Abstract
A loss of epidermal cohesion in pemphigus vulgaris (PV) results from autoantibody action on keratinocytes (KCs) activating the signaling kinases and executioner caspases that damage KCs, causing their shrinkage, detachment from neighboring cells, and rounding up (apoptolysis). In this study, we found that PV antibody binding leads to activation of epidermal growth factor receptor kinase, Src, p38 MAPK, and JNK in KCs with time pattern variations from patient to patient. Both extrinsic and intrinsic apoptotic pathways were also activated. Although Fas ligand neutralizing antibody could inhibit the former pathway, the mechanism of activation of the latter remained unknown. PV antibodies increased cytochrome c release, suggesting damage to mitochondria. The immunoblotting experiments revealed penetration of PVIgG into the subcellular mitochondrial fraction. The antimitochondrial antibodies from different PV patients recognized distinct combinations of antigens with apparent molecular sizes of 25, 30, 35, 57, 60, and 100 kDa. Antimitochondrial antibodies were pathogenic because their absorption abolished the ability of PVIgG to cause keratinocyte detachment both in vitro and in vivo. The downstream signaling of antimitochondrial antibodies involved JNK and late p38 MAPK activation, whereas the signaling of anti-desmoglein 3 (Dsg3) antibody involved JNK and biphasic p38 MAPK activation. Using KCs grown from Dsg3−/− mice, we determined that Dsg3 did not serve as a surrogate antigen allowing antimitochondrial antibodies to enter KCs. The PVIgG-induced activation of epidermal growth factor receptor and Src was affected neither in Dsg3−/− KCs nor due to absorption of antimitochondrial antibodies. These results demonstrated that apoptolysis in PV is a complex process initiated by at least three classes of autoantibodies directed against desmosomal, mitochondrial, and other keratinocyte self-antigens. These autoantibodies synergize with the proapoptotic serum and tissue factors to trigger both extrinsic and intrinsic pathways of cell death and break the epidermal cohesion, leading to blisters. Further elucidation of the primary signaling events downstream of PV autoantigens will be crucial for the development of a more successful therapy for PV patients.
Keywords: Antibodies, Apoptosis, Cytochromes/Cytochrome c, Proteases/Caspase, Protein/Adhesion, Signal Transduction, Signal Transduction/Protein Kinases/Serine/Threonine, Subcellular Organelles/Mitochondria
Introduction
Pemphigus vulgaris (PV)3 is an IgG autoantibody-mediated disease of skin and mucosa caused by a loss of epidermal cohesion and manifested by progressive blistering and non-healing erosions. The mechanism of detachment of keratinocytes (KCs) in PV, termed acantholysis, is a subject of intensive research. Elucidation of the pathophysiologic mechanism of acantholysis should facilitate development of novel pharmacologic approaches to prevent and treat blistering without systemic corticosteroids, a mainstay of treatment of PV patients. On the cell membrane of KCs, PV antibodies react with desmoglein 3 (Dsg3) and other self-antigens and elicit downstream signaling events causing cell shrinkage, detachment from neighboring cells, and rounding up (reviewed in Ref. 1). Recent studies have demonstrated that acantholysis in PV depends on phosphorylation of keratinocyte adhesion molecules and their internalization and cleavage by apoptotic enzymes (reviewed in Ref. 2). The exact signaling events and their order, however, remain obscure.
Acantholysis can be blocked both by inhibitors of signaling kinases, such as p38 MAPK (3), mammalian target of rapamycin (4), Src, and epidermal growth factor receptor (EGFR) kinase (2, 4–7) and by other tyrosine kinases, phospholipase C, calmodulin, and protein kinase C (8, 9), as well as inhibitors of executioner caspases (4, 10, 11). The inhibitors of EGFR, Src, p38 MAPK, and mammalian target of rapamycin, can also abolish PVIgG-dependent activation of the apoptotic pathways in KCs (4, 12). Therefore, it appears that the structural damage (acantholysis) and death (apoptosis) of KCs in PV are mediated by the same set of enzymes, indicating that acantholysis and apoptosis are inseparable in PV. This fact has justified introduction of the new term “apoptolysis” to distinguish the unique mechanism of autoantibody-induced keratinocyte damage in PV from other known forms of cell death (2). Thus, previous studies not only revealed the complexity of PVIgG signaling but also clearly demonstrated the therapeutic potential of kinase inhibitors and pathway modifiers. Elucidation of the signaling pathways mediating apoptolysis has salient clinical implications because understanding the death pathway of KCs in PV may be a key to development of novel treatments.
In addition to autoantibodies, PV serum contains the non-IgG substances that can cause a sharp reduction of keratinocyte viability and weaken intercellular adhesion strength (13), indicating that apoptolysis in PV encompasses both the antibody-mediated and the non-antibody-mediated mechanisms. The serum factors implicated in apoptolysis include Fas ligand (FasL) (14), tumor necrosis factor α (15, 16), interleukins 1, 6, 8, and 15 (15, 17–19), nitric oxide (9, 20), kallikreins, and other proteases (21). Using an organ culture model of PV, it has been demonstrated that PVIgG works together with FasL and tumor necrosis factor α to induce apoptolysis (22). These and some other mediators of inflammation were found to be elevated in skin and/or serum of PV patients (16, 19, 23–25).
An important role of the Fas system in PV apoptolysis is well documented. Original immunohistochemical studies revealed Fas receptor (FasR) on KCs of lesional epidermis (26). Subsequent studies demonstrated that PVIgG binding to KCs causes secretion of soluble FasL, increased production and cellular amounts of FasR and FasL (soluble and membranal), co-aggregation of FasL and FasR with caspase 8 in a membranal death-inducing signaling complex, and down-regulation of the apoptosis inhibitor FLIP-l (5, 10, 11, 14, 27, 28). The keratinocyte FasR can be also triggered by FasL expressed by the cytotoxic T cells (29) found in the skin of PV patients (30). Dr. Pincelli and co-workers (31) have recently reported that FasL neutralizing antibody can prevent PVIgG-induced apoptolysis both in vitro and in vivo. When PVIgG was added to KCs in the presence of anti-FasL neutralizing antibody, the cleavage of the intracellular portion of Dsg3 and its degradation decreased (2). The caspase 8 inhibitor, too, prevented both Dsg3 cleavage and cell detachment, implicating involvement of the extrinsic apoptotic pathway. Other studies demonstrated that binding of PVIgG to KCs also activates caspase 9 (11), suggesting that the intrinsic apoptotic pathway is also involved in the pathophysiology of apoptolysis.
In this study, we investigated the mechanisms leading to activation of the intrinsic apoptotic pathway upon PVIgG binding to KCs. We found that PV patients develop antibodies that penetrate KCs and react with mitochondrial proteins, thus triggering the intrinsic pathway of cell death. To dissect out the signaling pathways coupled by different antigen-antibody systems in PV, we employed PVIgG absorbed with the keratinocyte mitochondrial protein fraction as well as KCs grown from the skin of neonatal Dsg3−/− mice. The obtained results demonstrated that apoptolysis in PV is a complex process initiated by at least three classes of autoantibodies directed against desmosomal autoantigens, mitochondrial autoantigens, and other keratinocyte autoantigens. The autoantibodies synergize with the effects of proapoptotic serum and tissue factors, triggering both extrinsic and intrinsic pathways of cell death that break the epidermal cohesion and cause blistering.
MATERIALS AND METHODS
Chemicals and Kits
The purified mouse anti-human FasL monoclonal antibody was purchased from BD Biosciences. The cell-permeable inhibitors of predominantly caspase 3 (but also of caspase 6, caspase 7, caspase 8, and caspase 10) DEVD-CHO and Z-DEVD-FMK and the enzyme-linked immunosorbent assay kits for measuring phosphorylated Src, p38 MAPK, and JNK were from EMD Biosciences, Inc. (La Jolla, CA). The FACETM EGFR kit was purchased from Active Motif (Carlsbad CA). In the EGFR assay, we used the peroxidase conjugated affinity purified goat anti-rabbit IgG secondary antibody (Rockland Immunochemicals, Inc., Gilbertsville, PA) that does not cross-react with human IgG by enzyme-linked immunosorbent assay. The above kits were designed to work in both human and mouse species. Separate kits for mouse and human cytochrome c immunoassays were purchased from R&D Systems, Inc. (Minneapolis, MN). The kits for measurements of enzymatic activities of caspases 3 and 8 were from EMD Biosciences, and that of caspase 9 was from R&D Systems. All assays were performed following protocols provided by the manufacturers.
Pemphigus and Normal Human IgG Fractions
The PV serum samples were from six acute patients with active lesions on both oral mucosa and the skin. The pooled sera of healthy people were purchased from Lonza (Rockland, ME). This study was approved by the University of California Irvine Human Subjects Review Committee. The diagnosis of PV was made based on the results of comprehensive clinical and histological examinations and immunological studies, which included direct immunofluorescence of skin biopsies, indirect immunofluorescence of the sera of patients on various epithelial substrates, and immunoblotting following standard protocols. The titer of intercellular antibodies determined on monkey esophagus ranged from 1/640 to 1/2560. The presence of anti-Dsg1 and Dsg3 antibodies in each serum was established using the MESACUP Dsg1 and Dsg3 enzyme-linked immunosorbent assay test system (MBL International Corp., Nagoya, Japan). The index values for Dsg1 antibodies ranged from 64 to 136, and those for Dsg3 antibodies ranged from 82 to 176, i.e. they were unequivocally positive. The IgG fractions were isolated by fast protein liquid chromatography protein G affinity chromatography using the FPLC system purchased from Amersham Biosciences following the manufacturer's protocol, as detailed elsewhere (11). All obtained PVIgG fractions induced suprabasal acantholysis and skin blistering in 1-day-old BALB/c mice injected intradermally with 1 mg of IgG/g of body weight, following standard protocol of passive PV antibody transfer (32). In some experiments, the PVIgG was preabsorbed with mitochondrial protein fraction (see below) by incubation for 1 h at 37 °C followed by a 15-min centrifugation at 10,000 × g.
Morphometric Assay of Acantholysis in Vitro
The morphometric assay was performed in the monolayers of normal human KCs and monolayers of KCs grown from the epidermis of neonatal Dsg3−/− and Dsg3+/+ littermates of the progeny that resulted from mating of heterozygous C57/BL/6J-Dsg3bal/J strain mice (stock number 000519) purchased from The Jackson Laboratory (Bar Harbor, ME). The progeny was genotyped at Transnetyc, Inc. (Cordova, TN). This study had been approved by the University of California Irvine Review Committee on the Use of Animals in Research. The keratinocyte monolayers were exposed to 1 mg/ml test PVIgG for 48 h at 37 °C. The extent of cell detachment (percentage of acantholysis) was measured following the established protocol (33). Briefly, the images of at least three representative microscopic fields were recorded through a ×10 objective using a computer-linked inverted phase-contrast microscope (Axiovert 135, Carl Zeiss Inc., Thornwood, NY). The percentage of acantholysis in each field was computed by subtracting the percentage of the areas covered by the cells from the total area of the microscopic field, taken as 100%.
Morphometric Assay of Acantholysis in Vivo
One-day-old pups delivered by the BALB/c mice purchased from The Jackson Laboratory were used to investigate the effect of preabsorption with mitochondrial proteins on the extent of epidermal acantholysis induced by passive transfer of 1 mg/g of body weight of PVIgG. The pups were injected intradermally with test PVIgGs and examined 24 h later for the presence of skin erosions and blisters and Nikolskiy sign. The extent of epidermal acantholysis was measured microscopically, as described in the standard protocols (34, 35). Briefly, the euthanized animals were snap-frozen in liquid nitrogen, cross-sectioned at the umbilicus level, embedded into the OCT compound (Miles Scientific, Naperville, IL), stained by hematoxylin and eosin, and evaluated by light microscopy. Five random microscopic fields in each skin section were captured at magnification ×10, using a Macintosh computer attached to the Axiovert 135 inverted microscope. The images were printed, and the extent of acantholysis was computed directly on the prints by measuring the length of the areas in the epidermis in which suprabasal cell detachment spread along more than four adjacent basal cells.
Mitochondrial Fractionation of Human KCs
Mitochondria were isolated from the monolayers of human and murine KCs using the mitochondrial/cytosol fractionation kit from BioVision Research Products (Mountain View, CA) and following the manufacturer's instruction. Briefly, KCs were detached by a brief trypsinization, isolated by centrifugation, washed in phosphate-buffered saline, resuspended in the cytosol extraction buffer containing a mix of dithiothreitol and protease inhibitors, homogenized in an ice-cold tissue grinder, and centrifuged at 700 × g for 10 min at 4 °C. The supernatant was recentrifuged at 10,000 × g for 30 min at 4 °C, and the pelleted mitochondrial fraction was resuspended in 100 μl of the mitochondrial extraction buffer and used in experiments. The protein concentration was determined by a Bradford protein assay kit (Bio-Rad). We absorbed PVIgG with either human mitochondrial proteins, for experiments with human KCs, or mouse proteins in the in vitro assays with Dsg3−/− and Dsg3+/+ KCs and passive transfer experiments in neonatal mice.
Immunoblotting Assays
To determine whether anti-keratinocyte antibodies produced by PV patients can penetrate the cell and reach mitochondria, the monolayers of normal human KCs were incubated for 16 h with 1 mg/ml IgG from six PV patients versus normal human IgG (NIgG), after which the cells were thoroughly washed and used to isolate the mitochondrial fraction, as described above. The presence of IgGs in the mitochondrial fraction was assayed by immunoblotting with horseradish peroxidase-conjugated goat anti-human IgG γ- chain (Rockland Immunochemicals, Inc.) diluted 1:2,000, in accordance to a standard protocol (36). To determine whether PV antibodies can react directly with mitochondrial proteins, each lane of the 4–15% SDS-PAGE gel was loaded with 25 μg of mitochondrial fraction of intact human KCs, and the proteins were separated, transferred to the membrane, and incubated overnight at 4 °C with each of the six PV sera used in this study or normal human serum diluted 1:1000 in the Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE). The membranes were thrice washed in phosphate-buffered saline, stained with a goat IRDye® 800-conjugated affinity-purified anti-human IgG secondary antibody (Rockland Immunochemicals, Inc.) diluted 1:7000, and scanned on the LI-COR Odyssey IR imager. The images shown represent typical appearances of protein bands in the gels.
Statistical Analysis
All experiments were performed in duplicates or triplicates, and the results were expressed as mean ± S.D. Statistical significance was determined using Student's t test. Differences were deemed significant if the calculated p value was <0.05.
RESULTS
PV Antibodies Activate Both Extrinsic and Intrinsic Apoptotic Pathways in KCs
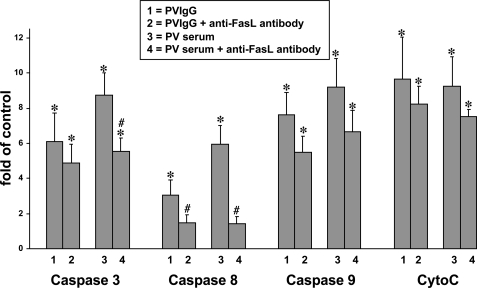
The roles of the FasL present in the serum of the patient versus an auto/paracrine FasL in the initiation of keratinocyte apoptolysis in PV were investigated by measuring the effect of FasL neutralizing antibody on the induction of the initiator caspases 8 and 9 as well as the effector caspase 3 in KCs treated with either pooled PV sera or pure PVIgG fractions. When compared with PVIgG, the whole PV serum exhibited a stronger ability to activate caspases 3 and 8 (Fig. 1). The addition of anti-FasL antibody significantly (p < 0.05) decreased the activity of caspase 8 elevated by both PV serum and PVIgG and the activity of caspase 3 elevated by PV serum. These results confirmed earlier reports that the FasL present in PV serum contributes to apoptolysis (14) and that binding of PV antibodies to KCs elicits production and secretion of auto/paracrine FasL (11, 27).
FIGURE 1.
Activation of both extrinsic and intrinsic apoptotic pathways in human KCs by PV antibodies. Normal human KCs grown to ∼80% confluence were incubated at 37 °C and 5% CO2 for 16 h in serum-free keratinocyte growth medium containing 1.6 mm Ca2+ supplemented with 1 mg/ml of the mixture of IgG fractions isolated from six PV patients ± 15 μg/ml anti-FasL antibody versus normal human IgG (control) or 20% of mixed heat-inactivated sera from the same six PV patients ± 15 μg/ml anti-FasL antibody versus pooled normal human serum (control), washed, homogenized, and then used to measure by enzyme-linked immunosorbent assay the activities of caspases 3, 8, and 9 and the concentration of cytochrome c (CytoC). The results are presented as mean ± S.D. -fold of control values in each group, taken as 1. Asterisk = p < 0.05 when compared with control; pound sign = p < 0.05 when compared with the treatment without anti-FasL antibody.
Both PVIgG and serum also activated the mitochondrial apoptotic pathway, manifested by elevation of cytochrome c and activation of caspase 9 (Fig. 1). In contrast to caspases 3 and 8, these events could not be prevented by the FasL-neutralizing antibody (p > 0.05). These results indicated that both extrinsic and intrinsic pathways of cell death can be triggered in KCs by PV antibodies and raised a question about the mechanism of activation of the intrinsic pathway.
PV Patients Develop Antimitochondrial Antibodies
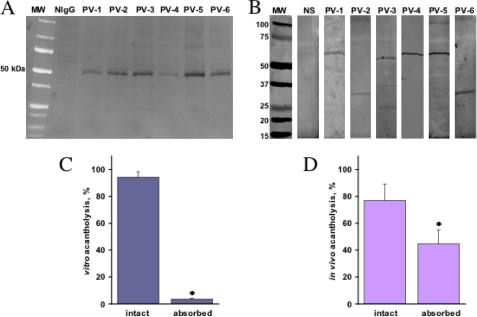
One of the probable mechanisms of activation of the intrinsic apoptotic pathway could be a mitochondrial damage by PV antibodies. Taking into consideration a preliminary report that IgGs from some pemphigus foliaceus patients added to cultured KCs were found in the subcellular mitochondrial fraction attached to the mitochondrial outer membrane (37), we hypothesized that PV antibodies, too, could damage mitochondria in KCs. To test this hypothesis, normal human KCs were incubated for 16 h with 1 mg/ml IgG from six PV patients versus NIgG (control), and the presence of IgGs in the mitochondrial fraction was assayed by immunoblotting. The PVIgGs from all six patients used in this study, but not NIgG, were visualized (Fig. 2A), indicating that PV antibodies entered the cells and reached mitochondria.
FIGURE 2.
Reactivities of PV antibodies with keratinocyte mitochondrial proteins. A, detection of PVIgGs in the subcellular mitochondrial fraction. Normal human KCs grown to ∼80% confluence were incubated at 37 °C and 5% CO2 for 16 h with 1 mg/ml IgGs from six PV patients, washed, and used to isolate the subcellular mitochondrial fraction as detailed under “Materials and Methods.” The obtained proteins were separated by SDS-PAGE and probed with horseradish peroxidase-conjugated anti-human IgG antibody that visualized PVIgGs at ∼50 kDa. MW, molecular size. B, evaluation of reactivities of IgG antibodies from six PV patients with mitochondrial proteins. The mitochondrial proteins were isolated from intact human KCs, separated by SDS-PAGE, and electroblotted as detailed under “Materials and Methods.” The blotting membranes were incubated with PV serum from each individual patient versus normal human serum (NS), washed, and stained with IRDye® 800-conjugated anti-human IgG. The apparent molecular size of the mitochondrial protein recognized by antibodies from the PV patient 1 (PV-1) is 60 kDa; for PV patient 2 (PV-2), the size is 30 kDa; for PV patient 3 (PV-3), the sizes are 25, 35, and 57 kDa; for PV patient 4 (PV-4), the size is 60 kDa; for PV patient 5 (PV-5), the size is 60 kDa; and for PV patient 6 (PV-6), the size is 30 kDa. The immunoblot analysis had a positive control of commercial antimitochondrial antibodies (not shown). C and D, effects of preabsorption of PV antibodies with the mitochondrial protein fraction on their acantholytic activity. The extent of acantholysis in vitro and in vivo was studied using the morphometric techniques described under “Materials and Methods.” C, the keratinocyte monolayers were exposed for 48 h to 1 mg/ml intact or preabsorbed PVIgG mixture from all six PV patients used in this study. D, the neonatal BALB/c mice were injected intradermally with 1 mg/g of body weight of the same PVIgG mixture, and the extent of acantholysis in mouse epidermis was measured 24 h after injection. In control experiments, absorption with the keratinocyte cytosolic fraction did not affect the acantholytic activities of test PVIgGs. Asterisk = p < 0.05 when compared with the effect of intact PVIgG.
Next, we investigated by immunoblotting whether PVIgGs found in the subcellular mitochondrial fraction could recognize mitochondrial proteins. We found that IgGs from all six PV patients, but not NIgG, reacted with mitochondrial antigens (Fig. 2B). The antimitochondrial antibodies produced by the PV patients 1, 4, and 5 recognized putative antigen(s) that migrated with the molecular size of ∼60 kDa. The antibodies from the PV patient 5 also recognized an ∼100-kDa protein. The PV patients 2 and 6 developed antibodies to the mitochondrial protein with an apparent molecular size of 30 kDa, whereas antibodies from the PV patient 3 reacted with the mitochondrial proteins showing the apparent molecular sizes of 25, 35, and 57 kDa (Fig. 2B).
Absorption of antimitochondrial antibodies inhibited the ability of PVIgG to induce acantholysis in the monolayers of human KCs (Fig. 2C) and also significantly (p < 0.05) reduced the extent of suprabasal acantholysis in the passive transfer model of PV in neonatal BALB/c mice (Fig. 2D). These results indicated that PV patients develop antibodies that can find their way to mitochondria in KCs and recognize a versatile group of mitochondrial proteins, which contributes to keratinocyte detachment and skin blistering.
Interrelationships between Activation of Signaling Kinases and Executioner Caspases in KCs Treated with Antibodies Produced by Different PV Patients
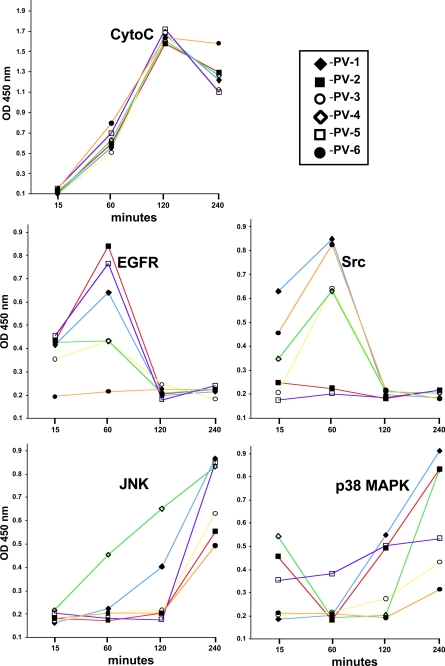
First, we performed a time course study to determine the time point at which IgG from a particular PV patient produces a maximal effect on the activities of signaling kinases and cytochrome c release. Although all test PVIgGs released cytochrome c equally efficiently, with a peak at 2 h, we found considerable variations in the onset of maximal activities of signaling kinases (Fig. 3). In the majority of cases, EGFR and Src peaked at 1 h and JNK peaked at 4 h after exposure (Fig. 3). The elevation of p38 MAPK activity caused by antibodies from some PV patients could be observed already at 15 min (i.e. the PV patients 2, 4, and 5), whereas the majority of test PVIgGs activated p38 MAPK after a prolonged incubation (Fig. 3).
FIGURE 3.
Time course analysis of kinase activities and cytochrome c (CytoC) release in human KCs treated with PVIgGs from individual patients. Normal human KCs grown to ∼80% confluence were incubated at 37 °C and 5% CO2 for the indicated periods of time in the keratinocyte growth medium containing 1.6 mm Ca2+ in the presence of 1 mg/ml IgG from each of the six PV patients used in this study and subjected to analysis of EGFR, Src, JNK, and p38 MAPK phosphorylation and cytochrome c release as detailed under “Materials and Methods.”
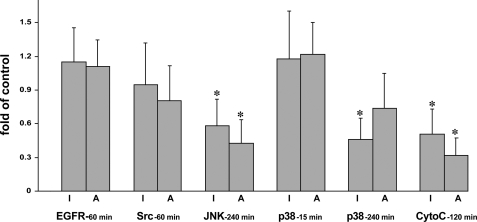
To elucidate the order of engagement of signaling kinases and proapoptotic enzymes in the apoptolysis induced by PV antibodies, we studied the effects of inhibitors of executioner caspases, DEVD-CHO and Z-DEVD-FMK, on cytochrome c release and activities of signaling kinases. Inhibition of executioner caspases did not significantly alter the PVIgG-induced EGFR and Src activities or an early p38 MAPK activity peak (p > 0.05) (Fig. 4). In contrast, the caspase inhibitors significantly (p < 0.05) decreased the activity of JNK and a late peak of p38 MAPK activity (Fig. 4), suggesting that activation of these signaling kinases resulted from, rather than led to, apoptolysis. Caspase inhibitors also decreased by ∼50% the PVIgG-dependent elevation of cytochrome c (Fig. 4).
FIGURE 4.
Effects of inhibitors (I) of executioner caspases and absorption (A) with mitochondrial proteins on the abilities of PV antibodies to activate signaling kinases and release cytochrome c (CytoC). Normal human KCs grown to ∼80% confluence were incubated at 37 °C and 5% CO2 in keratinocyte growth medium containing 1.6 mm Ca2+ in the presence of 1 mg/ml of the combinations of PVIgGs that exhibited the highest abilities to activate a particular type of kinase/elicit cytochrome c release at a specific time point in experiments shown on Fig. 3 ± a mixture of DEVD-CHO (10 μmol/liter) and Z-DEVD-FMK (10 μmol/liter) and used to measure the activities of EGFR (60 min; PV-2 + PV-5), Src (60 min; PV-1 + PV-6), JNK (240 min; PV-1 + PV-4), and p38 MAPK (15 and 240 min; PV-2 + PV-4 at both time points) kinases and cytochrome c release (120 min; pooled IgG from six PV patients). Alternatively, the same mixtures of PVIgGs were first preabsorbed with mitochondrial proteins as detailed under “Materials and Methods” and then added to keratinocyte monolayers without caspase inhibitors. The results are expressed as means ± S.D. -fold of the control values determined in the keratinocyte monolayers exposed to 1 mg/ml of the corresponding mixture of intact PVIgG without inhibitors, taken as 1. Asterisk = p < 0.05 when compared with control.
To elucidate the role of antimitochondrial antibodies in eliciting kinase signaling during apoptolysis of KCs, we measured the effect of preabsorption of test PVIgGs with mitochondrial protein fraction on their abilities to activate signaling kinases and release cytochrome c. Among the kinase activities tested, absorption with mitochondrial proteins significantly (p < 0.05) decreased the PVIgG-induced JNK activity and insignificantly (p > 0.05) decreased the late peak of p38 MAPK activity (Fig. 4), suggesting that these kinases were activated, at least in part, through the intrinsic pathway of cell death. Preabsorbed PVIgGs lost ∼70% of their ability to release cytochrome c (Fig. 4). The remaining activity might be due to a secondary mitochondrial damage mediated by effectors of the extrinsic cell death pathway. Taken together, these results indicated that PV antibodies induce apoptolysis through both extrinsic and intrinsic apoptotic pathways.
A Dsg3 Antibody Signaling
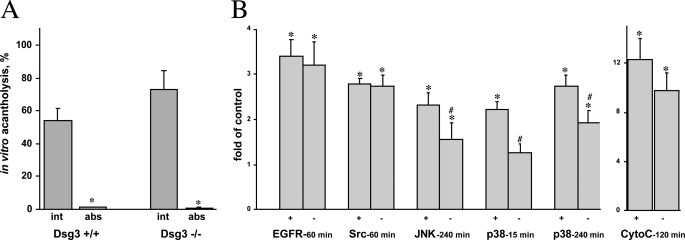
Taking into consideration an important role of Dsg3 antibodies in PV, we sought to determine whether and how the signaling events elicited by PV antibodies would change in the absence of the Dsg3 antigen on the keratinocyte cell membrane. First, we investigated the possibility that PV antibody-induced endocytosis of Dsg3 (38, 39) allows the antimitochondrial antibodies to enter KCs. To test this hypothesis, we evaluated how the absence of Dsg3 affects the ability to inhibit the acantholytic activity of PVIgG by preabsorption with murine mitochondrial proteins. The confluent monolayers of KCs grown from Dsg3+/+ or Dsg3−/− mice were exposed to intact versus preabsorbed PVIgG. The intact PVIgG caused ∼55% acantholysis in the monolayers of Dsg3+/+ KCs and ∼75% in the monolayers of Dsg3−/− KCs (Fig. 5A), as could be expected based on the important role of Dsg3 in mediating keratinocyte cell-cell adhesion. Eliminating the antimitochondrial antibodies abolished the acantholytic effects of PVIgG on Dsg3+/+ and Dsg3−/− KCs equally efficiently (Fig. 5A), indicating that Dsg3 was not involved in the acantholytic action of antimitochondrial autoantibodies.
FIGURE 5.
Role of Dsg3 in PV antibody signaling. A, the effect of absorption of antimitochondrial antibodies on the acantholytic activity of PVIgG. The intact (int) versus absorbed (abs) PVIgGs pooled from six PV patients were added to the confluent monolayers of KCs grown from the epidermis of neonatal Dsg3+/+ and Dsg3−/− littermate mice at the final concentration of 1 mg/ml and incubated for 48 h. Asterisk indicates significant, p < 0.05, differences between the extent of acantholysis in the monolayers treated with absorbed PVIgG and that in the respective monolayers of murine KCs treated with intact PVIgG. B, the effects of PV antibody on the activities of signaling kinases and cytochrome c (CytoC) release in the absence of Dsg3. The confluent monolayers of mouse KCs grown from the epidermis of neonatal Dsg3+/+ (+) versus Dsg3−/− (−) littermates were incubated with 1 mg/ml PVIgGs that had demonstrated the highest abilities to activate a particular type of kinase and release cytochrome c at a specific time point in experiments with human KCs (Fig. 3) and then were used to measure the activities of EGFR, Src, JNK, and p38 MAPK kinases and cytochrome c release as detailed under “Materials and Methods.” Results are expressed as -fold of control values determined in the corresponding Dsg3+/+ or Dsg3−/− monolayers treated with NIgG, taken as 1. Asterisk = p < 0.05 when compared with control; pound sign indicates significant, p < 0.05, differences from the values obtained in Dsg3+/+ KCs treated with the same PVIgGs mixes.
Next, we sought to elucidate a specific role of Dsg3 in PV antibody-induced signaling. Toward this goal, we compared the activities of signaling kinases and the cytochrome c release in Dsg3+/+ versus Dsg3−/− KCs treated with PVIgGs. Lack of Dsg3 did not affect the ability of PVIgG to activate EGFR and Src, but PVIgG-dependent activation of both JNK and p38 MAPK was significantly (p < 0.05) reduced (Fig. 5B). A decrease of cytochrome c in Dsg3−/− KCs was insignificant (p > 0.05). These results indicated that the pathophysiologic role of anti-Dsg3 antibody in PV autoimmunity is predominantly associated with the signaling events involving JNK and p38 MAPK, which may include both primary and secondary events mediating keratinocyte apoptolysis.
DISCUSSION
In this study, we demonstrated for the first time that PV patients develop antimitochondrial antibodies that penetrate KCs and react with several mitochondrial proteins. These autoantibodies are pathogenic because they activated the intrinsic pathway of cell death in KCs, and their absorption abolished the acantholytic activity of PVIgG. The antimitochondrial antibodies synergize with anti-keratinocyte antibodies of other specificities developed by PV patients and the effectors of extrinsic apoptotic pathway, such as FasL. PVIgG binding to keratinocyte plasma membrane launches an array of downstream signaling events subserved by signal transduction kinases, including Src, EGFR kinase, p38 MAPK, and JNK. Individual PVIgGs show unique time patterns of activation of these kinases. The preferential signaling pathway downstream of targeted self-antigens is apparently determined by a unique composition of the pool of anti-keratinocyte antibodies produced by each PV patient. Further elucidation of the primary signaling events elicited by pathogenic PV antibodies will be crucial for the development of a more successful therapy for PV patients.
The existence of antimitochondrial antibodies in PV patients was predicted by the “multiple hit” hypothesis (40). According to the most recent modification of this hypothesis (2), apoptolysis in PV results from the synergistic and cumulative effects of autoantibodies targeting keratinocyte antigens of different kinds, including molecules that mediate cell-to-cell adhesion and molecules that regulate cell survival and adhesion. Contribution of mitochondrial damage to apoptolysis in PV was suggested by an earlier finding of the elevated activity of caspase 9 in KCs exposed to PVIgG (11). Also, in the uninvolved skin of PV patients, edema and swelling of mitochondria with destruction of their cristae were observed (41), suggesting that mitochondrial damage precedes blister formation. The histochemical analysis of the lesional skin demonstrated abnormal activities of the mitochondrial enzymes oxidoreductase, adenosine triphosphatase, and NADH2-cytochrome c reductase (42–44). The balance between oxybiotic and anoxybiotic metabolism in the affected skin was drastically skewed toward the latter (44).
In the present study, the pathogenic role of antimitochondrial antibodies in PV was illustrated by: 1) almost complete inhibition of the ability of PVIgG to induce acantholysis in vitro and in vivo after preabsorption with the keratinocyte mitochondrial protein fraction and 2) ∼70% reduction of the cytochrome c release in KCs treated with preabsorbed versus intact PVIgG. The fact that adsorption of antimitochondrial antibodies dramatically decreased the acantholytic activity of PVIgG, in spite of the presence of other pathogenic antibodies such as anti-Dsg3 and anti-pemphaxin antibodies, can be explained through the multiple hit hypothesis that states that a constellation, rather than a single type, of antibodies is required to break the natural resistance of KCs. Our previous studies (45) demonstrated that although adsorption of a single type of pathogenic antibody eliminates the acantholytic activity of PVIgG, this autoantibody alone is not sufficient to induce acantholysis and skin blisters. However, adding it back to the preabsorbed PVIgG restores acantholytic activity of the latter (45). Thus, the pool of disease-causing PVIgG contains autoantibodies to a group of keratinocyte self-antigens, and it is the a synergistic action of these autoantibodies that breaks up the integrity of live epidermis and induces skin blistering.
Antimitochondrial antibodies from different PV patients recognized distinct combinations of the mitochondrial proteins with apparent molecular sizes of 25, 30, 35, 57, 60, and 100 kDa. This is in keeping with our previously published observations that PV patients develop antibodies to a large variety of epidermal antigens (32, 46) and earlier reports by other workers implicating in pemphigus autoimmunity the keratinocyte proteins with apparent molecular sizes of 12, 18, 47, 50, 52, 55, 59, 66, 67, 68, 75, 78, 102, 105, and 180/190 kDa, in addition to the 130-kDa Dsg3 and 160-kDa Dsg1 (reviewed in Ref. 46). Hence, the patient-to-patient variations in the repertoire of antimitochondrial antibodies observed in this study was not surprising. Most importantly, every PV serum tested had antimitochondrial antibodies, and all tested PVIgGs elicited cytochrome c release in a similar time pattern, in contrast with the effects of PVIgGs on signaling kinases that varied dramatically from patient to patient. Thus, although antimitochondrial antibodies appeared to be a common feature of PV autoimmunity, the fine mechanisms of antibody-induced keratinocyte detachment, death, and survival apparently vary among patients, which helps explain known patient-to-patient variations in clinical and immunopathological features of PV.
Although pemphigus mitochondrial antigens remain unknown, our previous observations of reactivities of PV antibodies with two new molecules similar to NADH dehydrogenase and taurine transporter (reviewed in Ref. 47) suggested them as good candidates. It is well known that mammalian mitochondria possess NADH dehydrogenase, the largest of the respiratory complexes containing at least 46 different proteins (48, 49). A recent study has revealed a putative mitochondrial taurine transporter responsible for cytoplasmic taurine uptake by mitochondria (50). The antigens targeted by antimitochondrial antibodies in other autoimmune diseases have been identified. In primary biliary cirrhosis, these are members of the 2-oxo-acid dehydrogenase complexes, of which the E2 subunit of pyruvate dehydrogenase complex is the major autoantigen (reviewed in Ref. 51). In myocarditis and dilated cardiomyopathy, the autoantibodies recognize the ADP/ATP carrier (52). IgA and IgM autoantibodies against the β-chain of the mitochondrial enzyme ATP synthase have been separately described in different cohorts of patients with celiac disease and nephrotic syndrome (53, 54).
We used immunoblot as the most reliable approach to determine the presence of autoantibodies in the subcellular mitochondrial fraction. It is noteworthy that Geoghagen and Jordon (37) conducted a pilot study that visualized by immunoelectron microscopy the antimitochondrial antibodies produced by pemphigus foliaceus patients. The presence of antimitochondrial antibodies within the pool of pemphigus antibodies explains well known observations of intracellular fluorescence of PVIgGs, both in tissue and in live cultured cells, that have usually been considered an epiphenomenon. Historically, several methodological approaches had been employed to demonstrate intracellular localization of autoantibodies, including regular and confocal immunofluorescent microscopy, immunoelectron microscopy, flow cytometry, and measurement of cellular/nuclear-associated radioisotope-labeled antibodies (reviewed in Ref. 55). Because the ability of intact autoantibodies to enter the cytosol or nuclei of living cells was disputed, the immunoblotting analysis was subsequently used to ultimately demonstrate the intracellular localization of penetrating IgG (56). In addition to antimitochondrial antibodies, autoantibodies of other specificities, such as anti-double-stranded DNA, anti-SSA/Ro, anti-SSB/La, anti-proteinase 3 (cANCA), anti-synaptosomal, anti-neuronal (anti-Hu), anti-guanylyl, and anti-ribosomal protein P antibodies, penetrated the cells (reviewed in Refs. 57 and 58). In most cases, the death of cells penetrated by autoantibodies was due to apoptosis (57–60). The downstream signaling resulting from the antimitochondrial antibody action in PV involved JNK, and to a lesser extent, p38 MAPK. Because these same kinases have been implicated in mediating Fas action (61), activation of Fas-mediated apoptotic pathway in PV could, in part, be a result of a positive feedback from the mitochondrial damage (62, 63).
The mechanism of cell entry of antimitochondrial antibodies in PV may involve their binding to annexins at the keratinocyte plasma membrane (45, 64). Annexins can relocate to the cytosol (65–67). Moreover, biochemical and immunocytochemical studies demonstrated the occurrence of annexin in mitochondria (68). Alternatively, instead of binding to an antigen on the plasma membrane, PV antibodies may be bound by Fc receptors expressed on human epidermal KCs (69) and then internalized. Besides KCs (56, 70), many other cell types can be penetrated by autoantibodies through antigen-mediated or Fc-mediated internalization pathways (reviewed in Refs. 55 and 58). In addition, a cell surface protein may act as a surrogate for the nominal mitochondrial antigen. We hypothesized that by analogy with the cell surface molecules calreticulin and myosin 1 that can serve as surrogate antigens for penetrating anti-double-stranded DNA antibodies (71, 72), Dsg3 might be a surrogate antigen for antimitochondrial antibodies in PV. Previous studies demonstrated that upon binding of pathogenic PVIgG, Dsg3 is internalized through a clathrin- and dynamin-independent endolysosomal pathway where it is degraded (38, 39) and that anti-Dsg3 antibody binding to KCs leads to activation of p38 MAPK (6). If internalization of Dsg3 would indeed provide a vehicle for antimitochondrial antibody entry of KCs, then lack of Dsg3 on the plasma membrane, as in Dsg3−/− KCs, would eliminate the ability to abolish acantholytic activity of PVIgG by preabsorption with mitochondrial proteins. In fact, preabsorption of antimitochondrial antibodies decreased acantholytic effects of PVIgG against both Dsg3+/+ and Dsg3−/− KCs, indicating that mechanisms other than Dsg3 internalization allowed antimitochondrial antibodies to find their way inside KCs.
Having discovered antimitochondrial antibodies in PV patients and demonstrated their pathogenic role, we asked how this new pathophysiologic pathway relates to the established mechanisms of apoptolysis in PV. Experiments with Dsg3−/− KCs demonstrated that the signaling of anti-Dsg3 antibody involves the same signaling events, such as activation of JNK and a late peak of p38 MAPK activity, as those produced by antimitochondrial antibodies. It is well known that the p38 MAPK signaling pathway can be stimulated by cellular stress and proinflammatory cytokines in a way that is similar to the JNK signaling pathway, and both can be involved in cell death via apoptosis (reviewed in Ref. 73). We found that inhibitors of the executioner caspases abolished activation of JNK and the late p38 MAPK peak, indicating that these activities were triggered by the cell injury caused by PV autoantibodies, such as collapse of the cytoskeleton and disassembly of desmosomes. The executioner caspases can cleave Dsg1–3 (2, 74, 75). Therefore, antimitochondrial and anti-Dsg3 antibodies have common mechanisms of signaling. In contrast to the signaling produced by antimitochondrial antibody, anti-Dsg3 antibody elicited two peaks of p38 MAPK activity, which supports the data recently reported by Dr. Rubenstein and co-workers (12). Neither antimitochondrial nor anti-Dsg3 antibodies activated the EGFR/Src pathway. This is in keeping with the previous report that gene silencing of Dsg1 and/or Dsg3 via RNA interference does not affect the ability of human KCs to respond to PVIgG by elevation of the EGFR and Src activities (6).
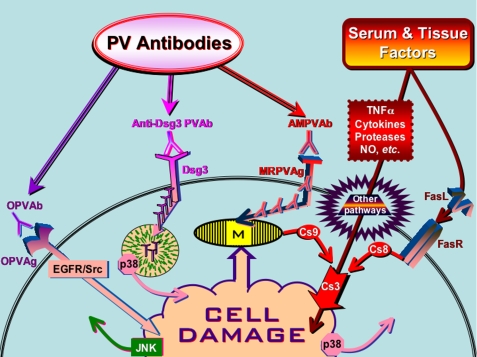
Thus, the results of this study and recent reports from other groups studying PV antibody signaling in KCs (reviewed in Ref. 2) clearly indicate that the process of apoptolysis mediating keratinocyte detachment and blistering in PV is rather complex. It relies upon a synergistic action of autoantibodies to several types of self-antigens, including adhesion molecules, mitochondrial proteins, other putative keratinocyte self-antigens like acetylcholine receptors, as well as serum and tissue factors, such as FasL, tumor necrosis factor α, cytokines, serine proteases, and nitric oxide. By acting altogether, these factors become able to overcome the natural resistance and activate both the extrinsic and the intrinsic cell death pathways in KCs (Fig. 6).
FIGURE 6.
Hypothetical scheme of signaling events mediating keratinocyte apoptolysis in PV. The abbreviations are: AMPVAb, anti-mitochondrial PV antibody; Cs, caspase; MRPVAg, mitochondria-related PV antigen; NO, nitric oxide; OPVAb, PV antibodies of other specificities; OPVAg, other types of putative PV antigens; PVAb, PV antibody; TNFα, tumor necrosis factor α; M, mitochondria.
Conclusions
This study determined the following. 1) The mechanism of apoptolysis in PV encompasses several tiers of events triggered through distinct antigen-antibody systems. Future studies should define the antigenic specificities and the mechanisms of cell penetration of antimitochondrial antibodies in PV. 2) The signaling pathways triggered by autoantibodies in PV vary from patient to patient because a unique composition of the pool of autoantibodies apparently determines the preferential signaling pathways downstream of targeted self-antigens. 3) Development of a new treatment of PV patients should be directed toward interruption of the key signaling events triggering apoptolysis.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant GM62136 (to S. A. G.). A preliminary account was presented at the 69th Annual Meeting of the Society for Investigative Dermatology, Montreal, Canada, May 6–9, 2009, and published in the form of an abstract (Chernyavsky, A. I., Arredondo, J., and Grando, S. A. (2009) J. Invest. Dermatol. 129, Suppl. S11 (Abstr. 64)).
This article was selected as a Paper of the week.
- PV
- pemphigus vulgaris
- KC
- keratinocyte
- FasL
- Fas ligand
- FasR
- Fas receptor
- EGFR
- epidermal growth factor receptor
- Z-DEVD-FMK
- benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone
- DEVD-CHO
- N-acetyl-Asp-Glu-Val-Asp-aldehyde
- MAPK
- mitogen-activated protein kinase
- JNK
- c-Jun N-terminal kinase
- NIgG
- normal human IgG.
REFERENCES
- 1.Bystryn J. C., Grando S. A. (2006) J. Am. Acad. Dermatol. 54, 513–516 [DOI] [PubMed] [Google Scholar]
- 2.Grando S. A., Bystryn J. C., Chernyavsky A. I., Frusić-Zlotkin M., Gniadecki R., Lotti R., Milner Y., Pittelkow M. R., Pincelli C. (2009) Exp. Dermatol. 18, 764–770 [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz P., Hu P., Warren S., Liu Z., Diaz L. A., Rubenstein D. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12855–12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pretel M., España A., Marquina M., Pelacho B., López-Picazo J. M., López-Zabalza M. J. (2009) Exp. Dermatol. 18, 771–780 [DOI] [PubMed] [Google Scholar]
- 5.Frusić-Zlotkin M., Raichenberg D., Wang X., David M., Michel B., Milner Y. (2006) Autoimmunity 39, 563–575 [DOI] [PubMed] [Google Scholar]
- 6.Chernyavsky A. I., Arredondo J., Kitajima Y., Sato-Nagai M., Grando S. A. (2007) J. Biol. Chem. 282, 13804–13812 [DOI] [PubMed] [Google Scholar]
- 7.Chernyavsky A. I., Arredondo J., Piser T., Karlsson E., Grando S. A. (2008) J. Biol. Chem. 283, 3401–3408 [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Carpintero I., España A., Pelacho B., López Moratalla N., Rubenstein D. S., Diaz L. A., López-Zabalza M. J. (2004) Br. J. Dermatol. 151, 565–570 [DOI] [PubMed] [Google Scholar]
- 9.Marquina M., España A., Fernández-Galar M., López-Zabalza M. J. (2008) Br. J. Dermatol. 159, 68–76 [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Brégégère F., Frusić-Zlotkin M., Feinmesser M., Michel B., Milner Y. (2004) Apoptosis 9, 131–143 [DOI] [PubMed] [Google Scholar]
- 11.Arredondo J., Chernyavsky A. I., Karaouni A., Grando S. A. (2005) Am. J. Pathol. 167, 1531–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H. E., Berkowitz P., Jolly P. S., Diaz L. A., Chua M. P., Rubenstein D. S. (2009) J. Biol. Chem. 284, 12524–12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirillo N., Lanza M., Femiano F., Gaeta G. M., De Rosa A., Gombos F., Lanza A. (2007) J. Cell Physiol. 212, 563–567 [DOI] [PubMed] [Google Scholar]
- 14.Puviani M., Marconi A., Cozzani E., Pincelli C. (2003) J. Invest. Dermatol. 120, 164–167 [DOI] [PubMed] [Google Scholar]
- 15.Ameglio F., D'Auria L., Cordiali-Fei P., Trento E., D'Agosto G., Mastroianni A., Giannetti A., Giacalone B. (1999) J. Biol. Regul. Homeost. Agents 13, 220–224 [PubMed] [Google Scholar]
- 16.Alecu M., Alecu S., Coman G., Gălăţescu E., Ursaciuc C. (1999) Roum. Arch. Microbiol. Immunol. 58, 121–130 [PubMed] [Google Scholar]
- 17.Bhol K. C., Desai A., Kumari S., Colon J. E., Ahmed A. R. (2001) Clin. Immunol. 100, 172–180 [DOI] [PubMed] [Google Scholar]
- 18.Narbutt J., Lukamowicz J., Bogaczewicz J., Sysa-Jedrzejowska A., Torzecka J. D., Lesiak A. (2008) Mediators Inflamm. 2008, 875394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baroni A., Perfetto B., Ruocco E., Greco R., Criscuolo D., Ruocco V. (2002) Dermatology 205, 116–121 [DOI] [PubMed] [Google Scholar]
- 20.Siebra M. X., Santos M. A., Almeida T. L., Leite A. C., Cunha F. Q., Rocha F. A. (2006) Braz. J. Med. Biol. Res. 39, 671–675 [DOI] [PubMed] [Google Scholar]
- 21.Grando S. A. (1992) J. Dermatol. Sci. 4, 95–97 [DOI] [PubMed] [Google Scholar]
- 22.Orlov M. D., Chernyavsky A. I., Arredondo J., Grando S. A. (2006) Autoimmunity 39, 557–562 [DOI] [PubMed] [Google Scholar]
- 23.Grando S. A., Glukhenky B. T., Drannik G. N., Epshtein E. V., Kostromin A. P., Korostash T. A. (1989) Arch. Dermatol. 125, 925–930 [PubMed] [Google Scholar]
- 24.Feliciani C., Toto P., Amerio P., Pour S. M., Coscione G., Shivji G., Wang B., Sauder D. N. (2000) J. Invest. Dermatol. 114, 71–77 [DOI] [PubMed] [Google Scholar]
- 25.Feliciani C., Toto P., Wang B., Sauder D. N., Amerio P., Tulli A. (2003) Exp. Dermatol. 12, 466–471 [DOI] [PubMed] [Google Scholar]
- 26.Sayama K., Yonehara S., Watanabe Y., Miki Y. (1994) J. Invest. Dermatol. 103, 330–334 [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Brégégère F., Soroka Y., Frusic-Zlotkin M., Milner Y. (2004) FEBS Lett. 567, 281–286 [DOI] [PubMed] [Google Scholar]
- 28.Frusic-Zlotkin M., Pergamentz R., Michel B., David M., Mimouni D., Brégégère F., Milner Y. (2005) Ann. N. Y. Acad. Sci. 1050, 371–379 [DOI] [PubMed] [Google Scholar]
- 29.Pacheco-Tovar M. G., Avalos-Díaz E., Vega-Memije E., Bollain-y-Goytia J. J., López-Robles E., Hojyo-Tomoka M. T., Domínguez-Soto L., Herrera-Esparza R. (2009) J. Eur. Acad. Dermatol. Venereol. 23, 697–701 [DOI] [PubMed] [Google Scholar]
- 30.Grando S. A., Glukhenky B. T., Drannik G. N., Kostromin A. P., Boiko Y. Ya., Senyuk O. F. (1989) Autoimmunity 3, 247–260 [DOI] [PubMed] [Google Scholar]
- 31.Lotti R. E. S., Truzzi F., Palazzo E., Marconi A. Y. K., Pincelli C. (2009) J. Invest. Dermatol. 129, Suppl.S14 (Abstr. 80) [Google Scholar]
- 32.Nguyen V. T., Ndoye A., Shultz L. D., Pittelkow M. R., Grando S. A. (2000) J. Clin. Invest. 106, 1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen V. T., Chernyavsky A. I., Arredondo J., Bercovich D., Orr-Urtreger A., Vetter D. E., Wess J., Beaudet A. L., Kitajima Y., Grando S. A. (2004) Exp. Cell Res. 294, 534–549 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen V. T., Arredondo J., Chernyavsky A. I., Kitajima Y., Pittelkow M., Grando S. A. (2004) J. Biol. Chem. 279, 2135–2146 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen V. T., Arredondo J., Chernyavsky A. I., Pittelkow M. R., Kitajima Y., Grando S. A. (2004) Arch. Dermatol. 140, 327–334 [DOI] [PubMed] [Google Scholar]
- 36.Chernyavsky A. I., Arredondo J., Wess J., Karlsson E., Grando S. A. (2004) J. Cell Biol. 166, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geoghegan W., Jordon R. (1992) J. Invest. Dermatol. 98, 589 (Abstr. 225) [DOI] [PubMed] [Google Scholar]
- 38.Calkins C. C., Setzer S. V., Jennings J. M., Summers S., Tsunoda K., Amagai M., Kowalczyk A. P. (2006) J. Biol. Chem. 281, 7623–7634 [DOI] [PubMed] [Google Scholar]
- 39.Delva E., Jennings J. M., Calkins C. C., Kottke M. D., Faundez V., Kowalczyk A. P. (2008) J. Biol. Chem. 283, 18303–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grando S. A. (2000) Dermatology 201, 290–295 [DOI] [PubMed] [Google Scholar]
- 41.Grando S. A., Terman A. K., Stupina A. S., Glukhenky B. T., Romanenko A. B. (1991) Clin. Exp. Dermatol. 16, 359–363 [DOI] [PubMed] [Google Scholar]
- 42.Tseraidis G. S., Bavykina E. A. (1971) Vestn. Dermatol. Venerol. 45, 8–12 [PubMed] [Google Scholar]
- 43.Tseraidis G. S., Bavykina E. A. (1972) Arkh. Patol. 34, 72–78 [PubMed] [Google Scholar]
- 44.Gheorghe E., Adumitresi C., Botnarciuc M., Manea M. (2005) Rom. J. Morphol. Embryol. 46, 73–78 [PubMed] [Google Scholar]
- 45.Nguyen V. T., Ndoye A., Grando S. A. (2000) J. Biol. Chem. 275, 29466–29476 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen V. T., Lee T. X., Ndoye A., Shultz L. D., Pittelkow M. R., Dahl M. V., Lynch P. J., Grando S. A. (1998) Arch. Dermatol. 134, 971–980 [DOI] [PubMed] [Google Scholar]
- 47.Grando S. A. (2006) Autoimmunity 39, 521–530 [DOI] [PubMed] [Google Scholar]
- 48.Carroll J., Fearnley I. M., Skehel J. M., Shannon R. J., Hirst J., Walker J. E. (2006) J. Biol. Chem. 281, 32724–32727 [DOI] [PubMed] [Google Scholar]
- 49.Fearnley I. M., Carroll J., Walker J. E. (2007) Methods Mol. Biol. 357, 103–125 [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. (2002) EMBO J. 21, 6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishibashi H., Shimoda S., Gershwin M. E. (2005) Semin Liver Dis. 25, 337–346 [DOI] [PubMed] [Google Scholar]
- 52.Schulze K., Becker B. F., Schultheiss H. P. (1989) Circ. Res. 64, 179–192 [DOI] [PubMed] [Google Scholar]
- 53.Stulík J., Hernychová L., Porkertová S., Pozler O., Tucková L., Sánchez D., Bures J. (2003) Proteomics 3, 951–956 [DOI] [PubMed] [Google Scholar]
- 54.Musante L., Candiano G., Bruschi M., Santucci L., Carnemolla B., Orecchia P., Giampuzzi M., Zennaro C., Sanna-Cherchi S., Carraro M., Oleggini R., Camussi G., Perfumo F., Ghiggeri G. M. (2005) Clin. Exp. Immunol. 141, 491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golan T. D. (1997) Leukemia 11, 6–9 [DOI] [PubMed] [Google Scholar]
- 56.Golan T. D., Sigal D., Sabo E., Shemuel Z., Guedj D., Weinberger A. (1997) Lupus 6, 18–26 [DOI] [PubMed] [Google Scholar]
- 57.Alascón-Segovia D. (1998) J. Autoimmun 11, 509–510 [DOI] [PubMed] [Google Scholar]
- 58.Lim P. L., Zouali M. (2006) Immunol. Lett. 103, 17–26 [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Argüelles A., Brito G. J., Reyes-Izquierdo P., Pérez-Romano B., Sánchez-Sosa S. (2007) J. Autoimmun 29, 281–286 [DOI] [PubMed] [Google Scholar]
- 60.Rivadeneyra-Espinoza L., Ruiz-Argüelles A. (2006) J. Autoimmun 26, 52–56 [DOI] [PubMed] [Google Scholar]
- 61.Chang H. Y., Nishitoh H., Yang X., Ichijo H., Baltimore D. (1998) Science 281, 1860–1863 [DOI] [PubMed] [Google Scholar]
- 62.Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K. J., Debatin K. M., Krammer P. H., Peter M. E. (1998) EMBO J. 17, 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grossmann J. (2002) Apoptosis 7, 247–260 [DOI] [PubMed] [Google Scholar]
- 64.Bastian B. C., Nuss B., Römisch J., Kraus M., Bröcker E. B. (1994) J. Dermatol. Sci. 8, 194–202 [DOI] [PubMed] [Google Scholar]
- 65.Barwise J. L., Walker J. H. (1996) J. Cell Sci. 109, 247–255 [DOI] [PubMed] [Google Scholar]
- 66.Mohiti J., Caswell A. M., Walker J. H. (1995) Mol. Membr. Biol. 12, 321–329 [DOI] [PubMed] [Google Scholar]
- 67.Podszywalow-Bartnicka P., Strzelecka-Kiliszek A., Bandorowicz-Pikula J., Pikula S. (2007) Acta Biochim. Pol. 54, 261–271 [PubMed] [Google Scholar]
- 68.Rainteau D., Mansuelle P., Rochat H., Weinman S. (1995) FEBS Lett. 360, 80–84 [DOI] [PubMed] [Google Scholar]
- 69.Tigalonowa M., Bjerke J. R., Livden J. K., Matre R. (1990) Acta Derm. Venereol. 70, 385–390 [PubMed] [Google Scholar]
- 70.Golan T. D., Gharavi A. E., Elkon K. B. (1993) J. Invest. Dermatol. 100, 316–322 [DOI] [PubMed] [Google Scholar]
- 71.Seddiki N., Nato F., Lafaye P., Amoura Z., Piette J. C., Mazié J. C. (2001) J. Immunol. 166, 6423–6429 [DOI] [PubMed] [Google Scholar]
- 72.Yanase K., Smith R. M., Puccetti A., Jarett L., Madaio M. P. (1997) J. Clin. Invest. 100, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho S. G., Choi E. J. (2002) J. Biochem. Mol. Biol. 35, 24–27 [DOI] [PubMed] [Google Scholar]
- 74.Lanza A., Cirillo N. (2007) Br. J. Dermatol. 156, 400–402 [DOI] [PubMed] [Google Scholar]
- 75.Cirillo N., Lanza M., De Rosa A., Cammarota M., La Gatta A., Gombos F., Lanza A. (2008) J. Cell Biochem. 103, 598–606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.