Abstract
JLP (JNK-associated leucine zipper protein) is a novel scaffolding protein involved in JNK signaling. Although it is known that JLP is highly expressed in brain, the biological function of JLP in neuronal systems remains unknown. Here, we report a novel interaction between JLP and SCG10 (superior cervical ganglia clone 10), which is a microtubule-destabilizing factor that is essential for neurite outgrowth. Inhibition of endogenous JLP expression using small interference RNA methodology strongly enhanced nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Our results show that JLP negatively regulates NGF-induced neurite outgrowth by decreasing the level of phosphorylated SCG10. Furthermore, inhibition of JNK phosphorylation by a small molecule inhibitor, SP600125, resulted in inhibition of SCG10 phosphorylation and inhibition of neurite growth. Taken together, our results suggest that JLP negatively regulates NGF-induced neurite outgrowth through a sequestering mechanism that results in an attenuation of NGF-induced SCG10 phosphorylation.
Keywords: Cytoskeleton/Microtubules, Cytoskeleton/Neurofilaments, Cytoskeleton/Tubulin, Neurobiology/Neuroscience, Phosphorylation/Kinases/Serine-Threonine, Phosphorylation/Serine/Threonine, Protein/Protein-Protein Interactions, Signal Transduction/Adapter Proteins
Introduction
Neurite outgrowth is accomplished by continuous reorganization of cytoskeletal proteins, of which, microtubules (MTs)4 play a critical role. Neuronal MTs exist in a highly dynamic state in growth cones of neurons. There, MTs form dense parallel arrays in the axon shaft and are oriented with their plus ends toward the growth cone (1–3). Although the minus ends of MTs are relatively stable, the plus ends of MTs undergo variable phases of polymerization and depolymerization, thereby affecting neurite extension. Thus, the rates of neurite elongation and growth cone advance are dependent on proper regulation of microtubule polymerization and depolymerization reactions (1–4).
SCG10, a neural-specific phosphoprotein, plays a critical role in neuronal development through its microtubule-destabilizing activity (4, 5). During neuronal development, SCG10 expression is highly up-regulated as neurons become differentiated (5, 6). It has been shown that SCG10 contains several serine residues that can be phosphorylated by multiple kinases, including c-Jun N-terminal kinase (JNK) (5). Recent studies have also shown that JNK-mediated phosphorylation controls the ability of SCG10 to mediate depolymerization of MTs. The microtubule-destabilizing activity of SCG10 is dependent on the phosphorylation status of the protein (7) and inhibition of JNK activity by small molecule inhibitors was found to abrogate the ability of SCG10 to mediate tubulin depolymerization. These observations suggest that SCG10 phosphorylation is a critical step in microtubule homeostasis and axondendritic growth during brain development (8).
JNKs participate in multiprotein cascades with upstream activators and downstream effectors. This kinase cascade is regulated spatiotemporally by scaffolding proteins such as the JIP/JSAP/JLP family (9, 10). The kinase activity of JNK is modulated by additional upstream kinases such as MKK4 and MKK7, and this signaling module appears to function as a complex that involves the participation of scaffolding proteins such as JIP1, JIP2, JIP3/JSAP1, JLP, SPAG9, JIP4, and POSH (10). Of these, JLP is highly expressed in the brain tissue and has been shown to bind to a number of signaling molecules, including JNK and to modulate their activity. JLP contains two leucine zipper domains (LZI and LZII), and a C-terminal domain (CTD), which are highly conserved as evidenced by their presence in the UNC-16 protein of Caenorhabditis elegans (11). The LZI domain of JLP interacts with MAX. The JLP LZII domain associates with kinesin light chain and mediates the subcellular localization of JLP (11, 12). However, the nature of proteins that bind to the CTD of JLP remains unknown, despite the fact that it is the most highly conserved domain.
To search for novel JLP-interacting protein(s), we performed a yeast two-hybrid screen with the CTD of JLP as the bait using a cDNA library derived from mouse brain. Here we report identification of SCG10 as a JLP-CTD interacting protein. To understand the biological significance of the interaction between JLP and SCG10, we specifically inhibited the endogenous expression of JLP using siRNA methodology in NGF-stimulated PC12 cells. Our studies indicate that JLP negatively regulates NGF-induced neurite outgrowth by inhibiting JNK-dependent phosphorylation of SCG10. This is the first indication that JLP mediates neurite outgrowth through the specific interaction with microtubule dynamics regulators, suggesting a novel role for the JIP/JLP family of scaffolding protein in neuronal differentiation and development.
EXPERIMENTAL PROCEDURES
Cell Culture
H1299 and COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. PC12 cells were obtained from the American Type Culture Collection (ATCC) and cultured on 100-mm Falcon tissue culture plates precoated with collagen and polylysine in RPMI 1640 medium supplemented with 8% fetal bovine serum and 8% horse serum.
Plasmids
The pSG5 plasmids expressing S-tagged WT-JLP, JLP deletion mutants, and JLP domain mutants have been described previously (11). Wild-type stathmin, SCG10, and SCLIP cDNAs were cloned into the pSG5 vector that contained a HA tag via EcoRI and MluI restriction sites. To make the SCG10 domain mutants, the following primers were used: for domain A, 5′-ATG GCT AAA ACA GCA ATG GCC and antisense, 5′-CGC CAA CGC GTT CTT TGG AGA AGC TAG AGT TCG; for domain B, sense 5′-CGC GAA TTC GCC ACC ATG AAG AAA GAC CTG TCT CTG GAG and antisense, 5′-CGC CAA CGC GTT GCC AGA CAG TTC AAC CTG CAG; and for domain C, sense 5′-CGC GAA TTC GCC ACC ATG ACC TAC GAC GAC ATG GAG GTG and antisense, 5′-CGC CAA CGC GTT GCC AGA CAG TTC AAC CTG CAG. All PCR products were cloned into the pSG5 vector that contained a HA tag via EcoRI and MluI restriction sites. All the constructs were confirmed by sequence analysis.
Generation of a Rabbit Polyclonal Anti-SCG10 Antibody
Because the N-terminal region of SCG10 is insoluble, a truncated form that is devoid of the first 35 amino acids was used as an antigen to raise a rabbit polyclonal anti-SCG10 antibody (Rockland Immunochemicals). The antibody was tested and immunoaffinity-purified for the immunoprecipitation studies.
Immunoprecipitation
Cell lysates were prepared in lysis buffer containing 25 mm HEPES (pH 7.6), 0.1% Triton X-100, 20 mm β-glycerophosphate, 300 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 0.2 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium fluoride, and protease inhibitors (apostatin, leupeptin, and pepstatin). For S-agarose precipitation, the cell lysates were incubated with S-protein agarose for 1 h at 4 °C. For anti-SCG10 or anti-JLP immunoprecipitation, the cell lysates were incubated with the anti-JLP or anti-SCG10 antibodies for 1 h at 4 °C, followed by a 1-h incubation with protein G-Sepharose at 4 °C. The precipitates were analyzed by SDS-PAGE and immunoblotted with the indicated antibodies.
siRNA Transfection and NGF Treatment
JLP siRNAs were chemically synthesized (Dharmacon). The sequences were as follows: for PC12 cell transfection, 5′-AACACTTGGAAAGAACCAAAC-3′; for H1299 cell transfection, 5′-AACACTTAGAAAGAACCAAAC-3′. For PC12 cells, 10 μl of the JLP siRNA (100 μm stock) was mixed with 25 μl of Lipofectamine 2000 reagent (Invitrogen); for H1299 cells, 6 μl of the JLP siRNA (100 μm) was mixed with 15 μl of Lipofectamine 2000 reagent. The mixtures were applied according to the manufacture's protocol. Two days after transfection, 100 ng/ml NGF was added to the transfected PC12 cells.
Neurite Outgrowth Analysis
Analyses of neurite extension were performed on digitized images of live cells taken under phase contrast illumination with an Olympus IMT2 inverted microscope linked to a digital camera. Images of random fields were taken. The number of differentiated cells was determined by visual examination of the field and counting cells that had at least one neurite with a length longer than the cell body diameter. More than 200 cells were examined in each sample. Neurite length was determined by manually tracing the length of the longest neurite of differentiated cells using the NIH ImageJ program. Experiments were repeated at least three times using cultures prepared on separate days. The p value was calculated using the student t test.
In Vivo Phosphorylation Analysis
PC12 cells were transfected with JLP siRNA and treated with NGF as described above. After 2 days, the culture medium was replaced with phosphate-free Dulbecco's modified Eagle's medium for 2 h. 3 mCi of [32P]orthophosphate was then added into each plate. Following a 2-h incubation, the radiolabeled cells were harvested and subjected to immunoprecipitation analysis with an anti-SCG10 antibody as described above. All results were quantitated using the Fuji Image Gauge program.
JNK Inhibitor Assay
PC12 cells (3 × 106/100-mm plate) were treated with vehicle (DMSO) or 10 μm of the specific JNK inhibitor SP600125 (Invitrogen) for 2 h before the NGF treatment. 10 μm of the inhibitor was applied based on a dose titration assay that showed that the inhibitor at 10 μm effectively inhibited JNK activity without reducing cell viability (supplemental Fig. 1).
RESULTS
JLP Interacts with SCG10/SCLIP
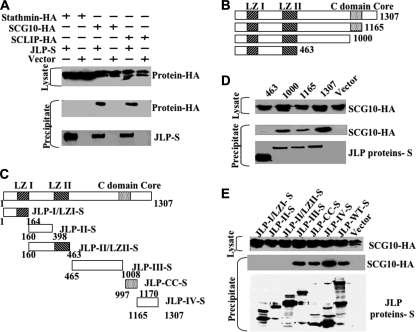
Our studies, described earlier, have shown that JLP is a scaffolding protein that regulates JNK/p38MAPK signaling (11). JLP contains two leucine zipper domains (LZI and LZII). JLP also contains a CTD that is highly conserved through evolution (11). The identity of cellular factors that bind to the CTD of JLP remains unknown, despite the fact that it is the most highly conserved domain. To search for proteins that interact with CTD of JLP, we performed a yeast two-hybrid screen with the CTD (amino acids 956–1161) as the bait using a mouse brain cDNA library. This screen identified SCG10 as one of the proteins that specifically interacted with the CTD of JLP (data not shown). Because SCG10 shares extensive homology with stathmin and SCLIP, we examined whether JLP interacts with these proteins in vivo. An immunoprecipitation assay using cell extracts derived from COS-7 cells expressing JLP and the indicated stathmin family member demonstrated that JLP interacts with SCG10 and SCLIP, but not stathmin (Fig. 1A). To map the SCG10 binding region(s) of JLP, we performed a number of immunoprecipitation assays in COS-7 cells using a set of deletion mutants (Fig. 1B) and a set of internal fragments of JLP (Fig. 1C). These studies showed that deletion mutant JLP-1000 was able to precipitate SCG10, whereas mutant JLP-463 failed to do so. This suggested to us that the region of JLP spanning amino acids 463–1000 is sufficient for SCG10 binding (Fig. 1D). In a similar assay, using internal fragments of JLP, we observed that JLP contained three individual domains that can mediate SCG10 binding: Domain III (amino acids 465–1008), C-domain core (amino acids 997–1170), and Domain IV (amino acids 1165–1307) (Fig. 1E), suggesting the presence of three different binding sites for this protein.
FIGURE 1.
Interaction between JLP and stathmin family members. A, cell lysates derived from COS-7 cells overexpressing JLP-S and HA-tagged stathmin, SCG10, and SCLIP were subjected to pulldown assays using S-protein-agarose beads, and the precipitates were analyzed by SDS-PAGE, followed by Western blotting with an anti-HA antibody (middle panel). The expression of HA-tagged stathmin, SCG10, and SCLIP, as well as the precipitated S-tagged JLP (JLP-S), are shown in the top and bottom panels. B and C, schematic representation of the deletion and domain mutants of JLP, respectively. D and E, domain mapping of the JLP-SCG10 interaction. A series of deletion mutants (B) and domain mutants (C) of JLP were co-transfected into COS-7 cells with HA-SCG10 (top panel) and subjected to precipitation using S-protein-agarose. Precipitates were analyzed by SDS-PAGE, followed by Western blotting with an anti-HA antibody. The expression of HA-SCG10 and the precipitated JLP mutants are shown in the top and bottom panels.
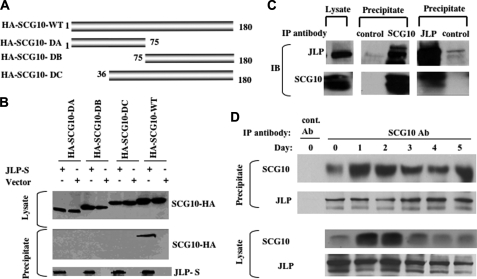
Conversely, to map the one or more regions of SCG10 that interact with JLP, immunoprecipitation assays were performed in COS-7 cells using a set of domain mutants of SCG10 (Fig. 2A). These results revealed that none of the domain mutants of SCG10 were able to interact with JLP, whereas the full length of SCG10 was able to do so (Fig. 2B). This fact, to some extent, explained why stathmin was unable to interact with JLP. The additional N-terminal domain of SCG10 seems to contribute to the interaction with JLP, although it has been shown to be a membrane association domain (13).
FIGURE 2.
SCG10 binds to JLP only in its full-length form. A, schematic representation of the domain mutants of SCG10. B, mapping JLP-interacting domain of SCG10. A series of domain mutants of SCG10 (top panel) were co-transfected into COS-7 cells with JLP-S and subjected to precipitation using S-protein-agarose. Precipitates were analyzed by SDS-PAGE, followed by Western blotting with an anti-HA antibody. The expression of HA-SCG10 mutants and the precipitated JLP are shown in the top and bottom panels. C, the interaction between JLP and SCG10 during NGF-induced differentiation of PC12 cells. An immunoprecipitation assay using an anti-SCG10 or JLP antibody was performed using cell lysates derived from the PC12 cells treated with NGF for 3 days. The immunoprecipitates together with the lysate were analyzed using the antibodies against JLP or SCG10. D, a time-course study of the interaction between JLP and SCG10. Immunoprecipitation assays were performed as in C using cell lysates derived from PC12 cells treated with NGF at various time points as indicated. The lysates were immunoprecipitated with control antibody (cont. Ab) or anti-SCG10 antibody (SCG10 Ab). Western blot analysis was performed on the lysates and the immunoprecipitates using specific antibodies against JLP and SCG10.
During NGF-induced differentiation of PC12 cells, the expression levels of SCG10 were shown to increase dramatically as a function of the differentiated phenotype (14), whereas the levels of JLP remain constant (data not shown). To determine whether JLP interacts with SCG10 during differentiation, we carried out an immunoprecipitation assay using NGF-treated PC12 cells. As predicted by our results obtained with COS-7 cells, these studies showed that immunoprecipitates of SCG10 contained detectable levels of JLP, suggesting that endogenous JLP interacted with endogenous SCG10 during NGF-induced differentiation of PC12 cells (Fig. 2C). A reciprocal experiment using anti-JLP antibodies for immunoprecipitation assay, followed by immunoblotting using anti-SCG10 antibodies, also shows interaction between JLP and SCG10. To determine the kinetics of the interaction during differentiation of PC12, we performed immunoprecipitation assays using anti-SCG10 antibodies on lysates from PC12 cells treated with NGF for various periods of time. The results presented in Fig. 2D show that SCG10 was induced in Day 1 after treatment of NGF and reached a maximum level on Day2, followed by subsequent decline in expression levels. Immunoprecipitation of SCG10 could co-precipitate JLP before NGF stimulation and during the whole course of differentiation induced by NGF. This suggests that SCG10 interacts with JLP before and after the treatment of NGF. The amount of JLP co-immunoprecipitated with SCG10 was similar during the course of differentiation, although higher amounts of SCG10 could be immunoprecipitated by the anti-SCG10 antibodies early in the differentiation course (Days 1 and 2). This suggests that the JLP-SCG10 interaction persists consistently throughout differentiation. However, we did not observe higher levels of JLP in immunoprecipitates derived from Day 1 and Day 2 lysates, which contained increased amounts of SCG10. This suggests that, during the course of differentiation, on Days 1 and 2, certain levels of SCG10 exist in an unbound state to mediate tubulin depolymerization.
Loss of JLP Enhances Neurite Outgrowth in the NGF-treated PC 12 Cells
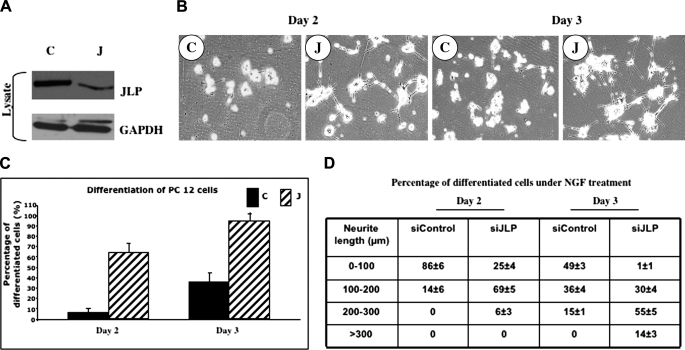
The biological function of JLP remains largely unknown in the neuronal tissue despite its high level of expression in the brain. Because SCG10 expression is up-regulated during neural development and regeneration, and because it has been shown to promote neurite outgrowth in NGF-treated PC12 cells, the interaction between JLP and SCG10 indicates that JLP may play a role in regulating neurite outgrowth and neuronal differentiation. To test this hypothesis, we knocked down the endogenous expression of JLP mRNA in PC12 cells using a specific siRNA against JLP. Treatment of PC12 cells with this siRNA resulted in ∼70% reduction in JLP levels when compared with control siRNA-transfected cells (Fig. 3A). Following treatment with NGF for 2–3 days, we examined the morphology of cells and found that treatment of PC12 cells with a JLP siRNA results in enhanced neurite growth. This suggests that loss of JLP expression promotes differentiation in these cells (Fig. 3B). By Day 2, >60% of the cells were differentiated in the JLP siRNA-transfected plates compared with ∼8% of those seen in the control plates (Fig. 3C). By Day 3, ∼95% of the JLP siRNA-transfected cells underwent differentiation, whereas only 35% of the control cells were differentiated at this time (Fig. 3C). Interestingly, measurement of the neurite length of the differentiated cells revealed that roughly 65% of the differentiated cells transfected with JLP siRNA contained neurites with a length ranging between 100 and 200 μm, whereas 93% of the differentiated cells in the control plates contained neurites shorter than 100 μm after 2 days of NGF treatment (Fig. 3D). On Day 3, 69% of the differentiated cells in the JLP siRNA-transfected plates contained neurites that were longer than 200 μm, whereas 15% of the control cells contained neurites with a length longer than 200 μm (Fig. 3D). Based on these observations, we conclude that loss of JLP strongly enhances neurite outgrowth in the NGF-treated PC12 cells.
FIGURE 3.
JLP regulates NGF-induced neurite outgrowth in PC12 cells. A, JLP protein levels in JLP siRNA-expressing cells (C, control siRNA; J, JLP siRNA). B, neurite outgrowth in NGF-treated PC12 cells in which endogenous JLP was down-regulated by its siRNA. The fields were randomly selected on the indicated day of NGF treatment. C, the percentage of differentiated PC12 cells undergoing NGF treatment. The differentiated cells were counted as described under “Experimental Procedures” (p < 0.001). D, neurite length of differentiated PC12 cells. The length of the longest neurite of each differentiated cell was measured using ImageJ software (National Institutes of Health).
Loss of JLP Promotes SCG10 Phosphorylation
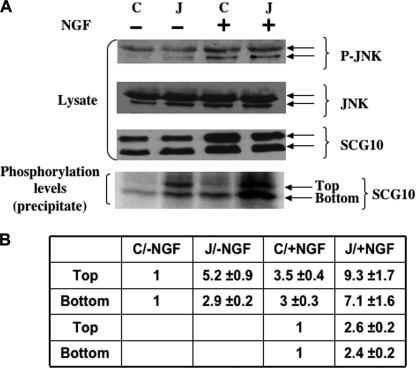
It has been reported that SCG10 can specifically regulate microtubule dynamics that are critical for neuronal differentiation and that this process is dependent on the phosphorylation status of SCG10 (7). Furthermore, stable overexpression of SCG10 has been found to promote neurite outgrowth in NGF-treated PC12 cells (15). To define the mechanism by which loss of JLP enhances neurite outgrowth, we examined whether JLP regulates SCG10 phosphorylation during NGF-induced differentiation. To that end, we incubated control and JLP siRNA-transfected cells with [32P]orthophosphate and determined the phosphorylation status of SCG10. Results presented in Fig. 4 show that, in JLP siRNA-expressing NGF-treated PC12 cells, SCG10 phosphorylation was increased ∼2.5-fold compared with the control cells (Fig. 4, A and B). In the absence of NGF stimulation, although the cells did not undergo differentiation, the phosphorylation of SCG10 in the JLP siRNA-transfected cells was also increased by >3-fold to that of the control cells (Fig. 4, A and B). These results suggest that inhibition of JLP expression results in an increase in phosphorylated SCG10, suggesting that JLP negatively regulates the phosphorylation status of this protein. Our results also showed that JNK activity induced by NGF stimulation was unaffected in JLP siRNA-treated cells (Fig. 4A), suggesting that the change in SCG10 phosphorylation is not due to the alteration of JNK activity.
FIGURE 4.
The role of JLP in regulating SCG10 phosphorylation in PC12 cells. A, the phosphorylation level of endogenous SCG10 in PC12 cells in response to NGF treatment. PC12 cells were transfected with control siRNA (C) or JLP siRNA (J), followed by NGF treatment. Two days after the treatment, the cultures were incubated with [32P]orthophosphate for 2 h. Lysates from the radiolabeled cells were subjected to immunoprecipitation analysis using an anti-SCG10 antibody. The immunoprecipitates were resolved by SDS-PAGE and subjected to autoradiography. B, quantitation of SCG10 phosphorylation in the presence or absence of JLP. The top and bottom SCG10 protein bands indicated in A are quantitated separately. The basal phosphorylation levels of SCG10 in intact PC12 cells were defined as 1 unit.
Inhibition of JNK Suppresses NGF-induced Neurite Outgrowth and SCG10 Phosphorylation
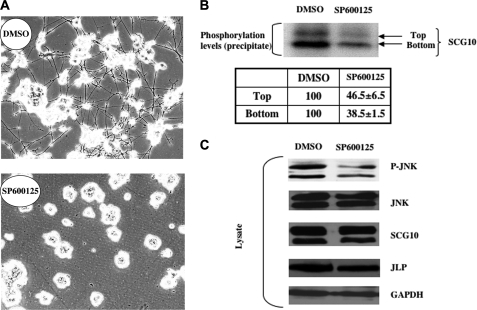
Mitogen-activated protein kinases have been shown to be involved in the NGF-signaling pathway regulating neuronal differentiation and neurite outgrowth (16). Our results show that JNK is activated during NGF-induced neuronal differentiation (Fig. 4A). To understand the role of JNK in the NGF-signaling pathway, we treated NGF-induced PC12 cultures with a small molecule inhibitor, SP600125, that can inhibit all three isoforms of JNK. Our results showed that the inhibition of JNK activity blocked NGF-induced neurite outgrowth (Fig. 5A), indicating that JNK is required for NGF-induced differentiation of PC12 cells. Because previous studies have shown that SCG10 can be phosphorylated by JNK in vitro (7), we hypothesized that JNK may regulate NGF-induced neurite outgrowth by phosphorylating SCG10. To address this question, we incubated control and JNK inhibitor-treated PC12 cells with [32P]orthophosphate and determined the phosphorylation status of SCG10. The results shown in Fig. 5B demonstrate that inhibition of JNK activity resulted in a decrease in the phosphorylation of SCG10 by 60%. These studies suggest that JNK-dependent phosphorylation of SCG10 is required for NGF-induced neurite outgrowth.
FIGURE 5.
JNK activity is required for the NGF-induced neurite outgrowth of PC12 cells. A, the effect of JNK inhibitor SP600125 on NGF-induced differentiation of PC12 cells. PC12 cells were incubated with vehicle (DMSO) or the JNK inhibitor (10 μm) 2 h before NGF treatment. Three days after the treatment, randomly selected fields were photographed in the indicated culture plates. B, changes in the phosphorylation level of SCG10 as a result of SP600125 treatment. PC12 cells were incubated with vehicle (DMSO) or the JNK inhibitor (10 μm) 2 h before NGF treatment. Three days after the treatment, the cultures were incubated with [32P]orthophosphate for 2 h. Lysates from the radiolabeled cells were immunoprecipitated using an anti-SCG10 antibody. The immunoprecipitates were resolved by SDS-PAGE and subjected to autoradiography. Phosphorylated levels of the top and bottom bands of SCG10 were quantitated. The phosphorylated levels of SCG10 of the control samples were set at 100. C, protein analysis of the lysates derived from the cells treated with vehicle (DMSO) or SP600125. PC12 cells were incubated with vehicle (DMSO) or the JNK inhibitor (10 μm) 2 h before NGF treatment. Three days after the treatment, the lysates were analyzed by Western blotting using specific antibodies against phospho-JNK, JNK, SCG10, JLP, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
DISCUSSION
Scaffolding proteins regulate signal transduction through specific protein-protein interactions. In the present study, we identified a microtubule-dynamics regulator SCG10 as a novel JLP-interacting protein using the yeast two-hybrid system as well as immunoprecipitation studies. This is the first indication of a JIP/JSAP/JLP scaffolding protein family member playing a role in regulating neuronal differentiation through interactions with neuronal growth-associated proteins.
Results presented in this study also show that the C-terminal domain of JLP interacts with SCG10 and SCLIP proteins but not with stathmin. Although there is extensive homology between stathmin and SCG10/SCLIP, stathmin does not contain the N-terminal region present in the SCG10 and SCLIP proteins. The N-terminal region of SCG10 is critical for directing it to Golgi membrane and transporting it to growth cones (13, 17, 18). Removal of this region completely abolished the interaction of SCG10 with JLP. Because SCG10 is an important regulator of microtubule dynamics involved in neuronal differentiation and development, we explored the functional consequences that result from the interaction between JLP and SCG10. Surprisingly, our results from studies using siRNA to JLP have indicated that JLP attenuates NGF-induced neurite extension and SCG10 phosphorylation. It is well established that JNK is activated in response to NGF stimulation (16). Our studies with the small molecule inhibitor SP600125 indicate that JNK activation is critical for NGF-induced neurite outgrowth in PC12 cells, which is consistent with an earlier observation that transfection of a dominant-negative mutant of JNK into PC12 cells results in an inhibition of neurite outgrowth in response to NGF treatment (19). Here, we show that JNK phosphorylates SCG10 during NGF stimulation, suggesting that JNK-dependent phosphorylation of SCG10 is a key event in NGF-induced neurite outgrowth. Thus, it appears that, in PC12 cells, JLP acts as a negative regulator of SCG10 phosphorylation. It is interesting to note that this regulation affects the rate of neurite outgrowth in NGF-treated PC12 cells. This is the first indication that a JIP family member regulates NGF-induced neurite outgrowth by interacting with microtubule dynamics regulators.
The conclusion that JLP negatively regulates NGF-induced SCG10-mediated and JNK-dependent neurite extension is strikingly different from our previous findings that JLP stimulates JNK activity in other cell types. In this context it is interesting to note that JLP did not alter NGF-induced activation of JNK. It appears that JLP modulates JNK-dependent phosphorylation of SCG10 and thereby NGF-induced neurite extension by sequestering JNK and/or SCG10 into a non-productive signaling complex. In fact, recent studies have shown that JLP can modulate physiological responses by sequestering active signaling components in different subcellular regions (20).
SCG10 is a neuronal-specific protein induced by NGF during differentiation of PC12 cells (21, 22). Its mRNA is highly induced in the early phase of differentiation induced by NGF, followed by subsequent decline in expression. Its protein expression also follows a similar pattern (Fig. 2D). Furthermore, SCG10 has been shown to accumulate in the growth cones of neurons (17, 18). Overexpression of SCG10 in PC12 cells strongly enhances neurite outgrowth in response to NGF, with an increase of the number of differentiated cells and with a more rapid kinetics of the neurite extension (15). JLP appears to negatively regulate the tubulin-depolymerizing activity of SCG10 by regulating the phosphorylation events mediated by JNK. These opposite effects of JLP and SCG10 on neurite outgrowth suggest that rapid synthesis and accumulation of SCG10 in the growth cones during early stages of differentiation (Days 1–3) may overcome the inhibitory effects of JLP, thereby stimulating the initiation of neurite outgrowth in the early phase of differentiation. When the levels of SCG10 decrease in the late phase of neuronal differentiation, JLP will reassert its inhibitory effects and stop further neurite outgrowth.
Previous studies have suggested that SCG10 expression is severely altered in neurological disorders such as Alzheimer disease and Down syndrome (5). Taken together, these studies implicate a modulatory role for JLP in SCG10-mediated neural growth and development. Our findings described here shed a new light on a novel mode of SCG10 regulation, providing a clue that could help identify novel therapeutic agents that could be used to treat human neurodegenerative disorders such as Alzheimer disease. Because JLP is highly expressed in the brain, including subcompartments such as the hippocampus (data not shown), our studies have provided a clue to understanding the biological function of JLP associated with the nervous system.
Supplementary Material
Acknowledgment
We thank Ramana V. Tantravahi for critical reading of the manuscript.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Fig. 1.
- MT
- microtubule
- JNK
- c-Jun N-terminal kinase
- CTD
- C-terminal domain
- siRNA
- small interference RNA
- NGF
- nerve growth factor
- HA
- hemagglutinin.
REFERENCES
- 1.Desai A., Mitchison T. J. (1997) Annu. Rev. Cell Dev. Biol. 13, 83–117 [DOI] [PubMed] [Google Scholar]
- 2.Dent E. W., Gertler F. B. (2003) Neuron 40, 209–227 [DOI] [PubMed] [Google Scholar]
- 3.Gordon-Weeks P. R. (2004) J. Neurobiol. 58, 70–83 [DOI] [PubMed] [Google Scholar]
- 4.Marshall C. J. (1994) Curr. Opin. Genet. Dev. 4, 82–89 [DOI] [PubMed] [Google Scholar]
- 5.Mori N., Morii H. (2002) J. Neurosci. Res. 70, 264–273 [DOI] [PubMed] [Google Scholar]
- 6.Ozon S., Byk T., Sobel A. (1998) J. Neurochem. 70, 2386–2396 [DOI] [PubMed] [Google Scholar]
- 7.Grenningloh G., Soehrman S., Bondallaz P., Ruchti E., Cadas H. (2004) J. Neurobiol. 58, 60–69 [DOI] [PubMed] [Google Scholar]
- 8.Tararuk T., Ostman N., Li W., Björkblom B., Padzik A., Zdrojewska J., Hongisto V., Herdegen T., Konopka W., Courtney M. J., Coffey E. T. (2006) J. Cell Biol. 173, 265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmarsh A. J. (2006) Biochem. Soc. Trans. 34, 828–832 [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran D. N., Kashef K., Lee C. M., Xu H., Reddy E. P. (2007) Oncogene 26, 3185–3202 [DOI] [PubMed] [Google Scholar]
- 11.Lee C. M., Onésime D., Reddy C. D., Dhanasekaran N., Reddy E. P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14189–14194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen Q., Lee C. M., Le A., Reddy E. P. (2005) J. Biol. Chem. 280, 30185–30191 [DOI] [PubMed] [Google Scholar]
- 13.Di Paolo G., Lutjens R., Pellier V., Stimpson S. A., Beuchat M. H., Catsicas S., Grenningloh G. (1997) J. Biol. Chem. 272, 5175–5182 [DOI] [PubMed] [Google Scholar]
- 14.Curmi P. A., Gavet O., Charbaut E., Ozon S., Lachkar-Colmerauer S., Manceau V., Siavoshian S., Maucuer A., Sobel A. (1999) Cell Struct. Funct. 24, 345–357 [DOI] [PubMed] [Google Scholar]
- 15.Riederer B. M., Pellier V., Antonsson B., Di Paolo G., Stimpson S. A., Lütjens R., Catsicas S., Grenningloh G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. (2002) Science 296, 1648–1649 [DOI] [PubMed] [Google Scholar]
- 17.Stein R., Mori N., Matthews K., Lo L. C., Anderson D. J. (1988) Neuron 1, 463–476 [DOI] [PubMed] [Google Scholar]
- 18.Lutjens R., Igarashi M., Pellier V., Blasey H., Di Paolo G., Ruchti E., Pfulg C., Staple J. K., Catsicas S., Grenningloh G. (2000) Eur. J. Neurosci. 12, 2224–2234 [DOI] [PubMed] [Google Scholar]
- 19.Gelderblom M., Eminel S., Herdegen T., Waetzig V. (2004) Int. J. Dev. Neurosci. 22, 559–564 [DOI] [PubMed] [Google Scholar]
- 20.Gantulga D., Tuvshintugs B., Endo Y., Takino T., Sato H., Murakami S., Yoshioka K. (2008) J. Biochem. 144, 693–700 [DOI] [PubMed] [Google Scholar]
- 21.Hannan A. J., Henke R. C., Weinberger R. P., Sentry J. W., Jeffrey P. L. (1996) Neuroscience 72, 889–900 [DOI] [PubMed] [Google Scholar]
- 22.Stein R., Orit S., Anderson D. J. (1988) Dev. Biol. 127, 316–325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.