Abstract
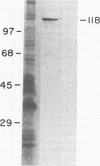
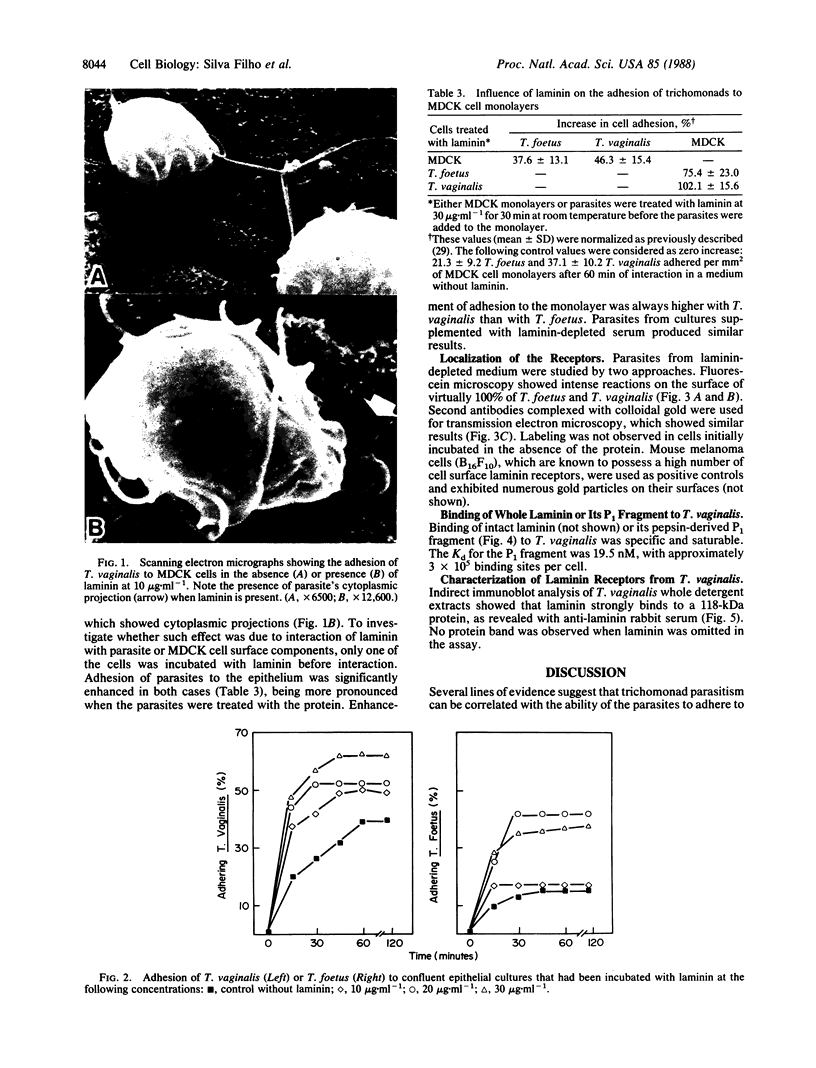
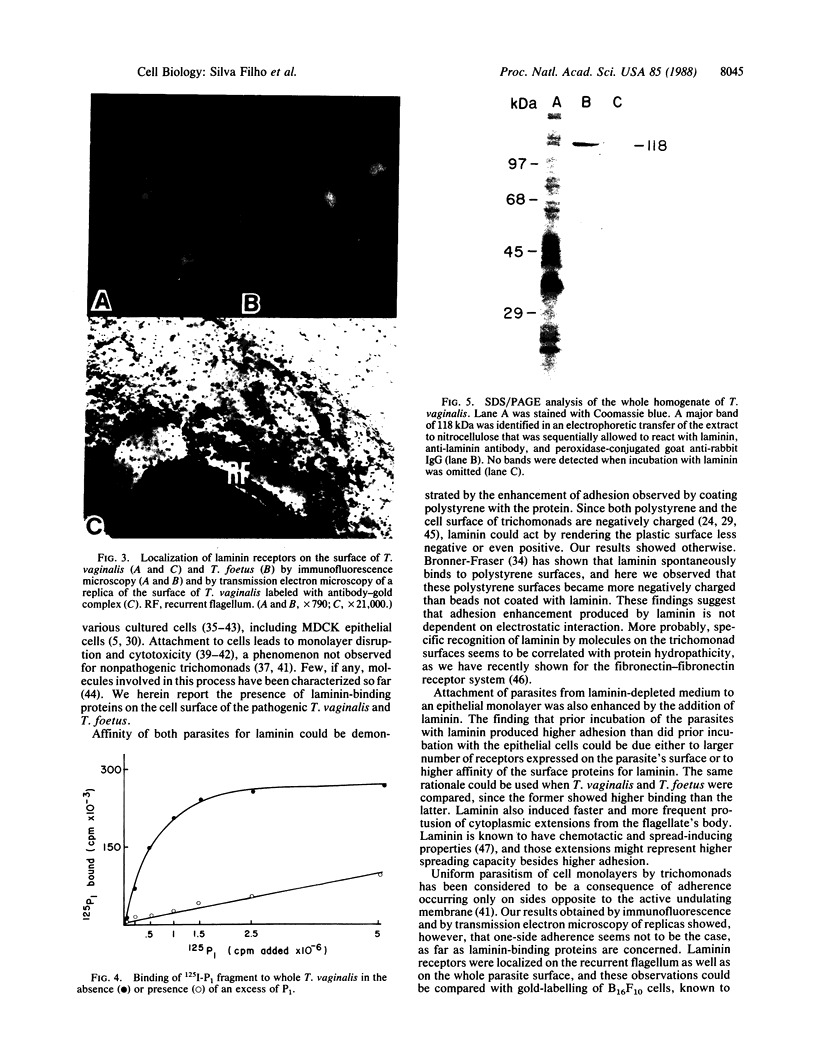
Adhesion is regarded as an important feature in the pathogenesis of various microorganisms. Ability to recognize extracellular matrix proteins, such as laminin or fibronectin, has been correlated with invasiveness. We report that laminin enhances the adhesion of the parasitic protozoa Trichomonas vaginalis and Tritrichomonas foetus to a polystyrene substrate and to the surface of epithelial cells (Madin-Darby canine kidney cell line) in vitro. The enhancement was higher for T. vaginalis than for T. foetus. Addition of anti-laminin antibodies to medium significantly inhibited the adhesion of parasites to polystyrene substrate. Indirect immunofluorescence and transmission electron microscopy of replicas of the parasite's surface labeled with antibody-gold complexes showed laminin-binding sites distributed over the parasite surface. Iodinated P1 fragment of laminin, which retains the laminin-binding site, binds saturably to the parasite surface with a Kd of 19.5 nM, for about 3 X 10(5) binding sites per cell. Immunoblotting analysis of whole parasite extracts showed that a protein of 118 kDa is responsible for laminin binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Garza G. E. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect Immun. 1988 Jan;56(1):28–33. doi: 10.1128/iai.56.1.28-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985 Dec;50(3):701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M., Nurcombe V., Edgar D., Paulsson M., Timpl R. The cellular interactions of laminin fragments. Cell adhesion correlates with two fragment-specific high affinity binding sites. J Biol Chem. 1987 Aug 25;262(24):11532–11538. [PubMed] [Google Scholar]
- Barthel J. S., Butt J. H., 2nd Paracentesis. Am Fam Physician. 1983 Sep;28(3):28–28. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur P., Savel J. Evaluation de la virulence des souches de Trichomonas vaginalis par l'étude de l'effet cytopathogène sur culture de cellules. C R Seances Soc Biol Fil. 1982;176(6):849–860. [PubMed] [Google Scholar]
- Brentani R. R., Ribeiro S. F., Potocnjak P., Pasqualini R., Lopes J. D., Nakaie C. R. Characterization of the cellular receptor for fibronectin through a hydropathic complementarity approach. Proc Natl Acad Sci U S A. 1988 Jan;85(2):364–367. doi: 10.1073/pnas.85.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretaña A., Avila J. L., Arias-Flores M., Contreras M., Tapia F. J. Trypanosoma cruzi and American Leishmania spp: immunocytochemical localization of a laminin-like protein in the plasma membrane. Exp Parasitol. 1986 Apr;61(2):168–175. doi: 10.1016/0014-4894(86)90149-9. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Latex beads as probes of a neural crest pathway: effects of laminin, collagen, and surface charge on bead translocation. J Cell Biol. 1984 Jun;98(6):1947–1960. doi: 10.1083/jcb.98.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant G., Rao C. N., Brentani M., Martins W., Lopes J. D., Martin S. E., Liotta L. A., Schiffmann E. A role for the laminin receptor in leukocyte chemotaxis. J Leukoc Biol. 1987 Mar;41(3):220–227. doi: 10.1002/jlb.41.3.220. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN R. T., MILLER N. F., LUDOVICI P. P., RILEY G. M. A study of Trichomonas vaginalis in human cell culture. Am J Obstet Gynecol. 1963 Apr 1;85:947–954. doi: 10.1016/s0002-9378(16)35599-5. [DOI] [PubMed] [Google Scholar]
- Cappuccinelli P., Varesio L. The effect of cytochalasin B, colchicine and vinblastine on the adhesion of Trichomonas vaginalis to glass surfaces. Int J Parasitol. 1975 Feb;5(1):57–61. doi: 10.1016/0020-7519(75)90098-3. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Cereijido M. Electrical properties of Madin-Darby canine kidney cells. Fed Proc. 1984 May 15;43(8):2230–2235. [PubMed] [Google Scholar]
- Chávez B., Knaippe F., Gonzalez-Mariscal L., Martínez-Palomo A. Giardia lamblia: electrophysiology and ultrastructure of cytopathology in cultured epithelial cells. Exp Parasitol. 1986 Jun;61(3):379–389. doi: 10.1016/0014-4894(86)90194-3. [DOI] [PubMed] [Google Scholar]
- Costa e Silva Filho F., Elias C. A., de Souza W. Further studies on the surface charge of various strains of Trichomonas vaginalis and Tritrichomonas foetus. Cell Biophys. 1986 Jun;8(3):161–176. doi: 10.1007/BF02788492. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- FROST J. K. Trichomonas vaginalis and cervical epithelial changes. Ann N Y Acad Sci. 1962 Sep 29;97:792–799. doi: 10.1111/j.1749-6632.1962.tb34689.x. [DOI] [PubMed] [Google Scholar]
- Heath J. P. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis. 1981 Apr;57(2):106–117. doi: 10.1136/sti.57.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard T. K., Malinoff H. L., Wicha M. S. Macrophages express a plasma membrane receptor for basement membrane laminin. Am J Pathol. 1986 May;123(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Junker J. L., Wilson M. J. Divergent expression of laminin and fibronectin in non-tumorigenic and transformed liver epithelial cells. J Cell Sci. 1985 Jun;76:213–223. doi: 10.1242/jcs.76.1.213. [DOI] [PubMed] [Google Scholar]
- KOSS L. G., WOLINSKA W. H. Trichomonas vaginalis cervicitis and its relationship to cervical cancer. A histocytological study. Cancer. 1959 Nov-Dec;12:1171–1193. doi: 10.1002/1097-0142(195911/12)12:6<1171::aid-cncr2820120613>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kennedy B. G., Lever J. E. Regulation of Na+,K+-ATPase activity in MDCK kidney epithelial cell cultures: role of growth state, cyclic AMP, and chemical inducers of dome formation and differentiation. J Cell Physiol. 1984 Oct;121(1):51–63. doi: 10.1002/jcp.1041210108. [DOI] [PubMed] [Google Scholar]
- Kitamura A. Attachment of Paramecium to polystyrene surfaces: a model system for the analysis of sexual cell recognition and nuclear activation. J Cell Sci. 1982 Dec;58:185–199. doi: 10.1242/jcs.58.1.185. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Ravdin J. I., Rein M. F. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun. 1985 Dec;50(3):778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulda J., Honigberg B. M. Behavior and pathogenicity of Tritrichomonas foetus in chick liver cell cultures. J Protozool. 1969 Aug;16(3):479–495. doi: 10.1111/j.1550-7408.1969.tb02304.x. [DOI] [PubMed] [Google Scholar]
- Leighton J., Brada Z., Estes L. W., Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969 Jan 31;163(3866):472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Regulation of dome formation in kidney epithelial cell cultures. Ann N Y Acad Sci. 1981;372:371–383. doi: 10.1111/j.1749-6632.1981.tb15489.x. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Lumsden W. H., Robertson D. H., McNeillage G. J. Isolation, cultivation, low temperature preservation, and infectivity titration of Trichomonas vaginalis. Br J Vener Dis. 1966 Sep;42(3):145–154. doi: 10.1136/sti.42.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Palomo A., González-Robles A., Chávez B., Orozco E., Fernández-Castelo S., Cervantes A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J Protozool. 1985 Feb;32(1):166–175. doi: 10.1111/j.1550-7408.1985.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Pindak F. F., Gardner W. A., Jr, Mora de Pindak M. Growth and cytopathogenicity of Trichomonas vaginalis in tissue cultures. J Clin Microbiol. 1986 Apr;23(4):672–678. doi: 10.1128/jcm.23.4.672-678.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen S. E., Nielsen M. H., Lind I., Rhodes J. M. Morphological studies of the cytotoxicity of Trichomonas vaginalis to normal human vaginal epithelial cells in vitro. Genitourin Med. 1986 Aug;62(4):240–246. doi: 10.1136/sti.62.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Rao C. N., Magnani J. L., Spitalnik S. L., Liotta L. A., Ginsburg V. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1306–1310. doi: 10.1073/pnas.82.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Bächinger H. P., Timpl R. Characterization of pepsin fragments of laminin in a tumor basement membrane. Evidence for the existence of related proteins. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1651–1660. doi: 10.1515/bchm2.1980.361.2.1651. [DOI] [PubMed] [Google Scholar]
- Rubin K., Johansson S., Hök M., Obrink B. Substrate adhesion of rat hepatocytes. On the role of fibronectin in cell spreading. Exp Cell Res. 1981 Sep;135(1):127–135. doi: 10.1016/0014-4827(81)90305-0. [DOI] [PubMed] [Google Scholar]
- SKACEL K. On the significance of trichomoniasis in praecancerous states of the uterine cervix. Neoplasma. 1957;4(3):297–303. [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Silva Filho F. C., Elias C. A., De Souza W. Role of divalent cations, pH, cytoskeleton components and surface charge on the adhesion of Trichomonas vaginalis to a polystyrene substrate. Mem Inst Oswaldo Cruz. 1987 Jul-Sep;82(3):379–384. doi: 10.1590/s0074-02761987000300009. [DOI] [PubMed] [Google Scholar]
- Smalheiser N. R., Schwartz N. B. Cranin: a laminin-binding protein of cell membranes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6457–6461. doi: 10.1073/pnas.84.18.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Wicha M. S., Huard T. K. Macrophages express cell surface laminin. Exp Cell Res. 1983 Feb;143(2):475–479. doi: 10.1016/0014-4827(83)90077-0. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Kühl U. Laminin and its receptor. Biochim Biophys Acta. 1985 Dec 17;823(2):147–160. doi: 10.1016/0304-419x(85)90010-1. [DOI] [PubMed] [Google Scholar]