Abstract
We have demonstrated previously that the Myc oncoprotein blocks cancer cell differentiation by forming a novel transcriptional repressor complex with histone deacetylase and inhibiting gene transcription of tissue transglutaminase (TG2). Moreover, induction of TG2 gene transcription and transamidase activity is essential for the differentiating effects of retinoids in cancer cells. Here, we show that two structurally distinct TG2 protein isoforms, the full-length (TG2-L) and the short form (TG2-S), exert opposing effects on cell differentiation. Repression of TG2-L with small interfering RNA, which did not affect TG2-S expression, induced dramatic neuritic differentiation in neuroblastoma cells. In contrast, overexpression of TG2-S or a GTP-binding-deficient mutant of TG2-L (R580A), both of which lack the GTP-binding Arg-580 residue, induced neuroblastoma cell differentiation, which was blocked by an inhibitor of transamidase activity. Whereas N-Myc repressed and retinoid activated both TG2 isoforms, repression of TG2-L, but not simultaneous repression of TG2-L and TG2-S, enhanced neuroblastoma cell differentiation due to N-Myc small interfering RNA or retinoid. Moreover, suppression of vasoactive intestinal peptide (VIP) expression alone induced neuroblastoma cell differentiation, and VIP was up-regulated by TG2-L, but not TG2-S. Taken together, our data indicate that TG2-L and TG2-S exert opposite effects on cell differentiation due to differences in GTP binding and modulation of VIP gene transcription. Our findings highlight the potential importance of repressing the GTP binding activity of TG2-L or activating the transamidase activity of TG2-L or TG2-S for the treatment of neuroblastoma, and possibly also other Myc-induced malignancies, and for enhancing retinoid anticancer effects.
Keywords: Cell/Differentiation, Diseases/Cancer/Neuroblastoma, Histones/Deacetylase, Oncogene/Myc, Protein/Structure, Vitamins and Cofactors/Retinoid, Transglutaminase
Introduction
Neuroblastoma, which originates from precursor neuroblasts of the sympathetic nervous system, is the commonest solid tumor in early childhood with a cure rate of only 40%, even with intensive chemoradiotherapy, retinoid differentiation therapy, surgery, and autologous bone marrow transplant (1, 2). N-Myc oncogene amplification and consequent overexpression of N-Myc mRNA and protein are seen as a clonal feature in 25–30% of tumors and correlate with poor prognosis in patients with neuroblastoma (3). The N-Myc oncoprotein exerts its effects by direct binding to cognate DNA sequences and modulating gene transcription (4), leading to a cell differentiation block, proliferation, malignant transformation, and tumor progression (3, 5).
Conventional therapy for neuroblastoma patients now includes a retinoid differentiation agent (1, 2). Unlike conventional chemoradiotherapy, differentiation therapy demonstrates minimal side effects on normal cells. However, resistance to retinoids eventually develops in more than 50% of neuroblastoma patients. Thus, a better understanding of tumor cell sensitivity and resistance to this type of agent may provide novel therapeutic targets for drug discovery.
One of the key factors involved in the neuroblastoma cell response to retinoids is tissue transglutaminase (TG2).2 Induction of TG2 transcription and its transamidase (protein cross-linking) activity is essential for retinoid-induced cell differentiation in neuroblastoma and other malignant cells (6–8). We have shown that Myc oncoproteins block tumor cell differentiation by recruiting histone deacetylase 1 protein to the gene promoter of TG2 and repressing TG2 gene transcription (9). TG2 is a multifunctional enzyme with both GTP binding and Ca2+-activated transamidase (transglutaminase) activities (for review, see Refs. 10, 11). Importantly, GTP binding inhibits transamidation, and conversely, transamidation due to Ca2+ binding represses GTP binding (12, 13). The biological function of TG2 is, therefore, dependent on whether transamidation or GTP binding is switched on, in a mutually exclusive way. Mutation of the critical GTP-binding residue Arg-580 to Ala increases the sensitivity of TG2 to calcium activation and results in disinhibition of intracellular transamidase activity (14, 15).
Two isoforms of TG2 mRNA and protein have been characterized (16, 17). Through exon skipping and intron retention, TG2 precursor mRNA is alternatively spliced to generate a short form (TG2-S) mRNA (16, 18), in addition to a full-length (TG2-L) mRNA. At the protein level, whereas the N-terminal 538 amino acids are shared, TG2-S contains 10 unique amino acids, and TG2-L contains 149 distinctive amino acids at the C terminus. Structurally, both TG2-L and TG2-S contain the transamidase active site catalytic triad (Cys-277, His-355, and Asp-358) (10, 19, 20); however, compared with TG2-L protein, TG2-S protein lacks the GTP-binding Arg-580 residue. Because different isoforms of a single gene due to alternative splicing may have similar or opposite functions (for review, see Ref. 21) and TG2-L and TG2-S exert opposite effects on cell viability (17), we carried out a series of experiments to define the roles of TG2-L and TG2-S in neuroblastoma cell differentiation and the mechanisms through which TG2-L and TG2-S exerted their effects.
EXPERIMENTAL PROCEDURES
Cell Culture
Human neuroblastoma BE(2)-C cell line was generously supplied by Dr. J. Biedler (Memorial Sloan-Kettering Cancer Center, New York, NY). Human neuroblastoma LAN-1 and LAN-2 cell lines were obtained from the American Type Culture Collection (Manassas, VA). All cells were cultured in Dulbecco's modified Eagle's medium supplemented with l-glutamine and 10% fetal calf serum. All-trans-retinoic acid (atRA) (Sigma) was solubilized in ethanol.
siRNA Transfection
The target sequence of the TG2-L siRNA corresponding to exon 11 of TG2 gene, which was specific for TG2-L, was 5′-AAAUACCGUGACUGCCUUA-3′. The target sequence of TG2-Total (TG2-T) siRNA corresponding to exon 7, which was shared by TG2-L and TG2-S, was 5′-GCAACCUUCUCAUCGAGUA-3′. The target sequence of vasoactive intestinal peptide gene (VIP) siRNA was 5′- CCCUAUUAUGAUGUAUCCA-3′. The siRNAs were custom synthesized by Applied Biosystems and transfected into cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions (22).
Plasmid Transfection
Plasmid expressing empty vector, human TG2-L cDNA or transamidation-deficient C277S mutant TG2-L cDNA was provided by Prof. G. Johnson (7). Plasmid expressing human GTP-binding-deficient R580A TG2-L mutant was generated by site-directed mutagenesis as described (23) using TG2-L cDNA cloned into the EcoRI-NotI sites of pcDNA3.1 as template, and plasmid expressing human TG2-S cDNA or VIP cDNA was purchased from GeneCopoeia (Germantown, MD) and OriGene (Rockville, MD), respectively, and confirmed by sequencing. A TG2-l-siRNA-resistant TG2-L expression construct was generated by site-directed mutagenesis as described (23) by mutating the siRNA target region to AAGTATCGCGATTGTCTCA. A VIP-siRNA-resistant VIP expression construct was similarly generated by site-directed mutagenesis by mutating the siRNA target region to CCATACTACGACGTCTCGA. Transient transfection was performed using Lipofectamine 2000 reagent according to the manufacturer's instructions (Invitrogen).
Semiquantitative Competitive RT-PCR
Semiquantitative competitive RT-PCR has been described previously (9). It involved determining a ratio between the expression level of a target gene and that of the housekeeping gene β-actin in total RNA samples. Fold induction or repression of a target gene was calculated by ascribing the ratio as 1.0 for control samples.
Immunoblot Analysis
Cells were lysed, and protein was extracted and analyzed by SDS-PAGE. Membranes were probed with polyclonal rabbit anti-TG2 (1:1000) (Thermo Scientific), monoclonal mouse anti-TG2 (1:300) (Santa Cruz Biotechnology), or mouse anti- VIP antibody (1:200) (Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated anti-rabbit or anti-mouse antiserum (1:2000) (Pierce), respectively. Membranes were finally reprobed with anti-β-actin antibody (Pierce) as a loading control.
Cell Growth and Differentiation Assays
Cell growth was analyzed by counting the number of live cells under the microscope, after cells were stained with trypan blue and loaded into a hemocytometer. Cell differentiation was assessed by analyzing neurite outgrowth. Cell images were captured under phase-contrast microscopy and stored, and neurite outgrowth was quantified with the method we have described (9).
Statistical Analysis
All data for statistical analysis were calculated as means ± S.E. Differences were analyzed for significance using a t test between two groups or analysis of variance among groups. A probability value of 0.05 or less was considered significant.
RESULTS
TG2-L and TG2-S Gene Expression Is Repressed by N-Myc Oncogene and Reactivated by Retinoid Differentiation Therapy
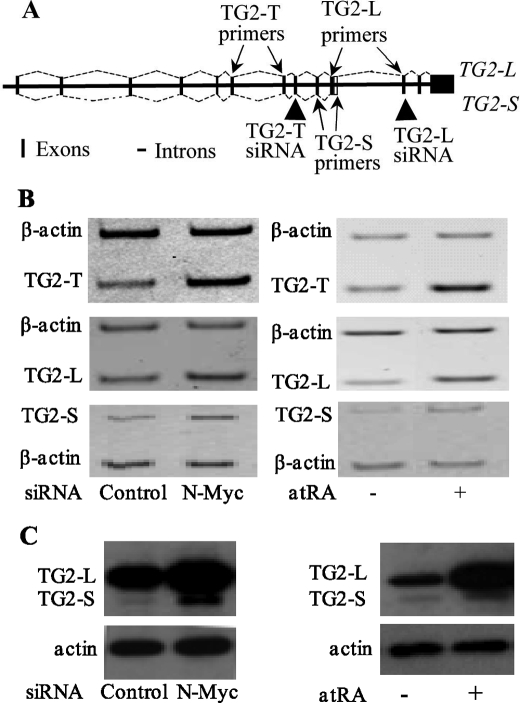
Through RT-PCR analysis using primers that target TG2 exons 4 and 6, which are shared by TG2-L and TG2-S, we have demonstrated previously that N-Myc oncoprotein represses expression of this mRNA sequence, which we called TG2-Total (TG2-T, TG2-L plus TG2-S) (9). Induction of TG2-T transcription and transamidation activity has been reported to be essential for retinoid-induced differentiation in neuroblastoma and leukemia cells (6–8). We therefore examined whether N-Myc oncoprotein and retinoid differentiation therapy modulated gene expression of both TG2-L and TG2-S. As indicated in Fig. 1A, the TG2-S mRNA contains 164 nucleotides after the 5′-splicing site of exon 10 that encodes 10 amino acids followed by a pre-mature stop codon. TG2-L mRNA contains a shorter exon 10 than TG2-S mRNA, but it has three extra downstream exons. We therefore designed specific reverse primers for TG2-L and TG2-S that target exon 11 of TG2-L and the 3′-end region of exon 10 of TG2-S, respectively (Fig. 1A). Consistent with our previous report, N-Myc siRNA reduced N-Myc mRNA and protein expression by about 80% (9). Semiquantitative competitive RT-PCR (Fig. 1B) and immunoblot (Fig. 1C) analysis revealed that transfection with N-Myc siRNA or treatment with 1 μm atRA up-regulated mRNA and protein expression of both TG2-L and TG2-S in the N-Myc-amplified neuroblastoma BE(2)-C cells.
FIGURE 1.
N-Myc oncoprotein represses, whereas the differentiation agent retinoid up-regulates, gene expression of TG2-L and TG2-S in neuroblastoma cells. A, schematic representation of human TG2 pre-mRNA shows the generation of TG2-L and TG2-S mRNA through the use of an alternative 5′ splice junction (donor site) in exon 10 leading to a change in the 3′ boundary of the exon. Arrows indicate the target exon to which a forward or reverse primer for TG2-T, TG2-L, or TG2-S was designed; arrowheads point to the target exon where siRNA against TG2-T or TG2-L was designed. Vertical bar indicates an exon, and a horizontal line indicates an intron. B and C, BE(2)-C neuroblastoma cells were transfected with scrambled control or N-Myc siRNA (left panels) or treated with vehicle control or 1 μm atRA (right panels) for 48 h. B, effect of N-Myc siRNA or atRA on the expression of TG2-L, TG2-S, or TG2-T mRNA was analyzed by semiquantitative competitive RT-PCR using PCR primers targeting TG2-L alone, TG2-S alone, or TG2-T together with primers targeting the housekeeping gene β-actin as a loading control. C, effect of N-Myc siRNA or atRA on the expression of TG2-L and TG2-S protein was analyzed by immunoblotting with a monoclonal anti-TG2 antibody together with an anti-actin antibody as a loading control.
Neuroblastoma Cell Differentiation Is Repressed by TG2-L Expression
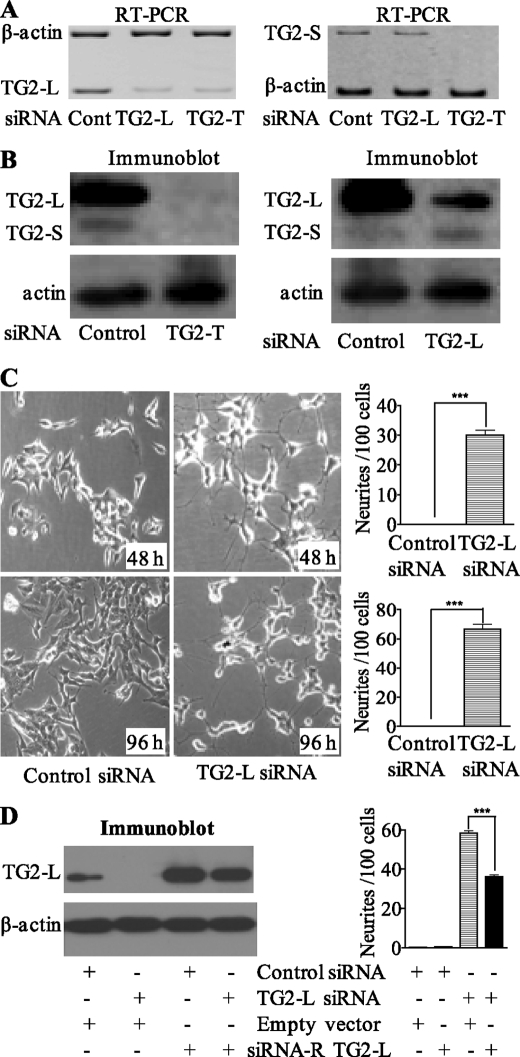
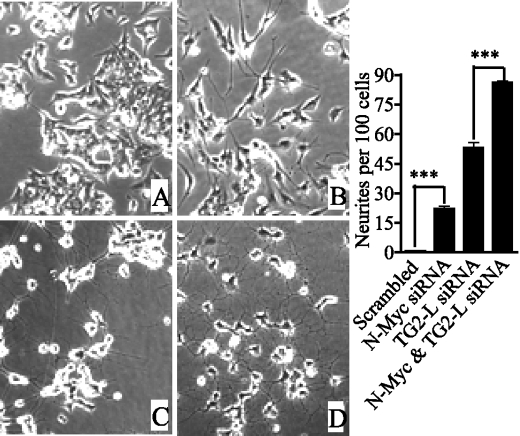
Different isoforms of a single gene due to alternative splicing can exert similar or opposite functions (for review, see Ref. 21). To assess the functional relevance of transcriptional modulation of TG2-L and TG2-S by N-Myc oncoprotein and retinoid differentiation therapy, we first examined whether endogenous TG2-L and TG2-S exert similar or opposite functions in neuroblastoma cells. We designed a TG2-L siRNA specifically targeting exon 11 of TG2-L and a TG2-T siRNA targeting exon 7 that was present in both TG2-L and TG2-S mRNA (Fig. 1A). Design of siRNA specifically targeting TG2-S was not successful due to the very small number of nucleotides unique to TG2-S and the unsuitability of the nucleotides for siRNA designing. As shown in Fig. 2, A and B, TG2-T siRNA repressed mRNA and protein expression of both TG2-L and TG2-S, whereas TG2-L siRNA repressed mRNA and protein expression of TG2-L but not TG2-S. Consistent with our published results (9), simultaneous repression of both TG2-L and TG2-S with TG2-T siRNA had no significant effect on differentiation of neuroblastoma BE(2)-C cells. However, TG2-L siRNA induced neurite outgrowth by 30-fold at 48 h after siRNA transfection and 60-fold another 48 h later (Fig. 2C). To confirm that the effect of TG2-L siRNA was not due to an off-target effect, we mutated six nucleotides of the TG2-L expression construct at the site of the TG2-L siRNA target region so that the mutant construct gives rise to a protein that is the same as endogenous TG2-L but is resistant to TG2-L siRNA. Immunoblot analysis showed that transfection of the siRNA-resistant TG2-L expression construct resulted in overexpression of TG2-L protein, even when co-transfected with TG2-L siRNA (Fig. 2D). Cell differentiation assays confirmed that transfection with the siRNA-resistant TG2-L expression construct reduced neuroblastoma cell differentiation due to TG2-L siRNA by ∼40% (Fig. 2D). These data suggest that TG2-L represses neuroblastoma cell differentiation and that TG2-S induces differentiation.
FIGURE 2.
Repression of TG2-L induces dramatic neuroblastoma cell differentiation. Neuroblastoma BE(2)-C cells were transfected with scrambled control (Cont) siRNA, TG2-T siRNA, TG2-L siRNA, and/or an siRNA-resistant TG2-L expression construct, followed by mRNA expression (A), protein expression (B and D), or cell differentiation studies (C and D). A, TG2-L and TG2-S mRNA expression was examined 48 h after siRNA transfection by competitive RT-PCR with primers specifically targeting TG2-L or TG2-S together with primers targeting the housekeeping gene β-actin as a loading control. B, TG2-L and TG2-S protein expression was examined 48 h after siRNA transfection by immunoblotting with a monoclonal anti-TG2 antibody together with an anti-actin antibody as a loading control. C, 48 and 96 h after transfection with scrambled control or TG2-L-specific siRNA, cell differentiation was assessed by analyzing neurite outgrowth. Cell images were captured under phase-contrast microscope and stored, and neurite outgrowth was quantified. D, BE(2)-C cells were co-transfected with scrambled control siRNA or TG2-L siRNA together with a construct encoding TG2-L siRNA-resistant (siRNA-R) TG2-L or a construct encoding empty vector. TG2-L protein expression was analyzed by immunoblotting, and cell differentiation was assessed by analyzing neurite outgrowth 96 h after transfections. ***, p < 0.001.
Opposing Role of TG2-L and TG2-S on Neuroblastoma Cell Differentiation Is Due to the GTP- binding Arg-580 Residue
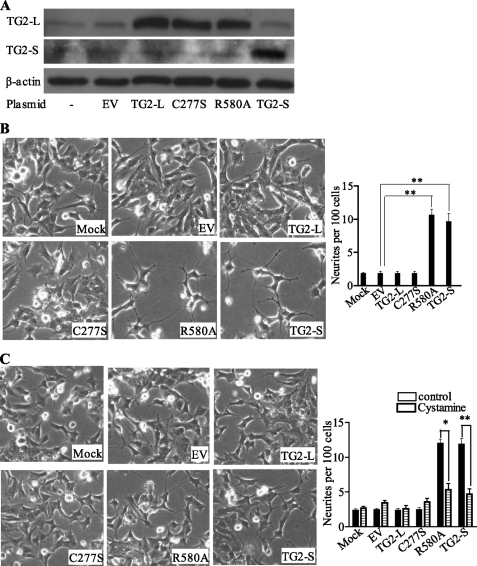
Because TG2-L contains, but TG2-S lacks, the GTP-binding Arg-580 residue that is required for maintaining the intracellular transamidase activity of TG2 latent (13, 14), we tested whether the Arg-580 residue is critical for the opposite effects of TG2-L and TG2-S on neuroblastoma cell differentiation. Neuroblastoma BE(2)-C cells were transfected with constructs encoding empty vector, TG2-S, TG2-L, transamidase-deficient mutant TG2-L (C277S), GTP-binding-deficient mutant TG2-L (R580A), or mock-transfected. Immunoblot analysis confirmed that transfection with the constructs led to overexpression of the target proteins (Fig. 3A). Analysis of neurite outgrowth showed no effect of TG2-L or TG2-L C277S overexpression in inducing cell differentiation. In contrast, overexpression of TG2-L R580A or TG2-S induced neurite outgrowth by more than 5-fold (Fig. 3B). Treatment of the transfected cells with the transamidation inhibitor, cystamine, blocked the neurite outgrowth induced by overexpression of TG2-L R580A (p < 0.05) or TG2-S (p < 0.01) (Fig. 3, B and C). These data indicate that the transamidase activity of TG2-S or TG2-L R580A induces neuroblastoma cell differentiation and that repression of cell differentiation by TG2-L is due to Arg-580-mediated inhibition of transamidase activity.
FIGURE 3.
Arg-580 GTP-binding residue of TG2 is critical for repressing neuroblastoma cell differentiation. BE(2)-C neuroblastoma cells were transfected with empty vector (EV), constructs encoding wild type TG2-L, transamidase-deficient mutant TG2-L (C277S), GTP-binding-deficient mutant TG2-L (R580A) or wild type TG2-S, or mock transfected. A, 72 h after transfection, protein was extracted and subjected to immunoblot analysis of TG2-L and TG2-S with an anti-TG2 antibody together with an antibody against β-actin as a loading control. B and C, 8 h after transfection with the different constructs, cells were treated with vehicle control (all images in B) or the transamidase inhibitor cystamine (20 μm) (all images in C) for 4 days. Cell images were captured under phase-contrast microscope and stored, and cell differentiation was assessed by analyzing neurite outgrowth. *, p < 0.05; **, p < 0.01.
TG2-L Blocks Neuroblastoma Cell Differentiation by Activating the Expression of VIP
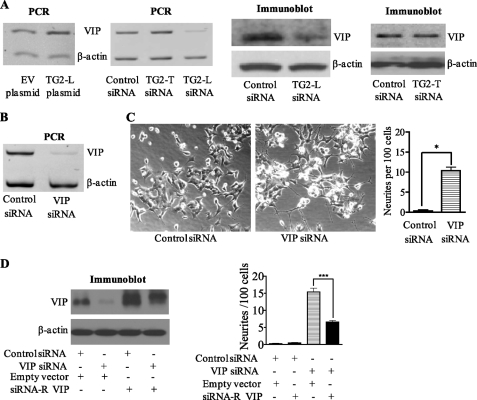
We have recently shown that TG2 modulates gene transcription by cross-linking and, consequently, inactivating the transcription factor Sp1 (24). To identify the downstream target responsible for TG2-L-induced neuroblastoma cell differentiation block, we reviewed the published cDNA microarray data that compared gene expression in neuroblastoma SY-5Y cells stably transfected with a TG2-L expression construct or control empty vector (25). Among the genes significantly modulated by TG2-L in SY-5Y cells, VIP was confirmed to be up-regulated by more than 100% in BE(2)-C cells transfected with a construct encoding TG2-L compared with cells transfected with a construct encoding empty vector (Fig. 4A). RT-PCR and immunoblot analyses also showed that in BE(2)-C cells, the expression of VIP was reduced by >80% after repression of TG2-L expression by TG2-L siRNA, but not after simultaneous repression of TG2-L and TG2-S expression by TG2-T siRNA (Fig. 4A). Further, repression of VIP gene expression with siRNA specifically targeting VIP (Fig. 4B) also induced marked neuritic differentiation (Fig. 4C). To confirm that the effect of VIP siRNA was not due to an off-target effect, we mutated six nucleotides of the VIP expression construct at the site of the VIP siRNA target region so that the mutant construct gives rise to a protein that is the same as endogenous VIP but is resistant to VIP siRNA. Immunoblot analysis showed that transfection of the siRNA-resistant VIP expression construct resulted in overexpression of VIP protein, even when co-transfected with VIP siRNA (Fig. 4D). Cell differentiation assays confirmed that transfection with the siRNA-resistant VIP expression construct reduced neuroblastoma cell differentiation due to VIP siRNA by ∼60% (Fig. 4D). These data indicate that activation of VIP gene expression is at least partly responsible for TG2-L-induced differentiation block in neuroblastoma cells.
FIGURE 4.
TG2-L blocks neuroblastoma cell differentiation by up-regulating VIP gene expression. A, BE(2)-C neuroblastoma cells were transfected with empty vector, a TG2-L-encoding construct, scrambled control siRNA, TG2-T siRNA, or TG2-L siRNA, followed by RNA or protein extraction. The effect of the siRNAs on the expression of VIP mRNA and protein was analyzed by competitive RT-PCR and immunoblotting, respectively, with the housekeeping gene β-actin as a loading control. B and C, BE(2)-C cells were transfected with scrambled control or VIP-specific siRNA. B, repression of VIP gene expression by VIP siRNA was confirmed by semiquantitative competitive RT-PCR. C, cell images were captured under phase-contrast microscope and stored, and the effect of VIP siRNA on cell differentiation was assessed by analyzing neurite outgrowth. D, BE(2)-C cells were co-transfected with scrambled control siRNA or VIP siRNA together with a construct encoding VIP siRNA-resistant (siRNA-R) VIP or a construct encoding empty vector. VIP protein expression was analyzed by immunoblotting, and cell differentiation was assessed by analyzing neurite outgrowth. *, p < 0.05; ***, p < 0.001.
Transcriptional Repression of TG2-L Inhibits N-Myc-induced Cell Differentiation Block
We have previously reported that N-Myc expression blocked neuroblastoma cell differentiation by transcriptional repression of TG2-T (9). Because N-Myc repressed mRNA and protein expression of both TG2-L and TG2-S (Fig. 1, B and C), we examined the effect of transcriptional repression of TG2-L or TG2-S on N-Myc-induced differentiation block in neuroblastoma cells. Consistent with our previous report, N-Myc siRNA induced neuroblastoma cell differentiation, and simultaneous repression of TG2-L and TG2-S expression with TG2-T siRNA partly blocked N-Myc siRNA-induced neuritic differentiation (9). In contrast, co-transfection of TG2-L siRNA with N-Myc siRNA co-operatively enhanced neuroblastoma cell differentiation (Fig. 5). This result suggests that transcriptional repression of TG2-L inhibits N-Myc-induced cell differentiation block and that transcriptional repression of TG2-S is at least partly responsible for N-Myc oncogene-induced cell differentiation block.
FIGURE 5.
Transcriptional repression of TG2-L inhibits N-Myc-induced cell differentiation block. Neuroblastoma BE(2)-C cells were transfected with scrambled control siRNA (A), N-Myc siRNA (B), TG2-L siRNA (C), or TG2-L siRNA plus N-Myc siRNA (D). Cell images were captured under phase-contrast microscope and stored 4 days after transfection, and cell differentiation was assessed by analyzing neurite outgrowth. ***, p < 0.001.
Transcriptional Activation of TG2-L Causes Resistance to the Anticancer Effects of Retinoid in a Range of Neuroblastoma Cells
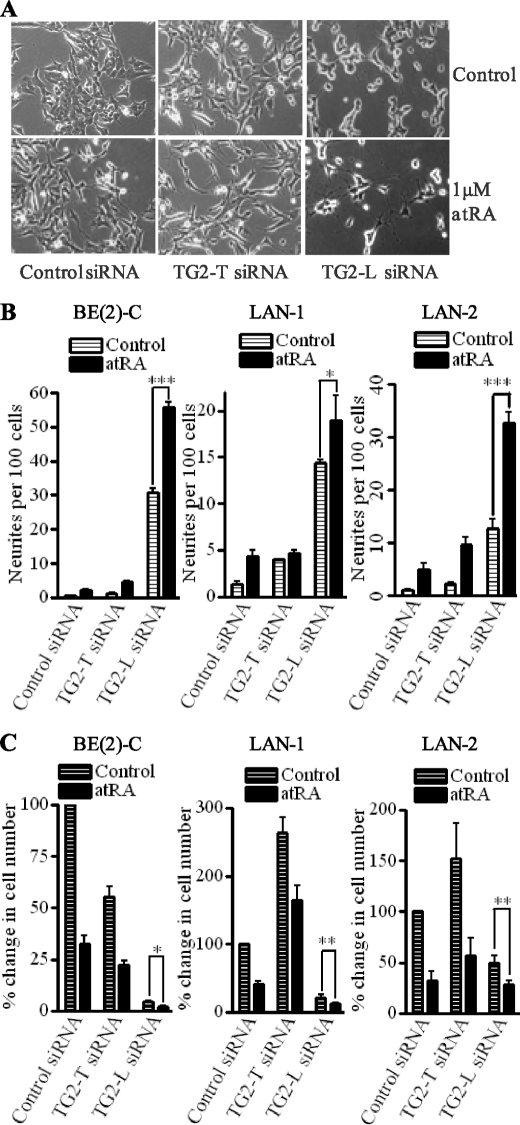
Because retinoid differentiation therapy induced gene expression of both TG2-L and TG2-S in neuroblastoma cells (Fig. 1, B and C), we next tested whether transcriptional activation of TG2-L rendered neuroblastoma cells resistant to the anticancer efficacy of retinoid. Whereas atRA, TG2-T siRNA, and a combination of atRA and TG2-T siRNA showed minor effects on cell differentiation, TG2-L siRNA induced neurite outgrowth by about 20-fold at 2 days (Fig. 6, A and left panel of B) and by about 40-fold at 4 days (supplemental Fig. 1) after treatment. Combining TG2-L siRNA and atRA treatment was even more effective in the induction of neuronal differentiation: inducing neurite outgrowth by 50–60-fold at both time points (p < 0.05) (Fig. 6, A and left panel of B, and supplemental Fig. 1). To assess whether the observation in BE(2)-C cells was a general feature of other neuroblastoma cell lines, we transfected N-Myc-amplified LAN-1 and LAN-2 cells with scrambled control, TG2-T siRNA or TG2-L siRNA, followed by treatment with vehicle control or 1.0 μm atRA for 4 days. Transfection of TG2-T siRNA had only limited effects on cell differentiation, whereas atRA induced neuritic outgrowth by about 4-fold. Importantly, TG2-L siRNA induced neurite outgrowth by 7-fold in LAN-1 and 14-fold in LAN-2 cells, respectively (Fig. 6B), and a combination of TG2-L siRNA and atRA co-operatively induced cell differentiation by about 19-fold in LAN-1 and 32-fold in LAN-2 cells.
FIGURE 6.
Combination of atRA and TG2-L siRNA, but not TG2-T siRNA, co-operatively induces neuroblastoma cell differentiation and consequently growth arrest in a range of neuroblastoma cells. A–C, neuroblastoma cell lines BE(2)-C (A and left panels in B and C), LAN-1 (middle panels in B and C), or LAN-2 (right panels in B and C) were transfected with scrambled control siRNA, TG2-T siRNA, or TG2-L siRNA, followed by treatment with vehicle control or 1.0 μm atRA. A, 2 days after treatment, representative images of BE(2)-C cells were captured under phase-contrast microscope and stored for quantification of neurites. B, cell differentiation was assessed by analyzing neurite outgrowth 2 days after treatment in BE(2)-C cells or 4 days after treatment in LAN-1 and LAN-2 cells. C, 4 days after treatment with control or atRA, BE(2)-C, LAN-1, and LAN-2 cells were stained with trypan blue and loaded into a hemocytometer. Cell growth was analyzed by counting the number of live cells under microscope. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Retinoids exert anticancer effects by induction of cell differentiation and growth arrest; thus, we tested whether the combination of TG2-L siRNA and atRA co-operatively repressed cell growth. As shown in Fig. 6, A and C, treatment with 1.0 μm atRA for 4 days decreased the number of viable BE(2)-C, LAN-1, and LAN-2 cells by 60–70%. TG2-T siRNA decreased the growth of BE(2)-C cells but increased the growth of LAN-1 and LAN-2 cells. The combination of atRA and TG2-L siRNA further decreased cell growth by about 50% in all three cell lines compared with TG2-L siRNA alone (p < 0.05). These data suggest that transcriptional activation of TG2-L renders neuroblastoma cells resistant to the anticancer effects of retinoid.
Finally, we tested whether overexpression of TG2-L partly blocked atRA-induced cell differentiation and growth inhibition. As shown in supplemental Fig. 2, transfection of a TG2-L expression construct reduced atRA-induced cell differentiation by ∼70% and atRA-induced cell growth arrest by ∼40% in BE(2)-C cells (p < 0.05). These data confirm that overexpression of TG2-L renders neuroblastoma cells resistant to atRA-induced anticancer effects.
DISCUSSION
The induction of TG2 gene transcription and transamidase activity is essential for tumor cell differentiation due to N-Myc oncogene repression (9) or retinoid differentiation therapy (6–8) in neuroblastoma and other malignant cells. In this study, we have shown that the two major protein isoforms of TG2 exert opposite effects: TG2-S induces, whereas TG2-L represses, neuroblastoma cell differentiation. This finding has important implications for studies of the role of TG2 expression in human cancer and for TG2 as an anticancer treatment target.
Although the transamidase catalytic triad and transition state-stabilizing residue are present in both TG2-L and TG2-S proteins, TG2-S lacks the Arg-580 residue that is critical for GTP binding and inhibition of transamidase activity (13–15). Our current data show that TG2-L with an Ala mutation of the Arg-580 residue induces, whereas wild-type TG2-L blocks, neuroblastoma cell differentiation and that inhibition of transamidase activity with a chemical inhibitor blocks the cell differentiation induced by both TG2-S and the R580A mutant TG2-L. Taken together, our data indicate that TG2-L blocks neuroblastoma cell differentiation through Arg-580-mediated GTP binding and inhibition of transamidation and that TG2-S induces cell differentiation through its transamidase activity not being inhibited due to the lack of Arg-580-mediated GTP binding.
We have recently found that TG2 modulates gene transcription by cross-linking the transcription factor Sp1 (24). Our current study reveals that repression of VIP with siRNA induces neuroblastoma cell differentiation and that repression of TG2-L, but not simultaneous repression of TG2-L and TG2-S, reduces VIP gene transcription. These data suggest that TG2-L blocks neuroblastoma cell differentiation at least partly by inducing VIP gene expression and provide the foundation for drug discovery of small molecule inhibitors of VIP for the treatment of neuroblastoma.
The N-Myc oncoprotein induces the initiation and progression of neuroblastoma, in part, by inhibiting cell differentiation (3), and N-Myc blocks neuroblastoma cell differentiation through recruiting histone deacetylase 1 protein to TG2 gene promoter and repressing TG2-T gene transcription (9). Our current study shows that repression of N-Myc gene expression reactivates the transcription of both TG2-L and TG2-S, that repression of TG2-L enhances N-Myc siRNA-induced cell differentiation, and that overexpression of TG2-S induces cell differentiation. These data suggest that transcriptional repression of TG2-L inhibits N-Myc-induced cell differentiation block and that N-Myc blocks neuroblastoma cell differentiation at least partly through transcriptional repression of TG2-S.
Treatment with retinoid improves event-free survival rate in neuroblastoma patients when it is administered after chemotherapy (2). However, most patients eventually develop resistance. In this study, we have demonstrated that treatment with retinoid induces gene expression of both TG2-L and TG2-S and that simultaneous repression of both TG2-L and TG2-S has a minor effect on retinoid-induced cell differentiation and growth inhibition, whereas repression of TG2-L significantly enhances retinoid-induced cell differentiation and growth arrest in all three neuroblastoma cell lines tested. These data suggest that retinoid-induced transcriptional activation of TG2-L renders neuroblastoma cells resistant to, whereas transcriptional activation of TG2-S contributes to, retinoid-induced anticancer effects. Our findings suggest that specific small molecule inhibitors of GTP binding by TG2-L or small molecule activators of transamidation by TG2-L or TG2-S may have therapeutic benefit in neuroblastoma, and possibly other cancer types, characterized by resistance to retinoid therapy.
In summary, neuroblastoma cell differentiation is repressed by maintaining the latency of transamidase activity through GTP binding to the Arg-580 residue of TG2-L. This in turn leads to the activation of VIP expression. Conversely, neuroblastoma cell differentiation is induced by the transamidase activity of TG2-S, which is not latent due to its lack of Arg-580. Furthermore, transcriptional repression of TG2-L by N-Myc inhibits, yet transcriptional repression of TG2-S by N-Myc is at least partly responsible for, N-Myc oncoprotein-induced cell differentiation block, and transcriptional activation of TG2-L by retinoid counteracts retinoid anticancer effects. Our findings highlight repression of the GTP binding activity of TG2-L or activation of the transamidase activity of TG2-L or TG2-S as novel drug development directions for the treatment of cancers overexpressing Myc oncoproteins and for overcoming cancer cell resistance to retinoid differentiation therapy.
Supplementary Material
This work was supported in part by National Health and Medical Research Council of Australia Grants 568753 and 459406. The authors were also supported by the Cancer Institute of New South Wales and National Health and Medical Research Council program grants and a Cancer Institute New South Wales Career Development Fellowship. The Children's Cancer Institute Australia for Medical Research is affiliated with University of New South Wales and Sydney Children's Hospital.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- TG2
- tissue transglutaminase
- TG2-L
- TG2 long isoform
- TG2-S
- TG2 short isoform
- TG2-T
- both TG2 long and short isoforms
- atRA
- all-trans-retinoic acid
- siRNA
- small interfering RNA
- VIP
- vasoactive intestinal peptide
- RT
- reverse transcription.
REFERENCES
- 1.Maris J. M., Hogarty M. D., Bagatell R., Cohn S. L. (2007) Lancet 369, 2106–2120 [DOI] [PubMed] [Google Scholar]
- 2.Matthay K. K., Villablanca J. G., Seeger R. C., Stram D. O., Harris R. E., Ramsay N. K., Swift P., Shimada H., Black C. T., Brodeur G. M., Gerbing R. B., Reynolds C. P. (1999) N. Engl. J. Med. 341, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 3.Brodeur G. M. (2003) Nat. Rev. Cancer 3, 203–216 [DOI] [PubMed] [Google Scholar]
- 4.Patel J. H., Loboda A. P., Showe M. K., Showe L. C., McMahon S. B. (2004) Nat. Rev. Cancer 4, 562–568 [DOI] [PubMed] [Google Scholar]
- 5.Pelengaris S., Khan M., Evan G. (2002) Nat. Rev. Cancer 2, 764–776 [DOI] [PubMed] [Google Scholar]
- 6.Singh U. S., Pan J., Kao Y. L., Joshi S., Young K. L., Baker K. M. (2003) J. Biol. Chem. 278, 391–399 [DOI] [PubMed] [Google Scholar]
- 7.Tucholski J., Lesort M., Johnson G. V. (2001) Neuroscience 102, 481–491 [DOI] [PubMed] [Google Scholar]
- 8.Balajthy Z., Csomós K., Vámosi G., Szántó A., Lanotte M., Fésüs L. (2006) Blood 108, 2045–2054 [DOI] [PubMed] [Google Scholar]
- 9.Liu T., Tee A. E., Porro A., Smith S. A., Dwarte T., Liu P. Y., Iraci N., Sekyere E., Haber M., Norris M. D., Diolaiti D., Della Valle G., Perini G., Marshall G. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18682–18687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorand L., Graham R. M. (2003) Nat. Rev. Mol. Cell Biol. 4, 140–156 [DOI] [PubMed] [Google Scholar]
- 11.Iismaa S. E., Mearns B. M., Lorand L., Graham R. M. (2009) Physiol. Rev. 89, 991–1023 [DOI] [PubMed] [Google Scholar]
- 12.Achyuthan K. E., Greenberg C. S. (1987) J. Biol. Chem. 262, 1901–1906 [PubMed] [Google Scholar]
- 13.Begg G. E., Holman S. R., Stokes P. H., Matthews J. M., Graham R. M., Iismaa S. E. (2006) J. Biol. Chem. 281, 12603–12609 [DOI] [PubMed] [Google Scholar]
- 14.Begg G. E., Carrington L., Stokes P. H., Matthews J. M., Wouters M. A., Husain A., Lorand L., Iismaa S. E., Graham R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Tucholski J., Gundemir S., Johnson Voll G. V. (2008) Int. J. Clin. Exp. Med. 1, 248–259 [PMC free article] [PubMed] [Google Scholar]
- 16.Fraij B. M., Birckbichler P. J., Patterson M. K., Jr., Lee K. N., Gonzales R. A. (1992) J. Biol. Chem. 267, 22616–22623 [PubMed] [Google Scholar]
- 17.Antonyak M. A., Jansen J. M., Miller A. M., Ly T. K., Endo M., Cerione R. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18609–18614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citron B. A., Suo Z., SantaCruz K., Davies P. J., Qin F., Festoff B. W. (2002) Neurochem. Int. 40, 69–78 [DOI] [PubMed] [Google Scholar]
- 19.Liu S., Cerione R. A., Clardy J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2743–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iismaa S. E., Holman S., Wouters M. A., Lorand L., Graham R. M., Husain A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12636–12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matlin A. J., Clark F., Smith C. W. (2005) Nat. Rev. Mol. Cell Biol. 6, 386–398 [DOI] [PubMed] [Google Scholar]
- 22.Liu T., Liu P. Y., Tee A. E., Haber M., Norris M. D., Gleave M. E., Marshall G. M. (2009) Eur. J. Cancer 45, 1846–1854 [DOI] [PubMed] [Google Scholar]
- 23.Murthy S. N., Iismaa S., Begg G., Freymann D. M., Graham R. M., Lorand L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2738–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsukawa H., Fukaya Y., Frampton G., Martinez-Fuentes A., Suzuki K., Kuo T. F., Nagatsuma K., Shimokado K., Okuno M., Wu J., Iismaa S., Matsuura T., Tsukamoto H., Zern M. A., Graham R. M., Kojima S. (2009) Gastroenterology 136, 1783–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J. S., Kim I. H., Kim S. Y. (2006) Front. Biosci. 11, 2774–2781 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.