Abstract
All members of the Oxa1/Alb3/YidC family have been implicated in the biogenesis of respiratory and energy transducing proteins. In Escherichia coli, YidC functions together with and independently of the Sec system. Although the range of proteins shown to be dependent on YidC continues to increase, the exact role of YidC in insertion remains enigmatic. Here we show that YidC is essential for the insertion of subunit K of the NADH:ubiquinone oxidoreductase and that the dependence is due to the presence of two conserved glutamate residues in the transmembrane segments of subunit K. The results suggest a model in which YidC serves as a membrane chaperone for the insertion of the less hydrophobic, negatively charged transmembrane segments of NuoK.
Keywords: Bioenergetics/Respiratory Chain, Membrane/Biogenesis, Membrane/Proteins, Organisms/Bacteria, Protein/Assembly, Protein/Targeting
Introduction
In Escherichia coli the inner membrane contains essential energy transducing complexes such as components of the electron transport chain. The majority of inner membrane proteins are inserted cotranslationally via the general secretory pathway otherwise known as the Sec system. In this pathway, the bacterial signal recognition particle targets ribosome-bound nascent chains to the SecYEG translocase via the signal recognition particle receptor FtsY. Membrane insertion of these proteins proceeds by a cotranslational “threading mechanism” in which the accessory protein YidC is postulated to play an important role in the clearance of transmembrane segments (TMSs)2 from the SecYEG channel (1, 2). A small subset of integral membrane proteins are targeted directly to YidC where they are integrated into the membrane in a Sec-independent manner. YidC belongs to the evolutionarily conserved Oxa1/Alb3/YidC family. Oxa-related proteins have been identified in all genomes sequenced to date and are postulated to have evolved before the divergence of the three major domains of life (3–5). Oxa1 (oxidase assembly) from yeast was the first member of this family to be described (6, 7). It was originally identified as an essential factor for the biogenesis of respiratory complexes in the mitochondrion, more specifically for the insertion of subunits of the cytochrome bc1 oxidase and ATP synthase (8). Alb3 is located in the thylakoid membranes of plant chloroplasts and involved in the biogenesis of light harvesting complexes (9). In E. coli it has been shown that YidC is essential for the insertion of subunit c and a of the F1F0 ATP synthase (Foc and Foa) (10, 11), subunit a of cytochrome o oxidase (CyoA) (12, 13), and MscL, the mechanosensitive channel of large conductance (14).
Although members of the family have all been implicated in membrane protein biogenesis of respiratory and energy transducing proteins, there is a great variance in substrate specificity within the family. For example Oxa1 proteins appear to have a varying role in the biogenesis of respiratory complexes I, III, IV, and V as illustrated by studies in Neurospora crassa, (15), Podospora anseria (16), HEK293 cells (17), and Saccharomyces cerevisiae (6, 7, 18–20). This highlights that although the general function of Oxa1/Alb3/YidC family proteins is known, each member plays a specific and in some cases yet to be identified role in the biogenesis of respiratory proteins.
In a recent study, we showed that YidC depletion in E. coli resulted in a cessation of growth under anaerobic conditions and that this growth defect may be in part due to reduced levels of the complex I homolog in bacteria, the NADH:ubiquinone reductase, or NADH dehydrogenase I, in the membrane (21). In particular, levels of the smallest membrane subunit K (NuoK) decreased. The aim of this study was to elucidate the role of YidC in NuoK membrane biogenesis and to determine the structural features of NuoK underlying this role. We found that in vitro synthesized NuoK requires both SecYEG and YidC for insertion and that two conserved negative charges in TMSs 2 and 3 determine the dependence of NuoK on YidC for insertion.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids
The YidC depletion strain E. coli FTL10 (22) was a generous gift of Frank Sargent (University of East Anglia, Norwich, UK). E. coli FTL10 YidC+ and YidC− strains were used as described (21). Strain E. coli SF100 was used for SecYEG overexpression and wild type and SecYEG+ inner membrane vesicles (IMVs) were prepared as described (23). E. coli BL21 (DE3) RosettaTM (Novagen) was used to prepare cell lysate for in vitro protein expression. Plasmids pEH1YidC (24) and pTrcYidC (25) were used for the overexpression of His-tagged and nontagged versions of YidC, respectively. Plasmid pETNuoK was constructed for in vitro expression of NuoK. The nuoK gene was PCR-amplified from E. coli genomic DNA and cloned into pET20b (Novagen) yielding pETNuoK. Glutamate residues Glu36 and/or Glu72 were substituted for lysines using the Stratagene QuikChange® site-directed mutagenesis kit with plasmid pETNuoK as template. This yielded plasmids pETNuoK E36K, pETNuoK E72K, and pETNuoK E36K,E72K, and the mutations were confirmed by sequencing. Plasmid pBSKFtsQ was used for in vitro expression of FtsQ (26). DNA manipulations were performed using E. coli DH5α to maintain plasmids and constructs.
Materials
Sodium fumarate was purchased from Sigma- Aldrich. Antiserum against YidC was raised in chickens against purified His-tagged YidC (Agrisera AB, Vännä, Sweden). Antisera against subunit K of the NADH dehydrogenase I (NuoK) was a generous gift from Takao Yagi (The Scripps Research Institute). Alkaline phosphatase-conjugated anti-chicken and anti-rabbit IgG were purchased from Sigma-Aldrich.
In Vitro Synthesis and Insertion Reactions
Synthesis reactions were carried out essentially as described by Saller et al. (25). The reactions were carried out for 30 min at 37 °C using T7 polymerase (Fermentas) and Easytag express protein labeling mix (PerkinElmer Life Sciences) in the presence of 10 μg of IMVs or proteo(liposomes). A small sample of the reaction was removed as a synthesis control, and in the case of NuoK constructs, the remainder was spun through a sucrose cushion consisting of 50 mm HEPES-KOH, pH 8, 0.5 mm phenylmethylsulfonyl fluoride, and 20% (w/w) sucrose to collect the membranes. The isolated membranes were resuspended in 50 mm HEPES-KOH, pH 8, and treated with 1.6 mg/ml proteinase K for 30 min on ice in the absence or presence of 0.1% SDS. For FtsQ, the reaction was treated with proteinase K following synthesis. The samples were trichloroacetic acid-precipitated and analyzed by SDS-PAGE and phosphorimaging.
Protein Determination and Western Blotting
The protein concentrations were determined with the DC protein assay (Bio-Rad) using bovine serum albumin as a standard. SDS-PAGE and immunoblot analyses were carried out according to methods previously described (27, 28). Signal capture and quantification were performed using the FUJIFILM LAS-4000 luminescent image analyzer.
Other Methods
YidC (24) and SecYEG (29) were purified and reconstituted in E. coli phospholipids (Avanti Polar Lipids, Alabaster, NY) at protein/lipid ratios of 0.125 and 0.055 (w/w), respectively, using Bio-Beads SM-2 (Bio-Rad).
RESULTS
Membrane Insertion of NuoK Is Adversely Affected by Decreased YidC and SecYEG Levels in the Membrane
NuoK is a 11-kDa protein containing three TMSs, connected by two short (0.3 and 1.2 kDa) loops, with a C terminus of 2 kDa (Fig. 1A). To monitor the requirements of NuoK insertion, NuoK was synthesized in vitro from plasmid pETNuoK. This resulted in the production of a 10–11-kDa protein visualized on SDS-PAGE (Fig. 1B, lane 1). The thick diffuse banding pattern is typically observed for hydrophobic proteins. When in vitro synthesis was performed in the presence of YidC+ IMVs, proteinase K treatment of NuoK resulted in a protein band ∼1.4 kDa smaller than full-length NuoK (Fig. 1B, lane 2). The fragment most likely represents digestion of the C-terminal residues (ΔC-NuoK). Solubilization of the IMVs with SDS prior to proteinase K digestion resulted in degradation of all full-length NuoK, whereas a weak signal of ΔC-NuoK remains likely because of protease protection by the SDS micelles around the three TMSs. We therefore conclude that the signal observed after proteinase K treatment represents membrane-inserted NuoK in its correct topology.
FIGURE 1.
Membrane insertion of NuoK is affected by YidC and SecYEG levels in the membrane. A, membrane topology of NuoK indicating the glutamate residues in TMSs 2 and 3. B and C, in vitro synthesis of NuoK were carried out at 37 °C for 30 min in the presence of 10 μg of IMVs. After the synthesis reaction, the membranes were collected through a sucrose cushion and resuspended in 50 mm HEPES-KOH, pH 8. Proteinase K was added to 1.6 mg/ml in the absence (lanes 2 and 5) or presence of SDS (lanes 3 and 6). The standards of 10% of the synthesis reactions are shown (lanes 1 and 4). The synthesis reactions were performed in the presence of YidC+ or YidC− IMVs (B) or wild type (WT) or SecYEG+ IMVs (C). D, NuoK insertion was quantified from the amount of protease-protected material in the presence of IMVs. The background signal of proteinase K-resistant NuoK observed when no liposomes were present was deducted from the data. All of the data points shown are the averages of three independent experiments. The bars indicate the standard errors of the mean. MW, molecular mass.
IMVs were isolated from YidC+ and YidC− strains, and YidC levels were monitored by Western blot to confirm YidC depletion (supplemental Fig. S1). When in vitro synthesis was performed in the presence of YidC+ and YidC− IMVs, NuoK insertion into IMVs was dramatically reduced in the YidC− strain when compared with the YidC+ (Fig. 1B). The effect of SecYEG overexpression on NuoK insertion was subsequently investigated. Overexpression of SecYEG (SecYEG+) greatly enhanced membrane insertion of NuoK when compared with wild type IMVs (Fig. 1C). Western blot analysis using an antibody directed against YidC indicated that overexpression of SecYEG did not affect YidC levels in the IMVs (supplemental Fig. S1).
The membrane insertion of NuoK observed in IMVs suggests involvement of both YidC and SecYEG. YidC depletion results in cells, and therefore IMVs, unable to generate a proton motive force (PMF) from ATP (10). It could therefore be the inability of the IMVs to generate a PMF that causes the observed reduction in NuoK insertion in YidC− IMVs. The PMF has been shown to play an essential role in the insertion of membrane proteins such as FtsQ (10) and the phage coat proteins M13 and Pf3 (30, 31). The role of the PMF in the insertion of in vitro synthesized NuoK was examined by the addition of the ionophores nigericin and valinomycin, which collapse the PMF (Fig. 2). Remarkably, insertion of NuoK into SecYEG+ IMVs was greatly enhanced following dissipation of the PMF (Fig. 2, upper panel, compare lanes 3 and 4). Insertion of the control protein FtsQ, which requires the PMF for insertion, was hampered by the addition of the ionophores (Fig. 2, lower panel, lanes 3 and 4). The observation that NuoK insertion is hampered in YidC− IMVs is therefore not a result of PMF dissipation.
FIGURE 2.
Insertion of NuoK is stimulated in the absence of a PMF. Upper panel, insertion assays with YEG+ IMVs were performed as described in the legend to Fig. 1 in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 3 μm nigericin/valinomycin (nig/val) to dissipate the PMF. As a control, insertion assays with in vitro synthesized FtsQ were performed (lower panel) in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 3 μm nigericin/valinomycin. The 10% standards of the synthesis reactions are shown (lanes 1 and 2). MW, molecular mass.
NuoK Interacts with YidC
Oxa1 has been isolated as a complex with in vitro synthesized Atp9 (homologous to Foc) as well as with the entire F1F0 ATP synthase, suggesting a role for Oxa1 in the assembly of the protein complex (20). In E. coli, in vitro synthesized Foc has been shown to copurify with YidC, suggesting that it too contacts its substrates and remains briefly associated with them (32). E. coli FTL10 was grown under anaerobic conditions in growth medium containing 10 mm fumarate and 0.5% (v/v) glycerol. Overexpression of YidC from plasmid pEH1YidC or pTrcYidC was induced with 0.5 mm isopropyl β-d-thiogalactopyranoside. When IMVs containing His-tagged YidC overexpressed from plasmid pEH1YidC were purified on a Ni2+-NTA column, NuoK was found to copurify with YidC (Fig. 3, lane 2). The recovery of NuoK on the Ni2+-NTA column was specific for the presence of His-tagged YidC because it was not recovered when the nontagged YidC was used as a control (Fig. 3, lane 1).
FIGURE 3.
NuoK associates with YidC. E. coli FTL10 was transformed with plasmids pEH1YidC and pTrcYidC, allowing the overexpression of His-tagged YidC (lane 2) and nontagged YidC (lane 1), respectively. Overexpression was performed under anaerobic growth conditions in media containing fumarate and glycerol. IMVs were isolated and solubilized in 2% N-dodecyl-β-d-maltoside whereupon YidC was purified by Ni2+-NTA chromatography. Elution fractions were run on SDS-PAGE, blotted onto polyvinylidene difluoride membrane, and probed with antibodies against NuoK. MW, molecular mass.
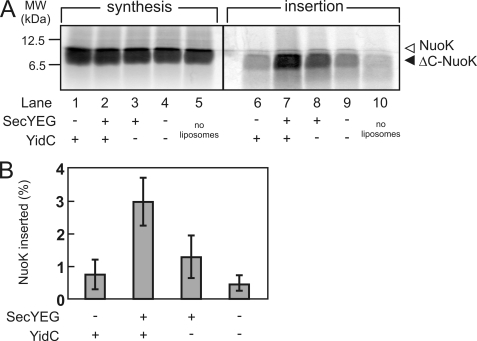
Both YidC and SecYEG Are Essential for NuoK Insertion into Proteoliposomes
To elucidate the minimal requirements for insertion of in vitro synthesized NuoK, insertion assays were performed with proteoliposomes containing purified YidC and SecYEG alone and both YidC and SecYEG (Fig. 4) (24). In the absence of (proteo)liposomes, almost all of the in vitro synthesized NuoK was degraded upon incubation with proteinase K (lane 10), whereas when NuoK was synthesized in the presence of empty liposomes, a small amount of proteinase K-resistant ΔC-NuoK remained (lane 9). A comparable amount of protease-resistant ΔC-NuoK was observed when NuoK was synthesized in the presence of YidC-containing proteoliposomes (lane 6) and could have arisen from interaction of the hydrophobic TMSs of NuoK with the (proteo)liposomes. A reproducible but slight increase in protease-protected NuoK was observed when SecYEG-containing proteoliposomes were present (lane 8), but it was only when both YidC and SecYEG were present that a high level of insertion was observed (lane 7). In agreement with the observation with IMVs, NuoK minimally requires both SecYEG and YidC for insertion in vitro.
FIGURE 4.
Both YidC and SecYEG are essential for NuoK insertion into proteoliposomes. A, NuoK insertion reactions were performed in the presence (lanes 1–4 and 6–9) or absence (lanes 5 and 10) of (proteo)liposomes. For the proteoliposomes, E. coli lipids were reconstituted with YidC and SecYEG at protein/lipid ratios of 0.125 and 0.055 (w/w), respectively. Insertion reactions (lanes 6–10) were performed by proteinase K digestion essentially as described in the legend to Fig. 1. The 5% standards of the synthesis reactions are shown (lanes 1–6). B, NuoK insertion was quantified from the amount of protease-protected material in the presence and absence of (proteo)liposomes. All of the data points shown are the averages of three independent experiments. The bars indicate the standard errors of the mean. MW, molecular mass.
Mutation of Glutamates at Positions 36 and 72 to Lysines Renders NuoK Independent of YidC for Insertion
YidC has been postulated to form a platform at which TMSs can be released into the membrane (33), and this may particularly be important for less hydrophobic or negatively charged TMSs (34). NuoK has two conserved glutamates at positions 36 and 72 in TMSs 2 and 3, respectively (Fig. 1A). Substitution of these glutamates results in a dramatic loss of function of the NADH dehydrogenase I (35, 36). To test whether YidC is specifically involved in the insertion of these membrane-embedded negative charges, we constructed NuoK mutant proteins in which the glutamates were substituted for lysines. Using the insertion assay described for wild type NuoK, the insertion of the mutants into YidC+ and YidC− IMVs was investigated (Fig. 5A). The mutants showed slight aberrant running behavior on SDS-PAGE. NuoK E36K (Fig. 5A, top panel, lane 1) and the double mutant NuoK E36K,E72K (Fig. 5A, bottom panel, lane 1) run at ∼12.5 kDa, whereas NuoK E72K (Fig. 5A, middle panel, lane 1) runs at 12 kDa. This behavior is likely caused by the alterations in the charge of the TMSs. In the presence of YidC+ IMVs, incubation with proteinase K produced a protected fragment ∼1.3 kDa smaller than full-length NuoK E36K (Fig. 5A, top panel, lane 2), which likely corresponds to protease digestion of the C terminus. When proteinase K digestion was performed in the presence of SDS, almost all of the NuoK E36K protein was digested (Fig. 5A, top panel, lane 3). We therefore concluded that the protease-protected fragment observed corresponds to inserted protein in the correct topology. When NuoK E36K is synthesized in the presence of YidC− IMVs, almost no protease-protected protein was observed (Fig. 5A, top panel, lane 5). The mutant protein is therefore still dependent on YidC for insertion. A similar result was obtained for the mutant protein NuoK E72K (Fig. 5A, middle panel, lane 1). The mutant protein containing the double substitution NuoK E36K,E72K did, however, exhibit a insertion requirement different from those with single substitutions (Fig. 5A, bottom panel). In the presence of YidC+ IMVs, incubation with proteinase K results in a fragment 2.5 kDa smaller than full-length NuoK E36K,E72K (Fig. 5A, bottom panel, lane 2). Most, but not all, of the material was digested by the protease if the IMVs were solubilized with SDS, indicating that the signal observed corresponded to inserted NuoK E36K,E72K (Fig. 5A, bottom panel, lane 3). The amount of inserted NuoK E36K,E72K did not change when the assay was performed in the presence of YidC− IMVs (Fig. 5A, bottom panel, lane 5). Therefore, it could be concluded that substitution of both glutamates for lysines renders NuoK independent of YidC for insertion.
FIGURE 5.
Mutation of glutamates at positions 36 and 72 to lysines renders NuoK independent of YidC for insertion. A, insertion reactions were performed as described in the legend of Fig. 1. Synthesis reactions were performed in the presence of YidC+ or YidC− IMVs, and proteinase K was added in the absence (lanes 2 and 5) or presence (lanes 3 and 6) of SDS. Synthesis reactions were performed with plasmids containing single mutants NuoK E36K (top panel) and NuoK E72K (middle panel) and the double mutant NuoK E36K,E72K (bottom panel). The 10% standards of the synthesis reactions are shown (lanes 1 and 4). B, NuoK insertion was quantified from the amount of protease-protected material in the presence of YidC+ (indicated in white) and YidC− (indicated in dark gray) IMVs. All of the data points shown are the averages of three independent experiments. The bars indicate the standard errors of the mean. C, NuoK insertion reactions were performed in the presence (lanes 1–4 and 6–9) or absence (lanes 5 and 10) of (proteo)liposomes reconstituted with or without purified SecYEG and YidC as indicated. The insertion reactions (lanes 6–10) were performed essentially as described in the legend to Fig. 1 using plasmids containing single substitutions NuoK E36K (top panel) and NuoK E72K (middle panel) and double substitutions NuoK E36K,E72K (bottom panel). 5% standards of the synthesis reactions are shown (lanes 1–5). D, NuoK insertion was quantified from the amount of protease-protected material in the presence and absence of (proteo)liposomes. The background signal of proteinase K-resistant NuoK observed when no liposomes were present was deducted from the data. All of the data points shown are averages of three independent experiments. The bars indicate the standard errors of the mean.
The Double Mutant NuoK E36K,E72K Requires Only SecYEG for Insertion into Proteoliposomes
Insertion of the mutants described above was investigated using proteoliposomes to determine the minimal requirements for insertion. The same set of (proteo)liposomes was used as for the wild type NuoK. The insertion patterns observed for the single mutants NuoK E36K and NuoK E72K, was comparable with those observed for wild type NuoK (Figs. 4 and 5). In the absence of (proteo)liposomes, almost all NuoK E36K synthesized was digested by externally added proteinase K (Fig. 5B, top panel, lane 10). Proteoliposomes containing YidC only and empty liposomes afforded a small amount of protection in the presence of proteinase K (Fig. 5B, top panel, lanes 6 and 9), whereas synthesis in the presence of proteoliposomes containing only SecYEG resulted in a very slight but reproducible increase in insertion (Fig. 5B, top panel, lane 8) compared with that observed for YidC only and empty (proteo)liposomes. Only when synthesis was performed in the presence of proteoliposomes containing both YidC and SecYEG was an appreciable amount of inserted NuoK E36K observed (Fig. 5B, top panel, lane 7). Also for the mutant protein NuoK E72K, insertion was only observed in proteoliposomes containing both YidC and SecYEG (Fig. 5B, middle panel, lane 7). The observations with proteoliposomes support those made with IMVs.
In the presence of (proteo)liposomes, proteinase K treatment of in vitro synthesized NuoK E36K,E72K resulted in the formation of three protein bands (Fig. 5B, bottom panel, lanes 6–9). The upper band corresponds to undigested full-length protein, whereas the two lower bands, 2 and 2.5 kDa smaller than the full-length NuoK E36K,E72K, correspond to C-terminal truncations. If the mutant protein were inserted in the inverse topology, proteinase K digestion would most likely result in two bands corresponding to the N-terminal two TMSs (5.3–6.6 kDa) and TMS 3 plus the C terminus (4.2–5.5 kDa). This is not observed. Alternatively, if proteinase K did not cleave the protein in the periplasmic loop between TMSs 2 and 3, only full-length protein would be observed. It is therefore not possible to deduce the topology of the full-length protein observed after proteinase K digestion. In the absence of (proteo)liposomes, most of the NuoK was digested (Fig. 5B, bottom panel, lane 10). Incubation with proteinase K in the presence of proteoliposomes containing only YidC or empty liposomes resulted in a slight increase in protease-protected protein (Fig. 5B, bottom panel, lanes 6 and 9). However, when NuoK E36K,E72K was synthesized in the presence of SecYEG-containing proteoliposomes (either alone or together with YidC), an appreciable amount of insertion was observed (Fig. 5B, bottom panel, lanes 7 and 8). This indicates that, in contrast to wild type NuoK and the single glutamate mutants, NuoK E36K,E72K requires only SecYEG for insertion in vitro.
DISCUSSION
All of the subunits of the bacterial complex I, NADH dehydrogenase I, are encoded by the nuo operon. In E. coli, NADH dehydrogenase I contains 13 subunits, NuoA through to NuoN with NuoC and NuoD fused to form one protein. If any of these subunits are absent, a functional enzyme complex cannot be formed (37). In a previous study, we showed that levels of the small membrane subunit NuoK are greatly reduced upon YidC depletion (21). A decrease in the levels of NuoK in the membrane could have been due to the absence of one of the other subunits under YidC-depleting conditions, but as we now demonstrate, YidC is directly involved in the insertion of NuoK. Using IMVs we showed that the levels of YidC and SecYEG affected the efficiency of NuoK insertion. The decrease in insertion efficiency in YidC− IMVs was not due to the impaired ability of the IMVs to generate a PMF because in the absence of a PMF, the efficiency of insertion of NuoK was even enhanced. It has been shown with M13 procoat Lep and CyoA derivatives that the presence of positive charges in the translocated loops of these derivatives created proteins that only inserted in the absence of a PMF (38, 39). NuoK contains one negatively charged residue in the periplasmic loop between TMSs 2 and 3, whereas the cytoplasmic loop between TMSs 1 and 2 and the C-terminal tail contain numerous positively charged amino acids. The protein topology therefore follows that predicted by the “positive inside rule” (40), and it is not immediately obvious why the PMF would inhibit protein insertion. NADH dehydrogenase I is the preferred NADH dehydrogenase under anaerobic growth conditions (41). Unlike NADH dehydrogenase II, the proton-pumping NADH dehydrogenase I is energy-conserving, which is needed under the “energy limited” conditions of growth without oxygen. It is possible that NuoK insertion is enhanced under such conditions when the PMF is reduced and NADH dehydrogenase I is needed.
Although the number of known YidC-dependent membrane proteins has increased during recent years, the exact mechanism of YidC in membrane protein insertion remains enigmatic. YidC has been shown to contact the TMSs of numerous integral membrane proteins in various cross-linking studies (24, 42, 43), and yet YidC is dispensable in the insertion of most of these proteins (26, 44, 45). Using in vitro insertion into proteoliposomes, we show that NuoK minimally requires both YidC and SecYEG for insertion. Subunit a of the cytochrome oxidase, CyoA, has also been shown to require both YidC and SecYEG for insertion in vitro (12). Based on the individual insertion requirements of constructs containing either the N- or C-terminal parts of CyoA, it has been suggested that SecYEG and YidC work sequentially in the insertion of CyoA with YidC inserting the N-terminal hairpin and SecYEG inserting the C-terminal TMS and long periplasmic C-terminal tail (13, 46). This strict sequential insertion is only alleviated when a long linker between TMSs 1 and 2 is introduced, after which the C terminus can insert independently of the N-terminal hairpin (39). Interestingly the introduction of numerous positive charges in the signal peptide and TMS 1 block the insertion of this protein via the YidC pathway but do not result in default insertion via the Sec translocon (39).
We observed that the presence of the negative charges in TM 2 and 3 determines the YidC-dependent insertion. NuoK contains single glutamates in TM2 and 3, which are required for high ubiquinone activity (35, 36). Substitution of the glutamates at positions 36 and 72 for lysines produced a protein that, like most studied integral membrane proteins, required only SecYEG for integration into the membrane. This supports a model in which the TMSs of YidC have a role in forming a membrane chaperone, assisting the integration of less hydrophobic, negatively charged TMSs, similar to that proposed for Oxa1 (34, 34). The presence of just one glutamate-containing TMS renders the entire protein still YidC-dependent, and it is only when both glutamates have been substituted for lysines that the protein can be inserted by SecYEG, unassisted by YidC. NuoK is one of many integral membrane respiratory proteins that contain membrane-negative charges. NuoA has a similar structure to NuoK with glutamates at positions 81 and 102 (TM2 and 3) and an asparate at position 79 (TM2). It would be of interest if these similar structural features necessitate YidC in the insertion process.
In the well studied substrate of YidC, Foc, mutation of an aspartate at position 61 (TM2) to a glycine produces an ATP synthase that contains an enzymatically active F1 part but no functional F0 (47). There were, however, detectable levels of the mutant Foc present in the membrane. Attempts to elucidate the insertion requirements for this mutant in vitro were unsuccessful because of the proteinase K-resistant nature of the protein.3 Substitution of a glycine for aspartate in TM1 of Foc results in a mutant form of the protein that is still dependent of YidC for insertion but that does not form an oligomeric ring structure as the wild type protein does (32, 48). Thus the introduction of a negative charge into a TMS is tolerated by the YidC-only mode of insertion. Of the other proteins shown to insert with the assistance of YidC, the mechanosensitive channel of large conductance, MscL, and the phage coat proteins M13 and Pf3 have membrane-located negative charges. CyoA, however, does not contain any membrane-negative charges. YidC is not essential for the insertion of FtsQ (26) and LepB (49), even though interaction between these proteins and YidC during membrane insertion has been observed (1, 24, 42, 43, 50). FtsQ does not contain any membrane-embedded charges, whereas LepB contains a glutamate in TM2. There must therefore be structural features other than membrane-located negative charges that confer YidC dependence to proteins.
YidC is remarkably resilient to single amino acid substitutions, and even when TMSs 4 and 5 were swapped for unrelated TMSs, YidC activity was retained (33). It has therefore been suggested that it is the presence of the C-terminal 5 TMSs and not the specific sequences of the hydrophobic stretches that is essential for YidC activity. Our data support the hypothesis that YidC serves as a platform from which TMSs are integrated into the membrane. Furthermore, YidC is essential for NuoK TMS integration when membrane-embedded negative charges are present. The involvement of YidC in the integration of such TMSs may provide an explanation as to its conserved function in the biogenesis of respiratory proteins that often contain essential-for-function negative charges providing a basis for future mutagenesis studies on other YidC substrates.
Supplementary Material
Acknowledgments
We thank Takao Yagi (The Scripps Research Institute) for the anti-NuoK serum. We also thank Manfred Saller and Wiktor Majczak for useful discussions and technical assistance.
This work was supported by a grant from the Netherlands Proteomics Centre.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
S. Kol and J. de Keyzer, unpublished data.
- TMS
- transmembrane segment
- IMV
- inner membrane vesicle
- PMF
- proton motive force
- NTA
- nitrilotriacetic acid.
REFERENCES
- 1.Scotti P. A., Urbanus M. L., Brunner J., de Gier J. W., von Heijne G., van der Does C., Driessen A. J., Oudega B., Luirink J. (2000) EMBO J. 19, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Gier J. W., Luirink J. (2001) Mol. Microbiol. 40, 314–322 [DOI] [PubMed] [Google Scholar]
- 3.Pohlschröder M., Hartmann E., Hand N. J., Dilks K., Haddad A. (2005) Annu. Rev. Microbiol. 59, 91–111 [DOI] [PubMed] [Google Scholar]
- 4.Yen M. R., Harley K. T., Tseng Y. H., Saier M. H., Jr. (2001) FEMS Microbiol. Lett. 204, 223–231 [DOI] [PubMed] [Google Scholar]
- 5.Luirink J., Samuelsson T., de Gier J. W. (2001) FEBS Lett. 501, 1–5 [DOI] [PubMed] [Google Scholar]
- 6.Bauer M., Behrens M., Esser K., Michaelis G., Pratje E. (1994) Mol. Gen. Genet. 245, 272–278 [DOI] [PubMed] [Google Scholar]
- 7.Bonnefoy N., Chalvet F., Hamel P., Slonimski P. P., Dujardin G. (1994) J. Mol. Biol. 239, 201–212 [DOI] [PubMed] [Google Scholar]
- 8.Altamura N., Capitanio N., Bonnefoy N., Papa S., Dujardin G. (1996) FEBS Lett. 382, 111–115 [DOI] [PubMed] [Google Scholar]
- 9.Moore M., Harrison M. S., Peterson E. C., Henry R. (2000) J. Biol. Chem. 275, 1529–1532 [DOI] [PubMed] [Google Scholar]
- 10.van der Laan M., Bechtluft P., Kol S., Nouwen N., Driessen A. J. (2004) J. Cell Biol. 165, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi L., Jiang F., Chen M., Cain B., Bolhuis A., Dalbey R. E. (2003) Biochemistry 42, 10537–10544 [DOI] [PubMed] [Google Scholar]
- 12.du Plessis D. J., Nouwen N., Driessen A. J. (2006) J. Biol. Chem. 281, 12248–12252 [DOI] [PubMed] [Google Scholar]
- 13.van Bloois E., Haan G. J., de Gier J. W., Oudega B., Luirink J. (2006) J. Biol. Chem. 281, 10002–10009 [DOI] [PubMed] [Google Scholar]
- 14.Facey S. J., Neugebauer S. A., Krauss S., Kuhn A. (2007) J. Mol. Biol. 365, 995–1004 [DOI] [PubMed] [Google Scholar]
- 15.Nargang F. E., Preuss M., Neupert W., Herrmann J. M. (2002) J. Biol. Chem. 277, 12846–12853 [DOI] [PubMed] [Google Scholar]
- 16.Sellem C. H., Lemaire C., Lorin S., Dujardin G., Sainsard-Chanet A. (2005) Genetics 169, 1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiburek L., Fornuskova D., Wenchich L., Pejznochova M., Hansikova H., Zeman J. (2007) J. Mol. Biol. 374, 506–516 [DOI] [PubMed] [Google Scholar]
- 18.Meyer W., Bauer M., Pratje E. (1997) Curr. Genet. 31, 401–407 [DOI] [PubMed] [Google Scholar]
- 19.Lemaire C., Hamel P., Velours J., Dujardin G. (2000) J. Biol. Chem. 275, 23471–23475 [DOI] [PubMed] [Google Scholar]
- 20.Jia L., Dienhart M. K., Stuart R. A. (2007) Mol. Biol. Cell 18, 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price C. E., Driessen A. J. (2008) J. Biol. Chem. 283, 26921–26927 [DOI] [PubMed] [Google Scholar]
- 22.Hatzixanthis K., Palmer T., Sargent F. (2003) Mol. Microbiol. 49, 1377–1390 [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann A., Manting E. H., Veenendaal A. K., Driessen A. J., van der Does C. (1999) Biochemistry 38, 9115–9125 [DOI] [PubMed] [Google Scholar]
- 24.van der Laan M., Houben E. N., Nouwen N., Luirink J., Driessen A. J. (2001) EMBO Rep. 2, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saller M. J., Fusetti F., Driessen A. J. (2009) J. Bacteriol. 191, 6749–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Laan M., Nouwen N., Driessen A. J. (2004) J. Biol. Chem. 279, 1659–1664 [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 28.Towbin H., Staehelin T., Gordon J. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Does C., de Keyzer J., van der Laan M., Driessen A. J. (2003) Methods Enzymol. 372, 86–98 [DOI] [PubMed] [Google Scholar]
- 30.Cao G., Dalbey R. E. (1994) EMBO J. 13, 4662–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrer J., Kuhn A. (1990) Science 250, 1418–1421 [DOI] [PubMed] [Google Scholar]
- 32.Kol S., Turrell B. R., de Keyzer J., van der Laan M., Nouwen N., Driessen A. J. (2006) J. Biol. Chem. 281, 29762–29768 [DOI] [PubMed] [Google Scholar]
- 33.Jiang F., Chen M., Yi L., de Gier J. W., Kuhn A., Dalbey R. E. (2003) J. Biol. Chem. 278, 48965–48972 [DOI] [PubMed] [Google Scholar]
- 34.Saint-Georges Y., Hamel P., Lemaire C., Dujardin G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13814–13819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kervinen M., Pätsi J., Finel M., Hassinen I. E. (2004) Biochemistry 43, 773–781 [DOI] [PubMed] [Google Scholar]
- 36.Kao M. C., Nakamaru-Ogiso E., Matsuno-Yagi A., Yagi T. (2005) Biochemistry 44, 9545–9554 [DOI] [PubMed] [Google Scholar]
- 37.Schneider D., Pohl T., Walter J., Dörner K., Kohlstädt M., Berger A., Spehr V., Friedrich T. (2008) Biochim. Biophys. Acta 1777, 735–739 [DOI] [PubMed] [Google Scholar]
- 38.Samuelson J. C., Jiang F., Yi L., Chen M., de Gier J. W., Kuhn A., Dalbey R. E. (2001) J. Biol. Chem. 276, 34847–34852 [DOI] [PubMed] [Google Scholar]
- 39.Celebi N., Dalbey R. E., Yuan J. (2008) J. Mol. Biol. 375, 1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Heijne G., Gavel Y. (1988) Eur. J. Biochem. 174, 671–678 [DOI] [PubMed] [Google Scholar]
- 41.Tran Q. H., Bongaerts J., Vlad D., Unden G. (1997) Eur. J. Biochem. 244, 155–160 [DOI] [PubMed] [Google Scholar]
- 42.Yu Z., Koningstein G., Pop A., Luirink J. (2008) J. Biol. Chem. 283, 34635–34642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck K., Eisner G., Trescher D., Dalbey R. E., Brunner J., Müller M. (2001) EMBO Rep. 2, 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuelson J. C., Chen M., Jiang F., Möller I., Wiedmann M., Kuhn A., Phillips G. J., Dalbey R. E. (2000) Nature 406, 637–641 [DOI] [PubMed] [Google Scholar]
- 45.van der Laan M., Urbanus M. L., Ten Hagen-Jongman C. M., Nouwen N., Oudega B., Harms N., Driessen A. J., Luirink J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5801–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celebi N., Yi L., Facey S. J., Kuhn A., Dalbey R. E. (2006) J. Mol. Biol. 357, 1428–1436 [DOI] [PubMed] [Google Scholar]
- 47.Wachter E., Schmid R., Deckers G., Altendorf K. (1980) FEBS Lett. 113, 265–270 [DOI] [PubMed] [Google Scholar]
- 48.Jans D. A., Fimmel A. L., Langman L., James L. B., Downie J. A., Senior A. E., Ash G. R., Gibson F., Cox G. B. (1983) Biochem. J. 211, 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Gier J. W., Scotti P. A., Sääf A., Valent Q. A., Kuhn A., Luirink J., von Heijne G. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houben E. N., Zarivach R., Oudega B., Luirink J. (2005) J. Cell Biol. 170, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.