Abstract
The ligand-activated transcription factor, aryl hydrocarbon receptor (AHR), is a novel inducer of adaptive Tregs. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), the most potent AHR ligand, induces adaptive CD4+CD25+ Tregs during an acute graft-versus-host (GvH) response and prevents the generation of allospecific cytotoxic T lymphocytes. TCDD also suppresses the induction of experimental autoimmune encephalitis in association with an expanded population of Foxp3+ Tregs. In this study, we show that chronic treatment of NOD mice with TCDD potently suppresses the development of autoimmune Type 1 diabetes in parallel with greatly reduced pancreatic islet insulitis and an expanded population of CD4+CD25+Foxp3+ cells in the pancreatic lymph nodes. When treatment with TCDD was terminated after 15 weeks (23 weeks of age), mice developed diabetes over the next 8 weeks in association with lower numbers of Tregs and decreased activation of AHR. Analysis of the expression levels of several genes associated with inflammation, T-cell activation and/or Treg function in pancreatic lymph node cells failed to reveal any differences associated with TCDD treatment. Taken together, the data suggest that AHR activation by TCDD-like ligands may represent a novel avenue for treatment of immune-mediated diseases.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; AHR; aryl hydrocarbon receptor; NOD mice; regulatory T cell; TCDD; Type 1 diabetes
Aryl hydrocarbon receptor (AHR), along with its nuclear-binding partner aryl hydrocarbon receptor nuclear translocator (ARNT), is a ligand-activated transcription factor that has recently been associated with the induction of regulatory T cells (Tregs) [1–4]. AHR-dependent Tregs were first described in an acute graft-versus-host (GvH) model following activation of AHR by the most potent known ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [1]. In this model, treatment of the F1 host with TCDD led to altered donor T-cell differentiation, resulting in the expression of high levels of CD25 along with glucocorticoid-induced TNF receptor (GITR), CD28 and cytotoxic T-lymphocyte antigen (CTLA)4 on the donor CD4+ T cells. The cells also showed potent suppressive function in vitro. This altered CD4+ T-cell differentiation occurred only if the donor CD4+ T cells expressed AHR [1,2]. Induction of Tregs was not dependent on the presence of pre-existing CD4+CD25+ T cells in the donor population, and the cells did not express Foxp3 [3], suggesting that AHR may represent a novel transcription factor for adaptive Treg differentiation.
The induction of the CD4+ Treg phenotype on day 2 of the GvH response preceded the inhibition of expansion of the donor CD8+ T-cell population and the suppression of GvH disease (GvHD) on days 10–12 in TCDD-treated mice [2,5], supporting the idea that AHR-induced Tregs are central to suppression of the GvH response by TCDD. To further address the disease relevance of AHR-induced Tregs, we investigated the effects of TCDD on the development of Type 1 diabetes in the NOD mouse model. NOD mice develop diabetes spontaneously due to a breakdown in self-tolerance to several antigens expressed on pancreatic islet cells and the infiltration of the pancreas by self-reactive T cells [6,7]. Among other changes, overt diabetes develops in parallel with an age-related decline in the number and function of CD4+CD25+Foxp3+ Treg cells [8,9]. In this report, we show that chronic treatment of NOD female mice with TCDD, at a concentration that produces no overt toxicity, completely suppressed the development of diabetes. Suppression of diabetes was dependent on the continued presence of TCDD and correlated with an expanded population of CD4+CD25+FoxP3+ T cells in the pancreatic lymph nodes. These findings are consistent with a recent report of suppression of another autoimmune disease, experimental autoimmune encephalitis, associated with TCDD-induced Tregs [4], and together support the hypothesis that AHR signaling is a novel pathway for the induction of Tregs.
Materials & Methods
Animals
Female NOD/LtJ mice, 6–7 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions at Oregon State University (OR, USA) laboratory animal facilities. The mice were housed as four per cage on a ventilated rack under sterile caging conditions and supplied with autoclaved deionized water and irradiated rodent chow.
TCDD treatment
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), obtained as a standard of 99% purity from Cambridge Isotope Laboratories, Inc., (Woburn, MA, USA) was dissolved in anisole and diluted in peanut oil. A similar solution of anisole in peanut oil was prepared as a vehicle (VEH) control for TCDD treatment. All mice were dosed by gavage (100 μl/10 g bodyweight [bw]). An initial loading dose of 50 μg TCDD/kg bw was given at 8 weeks of age. Thereafter, the animals were dosed biweekly with a maintenance dose of 15 μg TCDD/kg bw. Based on an estimated 3-week half-life for TCDD in mice [10], this dosing regimen resulted in a steady-state body burden of 30–40 μg TCDD/kg bw during the 23-week experimental time period (to 31 weeks of age).
Blood glucose assay
Blood glucose levels were measured weekly using an ACCU-CHEK Advantage monitor (Roche Diagnostics). Animals were terminated by an overdose of CO2 when nonfasting blood glucose exceeded 250 mg/dl for 2 consecutive days.
Genotyping of AHR alleles in NOD mice
Genomic DNA was extracted from the liver of NOD mice and stored at −20°C. Oligonucleotide primer pairs specific for murine AHR exon 10 were designed using vector NTI 8 and purchased from Sigma-Aldrich: 5′-CGCTGCCCTT CATGTTT GCTACCG-3′ (forward) and 5′-GCCCTGGCT GGCACTGAT ACATGGA-3′ (reverse). PCR amplification was carried out on a PTC-200 Peltier thermacycler (MJ Research). Production of the expected amplicon size was confirmed by gel electrophoresis, and PCR products were purified using QIAquick PCR Purification Kit (Qiagen). Primers and purified PCR products were submitted to the Center for Gene Research and Biotechnology Core Laboratories at Oregon State University for DNA sequencing.
Real-time PCR
RNA was isolated from liver and pancreatic tissue using RNEasy Mini Kit (Qiagen). cDNAs were prepared using SuperArray Reaction Ready™ First Strand cDNA Synthesis Kit. PCR reactions were performed using RT2 Real-Time™ SYBR Green/ROX Master Mix with gene-specific primers for Ccl5, Mip, Tak1, EphA8, Grzb, cyclophilin, Il4, Il6, Il17, Tgfb1, Tgfb3, Tnfa, Ifng, Cd69, Cd28, Cd25 and Actinb (SuperArray Bioscience Corp, MD, USA). Reactions were performed on an Applied Biosystems 7500 sequence-detection instrument. Results were analyzed using ABI 7500 System Software. The assay and data analysis were carried out as per the manufacturer’s instructions. Gene expression was normalized to Actinb expression.
Histological assessment of islet insulitis
Pancreata were frozen in optimal cutting temperature compound. A minimum of five 5 μm pancreatic sections, each 200 μm apart, were cut for each tissue block. Sections were fixed in formalin, and stained with hematoxylin and eosin (H & E). Each islet was scored as: no insulitis, peri-insulitis or intrainsulitis (<50% or >50%).
Lymph node cell preparation & flow cytometry
Cell suspensions of pancreatic lymph nodes (2–3 pooled per mouse) were prepared using Hanks’ Balanced Salt Solution (HBSS; Sigma) supplemented with 2.5% phosphate-buffered saline (FBS; Hyclone) and 15 mM (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid (HEPES). Cells were incubated with rat IgG (Jackson Immunoresearch) to block nonspecific labeling, followed by incubation with appropriate combinations of fluorochrome-labeled antibodies to surface CD4 and CD25 (BD Biosciences). For Foxp3 staining, surface-stained cells were fixed, permeabilized and labeled with anti-Foxp3 antibody according to the manufacturer’s protocol (eBioscience). Viability of fixed cells was determined by prestaining with ethidium monoazide bromide (Invitrogen). For unfixed cells, viability was determined by exclusion of 7-amino-actinomycin D (eBioscience). Negative gating controls were included for all staining sets using the fluorescence minus one (FMO) protocol. Data were collected using FC500 flow cytometer (Beckman Coulter). Data analyses, including software compensation, were performed using WinList software (Verity Software House).
Results
NOD mice express the AHRd allele
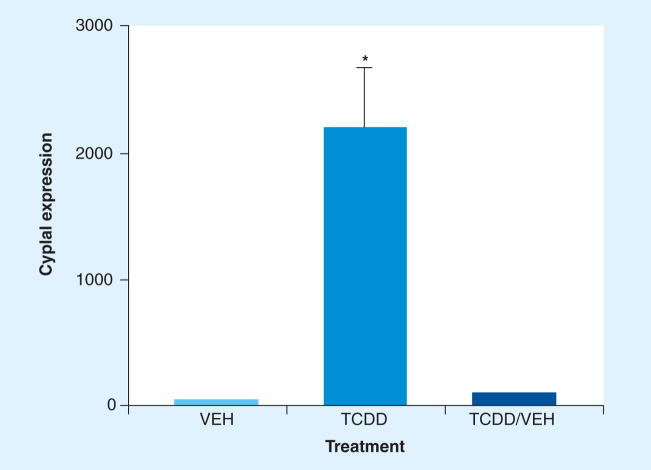
Certain strains of mice are more sensitive to the effects of TCDD due polymorphisms in the AHR gene that affect ligand-binding affinity. The AHRb allele, found in the prototypic ‘responder’ strain of C57Bl/6 mice, shows highest affinity for binding TCDD, whereas the AHRd allele, found in the prototypic ‘low responder’ DBA/2 strain, has tenfold lower binding affinity [11]. Thus, DBA/2 mice and other AHRd strains generally require at least a tenfold higher dose of TCDD to induce the same effects seen at lower doses in C57Bl/6 mice. The immunosuppressive potency of TCDD in mice varies in accordance with their AHR genotype [12]. In order to determine the level of responsiveness of NOD/ltr mice to TCDD, we sequenced polymorphic segments of exon 10 in the transactivation domain (TAD) of the AHR, which have been used to genotype various strains of mice [13]. As shown in Figure 1, the genomic sequences from NOD mice were identical to the sequences found in DBA/2 mice, indicating that NOD mice express the low affinity AHRd genotype. Based on this information, we used a dosing regimen calculated to achieve a constant body burden of 30–40 μg TCDD/kg bw over the 23-week experimental period. This dose rate was effective at maintaining elevated hepatic expression of Cyp1a1 message (Figure 2), which is directly regulated by AHR and is often used as a biomarker of exposure to AHR ligands [14]. Mice that were treated with TCDD for 15 weeks and then switched to VEH for the remaining 8 weeks of the experiment showed little induction of Cyp1a1 expression, suggesting the AHR was no longer activated. Based on a 3 week half-life of TCDD, the body burden of TCDD remaining in these mice was estimated at less than 4 μg TCDD/kg bw. At necropsy, no overt pathology was evident in any of the TCDD-treated mice. Histology revealed no TCDD-related pathology in the liver (data not shown).
Figure 1. Sequence analysis of exon 10 of AHR gene in NOD mice shows a low-affinity AHRd haplotype identical to that found in DBA/2 mice.
AHR: Aryl hydrocarbon receptor; NOD: Nonobese diabetic. Data adapted from [13].
Figure 2. Treatment of NOD mice with TCDD results in sustained activation of AHR as evidenced by a high level of hepatic Cyp1a1 message at 31 weeks of age.
Aryl hydrocarbon receptor (AHR) activation declines when TCDD treatment is terminated and is no longer apparent after 8 weeks of VEH treatment. NOD mice were dosed orally biweekly with VEH or TCDD until 31 weeks of age. At 23 weeks of age, half of the TCDD-treated mice were switched to VEH for the remaining 8 weeks. Liver samples were obtained from mice that survived to 31 weeks and processed for RNA. Cyp1a1 expression data represent fold-change relative to β-actin message from three, 12 and three mice in VEH, TCDD and TCDD/VEH groups, respectively.
NOD: Nonobese diabetic; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; VEH: Vehicle.
TCDD reversibly prevents the onset of diabetes
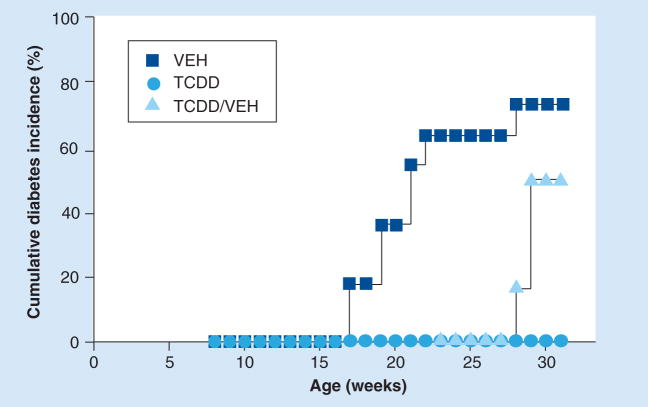
The influence of TCDD on the development of diabetes in NOD female mice is shown in Figure 3. The first cases of diabetes in VEH-treated mice occurred at 17 weeks of age. By 26 weeks of age, 73% (eight out of 11) of the VEH-treated mice developed diabetes, the final incidence in this group. In contrast, none of the mice that were treated with TCDD (zero out of 12) developed diabetes within 23 weeks of age. To determine if protection from diabetes required continuous treatment, half of the TCDD-treated group (six out of 12) was switched to VEH treatment at 23 weeks of age. After 5 weeks, the first case of diabetes occurred, with two more cases in the following week, for a final incidence of 50% (three out of six) at 31 weeks of age. None of the mice (zero out of six) that continued to be treated with TCDD developed diabetes. These data suggest that a sufficient body burden of TCDD must be maintained in order to prevent the development of diabetes in NOD mice.
Figure 3. TCDD-treated mice are protected from diabetes by continuous treatment with TCDD.
Groups of mice were treated with VEH (n = 11) or TCDD (n = 12) biweekly, beginning at 8 weeks of age. With no diabetes in any of the TCDD-treated mice at 23 weeks of age, half of the mice in the TCDD-treatment group were switched to VEH for the remainder of the study (TCDD/VEH). Blood glucose levels were measured weekly. Animals were terminated by an overdose of CO2 when nonfasting blood glucose level exceeded 250 mg/dl for 2 consecutive days. TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; VEH: Vehicle.
TCDD inhibits insulitis
Pancreata from TCDD-treated mice that survived without overt disease until 31 weeks of age were examined along with pancreata from mice that had been switched to VEH treatment at 23 weeks. Blood sugar levels at 31 weeks were 107 ± 6 in the TCDD-treated group (n = 6) compared with 128 ± 10 in the mice switched to VEH (n = 3) and 167 ± 22 in VEH-treated mice (n = 3).
In TCDD-treated mice (n = 4), 64% of islets showed no insulitis, 17% showed peri-insulitis and 19% showed more than 50% intra-insulitis. In TCDD-treated mice switched to VEH (n = 4), only 28% of islets showed no insulitis, 8% showed peri-insulitis, while 69% showed more than 50% intra-insulitis. Insufficient numbers of islets in sections from some animals precluded inclusion of their data.
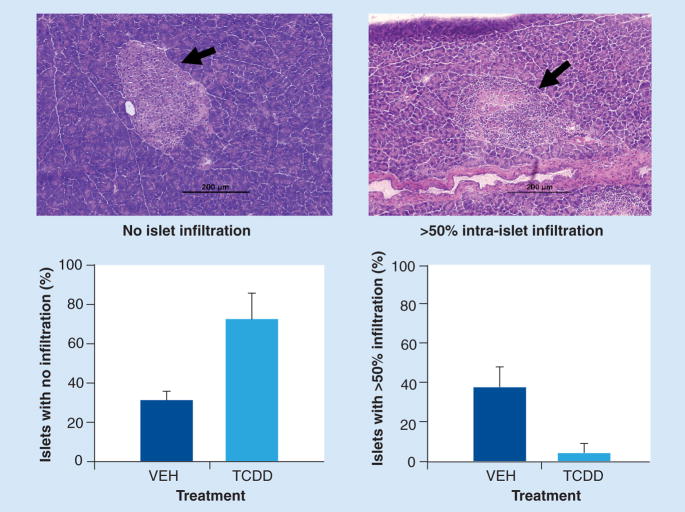
In a separate study, two groups of 12 NOD mice were treated biweekly with TCDD or VEH beginning at 8 weeks of age and ending at 15 weeks of age. During this time, two out of 12 VEH-treated mice were euthanized due to hyperglycemia and one VEH-treated mouse presented with a blood sugar level of 600 μg/dl on the day of termination. Blood sugar was within normal range in all other mice. Insulitis was examined in four representative nondiabetic mice per group, with dramatic differences observed between the groups. As shown in Figure 4, 73% of the islets from TCDD-treated mice showed no insulitis compared with 31% in VEH-treated mice. By contrast, only 6% of islets in TCDD-treated mice showed greater than 50% intra-insulitis compared with 38% in VEH-treated mice. Few islets in either group showed peri-insulitis (data not shown). These results suggest that TCDD suppresses the ongoing disease process by inhibiting the generation of self-reactive T effector cells and/or their migration into the pancreas.
Figure 4. NOD mice treated biweekly with TCDD from 8 to 15 weeks of age show greatly reduced insulitis.
Groups of 12 mice were treated with VEH or TCDD. At 15 weeks of age, pancreata were frozen in optimal cutting temperature compound. A minimum of five 5 μm pancreatic sections, each 200 μm apart, were cut for each tissue block. Sections were fixed in formalin and stained with hematoxylin and eosin for visualization and scoring of individual islets. Data reflect scores from four representative nondiabetic mice per treatment.
NOD: Nonobese diabetic; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; VEH: Vehicle.
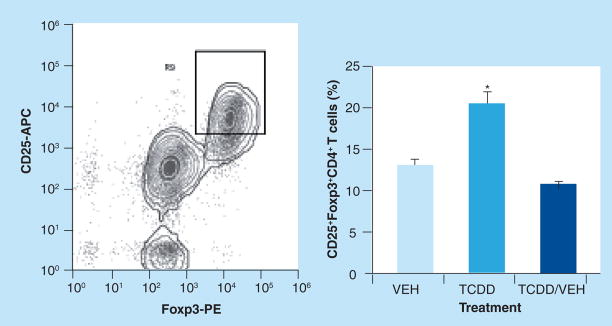
TCDD increases the frequency of CD4+CD25+Foxp3+ cells in pancreatic lymph nodes
Prevention of progressive insulitis and β-cell destruction in NOD mice has been shown to depend on the maintenance or expansion of a population of CD4+CD25+ Foxp3+ Tregs that suppresses the activation and/or effector functions of pathogenic T cells [8,9,16,17]. To determine if suppression of diabetes development by TCDD was associated with an altered frequency of Tregs, pancreatic lymph nodes were obtained from mice surviving to 31 weeks of age, and the expression of CD25 and Foxp3 on CD4+ T cells was examined by flow cytometry. As shown in Figure 5, continuous treatment with TCDD significantly increased the percentage of cells that coexpressed CD25 and Foxp3 within the CD4+ T-cell population, but did not alter the overall number or frequency of CD4+ or CD8+ T cells in the pancreatic lymph nodes (data not shown). Mice that had been treated with VEH for the final 8 weeks of the study did not show increased frequency of CD4+ CD25+Foxp3+ T cells at 31 weeks of age.
Figure 5. Frequency of CD4 +CD25+Foxp3+ T cells in the pancreatic lymph nodes is increased in NOD mice treated biweekly with TCDD until 31 weeks of age.
Mice removed from TCDD treatment 8 weeks earlier no longer show elevated frequency of CD4+CD25+ Foxp3+ T cells. Data represent mean ± SEM of three, 12 and three mice in VEH, TCDD and TCDD/VEH groups, respectively. Cells were stained for flow cytometric analysis as described in Materials and Methods. NOD: Nonobese diabetic; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; VEH: Vehicle.
CD25 expression was also examined on CD4+ cells in the pancreatic lymph nodes of 15-week old NOD mice. Treatment with TCDD resulted in a small but statistically significant increase in the frequency of CD4+ T cells that expressed CD25 (13.7 ± 0.9 % in TCDD [n = 12] vs 12.3 ± 0.7 % in VEH [n = 11]; p < 0.0004), suggesting that an increase in the Treg population may be emerging in the TCDD-treated mice at 15 weeks of age. Foxp3 expression was not measured by flow cytometry at 15 weeks but was examined by analysis of mRNA levels in pancreatic lymph node cells. However the message level of Foxp3 was not significantly different between VEH- and TCDD-treated cells at 15 weeks of age.
Changes in gene expression in pancreatic lymph node cells
With the goal of identifying changes in gene expression induced by TCDD that might implicate mechanisms underlying the suppression of diabetes development, we compared the expression levels of a variety of genes in the pancreatic lymph node cells of VEH- and TCDD-treated NOD mice at 15 weeks of age. Several genes associated with inflammation, T-cell activation and/or Treg function were examined (Ccl5, Mip, Tak1, EphA8, Grzb, cyclophilin, Il4, Il6, Il17, Tgfb1, Tgfb3, Tnfa, Ifng, Cd69, Cd28, Cd25 and Actinb), but none showed altered expression due to TCDD treatment.
Discussion
TCDD, acting through the AHR, is notable for its profound immunosuppressive potency. A single dose of TCDD, in the low μg/kg range, is effective in suppressing a variety of adaptive immune responses [18]. Previous studies have shown that T lymphocytes are key AHR-expressing targets for the immunosuppressive effects of TCDD. Using an allogeneic GvH model, we found that intrinsic activation of AHR in alloresponding donor CD4+ T cells by TCDD suppresses GvHD [5] and induces the generation of IL-10-producing CD4+ CD25+ Foxp3− Tregs [1,3]. These data suggest that activation of AHR in responding CD4+ T cells represents a unique pathway for the development of Tregs and a unique target for immunosuppressive therapies.
In the present studies, we used the NOD model of spontaneous autoimmune diabetes to determine the effect of chronic treatment with TCDD on disease development and the frequency of CD4+ T cells that expressed CD25 and/or Foxp3. The NOD model was considered ideal because the animals do not require intentional immunization to induce disease, and aberrant immunoregulation involving Tregs has been implicated in diabetes in both mice and humans [8,9,16,17]. Based on the finding that NOD mice express the low-affinity AHRd allele, we used a biweekly TCDD dosing regimen that was designed to maintain a relatively constant body burden of 35 μg TCDD/kg bw over the 24 week experimental period. This dosing regimen was shown to maintain activation of the AHR as demonstrated by an elevated Cyp1a1 message level in the liver of 31-week-old NOD mice. However, this dose of TCDD was not toxic as the bodyweight of TCDD-treated NOD mice continued to increase over the course of the study, and necropsy examination revealed no significant pathology. In addition, the cellularity of the pancreatic lymph nodes was not altered by TCDD treatment, indicating that this dosing regimen did not cause lymphocyte depletion. The lack of cytotoxicity is in keeping with the absence of direct cytotoxic effects of TCDD toward many cell types [19].
The most compelling result of TCDD treatment of NOD mice was the complete prevention of diabetes development in 100% of the animals through 31 weeks of age. Interestingly, however, the suppression of diabetes depended on continued dosing with TCDD. When TCDD treatment was stopped after 15 weeks (at 23 weeks of age), 50% of the mice developed diabetes. The onset of diabetes occurred when the body burden of TCDD had declined to levels that no longer activated the AHR, based on lack of Cyp1a1 gene expression in the liver. These results suggest that activation of AHR by TCDD does not induce long-term tolerance but that TCDD must be present in the cells to maintain AHR signaling activity.
Previous studies have suggested that the onset of diabetes in NOD mice is due to a general deficiency of Tregs [20,21]. Evidence also suggests that NOD Tregs are functionally impaired, requiring higher cell numbers to suppress disease compared with other autoimmune models [22,23]. The significantly increased frequency of CD4+CD25+Foxp3+ T cells in the pancreatic lymph nodes of TCDD-treated mice supports the hypothesis that activation of the AHR in CD4+ T cells increased the frequency of Treg cells that suppressed the development of disease. It is not yet known if AHR activation in NOD mice induces a novel type of Treg or if it expands the existing pool of natural Foxp3+ Tregs. In the GvH model, Tregs induced by AHR activation expressed high levels of CD25 and CTLA4 but did not express Foxp3 [3]. However, since all of the CD4+CD25+ cells in NOD mice also expressed Foxp3, underlying differences between the two models are not apparent. Interestingly, Quintana et al. recently reported that AHR regulates Foxp3 expression via direct binding to AHR-response elements in the promoter region of the Foxp3 gene [4]. However, these results are confounded by the need for very high concentrations of TCDD to induce Foxp3 expression in vitro (100 nM compared with a dissociation constant [Kd] of 0.27 nM for TCDD binding to AHRb) [24]. Even at 100 nM TCDD, the percentage of Foxp3+ cells was only 13% compared with 60% when 2.5 ng/ml TGF-β1 was used. At a minimum, these results suggest that the conditions under which AHR activation induces Foxp3 expression may differ depending on the model system, and that Foxp3 expression is not essential for the regulatory functions of AHR-induced Tregs.
We did not identify any candidate genes that were differentially regulated by AHR activation in the pancreatic lymph nodes of 15-week-old NOD mice prior to the onset of overt diabetes. This lack of change in gene expression might reflect a reduced sensitivity to detect subtle changes due to the use of RNA from the whole lymph node tissues rather than purified CD4+ T cells. It is also possible that the lack of change reflects an alternative site of action for TCDD. In this regard, it is interesting to note that higher levels of AHR message were reported in pancreatic Tregs compared with pancreatic T-effector cells, which coexist in the pancreatic islets prior to disease onset [25]. One possibility is that TCDD activates AHR in islet Tregs to enhance their suppressive effects on T-effector cells, leading to inhibition of ongoing insulitis. This possibility would be consistent with the greatly reduced severity of insulitis present in the pancreas of TCDD-treated mice. Remarkably, the islet CD4+ CD25+CD69− Tregs described by Herman et al. [25] overexpressed several of the same genes that we found elevated in AHR-induced Tregs in the GvH model, including Blimp-1, Ccr5, Ctla4, Gzmb, Il10 and Il2ra [3]. Furthermore, Tang et al. recently reported that diabetes in the NOD mouse was associated with a progressive decline in the ratio of CD25+ Tregs to T-effector cells in inflamed islets but not in pancreatic lymph nodes, and that treatment of mice with low doses of IL-2 promoted Treg survival and protected the mice from developing diabetes [26]. Since the IL-2 gene is known to be regulated by AHR activation in T cells [27; Funatake & Kerkvliet, Unpublished Data], the possibility that TCDD works through increasing local production of IL-2 in the pancreas merits attention.
Understanding the mechanisms involved in AHR-mediated effects on Treg development and function is an important goal that may reveal new strategies for the treatment of Type 1 diabetes as well as other immune-mediated diseases. The capability to intentionally induce Tregs by administering an exogenous AHR ligand that mimics TCDD’s effects is a particularly worthwhile goal. However, screening AHR ligands for TCDD-like effects will be an essential undertaking given recent reports that show another high-affinity AHR ligand, 6-formylindolo[3,2-b]carbazole (FICZ), a tryptophan-derived photoproduct of UV irradiation, does not promote the differentiation of Tregs but instead induces Th17 cells and promotes development of experimental autoimmune encephalitis [4,28]. It is not clear how different agonistic AHR ligands might alter the behavior of the AHR, apart from the known capability of some AHR ligands (unlike TCDD) to induce their own metabolism to active metabolite(s) with different activities [29]. Rapid metabolism of FICZ by AHR-inducible enzymes has been reported [30]. Alternatively, it is possible that different ligands alter the AHR’s conformation, allowing it to bind to DNA differently or to recruit different coactivators or corepressors and thus alter gene expression differently than TCDD. Given the potent suppression of autoimmune diabetes by TCDD, the influence of alternative ligands on AHR activation and gene induction is an important area of research deserving of further study.
Conclusion
The findings suggest that AHR-mediated effects on Treg development and function may reveal new strategies for therapeutic treatment of Type 1 diabetes and highlight the importance of discovering alternative ligands for AHR activation.
Executive summary
Chronic treatment of nonobese diabetic mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) potently suppresses the development of autoimmune Type 1 diabetes.
TCDD treatment greatly reduced pancreatic islet insulitis and expanded the population of CD4+CD25+Foxp3+ cells in pancreatic lymph nodes.
Following termination of treatment, mice developed diabetes over the next 8 weeks in association with lower numbers of T regulatory cells and decreased activation of aryl hydrocarbon receptors.
Acknowledgments
We gratefully acknowledge the Environmental Health Sciences Center at OSU for support of the flow cytometry facility, and Nikki B Marshall for helpful discussions.
Financial & competing interests disclosure
This research was funded by NIH Grant numbers P01-ES00040 and P30-ES00210. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪▪.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. First demonstration and initial characterization of a CD4+CD25+ T regulatory (Treg)-like cell induced by engagement of aryl hydrocarbon receptor (AhR) via 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment. [DOI] [PubMed] [Google Scholar]
- 2▪.Funatake CJ, Marshall NB, Kerkvliet NI. 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T cells toward a regulatory T cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T cells. J Immunotoxicol. 2008;5:81–91. doi: 10.1080/15476910802019037. First paper to exhibit AHR-dependent effects on CD8+ differentiation into a regulatory phenotype induced by TCDD. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. Extensive phenotypic and functional characterization of the novel subset of CD4+ Tregs induced by TCDD compared to conventional Tregs, including expression of Foxp3 and a role for IL-12R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Quintana FJ, Basso AS, Iglesias AH, et al. Control of Treg and Th17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. First report demonstrating a differential role for different AHR ligands in the suppression or exacerbation of experimental autoimmune encephalomyelitis through the induction of Tregs versus Th17, respectively. Concurrent companion publication along with [28] [DOI] [PubMed] [Google Scholar]
- 5.Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol Appl Pharmacol. 2002;185:146–152. doi: 10.1006/taap.2002.9537. [DOI] [PubMed] [Google Scholar]
- 6.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 8.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 9.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFβ1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos. 1983;11:397–403. [PubMed] [Google Scholar]
- 11.Okey AB, Vella LM, Harper PA. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1–450 by 3-methylcholanthrene. Mol Pharmacol. 1989;35:823–830. [PubMed] [Google Scholar]
- 12.Kerkvliet NI, Baecher-Steppan L, Smith BB, et al. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fundam Appl Toxicol. 1990;14:532–541. doi: 10.1016/0272-0590(90)90257-k. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RS, Penn SG, Holden K, Bradfield CA, Rank DR. Sequence variation and phylogenetic history of the mouse Ahr gene. Pharmacogenetics. 2002;12:151–163. doi: 10.1097/00008571-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of Cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol. 2007;71:1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 15.Andre I, Gonzalez A, Wang B, et al. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci USA. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Gregg RK, Jain R, Schoenleber SJ, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. Demonstrates a link in susceptibility or sustained resistance to diabetes and the developmental and functional decline of Treg activity to the expression of membrane bound form of TGF-β. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence BP, Kerkvliet NI. Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbons. In: Leubke RHR, Kimber I, editors. Immunotoxicology and Immunopharmacology. CRC Press; Boca Raton, FL, USA: 2006. pp. 239–258. [Google Scholar]
- 19.Knutson JC, Poland A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: failure to demonstrate toxicity in twenty-three cultured cell types. Toxicol Appl Pharmacol. 1980;54:377–383. doi: 10.1016/0041-008x(80)90163-5. [DOI] [PubMed] [Google Scholar]
- 20▪.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. Demonstrates an essential role for B7/CD28 costimulation in the development and homeostasis of Tregs for control of spontaneous autoimmune diseases in nonobese diabetic (NOD) mice. [DOI] [PubMed] [Google Scholar]
- 21.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 22▪.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. First to demonstrate the phenotypic and functional characteristics of protective CD4+CD62L+ cells that differ from other cell types reported to control diabetes in NOD mice. [DOI] [PubMed] [Google Scholar]
- 23.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced Type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 24.Ema M, Ohe N, Suzuki M, et al. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- 25.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon MS, Esser C. The murine IL-2 promoter contains distal regulatory elements responsive to the Ah receptor, a member of the evolutionarily conserved bHLH-PAS transcription factor family. J Immunol. 2000;165:6975–6983. doi: 10.4049/jimmunol.165.12.6975. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links Th17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. First demonstration that AHR-dependent induction of a Th17 response leads to accelerated onset and increased pathology of experimental autoimmune encephalomyelitis in mice. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 2004;149:151–164. doi: 10.1016/j.cbi.2004.08.005. [DOI] [PubMed] [Google Scholar]