Abstract
The RNA-processing exosome contains ribonucleases that degrade aberrant RNAs in archael and eukaryotic cells. In Saccharomyces cerevisiae, the nuclear/nucleolar 3′–5′ exoribonuclease Rrp6 distinguishes the nuclear exosome from the cytoplasmic exosome. In vivo, the TRAMP complex enhances the ability of the nuclear exosome to destroy some aberrant RNAs. Previous reports showed that purified TRAMP enhanced RNA degradation by the nuclear exosome in vitro. However, the exoribonucleolytic component(s) of the nuclear exosome enhanced by TRAMP remain unidentified. We show that TRAMP does not significantly enhance RNA degradation by purified exosomes lacking Rrp6 in vitro, suggesting that TRAMP activation experiments with nuclear exosome preparations reflect, in part, effects on the activity of Rrp6. Consistent with this, we show that incubation of purified TRAMP with recombinant Rrp6 results in a 10-fold enhancement of the rate of RNA degradation. This increased activity results from enhancement of the hydrolytic activity of Rrp6 because TRAMP cannot enhance the activity of an Rrp6 mutant lacking a key amino acid side chain in its active site. We observed no ATP or polyadenylation dependence for the enhancement of Rrp6 activity by TRAMP, suggesting that neither the poly(A) polymerase activity of Trf4 nor the helicase activity of Mtr4 plays a role in the enhancement. These findings identify TRAMP as an exosome-independent enhancer of Rrp6 activity.
Keywords: Nucleic Acid, Protein/RNA Interactions, RNA, RNA/Nuclear RNA, RNA/Processing, RNA/Turnover, RNA Exosome, Rrp6
Introduction
Eukaryotic cells contain quality-control systems that monitor RNA biogenesis. These systems feature ribonucleases that prevent the accumulation of nonfunctional RNAs as well as regulate normal mRNAs and repress viral and parasitic RNAs (1). The exosome, a highly conserved RNA-processing protein complex, plays a key role in RNA surveillance by providing the major 3′–5′ exoribonucleolytic activity in eukaryotes (2). Localized in the nucleus and cytoplasm, the exosome degrades aberrant noncoding and coding RNAs and catalyzes the accurate 3′ end formation of rRNAs, small nuclear RNAs, and small nucleolar RNAs (3, 4). In Saccharomyces cerevisiae, the exosome is composed of a nine-subunit core complex and the nuclear/cytoplasmic endo-exoribonuclease Dis3/Rrp44. These components interact with the nuclear riboexonuclease Rrp6 to form the nuclear exosome. Structure and function studies on the exosome from yeast and humans showed that its structural integrity requires a nine-subunit core, which is similar to the archael exosome and bacterial polynucleotide phosphorylase (5, 6). Despite this similarity to these exonuclease-competent complexes, the yeast and human core exosome appear to possess no catalytic activity. Indeed, all of the catalytic activity resides in Dis3/Rrp44 and Rrp6 (7, 8).
The ability of the exosome to distinguish between classes of RNA appears to be driven, in part, by protein co-factors and/or covalent modifications to the RNA (9–12). Unlike mRNA, the addition of poly(A) tail to noncoding RNAs destabilizes the transcripts. In yeast, aberrant noncoding RNAs are recognized and polyadenylated by the poly(A) polymerases Trf4 and Trf5p, which function in complexes termed TRAMP4 and TRAMP5, respectively, and contain the RNA helicase Mtr4 and the zinc knuckle proteins Air1 and Air2 (13–15). This recognition and poly(A) addition result in the recruitment of the nuclear exosome and subsequent hydrolysis of the RNA. The initial characterization of this pathway showed that the nuclear exosome and TRAMP participate in the surveillance and degradation of pre-tRNAiMet (16, 17). Subsequent work showed that this pathway also degrades rRNA and small nuclear/small nucleolar RNAs as well as regulating transcription from unannotated and/or silenced regions of the genome (4, 14, 18–22).
Biochemical analysis of TRAMP function in the presence of purified exosomes corroborates in vivo observations because TRAMP enhances the degradation of RNA substrates by the exosome in vitro (14, 15, 23). However, these experiments did not identify the component(s) of the exosome enhanced by TRAMP. The current model for RNA surveillance by the nuclear exosome implies that Rrp6 activity acts in concert with the core exosome complex, either through physical association or close coupling. Indeed, affinity purifications of core exosome subunits co-purify all of the other core polypeptides, as well as Dis3/Rrp44 and Rrp6, and affinity purification of Rrp6 co-purifies all of the core subunits and Dis3/Rrp44 (24, 25). Therefore, it is likely the purified core exosomes and nuclear exosomes used in these in vitro experiments contained Rrp6. The inactivity of the nine-subunit core of the exosome indicates that the observed RNA degradation results from the action of the hydrolytic exoribonucleases Dis3/Rrp44 and/or Rrp6 present in the exosome preparations. Moreover, recent findings suggest that Rrp6 can act independently of the core exosome and Dis3/Rrp44 in the degradation of poly(A)+ rRNAs (26). Thus, we undertook a series of experiments to identify the component(s) of the exosome that is the target of TRAMP enhancement.
Here, we report that the TRAMP complex in vitro does not significantly enhance RNA degradation by purified core exosomes containing Dis3/Rrp44 but lacking Rrp6. Instead, we observe a 10-fold enhancement of recombinant Rrp6 catalytic activity by TRAMP. The enhancement by TRAMP requires no ATP or polyadenylation activity, suggesting that neither the poly(A) polymerase activity of Trf4 nor the helicase activity of Mtr4 plays a role in the enhancement. These results suggest that Rrp6 is a direct target of TRAMP enhancement.
EXPERIMENTAL PROCEDURES
Purification of GST-Rrp6 and TRAMP and the Core Exosome
GST2 -Rrp6 was purified from Escherichia coli (XL1) carrying the pRST66H plasmid as described previously (27). TRAMP was purified from 2 liters of a TRF4-TAP strain (YSB1065, MATa his3-Δ0 leu2-Δ0 met15-Δ0 ura3-Δ0 TRF4-TAP-HIS3MX6) grown to an A600 of 2.0–3.0 in yeast extract, peptone with dextrose (YPD) at 30 °C. Cells were collected by centrifugation at 7500 × g and resuspended in 10 m K-HEPES, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 0.5 mm dithiothreitol, 2 mm benzamidine, 0.5 mm phenylmethylsulfonyl fluoride, 1 mm leupeptin, 2 mm pepstatin, 4 mm chymostatin, and 2.6 mm aprotinin, and disrupted by two passes through the French press at 15,000 pounds/square inch. The cell lysate was cleared by centrifugation at 34,000 × g for 22 min and then at 75,000 × g for 60 min. The supernatant was dialyzed against 20 mm K-HEPES, pH 7.9, 50 mm KCl, 0.2 mm EDTA, 20% glycerol, 0.5 mm phenylmethylsulfonyl fluoride, and 2 mm benzamidine for 3.5 h at 4 °C. Extracts were bound to 0.2 ml of IgG beads (Amersham Biosciences) for 2 h at 4 °C with gentle rocking, and the beads were washed with 50 ml of 10 mm Tris-Cl, pH 8.0, 150 mm NaCl, and 0.1% Nonidet P-40 at 4 °C. TRAMP complexes were released from beads with 70 units of AcTEV enzyme (Invitrogen) in 10 mm Tris-Cl, pH 8.0, 150 mm NaCl, 0.1% Nonidet P-40, 0.5 mm EDTA, and 1 mm dithiothreitol for 3.5 h at 14 °C with gentle rocking. High salt wash TRAMP was prepared by washing the IgG-bound TRAMP with 40 ml of 10 mm Tris-Cl, pH 8.0, 1 m NaCl, and 0.1% Nonidet P-40 at 4 °C, followed by reequilibration with 10 mm Tris-Cl, pH 8.0, 150 mm NaCl, and 0.1% Nonidet P-40 at 4 °C and release with AcTEV protease as described above. The core exosome was purified from 2 liters of an RRP46-TAP strain (YSB239, MATa ade2-1 his3-11,5 leu2-3,112 trp1-1 ura3-1 RRP46-TAP pep4::HIS3 rrp6::LEU2) grown to an A600 of 2.0–3.0 in YPD at 30 °C following the protocol for Trf4-TAP described above except that the core exosome was further purified by adsorption and elution from calmodulin beads as described previously (28). Western blot analysis was performed as described previously (29) with polyclonal anti-Mtr4 (1:1000) and anti-Rrp4p (1:1000), generously provided by Dr. David Tollervey (University of Edinburgh) and Dr. Jesus de la Cruz (Universite de Sevilla), respectively.
Assay of GST-Rrp6, TRAMP, and the Core Exosome
Exonuclease and polyadenylation assays were carried out in 10-μl reaction mixtures at 30 °C in 10 mm Tris-Cl, pH 7.5, 12.5 mm KOAc, 5 mm Mg2OAc, 1 mm dithiothreitol, 500 μm ATP, 5% glycerol, and 3 fmol of 5′ [32P]RNA. The reactions were stopped by the addition of 2 μl of 250 mm EDTA, 4% SDS, and 4 μg/ml proteinase K and incubation at 37 °C for 15 min. Products were separated by electrophoresis on 8% polyacrylamide and 8 m urea gel and analyzed by storage phosphorimaging. The synthetic 7 S template (RNA substrate) was PCR-amplified from genomic DNA using OSB 617 (5′-ATGATTACGCCAAGCTATTTAGGTGACACTATAGTTGACCTCAAATCAGGTACGTTAGTGCCTGTTTGAGCGT-3′) and OSB 618 (5′-ACTCACTACCAAACAGAATG-3′) as primers, and the PCR product was gel-purified from 0.8% agarose gel. The two guanosines at positions 1 and 4 at the 5′ end of the pre-7 S RNA were selectively labeled by carrying out SP6 RNA polymerase transcription of the template in the presence of 5′ [α-32P]GTP, CTP, and TTP, and chasing the stalled transcription complex by the addition of ATP and excess unlabeled GTP (29). The RNA substrate used in the experiment shown in Fig. 4B was synthesized with SP6 RNA polymerase using 5′ [α-32P]GTP and pSP64 poly(A) cut with EcoRI as described previously (30).
FIGURE 4.
TRAMP enhances of the exoribonuclease activity of Rrp6. A, GST-Rrp6 (0.3 pmol) or GST-Rrp6-6p was incubated in a 10-μl reaction with or without TRAMP (0.4 pmol) in the presence of 3 fmol of 5′ [32P]RNA substrate for 60 min at 30 °C and analyzed as described in the legend to Fig. 1. The panel is a composite of lanes taken from a single experiment. The values listed below the lane numbers represent the percentage of the RNA substrate remaining at the end of incubation. B, GST-Rrp6 and GST-Rrp6 + TRAMP were incubated as above with an RNA substrate uniformly labeled with 5′ [α-32P]guanosine, and the reaction products were separated by thin layer chromatography. The panel is a composite of lanes taken from a single experiment. The values listed below the lane numbers represent amount of product produced.
RESULTS
TRAMP Complex Does Not Enhance RNA Degradation by the Core Exosome Containing Dis3/Rrp44 but Lacking Rrp6 in Vitro
Previous characterizations of the role that the TRAMP complex plays in RNA degradation did not distinguish between a role for the core exosome and Rrp6 in general RNA or pre-tRNAiMet degradation, although recent evidence suggests that the core exosome component Dis3/Rrp44 and TRAMP suffice for recognition and degradation of pre-tRNAiMet (14, 15, 23). The significant accumulation of RNAs polyadenylated by TRAMP in Rrp6-deficient strains indicates a critical role for the catalytic activity of Rrp6 in their degradation (31, 32). Furthermore, there is a greater accumulation of some RNA species polyadenylated by TRAMP in Rrp6-deficient cells compared with cells depleted of core exosome components (4, 21), and strains defective for the interaction of Rrp6 with the exosome continue to degrade some Rrp6-specific RNAs (26).
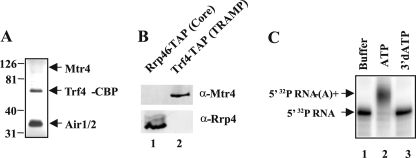
We purified the TRAMP complex from yeast using the tandem affinity method of Vanacova et al. (15). The strain used carries a chromosomally encoded TRF4-TAP gene fusion in which the coding sequence of Trf4 is fused at its carboxyl terminus to a tandem affinity tag containing the calmodulin-binding protein (CBP) and protein A, allowing purification of TRAMP on IgG beads and subsequent release of Trf4-CBP by cleavage of the affinity tag with the tobacco etch virus protease (Fig. 1A). Similar to other TRAMP preparations, ours appears to contain substoichiometric levels of Mtr4 compared with Trf4 and Air1 or Air2 (Fig. 1A) (21). Western blot analysis of this preparation shows that it contains Mtr4, but not the core exosome as indicated by the absence of Rrp4 (Fig. 1B). Incubation of this purified complex with a 5′ 32P-labeled RNA substrate corresponding to the 3′ 154 nucleotides of the 7 S pre-rRNA results in the polyadenylation of the RNA, as expected (Fig. 1C, lane 2). In contrast, substitution of 3′-dATP for ATP results in no observable change in RNA mobility, consistent with the fact that addition of 3′-dATP to RNA blocks subsequent elongation of the tail by poly(A) polymerases (Fig. 1C, lane 3).
FIGURE 1.
TRAMP polyadenylates a synthetic 7 S RNA in vitro. A, silver-stained polyacrylamide gel of 1.4 μg of TRAMP purified from a TRF4-TAP strain. The positions of molecular mass markers are shown on the left, and the identities of TRAMP subunits are shown on the right. B, Western blot comparison of 1 μg of affinity-purified TRAMP (lane 1) and 1.2 μg of affinity-purified core exosome (lane 2) using polyclonal antibodies to Rrp4 and Mtr4. C, TRAMP (0.3 pmol) incubated in a 10-μl reaction in the presence of 3 fmol of 5′ [32P]RNA substrate and either buffer, ATP (500 μm) or 3′-dATP (500 μm) for 60 min at 30 °C. The products and substrates were separated by denaturing PAGE and analyzed by storage phosphorimaging. The panel is a composite of lanes taken from a single experiment.
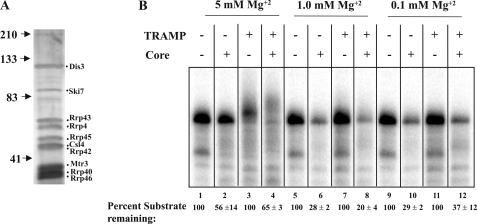
Next, we tested the ability of this complex to enhance the activity of the core exosome in vitro. We purified core exosomes from an rrp6-Δ strain that contained a TAP-tagged Rrp46 (Fig. 2A). Core exosomes were purified in a manner similar to TRAMP; however, they were washed with buffer containing a higher concentration of NaCl (500 mm versus 150 mm, respectively). Recent reports indicated that Dis3/Rrp44 provides the only active exonuclease activity in the yeast core exosome and that this enzyme works most efficiently at low Mg2+ levels (7, 8, 33). Indeed, we found that our core exosome preparation catalyzed RNA degradation far better at 1 mm and 0.1 mm Mg2+ than at 5 mm Mg2+ (Fig. 2B, compare lanes 2 with 6 and 10). We observed only slight enhancement of core exosome degradation by TRAMP under these conditions (Fig. 2B, compare lanes 6 and 8, and 10 and 12). The modest enhancement of activity by TRAMP may reflect the fact that it appears most active at the Mg2+ level (5 mm) where the core exosome exhibits minimal activity (Fig. 2B, lanes 2 and 4).
FIGURE 2.
TRAMP does not enhance RNA degradation by the core exosome. A, silver-stained polyacrylamide gel of 2.5 μg of core exosome purified from an RRP46-TAP strain. The positions of molecular mass markers are shown on the left, and the inferred identities of exosome subunits are shown on the right. B, TRAMP (0.4 pmol) and/or core exosome (0.4 pmol) incubated in a 10-μl reactions in the presence of 3 fmol of 5′ [32P]RNA substrate, ATP (500 μm), and the indicated concentrations of Mg2+ (Mg2OAc) for 60 min at 30 °C and analyzed as Fig. 1. The values listed below the lane numbers represent the average ± S.E. for the amount of substrate remaining after incubation and are calculated from the results of two independent experiments.
TRAMP Complex Enhances Rrp6-catalyzed RNA Degradation in Vitro
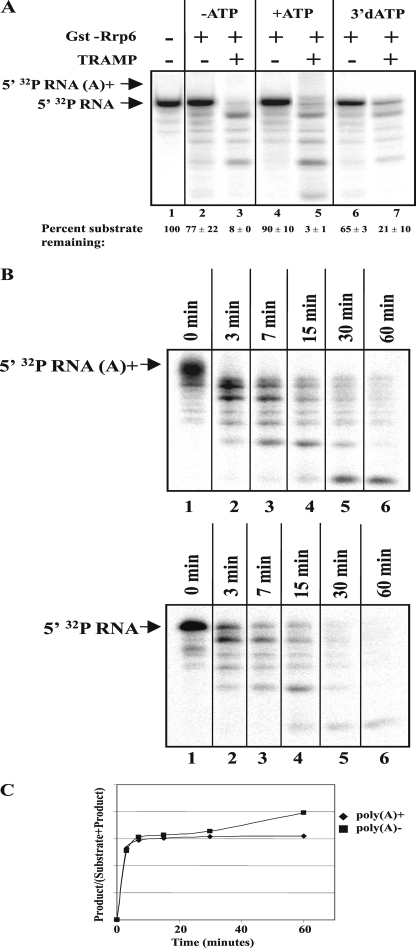
The inability of the TRAMP complex to enhance RNA degradation by Dis3/Rrp44 and the core exosome in vitro suggested that Rrp6 might be a direct target of the TRAMP complex (Fig. 2). Accordingly, we tested the ability of TRAMP to alter the activity of GST-Rrp6 purified from E. coli (Fig. 3A). Our previous experiments showed that GST-Rrp6 functions normally in vitro and in vivo in yeast (29). Titration of GST-Rrp6 in the presence of a fixed amount of TRAMP indicates that, at levels of GST-Rrp6 showing moderate levels of RNA degradation activity, the addition of TRAMP significantly enhances hydrolysis of the RNA (Fig. 3B, compare lanes and 10, and 11 and 12). Quantification of the amount of Rrp6-catalyzed RNA degradation in the absence and presence of TRAMP suggests that it enhances the activity of Rrp6 about 10-fold (Fig. 3B, compare lane 5 with 12).
FIGURE 3.
TRAMP enhances RNA degradation by recombinant Rrp6. A, Coomassie Blue-stained SDS-PAGE analysis of 2.75 μg of GST-Rrp6 purified from E. coli. The positions of molecular size markers are indicated at left. B, indicated quantities of GST-Rrp6 incubated with 0.3 pmol of TRAMP and 3 fmol of 5′ [32P]RNA substrate for 60 min at 30 °C. The reactions were stopped, and the products were separated by denaturing PAGE. The gel was visualized and quantitated by storage phosphorimaging. The values listed below the lane numbers represent the amount of RNA substrate remaining at the end of incubation. C, time course analysis of the effect of TRAMP on GST-Rrp6 degradation activity. TRAMP (0.3 pmol) and Rrp6 (0.3 pmol) were incubated with 3 fmol of 5′ [32P]RNA substrate at 30 °C for the indicated amounts of time and analyzed as in B. D, graphic display of the average rate of product formation by GST-Rrp6 in the presence and absence of TRAMP from the experiment shown in C and two additional measurements. Product was defined as RNAs shorter than the substrate and was quantified by storage phosphorimaging analysis. Error bars represent the S.D. from the three measurements.
We quantitatively assessed the TRAMP enhancement of Rrp6 activity by measuring the initial rate of RNA degradation with and without TRAMP. The results show three important aspects of the reaction. First, little RNA degradation occurs during the 60-min incubation in the presence of Rrp6 alone (Fig. 3C, lane 17). Second, in the presence of TRAMP alone, the RNA substrate increases in length, consistent with distributive polyadenylation of the substrate (Fig. 3C, lane 16). Third, the combination of TRAMP and GST-Rrp6 results in significantly enhanced degradation of the RNA substrate compared with GST-Rrp6 alone (Fig. 3C, compare lanes 17 and 18). Graphical analysis of the reaction rate indicates that TRAMP causes a 10-fold increase in the initial rate of RNA degradation by GST-Rrp6 (Fig. 3D). The ability of TRAMP to enhance GST-Rrp6 and some exosome preparations containing Rrp6 further implicates Rrp6 as a direct target of TRAMP (14, 15).
Next, we tested the possibility that the observed enhancement of Rrp6 activity by TRAMP reflected the activation of a latent nuclease activity in the TRAMP preparation. Accordingly, we assayed the ability of TRAMP to enhance the activity of a catalytically inactive Rrp6 mutant, Rrp6-6. This enzyme contains a Y361A mutation in its active site that inactivates the protein in vivo and in vitro (27) (Fig. 4, compare lanes 2 and 4). Addition of TRAMP to GST-Rrp6-6 does not result in RNA degradation, but does result in polyadenylation of the RNA by TRAMP (Fig. 4, lanes 4 and 5). Thus, Rrp6 does not activate a nuclease activity nor inhibit the polyadenylation activity of TRAMP.
We tested the generality and mechanism of TRAMP enhancement of Rrp6 and the mechanism of degradation in an assay containing a different 85-nucleotide RNA substrate uniformly labeled with 5′ [α-32P]guanosine. Previous studies with internally labeled RNA indicated that Rrp6 produces 5′-phosphorylated mononucleotides indicative of a hydrolytic mechanism of exonuclease activity (29). In the present experiment, thin layer chromatography of the reaction products shows that TRAMP enhances the production of 5′ [32P]guanosine by Rrp6 (Fig. 4B). This supports the conclusion that TRAMP enhances the hydrolytic exonuclease activity of Rrp6.
TRAMP Enhancement of Rrp6 Does Not Require ATP or Polyadenylation of the RNA Substrate
The TRAMP complex contains Trf4, an ATP-dependent poly(A) polymerase, and Mtr4, an ATP-dependent RNA helicase. We asked whether the enhancement of Rrp6 activity by TRAMP requires the presence of ATP or the addition of a poly(A) tail. The absence of ATP had little effect on the observed ability of TRAMP to enhance RNA degradation by GST-Rrp6 (Fig. 5A). Thus, it appears that neither ATP-dependent helicase activity nor polyadenylation of the RNA substrate is required for the observed enhancement. These findings agree with similar observations made by LaCava et al. (14) for the enhancement of exosome activity by TRAMP in vitro.
FIGURE 5.
TRAMP enhancement of the nuclease activity of Rrp6 does not require ATP or the presence of a poly(A) tail. A, GST-Rrp6 (0.3 pmol) was incubated with or without TRAMP (0.3 pmol in a 10-μl reaction in the presence of 3 fmol of 5′ [32P]RNA substrate and either buffer, ATP (500 μm), or 3′-dATP (500 μm) for 60 min at 30 °C and analyzed as described in the legend of Fig. 3. The values listed below the lane numbers represent the average ± S.E. for the amount of substrate remaining after incubation and are calculated from the results of two independent experiments. B, 5′ [32P]RNA substrate was preincubated with TRAMP in the presence (upper panel) or the absence (lower panel) of ATP. The reactions were phenol-extracted to remove TRAMP, and the RNA substrate was concentrated by ethanol precipitation. The RNAs were then incubated in the presence of GST-Rrp6 (1 pmol) for 60 min and analyzed as described in the legend of Fig. 3. C, graphic display of the rate of product formation by GST-Rrp6 for the poly(A)+ and poly(A)− substrates in the experiments shown in B. Product was defined as RNAs shorter than the substrate and was quantified by storage phosphorimaging analysis.
Consistent with these conclusions, inclusion of the poly(A) polymerase inhibitor 3′-dATP did not abolish TRAMP-enhanced RNA degradation by Rrp6 (Fig. 5A, lane 6). Nevertheless, addition of 3′-dATP did slightly inhibit degradation of the RNA. Additional experiments showed that extended preincubation of the substrate with TRAMP and 3′-dATP converted the RNA to a form largely resistant to subsequent treatment with Rrp6 (supplemental Fig. 1). This suggests that the absence of a 3′-hydroxyl on the RNA inhibits the exonuclease activity of Rrp6, a finding consistent with previous experiments showing that a 3′-phosphate renders an RNA substrate resistant to Rrp6 (29).
Previous studies of TRAMP enhancement of exosome activity suggested that the presence of a poly(A) tail might recruit the exosome to the substrate, thereby increasing the rate of degradation (14, 15). We tested the role of a poly(A) tail in Rrp6 activity by first polyadenylating the RNA substrate with TRAMP and then removing TRAMP prior to incubation of the RNA with Rrp6. The results show that polyadenylated and unadenylated substrates are degraded at similar rates (Fig. 5B). These results indicate that enhancement of Rrp6 activity by TRAMP requires neither the ATP-dependent activities of Trf4 and Mtr4 nor the presence of a poly(A) tail on the RNA substrate.
TRAMP Depleted of Mtr4 Enhances RNA Degradation by Rrp6
Previous studies showed that a high salt wash during TRAMP preparation removed Mtr4 from the complex (14, 15). Accordingly, we washed our TRAMP preparation extensively with buffer containing 1 m NaCl prior to elution of the complex from IgG beads. Western blot analysis revealed the presence of Trf4-CBP, but detected no Mtr4 (Fig. 6A). In agreement with previous work, we found that Trf4 and Air1 + 2p are sufficient for polyadenylation of an RNA substrate (Fig. 6B, lane 4) (15, 21). The high salt wash TRAMP (HSW TRAMP) enhanced the degradation of RNA by Rrp6 as efficiently as TRAMP containing Mtr4 (Fig. 6B, compare lanes 3 and 6). Moreover, both TRAMP preparations proved equally effective in enhancing Rrp6 degradation in the absence of ATP (Fig. 6C). These findings suggest that Mtr4 does not play a critical role in the ability of TRAMP to enhance the activity of Rrp6 with this RNA substrate.
FIGURE 6.
TRAMP depleted of Mtr4 enhances RNA degradation by Rrp6. A, Western blot comparison of 0.7 μg of affinity-purified TRAMP and 0.7 μg of affinity-purified high salt wash (HSW) TRAMP using polyclonal antibodies to Mtr4 and CBP that detects the Trf4-CBP fusion protein. B and C, GST-Rrp6 (0.3 pmol) incubated with or without TRAMP (0.4 pmol) or high salt wash TRAMP (HSW TRAMP) (0.4 pmol) in a 10-μl reaction in the presence of 3 fmol of 5′ [32P]RNA substrate in either ATP (500 μm) (B) or buffer (C) for 60 min at 30 °C and analyzed as described in the legend to Fig. 1. The values listed below the lane numbers in B and C represent the amount of substrate remaining after incubation.
DISCUSSION
The evidence reported here suggests that the TRAMP complex enhances the activity of the exoribonuclease Rrp6. We found that RNA degradation by purified core exosomes containing Dis3/Rrp44, but lacking Rrp6, is not significantly enhanced by TRAMP in vitro. In contrast, TRAMP enhanced the rate of RNA degradation by recombinant GST-Rrp6. This increased activity results from enhancement of the hydrolytic activity of Rrp6 because TRAMP does not hydrolyze RNA alone and cannot enhance the activity of an Rrp6 mutant lacking a key amino acid side chain in its active site. Thus, these findings suggest that Rrp6 may be a direct target of TRAMP during RNA surveillance in S. cerevisiae.
Previous reports showed that purified TRAMP enhanced RNA degradation by the nuclear exosome in vitro (14, 15). However, those experiments did not identify the exoribonucleolytic component(s) of the nuclear exosome enhanced by TRAMP. Our findings indicate that the TRAMP complex significantly enhances the activity of Rrp6 in vitro, but not the activity of exosomes containing Dis3/Rrp44 but lacking Rrp6. The 10-fold enhancement of Rrp6 activity observed in the absence of the core exosome compares well with the magnitude of enhancement by TRAMP of core exosomes containing Rrp6 and Dis3/Rrp44 (14). Moreover, the Mg2+ concentrations used in the previous experiments should significantly inhibit Dis3/Rrp44 activity because our findings, and the experiments of others, showed that Dis3/Rrp44 is active only at much lower concentrations (<1 mm) of the cation (7, 23, 33). Under these conditions, polyadenylation of RNA substrates by TRAMP occurs very slowly (Fig. 2) (23). Nevertheless, previous experiments show that recombinant Dis3/Rrp44 binds hypomodified tRNAiMet under these conditions, and, although only modest polyadenylation by TRAMP occurs, it does enhance degradation of the RNA by Dis3/Rrp44 (23). The fact that TRAMP enhances Dis3/Rrp44 degradation of purified hypomodified tRNAiMet in the absence of the core exosome but has little effect on the rate of core exosome and Dis3/Rrp44 degradation of our 7 S RNA substrate suggests that the presence of the core exosome and/or the nature of the RNA substrate has significant effects on the activity of Dis3/Rrp44. Indeed, studies in vitro with several model RNA substrates in the presence of Dis3/Rrp44, or the core exosome plus Dis3/Rrp44 showed that the presence of the core exosome inhibits Dis3/Rrp44 activity (8). In contrast, reactions containing the core exosome, Dis3/Rrp44p and Rrp6 exhibit the RNA degradation specificity of Rrp6.
We observed no ATP or polyadenylation dependence for the enhancement of Rrp6 activity by TRAMP, suggesting that neither the poly(A) polymerase activity of Trf4 nor the helicase activity of Mtr4 plays a role in the enhancement. Moreover, TRAMP depleted of Mtr4 enhanced Rrp6 as well as TRAMP containing Mtr4. Although we cannot exclude effects due to residual Mtr4, it seems likely that it plays no significant role in enhancing Rrp6 activity. Rougemaille et al. (19) showed that TRAMP-dependent degradation of HSP101 mRNA in vivo required the presence of Trf4, but not its catalytic activity, suggesting that Trf4 polyadenylation activity is not required for efficient mRNA degradation by the nuclear exosome. Similarly, global changes in RNA transcript levels caused by the absence of Trf4 do not appear to result from loss of its polyadenylation activity (34). Experiments in vitro also showed that TRAMP enhanced RNA degradation by the exosome in the absence of ATP (14). In contrast, other work showed that efficient degradation of tRNAiMet by the nuclear exosome required catalytically active Trf4 and the presence of Mtr4, which require ATP for their polyadenylation and helicase activities, respectively (15, 17). The different results in vitro obtained by the two groups may reflect combinations of different exosome preparations and RNA substrates that result in different degrees of dependence on polyadenylation and helicase activities of TRAMP. Indeed, TRAMP demonstrates a clear preference for the polyadenylation of hypomodified tRNAiMet, possibly reflecting tertiary structural differences between it and the fully modified tRNAiMet (15). These findings may reflect separable roles of TRAMP components in RNA substrate recognition and acceleration of substrate degradation. The former activity may result from the RNA binding activities of the Air proteins and the latter from the ATP-dependent activities of Trf4 and Mtr4. The evidence to date suggests that Mtr4 and Trf4 enzymatic activities may be dispensable for some RNA substrates.
In the present study, we used recombinant Rrp6 to study the degradation of an RNA containing the 3′ end sequence of 7 S pre-rRNA that is predicted to contain considerable secondary structure. The degradation pattern of these 5′ 32P-labeled RNAs shows a reproducible collection of intermediate products that may reflect pausing or release of Rrp6 from the RNA at base-paired portions of the substrate. The enhancement of degradation by TRAMP does not significantly alter this pattern, suggesting that TRAMP does not increase the processivity of Rrp6. Instead, we favor the idea that a component of TRAMP enhances the RNA binding ability of Rrp6, thereby accelerating substrate degradation. Rrp6 interacts with complexes containing the TRAMP components, thus its association with the putative RNA-binding proteins Air1 or Air2 could serve to enhance RNA binding in an ATP-independent manner (12, 24, 25).
In summary, we presented evidence that the TRAMP complex does not enhance the activity of the core exosome and Dis3/Rrp44 in vitro. TRAMP does significantly enhance the activity of the nuclear exosome component Rrp6. Our studies in vitro complement recent observations in vivo indicating that degradation of some rRNA intermediates require Rrp6, but not the core exosome and Dis3/Rrp44 (4, 26). These studies support an exosome-independent role for Rrp6 in RNA surveillance and suggest that enhancement of the degradation of specific transcripts may not require all of the activities of the TRAMP complex.
Supplementary Material
Acknowledgments
We appreciate the gifts of antibodies from David Tollervey (Rrp4, University of Edinburgh) and Jesus de la Cruz (Mtr4, Universidad de Sevilla). We thank Eric Phizicky, Yi-tao Yu, Tom Eickbush, and Jason Hoskins for advice.
This work was supported, in whole or in part, by National Institutes of Health Grant GM-59898. This work was also supported by National Science Foundation Grant MCB-0817324.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- GST
- glutathione S-transferase
- TAP
- tandem affinity protein
- CBP
- calmodulin-binding protein.
REFERENCES
- 1.Doma M. K., Parker R. (2007) Cell 131, 660–668 [DOI] [PubMed] [Google Scholar]
- 2.Vanacova S., Stefl R. (2007) EMBO Rep. 8, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler J. S. (2002) Trends Cell Biol. 12, 90–96 [DOI] [PubMed] [Google Scholar]
- 4.Houseley J., Tollervey D. (2006) EMBO Rep. 7, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorentzen E., Conti E. (2005) Mol. Cell 20, 473–481 [DOI] [PubMed] [Google Scholar]
- 6.Lorentzen E., Walter P., Fribourg S., Evguenieva-Hackenberg E., Klug G., Conti E. (2005) Nat. Struct. Mol. Biol. 12, 575–581 [DOI] [PubMed] [Google Scholar]
- 7.Dziembowski A., Lorentzen E., Conti E., Séraphin B. (2007) Nat. Struct. Mol. Biol. 14, 15–22 [DOI] [PubMed] [Google Scholar]
- 8.Liu Q., Greimann J. C., Lima C. D. (2006) Cell 127, 1223–1237 [DOI] [PubMed] [Google Scholar]
- 9.Mullen T. E., Marzluff W. F. (2008) Genes Dev. 22, 50–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayne E. H., White S. A., Allshire R. C. (2007) Cell 129, 651–653 [DOI] [PubMed] [Google Scholar]
- 11.Arigo J. T., Eyler D. E., Carroll K. L., Corden J. L. (2006) Mol. Cell 23, 841–851 [DOI] [PubMed] [Google Scholar]
- 12.Vasiljeva L., Buratowski S. (2006) Mol. Cell 21, 239–248 [DOI] [PubMed] [Google Scholar]
- 13.Kadaba S., Wang X., Anderson J. T. (2006) RNA 12, 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., Jacquier A., Tollervey D. (2005) Cell 121, 713–724 [DOI] [PubMed] [Google Scholar]
- 15.Vanácová S., Wolf J., Martin G., Blank D., Dettwiler S., Friedlein A., Langen H., Keith G., Keller W. (2005) PLoS Biol. 3, e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadaba S., Krueger A., Trice T., Krecic A. M., Hinnebusch A. G., Anderson J. (2004) Genes Dev. 18, 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Jia H., Jankowsky E., Anderson J. T. (2008) RNA 14, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houseley J., Kotovic K., El Hage A., Tollervey D. (2007) EMBO J. 26, 4996–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rougemaille M., Gudipati R. K., Olesen J. R., Thomsen R., Seraphin B., Libri D., Jensen T. H. (2007) EMBO J. 26, 2317–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chekanova J. A., Gregory B. D., Reverdatto S. V., Chen H., Kumar R., Hooker T., Yazaki J., Li P., Skiba N., Peng Q., Alonso J., Brukhin V., Grossniklaus U., Ecker J. R., Belostotsky D. A. (2007) Cell 131, 1340–1353 [DOI] [PubMed] [Google Scholar]
- 21.Wyers F., Rougemaille M., Badis G., Rousselle J. C., Dufour M. E., Boulay J., Régnault B., Devaux F., Namane A., Séraphin B., Libri D., Jacquier A. (2005) Cell 121, 725–737 [DOI] [PubMed] [Google Scholar]
- 22.Wang S. W., Stevenson A. L., Kearsey S. E., Watt S., Bähler J. (2008) Mol. Cell. Biol. 28, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider C., Anderson J. T., Tollervey D. (2007) Mol. Cell 27, 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krogan N. J., Peng W. T., Cagney G., Robinson M. D., Haw R., Zhong G., Guo X., Zhang X., Canadien V., Richards D. P., Beattie B. K., Lalev A., Zhang W., Davierwala A. P., Mnaimneh S., Starostine A., Tikuisis A. P., Grigull J., Datta N., Bray J. E., Hughes T. R., Emili A., Greenblatt J. F. (2004) Mol. Cell 13, 225–239 [DOI] [PubMed] [Google Scholar]
- 25.Gavin A. C., Bösche M., Krause R., Grandi P., Marzioch M., Bauer A., Schultz J., Rick J. M., Michon A. M., Cruciat C. M., Remor M., Höfert C., Schelder M., Brajenovic M., Ruffner H., Merino A., Klein K., Hudak M., Dickson D., Rudi T., Gnau V., Bauch A., Bastuck S., Huhse B., Leutwein C., Heurtier M. A., Copley R. R., Edelmann A., Querfurth E., Rybin V., Drewes G., Raida M., Bouwmeester T., Bork P., Seraphin B., Kuster B., Neubauer G., Superti-Furga G. (2002) Nature 415, 141–147 [DOI] [PubMed] [Google Scholar]
- 26.Callahan K. P., Butler J. S. (2008) Nucleic Acids Res. 36, 6645–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips S., Butler J. S. (2003) RNA 9, 1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 29.Burkard K. T., Butler J. S. (2000) Mol. Cell. Biol. 20, 604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler J. S., Sadhale P. P., Platt T. (1990) Mol. Cell. Biol. 10, 2599–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis C. A., Ares M., Jr. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3262–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuai L., Fang F., Butler J. S., Sherman F. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8581–8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorentzen E., Basquin J., Tomecki R., Dziembowski A., Conti E. (2008) Mol. Cell 29, 717–728 [DOI] [PubMed] [Google Scholar]
- 34.San Paolo S., Vanacova S., Schenk L., Scherrer T., Blank D., Keller W., Gerber A. P. (2009) PLoS Genet 5, e1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.