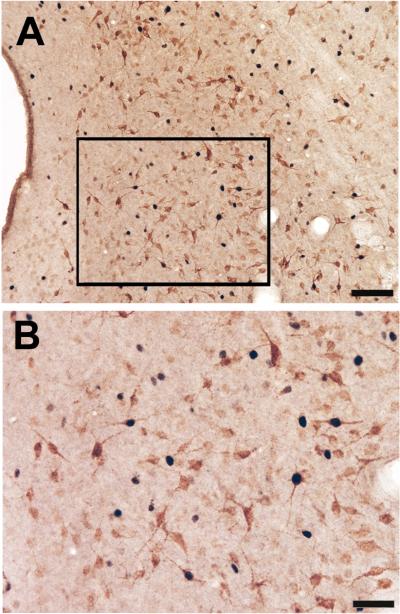
Figure 6.
Color photomicrograph showing a low (A) and high (B) power example of single and double labeled Fos and FG labeled cells in the PAG of a male rat. Scale bar = 100 μm in panel A; scale bar = 50 μm in panel B.
Figure 7.
Percentage of PAG-RVM neurons that were activated by persistent inflammatory pain (FG+Fos) in males (left) and females (right) throughout the rostrocaudal axis of PAG.
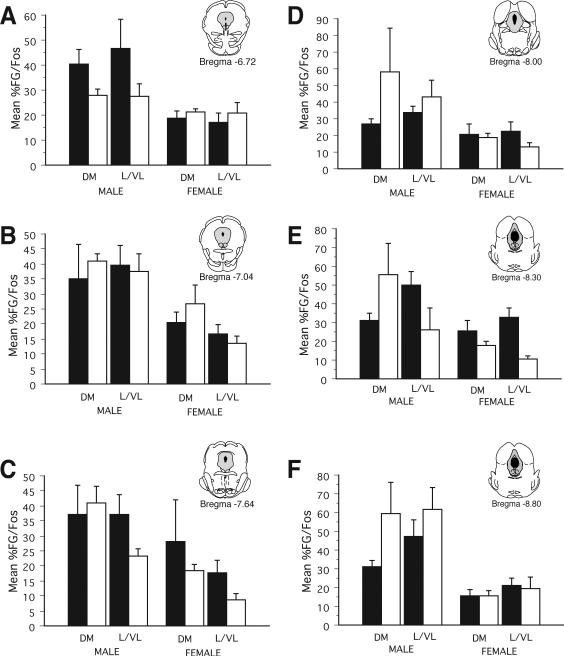
Figure 10.
Percentage of PAG-RVM neurons expressing CFA-induced Fos (%FG/Fos) following saline (black bars) or morphine (4.5 mg/kg; s.c.; white bars) in male (left) and female (right) rats for six rostrocaudal levels of PAG.
As females had significantly more FG+ cells in comparison to males (see Figure 2), we also determined the percentage of CFA-induced Fos+ cells that were present in PAG-RVM cells. As no differences were noted in the number or distribution of Fos in males versus females (see Figure 4), this would provide an indication of the percentage of PAG cells activated by persistent inflammatory pain that were localized in PAG-RVM output neurons. As shown in Figure 8, CFA-induced Fos preferentially engaged a higher percentage of PAG-RVM output neurons in males in comparison to females. An indication of the degree of variability in the percentile data is shown in Figure 11. Similar to what was noted in Figure 5, this held true for all rostrocaudal levels of PAG and for each region examined. Together, these data indicate that while females have significantly more PAG-RVM output neurons, this circuit is preferentially engaged in males in comparison to females during persistent pain states.
Figure 8.
Percentage of Fos+ PAG neurons that were retrogradely labeled from the RVM (Fos+FG) in males (left) and females (right) throughout the rostrocaudal axis of PAG.
Figure 11.
Percentage of CFA-induced Fos positive neurons that were retrogradely labeled from the RVM (%Fos/FG) following saline (black bars) or morphine (4.5 mg/kg; s.c.; white bars) in male (left) and female (right) rats for six rostrocaudal levels of PAG.
Morphine differentially suppresses persistent pain-induced activation of PAG-RVM neurons
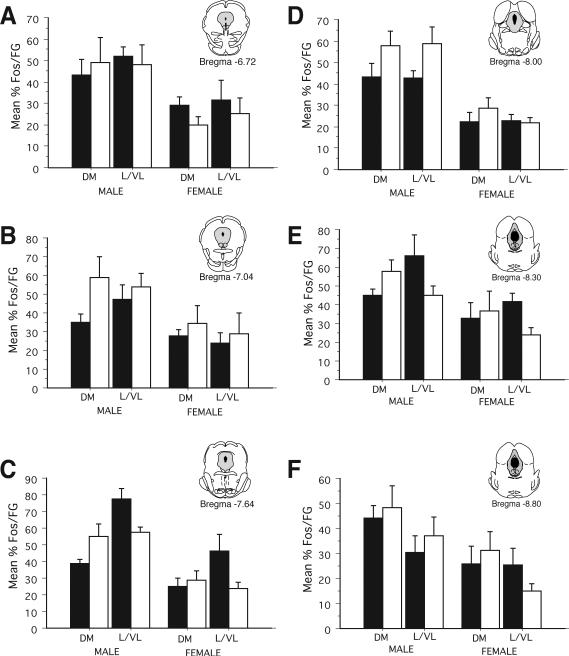
The above studies demonstrate that while persistent inflammatory pain activates a comparable number of PAG neurons in males and females, PAG-RVM output neurons were activated to a greater degree in males in comparison to females. The next series of experiments examined the ability of morphine to suppress CFA-induced Fos in males versus females. Systemic morphine administration (4.5 mg/kg, s.c.) attenuated CFA-induced Fos at all rostrocaudal levels of PAG in males (Figure 9); by contrast, comparable numbers of CFA-induced Fos+ cells were noted for saline versus morphine treated females. An ANOVA revealed significant main effects of sex (F(1,225)=5.70, p<0.05), treatment (F(1, 225)=20.84, p<0.01), PAG subdivision (F(1, 225)=16.65, p<0.01, and level (F(5, 225)=5.06, p<0.01). In addition, a significant sex by treatment interaction was noted (F(1, 225)=4.72, p<0.05). Across all levels, morphine administration resulted in a 26%-46% reduction in Fos expression in males; by contrast only a 0-19% reduction in the number of Fos+ cells was noted in females.
Figure 9.
Distribution of CFA-induced Fos (black dots) following saline (black) or morphine (white) administration in males (left) and females (right) along the rostrocaudal axis of PAG. Each black circle represents one Fos+ cell. Bar graphs show mean number of Fos+ cells (± S.E.M.) for each treatment group broken down separately for the dorsomedial and lateral/ventrolateral PAG.
We next examined if morphine was preferentially acting on PAG-RVM neurons to suppress CFA-induced Fos. Figure 10 shows the percentage of PAG-RVM output neurons that expressed CFA-induced Fos following administration of either saline or morphine (4.5 mg/kg, s.c.) for males and females. An ANOVA on the number of FG+Fos labeled cells revealed significant main effects of sex (F(1,228)=60.57, p<0.01), treatment (F(1,228)=44.78, p<0.01), and PAG subdivision (F(1,228)=21.32, p<0.01), as well as a significant sex by treatment interaction (F(1,228)=8.15, p<0.01. In addition, a significant treatment by PAG subdivision by level interaction was noted (F(5,228)=2.42, p<0.05), indicating that the effect of morphine on CFA-induced Fos in PAG-RVM output neurons was variable and both level and region dependent. For example, in males, morphine administration reduced the percentage of dual labeled cells in both the dorsomedial and lateral/ventrolateral PAG within the rostral pole of PAG (−6.72). More caudally, morphine administration either had no effect or actually increased the percentage of dual labeled cells in the dorsomedial PAG in comparison to saline treated animals. Similar level and region dependent effects were noted for the lateral/ventrolateral PAG. In females, the percentage of PAG-RVM neurons that expressed CFA-induced Fos following morphine administration was comparable across levels.
As morphine significantly reduced the number of CFA-induced Fos+ neurons in males but not females, comparing the percent change in the activation of the PAG-RVM circuit (%FG that contained Fos) for morphine versus saline treated males and females is potentially biased. Therefore, we also examined the percentage of Fos+ neurons that were present in PAG-RVM neurons (%Fos in FG neurons) following morphine or saline treatment. A decrease in the %Fos present in PAG-RVM output neurons would suggest that the observed morphine-induced decreases in Fos expression (see Figure 4) were due to an inhibitory action on PAG-RVM output neurons. By contrast, if morphine was primarily acting on intrinsic PAG neurons, then the percentage of Fos present in FG+ neurons should be comparable for morphine and saline treated animals or increased in morphine treated animals, presumably due to the removal of a tonic inhibitory influence. The results are presented in Figure 11. Similar to what was noted in Figure 10, the effects of morphine on CFA-induced Fos in PAG-RVM neurons was variable across levels and PAG regions. For example, within the lateral/ventrolateral region of PAG of males, morphine increased (Bregma –8.00), decreased (Bregma –8.30) or had no effect (Bregma –8.80) on the percentage of Fos localized in PAG-RVM neurons. In females, the percentage of Fos present in PAG-RVM neurons was comparable for saline versus morphine treated animals. Together, these results suggest the influence of multiple mechanisms of morphine action on persistent pain induced Fos, or alternatively, natural variability across PAG regions and levels.
Discussion
There are now well-established sex differences in the analgesic effects of morphine; however, the CNS mechanisms underlying the sexually dimorphic actions of morphine are unknown. As the PAG, and its descending projections to the RVM, constitute an essential circuit for opioid analgesia, the present studies were conducted to determine if the PAG-RVM pathway is sexually dimorphic in its anatomical organization and in its activation during persistent inflammatory pain. The results of the present study demonstrate profound sex differences in both the anatomical and functional organization of the PAG-RVM pathway. The action of morphine on PAG neurons was also found to be sexually dimorphic. Together, these results suggest that the PAG-RVM pathway may provide the anatomical basis for observed sex differences in morphine analgesia.
Sexually Dimorphic PAG-RVM Circuit: Functional Implications
Retrograde tracer injection into the RVM produced dense retrograde labeling within the dorsomedial, lateral and ventrolateral regions of PAG; these results are consistent with previous anatomical studies characterizing PAG outputs to the medulla (van Bockstaele et al., 1991). Interestingly, while no sex differences were noted in the distribution of PAG-RVM output neurons, females had significantly more retrogradely labeled cells in comparison to males. The most prominent sex difference in retrograde labeling was observed within the lateral/ventrolateral region of the PAG, where at some rostrocaudal levels, females had almost twice the number of retrogradely labeled neurons in comparison to males. These studies are the first to demonstrate a sexually dimorphic organization of a primary endogenous pain pathway.
The lateral/ventrolateral region of PAG has been implicated in a variety of physiological functions, including the cardiovascular regulation (Bandler et al., 1991; Murphy et al., 1995), initiation of the ‘fight or flight’ response (Bandler and Carrive, 1988; Carrive et al., 1988; Zhang et al., 1990), vocalization (Larson and Kistler, 1984; Larson, 1985; Zhang et al., 1994; Jurgens, 2002; Dujardin and Jurgens, 2005) and reproductive behavior (McCarthy et al., 1991; Ogawa et al., 1991; Ogawa et al., 1992; Murphy and Hoffman, 2001). In addition, this region is a critical site for both stimulation-produced and morphine-induced analgesia (Reynolds, 1969; Behbehani and Fields, 1979; Fardin et al., 1984a; Fardin et al., 1984b; Morgan et al., 1991; Morgan et al., 1992; Zhang et al., 1998). Given that morphine produces a greater degree of analgesia in males in comparison to females (Cicero et al., 1996; Craft et al., 1996; Bartok and Craft, 1997; Cicero et al., 1997; Boyer et al., 1998; Tershner et al., 2000; Barrett et al., 2001; Cepeda and Carr, 2003; Stoffel et al., 2003; Stoffel et al., 2005; Wang and Murphy, 2005) and the PAG-RVM pathway is a critical circuit for opioid analgesia, we had initially hypothesized that if this circuit was sexually dimorphic, retrograde labeling from the RVM would be more prominent in males in comparison to females. Therefore, subsequent experiments were conducted to examine the ‘activation’ of this circuit by a persistent noxious stimulus. Intraplantar injection of the bacterium Complete Freund's adjuvant is a well-established model of persistent inflammatory pain (Millan et al., 1988; Stein et al., 1988a). Twenty-four hours following injection, edema and hyperalgesia are present in the injected paw. We have previously used this model and reported no sex differences in either the degree of edema produced by intraplantar CFA, or in the withdrawal latencies following application of a noxious thermal stimulus (Loyd et al., 2005; Wang and Murphy, 2005). Hyperalgesia in animal models of inflammatory pain is closely linked to activation of PAG-RVM descending modulatory circuits (Morgan et al., 1991; Williams et al., 1995) and involve both facilitation and inhibition of inflammatory pain (Guan et al., 2002; Miki et al., 2002; Ren and Dubner, 2002; Guan et al., 2003). In the present study, persistent inflammatory pain induced extensive Fos expression throughout the rostrocaudal axis of the PAG, indicating comparable activation of the PAG in males and females. Interestingly, while no differences were noted in either the number or distribution of Fos between males and females, persistent inflammatory pain preferentially activated the PAG-RVM circuit in males and not females. Thus, while females have almost twice the number of PAG-RVM output neurons in comparison to males, this descending modulatory circuit is not being ‘engaged’ to the same degree by persistent inflammatory pain in both sexes. As activation of the PAG and its descending outputs to the RVM inhibits dorsal horn neuronal responses to acute noxious stimuli (Carstens et al., 1979; Gray and Dostrovsky, 1983; Aimone et al., 1987; Jones and Gebhart, 1988; Waters and Lumb, 1997; Budai and Fields, 1998), the results of the present study may account for reported sex differences in nociceptive thresholds (Berkley, 1997; Fillingim, 2000; Fillingim and Ness, 2000; Barrett et al., 2002).
The adaptive significance of a sexually dimorphic PAG-RVM circuit is not clear. As mentioned above, the PAG is involved in a variety of physiological functions, and in females, this region is essential for the display of lordosis, the primary motor component of female reproductive behavior (McCarthy et al., 1991; Ogawa et al., 1991). Electrolytic lesions of the PAG, or direct administration of GABA antagonists, significantly attenuate lordosis, while stimulation of this region facilitates it (Sakuma and Pfaff, 1979a; Sakuma and Pfaff, 1979b; Sakuma and Pfaff, 1980; McCarthy et al., 1991; Kondo et al., 1993). In addition, stimulation of the PAG has been shown to provide excitatory drive over RVM pre-motoneurons that innervate the axial muscles necessary for the display of lordosis (Cottingham et al., 1987; Cottingham et al., 1988). Together, this suggests that in females, PAG outputs to the RVM may be specialized for the modulation of somatosensory information essential for the appropriate display of lordosis (Rose, 1975; Robbins et al., 1990; Robbins et al., 1992), and specifically, may serve to increase somatosensory thresholds during reproductive behavior (Crowley et al., 1976; Whipple and Komisaruk, 1985; Komisaruk and Whipple, 1986). In support, the PAG receives extensive projections from the lumbosacral spinal cord (Vanderhorst et al., 1996; Mouton et al., 2005), and spino-PAG afferents terminate preferentially in PAG regions containing dense retrograde labeling from the RVM (Murphy, unpublished observations). Therefore, activation of the lumbosacral spinal cord by peripheral afferents during mounting by a male may function to activate the PAG-RVM inhibitory pathway, thereby increasing sensory thresholds and allowing for appropriate postural adjustments necessary for successful intromission by the male.
Sexually Dimorphic Effect of Opiates on the PAG-RVM Circuit
Systemic morphine administration significantly attenuated CFA-induced Fos in males, but not females. These results are consistent with previous studies reporting significant sex differences in the antihyperalgesic effects of systemic morphine in persistent pain models (Cook and Nickerson, 2005; Wang and Murphy, 2005). Interestingly, while morphine clearly suppressed CFA-induced Fos in males, the effect of morphine on Fos expression in PAG-RVM neurons was highly variable. Indeed, depending on the PAG region and rostrocaudal level, administration of morphine either increased, decreased, or had no effect on the percentage of PAG-RVM neurons expressing Fos (%FG/Fos) in comparison to saline treated animals. Similarly, analysis of the percentage of Fos present in FG neurons (%Fos/FG) following morphine or saline treatment provided no additional clear insight. Two primary mechanisms have been proposed to account for morphines’ action in the PAG. First, morphine has been shown to hyperpolarize GABAergic interneurons that are presynaptic to PAG-RVM output neurons; this hyperpolarization results in the disinhibition of PAG output neurons to the RVM resulting in a net excitatory effect (Behbehani and Fields, 1979; Yaksh and Tyce, 1979; Cheng et al., 1986; Moreau and Fields, 1986; Chieng and Christie, 1994; Commons et al., 2000; Tortorici and Morgan, 2002). In the present study, this mechanism of morphine action would be reflected as a decrease in Fos expression in intrinsic (presumably GABAergic) neurons. The removal of tonic GABAergic inhibition would result in the net activation of PAG-RVM neurons, thereby inducing Fos and increasing the percentage of Fos/FG neurons in morphine treated animals in comparison to saline.
Morphine has also been shown to directly inhibit PAG-RVM neurons (Osborne et al., 1996). Anatomical studies by Commons et al. (2002) and Wang and Wessendorf (Wang and Wessendorf, 2002) estimated that between 27-50% of PAG neurons retrogradely labeled from the RVM contain MOR; these dual labeled cells were localized primarily within the caudal lateral/ventrolateral PAG. In the present study, a direct inhibitory mechanism of morphine action would be reflected as a decrease in the percentage of FG+Fos labeled cells following opioid administration.
A precise mechanism of morphine action (presynaptic versus direct) cannot be discerned in the present study, and the results suggest that both mechanisms contributed to the suppression of persistent pain induced Fos in the PAG of males. The variability noted in the action of morphine on PAG-RVM neurons is not easily attributable to level and region-dependent differences in PAG mu opioid receptor expression. Mu opioid receptors are localized throughout the rostral-caudal axis of PAG, with low to moderate levels of expression noted in the rostral two-thirds of PAG (Bregma –6.72 to –8.00) (Duncan and Murphy, 2005). The highest level of MOR expression is within the caudal ventrolateral PAG (Bregma –8.30 to −8.80), and in the present study, the effects of morphine on Fos expression in PAG-RVM neurons were quite variable at this level.
It is important to consider that while lesions of the PAG or intra-PAG naloxone significantly attenuate the effects of systemic morphine, they do not block it completely. This suggests the involvement of additional loci, including both central (Jensen and Yaksh, 1986; Heinricher et al., 1994) and peripheral (Stein et al., 1988b) sites of morphine action. Thus, in addition to a direct action within the PAG, morphine would also reduce incoming noxious input to the cord at the primary afferent synapse, which would ultimately reduce noxious stimulus-induced Fos in PAG.
There was no effect of morphine on CFA-induced Fos in females. These results concur with our recent data indicating that the ED50 for either systemic (Loyd et al., 2005) or intra-PAG (Loyd et al., 2005) morphine is approximately two times higher in females in comparison to males in a model of persistent inflammatory pain. Similar findings for intra-PAG morphine administration have been reported in studies utilizing acute pain models (Rossi et al., 1994; Krzanowska and Bodnar, 1999; Krzanowska and Bodnar, 2000).
Interestingly, recent studies report that systemic morphine simultaneously activates both pain inhibitory as well as pain facilitatory systems (Vanderah et al., 2001a; Vanderah et al., 2001b). Specifically, in rats, sub-analgesic doses of morphine (0.002-0.2 mg/kg) result in a hyperalgesic response, and this effect is significantly more pronounced in females in comparison to males (Holtman and Wala, 2005). While the mechanism(s) and neural substrates underlying morphine induced hyperalgesia are unknown, it is interesting to speculate that in females, morphine may concurrently activate both pain facilitatory and inhibitory circuits within the PAG, thereby resulting in no change in CFA-induced Fos. Further studies are obviously warranted.
Unfortunately, to date, all studies examining the antinociceptive mechanisms of morphine action in the PAG have been conducted exclusively in males; therefore, it is not known whether morphine administration has the same physiological effect on PAG neurons in females. The PAG contains a large population of estrogen receptors (ERα) (Murphy and Hoffman, 1999; Murphy and Hoffman, 2001), and in both males and females, approximately 20-25% of PAG-RVM neurons contain ERα (Loyd and Murphy, 2004a). Estrogen binding to its receptor within PAG-RVM neurons may be involved in ligand-dependent transcriptional events, thereby influencing the expression of various neurotransmitters and/or membrane properties (Zakon and Dunlap, 1999). Alternatively, as the gene encoding Fos contains an estrogen response element (Loose-Mitchell et al., 1988; Wang et al., 2003), changes in cycle status may have potentially influenced the effect of morphine on CFA-induced Fos in females, particularly if the animals were in similar stages of estrous. In the present study, the phase of the estrous cycle was not determined. However, if cycle status differentially affected Fos expression, then the degree of variability in CFA-induced Fos should be greater in females in comparison to males; however, this was not observed in the present study. Alternatively, the mu opioid receptor is a member of the inhibitory G protein-coupled receptor superfamily, and in hypothalamic slices, estrogen has been shown to rapidly uncouple the mu receptor from G protein-gated inwardly rectifying potassium channels resulting in a reduced hyperpolarization by MOR agonists (Kelly et al., 2003; Malyala et al., 2005). Estrogen has also been shown to result in MOR internalization, thereby limiting the amount of receptor available for exogenous ligand binding (Eckersell et al., 1998). Lastly, we have recently reported significant sex differences in mu opioid receptor protein levels within the caudal lateral/ventrolateral PAG, with intact males have 11% higher levels of MOR protein in comparison to diestrus females (Duncan and Murphy, 2005). Together, these studies may provide several direct mechanisms for the reduced potency of mu agonists noted in females.
Summary
There are now well-established sex differences in the analgesic effects of morphine; however, the CNS mechanisms underlying the sexually dimorphic actions of morphine are unknown. The PAG, and its descending projections to the RVM and dorsal horn of the spinal cord, constitute an essential neural circuit for opioid-based analgesia. The results of the present study demonstrate for the first time that the PAG-RVM pathway is sexually dimorphic in its anatomical organization and in its activation during persistent inflammatory pain. The actions of morphine on PAG neurons were also found to be sexually dimorphic. Together, these results suggest that the PAG-RVM pathway may provide the anatomical basis for observed sex differences in morphine analgesia.
Acknowledgements
This work was supported by NIH DA16272 and P50 AR49555 grants awarded to Anne Z. Murphy, Ph.D. The authors acknowledge the superb technical assistance of Ryan Castaneira and Shelby Suckow. The authors would also like to thank Matthew Ennis for helpful comments on an earlier version of this manuscript.
Grant Support: NIH grants DA16272 and AR49555 to AZM.
Literature Cited
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. doi: 10.1002/cne.902010211. [DOI] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl) 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology (Berl) 2002;160:170–181. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Marley NJ, O'Keefe J, Clanton CH. Reversal of morphine and stimulus-produced analgesia by subtotal spinal cord lesions. Pain. 1977;3:43–56. doi: 10.1016/0304-3959(77)90034-3. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivisions. J Comp Neurol. 1985;237:445–459. doi: 10.1002/cne.902370403. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Shepard RD. The midbrain periaqueductal gray in the rat. II. A Golgi analysis. J Comp Neurol. 1985;237:460–475. doi: 10.1002/cne.902370404. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983;261:132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav. Brain Sciences. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Williams CL, Lee SJ, Pasternak GW. Role of mu 1-opiate receptors in supraspinal opiate analgesia: a microinjection study. Brain Res. 1988;447:25–34. doi: 10.1016/0006-8993(88)90962-6. [DOI] [PubMed] [Google Scholar]
- Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796:315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- Budai D, Fields HL. Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol. 1998;79:677–687. doi: 10.1152/jn.1998.79.2.677. [DOI] [PubMed] [Google Scholar]
- Cameron A, Khan I, Westlund K, Willis W. The efferent projections of the periaqueductal gray in the rat: A PHA-L study. II. Descending projections. J Comp Neurol. 1995b;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Anatomical evidence that hypertension associated with the defence reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res. 1988;460:339–345. doi: 10.1016/0006-8993(88)90378-2. [DOI] [PubMed] [Google Scholar]
- Carstens E, Yokota T, Zimmermann M. Inhibition of spinal neuronal responses to noxious skin heating by stimulation of mesencephalic periaqueductal gray in the cat. J Neurophysiol. 1979;42:558–568. doi: 10.1152/jn.1979.42.2.558. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Chance WT, Krynock GM, Rosecrans JA. Effects of medial raphe and raphe magnus lesions on the analgesic activity of morphine and methadone. Psychopharmacology (Berl) 1978;56:133–137. doi: 10.1007/BF00431838. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Fields HL, Heinricher MM. Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Res. 1986;375:57–65. doi: 10.1016/0006-8993(86)90958-3. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma- aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Cottingham SL, Femano PA, Pfaff DW. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol. 1987;97:704–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- Cottingham SL, Femano PA, Pfaff DW. Vestibulospinal and reticulospinal interactions in the activation of back muscle EMG in the rat. Exp Brain Res. 1988;73:198–208. doi: 10.1007/BF00279673. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kalivas P, Stratmann J. Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharmacol. 1996;7:764–778. [PubMed] [Google Scholar]
- Crowley WR, Jacobs R, Volpe J, Rodriguez-Sierra JF, Komisaruk BR. Analgesic effect of vaginal stimulation in rats: modulation by graded stimulus intensity and hormones. Physiol Behav. 1976;16:483–488. doi: 10.1016/0031-9384(76)90328-0. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jurgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Murphy AZ. Sex-linked differences in mu opiate receptor distribution in the rat brain. Soc. Neuroscience Abstr. 2005 [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receeptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984a;306:105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984b;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Fillingim R. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Gerhart KD, Yezierski RP, Wilcox TK, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray or adjacent midbrain reticular formation. J Neurophysiol. 1984;51:450–466. doi: 10.1152/jn.1984.51.3.450. [DOI] [PubMed] [Google Scholar]
- Gray BG, Dostrovsky JO. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:932–947. doi: 10.1152/jn.1983.49.4.932. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou SP, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104:401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Guo X, Tang XC. Roles of periaqueductal gray and nucleus raphe magnus on analgesia induced by lappaconitine, N-deacetyllappaconitine and morphine. Zhongguo Yao Li Xue Bao. 1990;11:107–112. [PubMed] [Google Scholar]
- Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Anatomical and functional connections of neurons of the rostral medullary raphe of the rabbit. Brain Res. 1988;453:176–182. doi: 10.1016/0006-8993(88)90156-4. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortotici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Holstege G, Cowie RJ. Projections from the rostral mesencephalic reticular formation to the spinal cord. An HRP and autoradiographical tracing study in the cat. Exp Brain Res. 1989;75:265–279. doi: 10.1007/BF00247933. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Wala EP. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. III. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by microinjection technique. Brain Res. 1986;372:301–312. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res. 1988;460:281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- Jones SL, Light AR. Serotoninergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. J. Comp. Neurol. 1992;322:599–610. doi: 10.1002/cne.903220413. [DOI] [PubMed] [Google Scholar]
- Jurgens U. A study of the central control of vocalization using the squirrel monkey. Med Eng Phys. 2002;24:473–477. doi: 10.1016/s1350-4533(02)00051-6. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B. Vaginal stimulation-produced analgesia in rats and women. Ann N Y Acad Sci. 1986;467:30–39. doi: 10.1111/j.1749-6632.1986.tb14616.x. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Koizumi T, Arai Y, Kakeyama M, Yamanouchi K. Functional relationships between mesencephalic central gray and septum in regulating lordosis in female rats: effect of dual lesions. Brain Res Bull. 1993;32:635–638. doi: 10.1016/0361-9230(93)90166-9. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicted from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Analysis of sex and gonadectomy differences in B-endorphin antinociception elicted from the ventrolateral periaqueductal gray in rats. Eur. J. Pharm. 2000;392:157–161. doi: 10.1016/s0014-2999(00)00110-2. [DOI] [PubMed] [Google Scholar]
- Larson CR. The midbrain periaqueductal gray: a brainstem structure involved in vocalization. J Speech Hear Res. 1985;28:241–249. doi: 10.1044/jshr.2802.241. [DOI] [PubMed] [Google Scholar]
- Larson CR, Kistler MK. Periaqueductal gray neuronal activity associated with laryngeal EMG and vocalization in the awake monkey. Neurosci Lett. 1984;46:261–266. doi: 10.1016/0304-3940(84)90109-5. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell DS, Chiappetta C, Stancel GM. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988;2:946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Stezhka VV. Neurones in the dorsolateral periaqueductal grey matter in coronal slices of rat midbrain: electrophysiological and morphological characteristics. Exp Brain Res. 1999;124:53–58. doi: 10.1007/s002210050599. [DOI] [PubMed] [Google Scholar]
- Loyd D, Murphy AZ. Sex differences in the anatomical and functional organization of the PAG-RVM circuit. Soc. Neuroscience Abstr. 2004a [Google Scholar]
- Loyd D, Murphy AZ. Sex Differences of Organization and Activation of the Midbrain Periaqueductal Gray – Nucleus Raphe Magnus Pathway. Soc. Neurosci. Abstr. 2004b [Google Scholar]
- Loyd D, Wang X, Murphy AZ. Morphine injection into the periaqueductal gray produces a sexually dimorphic response in a model of persistent inflammatory pain. Soc. Neuroscience Abstr. 2005 [Google Scholar]
- Ma QP, Han JS. Naloxone blocks the release of opioid peptides in periaqueductal gray and N. accumbens induced by intra-amygdaloid injection of morphine. Peptides. 1991;12:1235–1238. doi: 10.1016/0196-9781(91)90200-9. [DOI] [PubMed] [Google Scholar]
- Malyala A, Kelly MJ, Ronnekleiv OK. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Connections of midbrain periaqueductal gray in the monkey. II. Descending efferent projections. J Neurophysiol. 1983;49:582–594. doi: 10.1152/jn.1983.49.3.582. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Pfaff DW, Schwartz-Giblin S. Midbrain central gray GABAA receptor activation enhances, and blockade reduces, sexual behavior in the female rat. Exp Brain Res. 1991;86:108–116. doi: 10.1007/BF00231045. [DOI] [PubMed] [Google Scholar]
- Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Czlonkowski A, Morris B, Stein C, Arendt R, Huber A, Hollt V, Herz A. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35:299–312. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- Miller PL, Ernst AA. Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. South Med J. 2004;97:35–41. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- Mohrland JS, Gebhart GF. Effect of selective destruction of serotonergic neurons in nucleus raphe magnus on morphine-induced antinociception. Life Sci. 1980;27:2627–2632. doi: 10.1016/0024-3205(80)90551-2. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Res. 1991;545:17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47:863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- Mouton LJ, Klop EM, Holstege G. C1-C3 spinal cord projections to periaqueductal gray and thalamus: a quantitative retrograde tracing study in cat. Brain Res. 2005;1043:87–94. doi: 10.1016/j.brainres.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Ennis M, Rizvi TA, Behbehani MM, Shipley MT. Fos expression induced by changes in arterial pressure is localized in distinct, longitudinally organized columns of neurons in the rat midbrain periaqueductal gray. J Comp Neurol. 1995;360:286–300. doi: 10.1002/cne.903600207. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of androgen and estrogen receptor containing neurons in the male rat periaqueductal gray. Horm. Beh. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kow L-M, McCarthy MM, Pfaff DW, Schwartz-Giblin S. Midbrain PAG control of female reproductive behavior: In vitro electrophysiological characterization of actions of lordosis-relevant substances. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 211–238. [Google Scholar]
- Ogawa S, Kow LM, Pfaff DW. Effects of lordosis-relevant neuropeptides on midbrain periaqueductal gray neuronal activity in vitro. Peptides. 1992;13:965–975. doi: 10.1016/0196-9781(92)90058-b. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol. 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit HK. Effects of raphe magnus and raphe pallidus lesions on morphine-induced analgesia and spinal cord monoamines. Pharmacol Biochem Behav. 1980;13:705–714. doi: 10.1016/0091-3057(80)90015-5. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Anderson EG. Morphine analgesia: blockade by raphe magnus lesions. Brain Res. 1975;98:612–618. doi: 10.1016/0006-8993(75)90380-7. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Robbins A, Pfaff DW, Schwartz-Giblin S. Reticulospinal and reticuloreticular pathways for activating the lumbar back muscles in the rat. Exp Brain Res. 1992;92:46–58. doi: 10.1007/BF00230382. [DOI] [PubMed] [Google Scholar]
- Robbins A, Schwartz-Giblin S, Pfaff DW. Ascending and descending projections to medullary reticular formation sites which activate deep lumbar back muscles in the rat. Exp Brain Res. 1990;80:463–474. doi: 10.1007/BF00227988. [DOI] [PubMed] [Google Scholar]
- Rose JD. Responses of midbrain neurons to genital and somatosensory stimulation in estrous and anestrous cats. Exp Neurol. 1975;49:639–652. doi: 10.1016/0014-4886(75)90049-7. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Mu and delta opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res. 1994;665:85–93. doi: 10.1016/0006-8993(94)91155-x. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am J Physiol. 1979a;237:R278–284. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol. 1979b;237:R285–290. doi: 10.1152/ajpregu.1979.237.5.R285. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Covergent effects of lordosis-relevant somatosensory and hypothalamic influences on central gray cells in the rat mesencephalon. Exp Neurol. 1980;70:269–281. doi: 10.1016/0014-4886(80)90026-6. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Fu QG, Zimmermann M. Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. J Neurophysiol. 1987a;58:327–341. doi: 10.1152/jn.1987.58.2.327. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Maisch B, Zimmermann M. Raphe magnus-induced descending inhibition of spinal nociceptive neurons is mediated through contralateral spinal pathways in the cat. Neurosci Lett. 1987b;76:168–172. doi: 10.1016/0304-3940(87)90710-5. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kubota A, Iwama T, Wada T, Yasui M, Fujibayashi K, Takagi H. Comparison of analgesic potencies of mu, delta and kappa agonists locally applied to various CNS regions relevant to analgesia in rats. Life Sci. 1983;33:689–692. doi: 10.1016/0024-3205(83)90596-9. [DOI] [PubMed] [Google Scholar]
- Shah Y, Dostrovsky JO. Electrophysiological evidence for a projection of the periaqueductal gray matter to nucleus raphe magnus in cat and rat. Brain Res. 1980;193:534–538. doi: 10.1016/0006-8993(80)90183-3. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988a;31:455–451. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Yassouridis A, Herz A. Antinociceptive effects of mu- and kappa-agonists in inflammation are enhanced by a peripheral opioid receptor-specific mechanism. Eur J Pharmacol. 1988b;155:255–264. doi: 10.1016/0014-2999(88)90511-0. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershner SA, Mitchell JM, Fields HL. Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain. 2000;85:153–159. doi: 10.1016/s0304-3959(99)00257-2. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM. Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. J Neurophysiol. 2002;88:1707–1715. doi: 10.1152/jn.2002.88.4.1707. [DOI] [PubMed] [Google Scholar]
- van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J. Comp. Neurol. 1991;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr., Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001a;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr., Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001b;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Mouton LJ, Blok BF, Holstege G. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J Comp Neurol. 1996;376:361–385. doi: 10.1002/(SICI)1096-9861(19961216)376:3<361::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109:619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Wang LH, Yang XY, Zhang X, Mihalic K, Xiao W, Farrar WL. The cis decoy against the estrogen response element suppresses breast cancer cells via target disrupting c-fos not mitogen-activated protein kinase activity. Cancer Res. 2003;63:2046–2051. [PubMed] [Google Scholar]
- Wang X, Murphy AZ. Sex differences in the antihyperalgesic and antinociceptive effects of morphine in a rat model of persistent inflammatory pain. A Journal Physiology. 2005 [Google Scholar]
- Waters AJ, Lumb BM. Inhibitory effects evoked from both the lateral and ventrolateral periaqueductal grey are selective for the nociceptive responses of rat dorsal horn neurones. Brain Res. 1997;752:239–249. doi: 10.1016/s0006-8993(96)01462-x. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Whipple B, Komisaruk BR. Elevation of pain threshold by vaginal stimulation in women. Pain. 1985;21:357–367. doi: 10.1016/0304-3959(85)90164-2. [DOI] [PubMed] [Google Scholar]
- Wilcox RE, Mikula JA, Levitt RA. Periaqueductal gray naloxone microinjections in morphine-dependent rats: hyperalgesia without “classical” withdrawal. Neuropharmacology. 1979;18:639–641. doi: 10.1016/0028-3908(79)90118-7. [DOI] [PubMed] [Google Scholar]
- Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res. 1995;690:207–216. doi: 10.1016/0006-8993(95)00554-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Tyce GM. Microinjection of morphine into the periaqueductal gray evokes the release of serotonin from spinal cord. Brain Res. 1979;171:176–181. doi: 10.1016/0006-8993(79)90747-9. [DOI] [PubMed] [Google Scholar]
- Zakon HH, Dunlap KD. Sex steroids and communication signals in electric fish: a tale of two species. Brain Behav Evol. 1999;54:61–69. doi: 10.1159/000006612. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Bandler R, Carrive P. Flight and immobility evoked by excitatory amino acid microinjection within distinct parts of the subtentorial midbrain periaqueductal gray of the cat. Brain Res. 1990;520:73–82. doi: 10.1016/0006-8993(90)91692-a. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Du LN, Wu GC, Cao XD. Modulation of intrathecal morphine-induced immunosuppression by microinjection of naloxone into periaqueductal gray. Zhongguo Yao Li Xue Bao. 1998;19:519–522. [PubMed] [Google Scholar]