Abstract
The endonuclease ERCC1-XPF incises the damaged strand of DNA 5′ to a lesion during nucleotide excision repair (NER) and has additional, poorly characterized functions in interstrand cross-link repair, double-strand break repair, and homologous recombination. XPA, another key factor in NER, interacts with ERCC1 and recruits it to sites of damage. We identified ERCC1 residues that are critical for the interaction with XPA and assessed their importance for NER in vitro and in vivo. Mutation of two conserved residues (Asn-110 and Tyr-145) located in the XPA-binding site of ERCC1 dramatically affected NER but not nuclease activity on model DNA substrates. In ERCC1-deficient cells expressing ERCC1N110A/Y145A, the nuclease was not recruited to sites of UV damage. The repair of UV-induced (6-4)photoproducts was severely impaired in these cells, and they were hypersensitive to UV irradiation. Remarkably, the ERCC1N110A/Y145A protein rescues the sensitivity of ERCC1-deficient cells to cross-linking agents. Our studies suggest that ERCC1-XPF engages in different repair pathways through specific protein-protein interactions and that these functions can be separated through the selective disruption of these interactions. We discuss the impact of these findings for understanding how ERCC1 contributes to resistance of tumor cells to therapeutic agents such as cisplatin.
Keywords: DNA/Damage, DNA/Nuclease, DNA/Repair, ERCC1-XPF, Nucleotide Excision Repair, Xeroderma Pigmentosum
Introduction
The preservation of the genetic information contained in DNA is essential for proper cell function and is ensured by multiple DNA repair pathways. Among these, nucleotide excision repair (NER)2 clears the genome of bulky, helix-distorting DNA lesions, such as those formed by UV light, environmental mutagens, and antitumor agents (1, 2). Two subpathways of NER exist that differ in their method of damage recognition. In transcription-coupled NER, lesions in the transcribed strand of genes block the progression of RNA polymerase II, triggering NER (3). In global genome NER, helix-distorting lesions anywhere in the genome are recognized by the XPC-RAD23B heterodimer (4), in some cases with the help of UV-DDB (5). The subsequent steps of NER are believed to be similar for both subpathways and occur by the sequential assembly of the NER proteins at the site of the lesion (6–8). Recruitment of TFIIH containing two helicase subunits XPB and XPD leads to the separation of the damaged and undamaged DNA strands (9, 10). This enables subsequent NER factors to bind, including XPA, the single-stranded binding protein RPA, and the endonuclease XPG (7). The last factor to be recruited to this preincision complex is the endonuclease ERCC1-XPF (6, 11). Once ERCC1-XPF is properly positioned on the DNA, via its interactions with XPA and RPA (12, 13), it incises the damage strand 5′ to the lesion followed by XPG making the 3′ incision (14), allowing the replicative DNA polymerases and associated factors to fill the gap and restore the original DNA sequence (15–17).
Inherited defects in NER cause xeroderma pigmentosum (XP) characterized by extreme photosensitivity and risk of skin cancer (1). Genetic defects in NER factors underlie additional disorders. Mutations in XPB, XPD, TTD-A, and XPG (all associated with TFIIH) can cause Cockayne syndrome, combined XP/Cockayne syndrome, or trichothiodystrophy, characterized by severe developmental defects and neurodegeneration, due to the additional role of these gene products in transcription (18). Mutations in ERCC1 cause cerebro-oculo-facio-skeletal syndrome, whereas mutations in XPF that severely affect protein expression cause accelerated aging (XFE progeroid syndrome (19, 20)). Mice completely deficient in ERCC1-XPF also age rapidly and have a dramatically reduced life span (21–23). The severe phenotype caused by deficiency of ERCC1-XPF, relative to XP, has been ascribed to the additional role of this NER factor in the repair of interstrand cross-links (ICLs) (19).
The recruitment of ERCC1-XPF to various DNA repair pathways is predicted to be mediated through specific protein-protein interactions. The interaction between XPA and ERCC1 is essential for NER involving a region of XPA encompassing three consecutive glycines (residues 72–74) (24–26). The structural basis for the interaction between XPA and ERCC1 was recently established (27, 28). A short and unstructured peptide of XPA, XPA67–80, including the three aforementioned glycine residues, undergoes a disorder-to-order transition upon binding to the central domain of ERCC1 (see Fig. 1). This 14- amino acid stretch of XPA is necessary and sufficient to mediate its interaction with ERCC1, and the XPA67–80 peptide can inhibit NER activity in vitro (27).
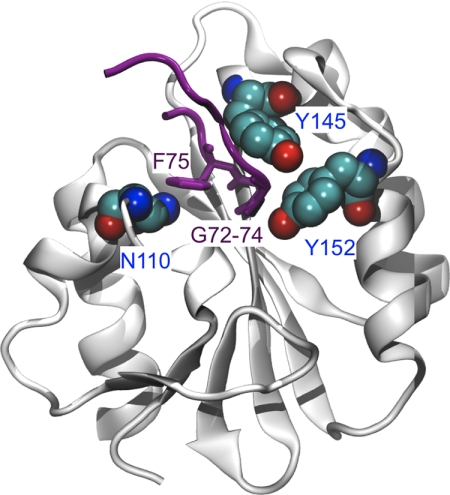
FIGURE 1.
Structure of an XPA peptide bound to the central domain of ERCC1. ERCC1 is shown in gray, and the XPA peptide with the highly conserved residues 72–75 (GGGF) is shown in purple. Residues selected for site-directed mutagenesis in ERCC1 (Asn-110, Tyr-145, and Tyr-152) are highlighted in atom color. The picture is adapted from Ref. 27.
These findings prompted us to investigate how the XPA-binding region of ERCC1 contributes to NER activity and other DNA repair functions of ERCC1-XPF. Herein, we report mutations in the central domain of ERCC1 that severely impact NER activity in vitro and in vivo. Importantly, these mutations did not affect the ability of ERCC1-XPF to function in other DNA repair pathways. Our studies suggest that the roles of ERCC1-XPF can be separated by disrupting specific protein interactions that target the endonuclease to different DNA repair pathways.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Site-directed mutagenesis was used to introduce point mutations in pFastBac1-ERCC1 (QuikChange kit) as described in Ref. 29. ERCC1 wild-type, ERCC1N110A, ERCC1Y145A, ERCC1Y152A, ERCC1N110A/Y145A, and ERCC1Y145A/Y152A were co-expressed with wild-type XPF in Sf9 insect cells. The heterodimers were purified over nickel affinity, size-exclusion, and heparin chromatography as described (29). Protein concentrations ranged from 0.1 to 0.3 mg/ml.
Nuclease Assay
Nuclease assays of wild-type and mutant ERCC1-XPF on a stem-loop substrate were carried out as described (29). See supplemental material for details.
In Vitro NER Assay
XPF-deficient cell extracts and the plasmid containing a 1,3-intrastrand cisplatin adduct were prepared, and NER assays were carried out as described (30, 31). See supplemental material for details.
Cell Culture
Human fibroblast cells XP2YO (XPF-deficient, GM08437) were cultured at 37 °C in a 5% CO2 humidified incubator in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Chinese hamster ovary cells (CHO) AA8 (wild-type), UV20 (ERCC1-deficient), and UV20 transduced with a lentiviral vector expressing wild-type or mutant hERCC1 were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Atlanta Biologicals), 1% penicillin-streptomycin, and 1% non-essential amino acids (Invitrogen).
Lentiviral Cell Transduction
The cDNA of wild-type ERCC1, ERCC1Y145A, ERCC1N110A, and ERCC1N110A/Y145A were cloned into the lentiviral vector pWPXL by substituting the green fluorescent protein cDNA. 293T cells were co-transfected with the lentiviral vector containing the different constructs, the packaging plasmid psPAX2, and the envelope plasmid pMD2G. Details of the vectors, the production of high titer viruses, and lentiviral transduction can be found through LentiWeb and in Refs. 32 and 33). UV20 CHO cells at 50% confluency were infected with the viral particles containing the different ERCC1 recombinant constructs with a multiplicity of infection of 10 and cultured as described above. The transduction efficiency was assessed by immunofluorescence.
Local UV Irradiation and Immunofluorescence
Cells were seeded and cultured on glass coverslips and processed as described (6). Briefly, cells were covered with a polycarbonate filter with 5-μm pores (Millipore) and irradiated with 120 J/m2 using a UV-C lamp (EL series, UVP, model UVLS-28). After a recovery period, the cells were washed with PBS, permeabilized with 0.2% Triton X-100 in PBS for 30 s, and then fixed with 3% paraformaldehyde containing 0.2% Triton X-100 for 15 min at room temperature. Cells were washed with PBS containing 0.1% Triton five times. To detect (6-4)PP, cells were treated with 0.07 m NaOH in PBS for 5 min and washed. Cells were blocked with PBS+ (PBS containing 0.15% glycine and 0.5% bovine serum albumin) for 30 min and then incubated with the primary antibodies (mouse monoclonal antibody 64M-2 against (6-4)PP (kindly provided by Stuart Clarkson, Geneva, Switzerland), 1:400; rabbit polyclonal antibody FL-297 against ERCC1 (Santa Cruz Biotechnology), 1:300, diluted in PBS+) for 2 h under dark and humid conditions. The samples were embedded in VECTASHIELD mounting medium (Vector Laboratories) containing 4′-6′-diamino-2-phenylindole at a concentration of 0.1 mg/ml. Cells were analyzed using a confocal microscope (Zeiss LSM 510). For quantification, at least 100 cells were analyzed and counted each in three independent experiments.
Clonogenic Survival Assays
Exponentially growing cells were plated in triplicate in 6-cm dishes at 250–5,000 cells/plate depending on the cell line and dose of genotoxin used. After allowing the cells to adhere overnight, they were exposed transiently to genotoxins. For UV, the medium was removed; the cells were washed with PBS and irradiated with UV-C (254 nm), and then the medium was replenished. For mytomycin C or cis-diamminedichloroplatinum(II), the cells were treated with medium containing the drug for 1 h at 37 °C and then washed twice with PBS and incubated in drug-free medium. To measure ionizing radiation sensitivity, cells were irradiated with a 137Cs source. 4 days after exposure, the cultures were fixed and stained with 50% methanol, 7% acetic acid and 0.1% Coomassie Blue. Colonies (defined as ≥20 cells) were counted using a Nikon stereomicroscope with ×10 eyepiece. The data were plotted as the number of colonies that grew on the treated plates relative to untreated plates ± S.E. for a minimum of two independent experiments.
Immunoblotting
Cells were trypsinized, washed with PBS, and lysed with 100 μl of NETT buffer (100 mm NaCl, 50 mm Tris base, pH 7.5, 5 mm EDTA, pH 8.0, 0.5% Triton X-100) containing CompleteTM mini protease inhibitor mixture (Roche Molecular Biochemicals). 60 μg of protein was resolved by SDS-PAGE (8%) and transferred to a nitrocellulose membrane. ERCC1 was detected with antibody D-10 (for both human and hamster, 1:100, Santa Cruz Biotechnology), or FL-297 (human only, 1:200, Santa Cruz Biotechnology). Tubulin (1:200, Abcam) was used as a loading control. Secondary antibodies used were: goat anti-rabbit IgG horseradish peroxidase (1:5000, Promega) and goat anti-mouse IgG horseradish peroxidase (1:5000, Promega).
RESULTS
A short polypeptide from XPA (residues 67–80) binds in a groove on the central domain of ERCC1 spanning residues 105–160. Mutations that alter the strictly conserved XPA residues Gly-72, Gly-73, Gly-74, or Phe-75 abolish NER activity in vitro (27). To study the functional role of the XPA-binding groove of ERCC1 in NER and possibly ICL and DSB repair, we set out to design mutations in ERCC1 that would disrupt the interaction with XPA. Inspection of the XPA-binding site suggested that three absolutely conserved residues in ERCC1 might be important for this interaction (supplemental Fig. 1); ERCC1 residue Asn-110 packs against XPA residue Phe-75, ERCC1 Tyr-145 contacts XPA Gly-74, and ERCC1 Tyr-152 contacts XPA residues Gly-73 and Gly-72 (Fig. 1). These ERCC1 residues are located on three different aspects of the pocket that accepts the XPA peptide, and their side chains contribute a substantial portion of the binding surface. ERCC1 residues Asn-110, Tyr-145, and Tyr-152 were mutated to alanine using site-directed mutagenesis, and we additionally generated the double mutants N110A/Y145A and Y145A/Y152A.
Mutations in the XPA-binding Domain of ERCC1 Affect NER but Not the Nuclease Activity of ERCC1-XPF
The mutant ERCC1 proteins were co-expressed with wild-type XPF in Sf9 insect cells, and the resulting heterodimers were purified in three chromatographic steps using nickel-nitrilotriacetic acid, size exclusion, and heparin columns (29). All of the mutant ERCC1 proteins eluted from the size exclusion column as proper heterodimers (supplemental Fig. 2A), indicating that mutations in the XPA-binding domain of ERCC1 did not disrupt the protein fold of ERCC1 or its interaction with XPF. All the proteins were judged to be >95% pure (supplemental Fig. 2B).
We first tested the effects of the ERCC1 N110A, Y145A, and Y152A mutations on the ability of ERCC1-XPF to incise DNA. The ERCC1 mutant protein complexes were incubated with a stem-loop DNA substrate in the presence of MnCl2 (34), and the release of a 10-mer oligonucleotide product was detected by denaturing PAGE analysis (Fig. 2A). ERCC1-XPF heterodimers containing mutant or wild-type ERCC1 subunits processed this model DNA substrate with similar efficiencies, cleaving at the single-stranded/double-stranded DNA junction of the stem loop. These results indicate that mutation of the Asn-110, Tyr-145, and Tyr-152 residues in ERCC1 does not affect the DNA binding and nuclease activities of the ERCC1-XPF heterodimer.
FIGURE 2.
Mutations in the XPA-binding domain of ERCC1 affect NER activity but not nuclease activity in vitro. A, incision of a stem loop substrate by wild-type and mutant ERCC1-XPF. A 5′-32P-labeled stem-loop DNA substrate (6.7 nm) was incubated with 6.7 nm (lanes 2, 4, 6, 8, 10, and 12) or 26.8 nm (lanes 3, 5, 7, 9, 11, and 13) ERCC1-XPF in the presence of 0.4 mm MnCl2. B, NER activity of wild-type and mutant ERCC1-XPF. A plasmid containing a site-specific 1,3-intrastrand cisplatin DNA cross-link (50 ng) was incubated with a whole cell extract from ERCC1-XPF-deficient cells (XP2YO) complemented with recombinant ERCC1-XPF containing the indicated mutations in ERCC1 (N110A, Y145A, Y152A) or XPF (D720A). The excised DNA fragments of 24–32 nucleotides were detected by annealing a complementary oligonucleotide containing a non-complementary 4G overhang and filling in with [α-32P]dCTP. Protein concentrations of ERCC1-XPF were 13.4 nm (lanes 2, 4, 6, 8, 10, 12, and 14) and 53.6 nm (lanes 3, 5, 7, 9, 11, 13, and 15). A labeled low molecular weight DNA ladder (New England Biolabs) was used as a marker. The position of a 25-mer is indicated.
We next tested the effects of these mutations on NER activity in cell-free extracts. A plasmid containing a site-specific 1,3-intrastrand cisplatin DNA cross-link was incubated with a cell-free extract from XPF-deficient cells (XP-F cells are devoid of both XPF and ERCC1 proteins (35–37)) that was complemented with recombinant ERCC1-XPF proteins containing wild-type or mutant ERCC1 subunits (30). The unsupplemented XP-F cell extract lacked detectable NER activity (Fig. 2B, lane 1). The addition of ERCC1WT-XPF protein to the extract restored NER activity, generating the characteristic excision products of 24–32 nucleotides in length (Fig. 2B, lanes 2 and 3). All mutations in the XPA-binding site of ERCC1 led to a decrease in NER activity, with the strongest effects observed for the ERCC1N110A single, ERCC1Y145A/Y152A, and ERCC1N110A/Y145A double mutants (Fig. 2B, lanes 4–13). The activity of ERCC1N110A/Y145A was only slightly above the background level measured with the catalytically inactive mutant XPFD720A (14) (Fig. 2B, lanes 14 and 15). These results demonstrate that an intact XPA-binding pocket in ERCC1 is required for NER activity in vitro.
XPA-binding Mutants of ERCC1 Do Not Localize to Sites of UV Damage
Having established that the XPA-interaction mutants of ERCC1 are defective in NER in vitro, we assessed whether they prevent the recruitment of ERCC1-XPF to sites of UV damage in living cells. Based on the in vitro results (Fig. 2), we chose the ERCC1N110A, ERCC1Y145A, and ERCC1N110A/Y145A mutants for the cellular studies. ERCC1-deficient UV20 CHO cells (38) were transduced with recombinant lentiviral vectors expressing mutant and wild-type ERCC1 cDNAs (14, 31). Immunoblotting revealed that the transduced UV20 cells stably expressed human ERCC1 protein (Fig. 3A).
FIGURE 3.
Mutations in the XPA-binding domain of ERCC1 affect its recruitment to sites of UV damage. A, expression levels of ERCC1 in transduced UV20 cells. Transduced cells express human ERCC1 tagged with hemagglutinin. Note that human ERCC1 has a slower mobility than the CHO protein due to larger size (297 amino acids versus 293 amino acids) and the presence of the hemagglutinin tag. Tubulin was used as a loading control. B, ERCC1-deficient CHO cells were transduced with wild-type or mutant ERCC1 and irradiated with UV light (120 J/m2) through a polycarbonate filter with 5-μm pores and then fixed and stained for ERCC1 (green) and (6-4)PP (red). DAPI, 4′-6′-diamino-2-phenylindole. C, graphical representation of the percentage of co-localization of ERCC1 with (6-4)PP in UV20 cells expressing various mutants of ERCC1. Data represent the average of at least three independent experiments ± S.D. (error bars). 100 cells were counted for each experiment.
UV20 cells expressing wild-type and mutant ERCC1 were UV-irradiated through a polycarbonate filter with 5-μm pores to generate a pattern of localized DNA damage that could be visualized by immunofluorescence microscopy (6). 1 h after UV irradiation, the cells were fixed, and the presence of (6-4)photoproducts ((6-4)PP) and of ERCC1 at sites of UV damage was monitored by immunofluorescence. As expected, UV20 cells expressing wild-type ERCC1 showed nearly complete (∼90%) co-localization of the nuclease with sites of (6-4)PP (Fig. 3B). By contrast, the single mutants ERCC1N110A or ERCC1Y145A showed less efficient co-localization with UV lesions. ERCC1N110A was detected at 23%, and ERCC1Y145A was detected at only 9% of the sites of UV damage (Fig. 3C). ERCC1N110A/Y145A was observed at sites of UV damage in less than 3% of the cases (Fig. 3, B and C), even if this protein accumulates in the nucleus. These results further support the conclusion that the reduced NER activity of these ERCC1 mutants is due to their failure to interact with XPA.
UV Lesions Persist in Cells Expressing ERCC1N110A/Y145A
To further test the conclusion that the recruitment of ERCC1N110A/Y145A to NER complexes was impaired, we measured the rate of UV lesion repair in ERCC1-deficient UV20 cells and UV20 cells expressing either wild-type ERCC1 or ERCC1N110A/Y145A. Cells were UV-irradiated to generate sites of local DNA damage and fixed at various time points after irradiation, and the amount of damage remaining was assessed by immunodetection of (6-4)PP. Immediately after UV irradiation, 45–55% of the cells contained (6-4)PP, indicating the fraction of cells where a filter pore overlapped with the nucleus (Fig. 4A). At 24 h, 15% of the UV20 cells still stained positively for (6-4)PP (Fig. 4B). This defines the rate of removal of (6-4)PPs from the genome in the absence of NER. (6-4)PPs are likely diluted out during cell division in 24 h (UV20 cells have a doubling time of ∼12 h) or are perhaps eventually removed by other pathways such as homologous recombination. Expression of wild-type ERCC1 in UV20 cells led to a dramatic increase in the rate of (6-4)PP removal, with only 5% of nuclei containing foci 4 h after irradiation and 0% by 8 h after irradiation. By contrast, the slope of the curve indicating removal of (6-4)PP in cells expressing ERCC1N110A/Y145A closely resembled that of untransduced UV20 cells, indicating that NER is severely affected if the XPA-ERCC1 interaction is disrupted.
FIGURE 4.
UV damage persists in UV20 cells expressing ERCC1N110A/Y145A but not wild-type ERCC1. A, untransduced UV20 cells or cells expressing wild-type ERCC1 or ERCC1N110A/Y145A were UV-irradiated as described in the legend for Fig. 3, cultured for 0, 1, 2, 4, 8, or 24 h following UV irradiation, and then fixed and stained for (6-4)PP. B, graphic representation of the percentage of cells with persistent (6-4)PP at various time points. Data represent the average of at least three independent experiments ± S.D. (error bars). 100 cells were counted for each experiment.
Mutations in the XPA-binding Domain of ERCC1 Inhibit NER but Not ICL or DSB Repair
Having established the importance of the XPA-binding domain of ERCC1 for NER, we wished to determine whether this domain is also important for other DNA repair activities of ERCC1-XPF. To test this, UV20 cells expressing wild-type ERCC1, ERCC1N110A, ERCC1Y145A, or ERCC1N110A/Y145A were tested for their sensitivity to UV irradiation, cross-linking agents (mitomycin C and cisplatin), and ionizing radiation by clonogenic survival assays (Fig. 5). As expected, untransduced UV20 cells were hypersensitive to all of these genotoxins relative to parental wild-type AA8 cells. Furthermore, expression of wild-type ERCC1 corrected the sensitivity to all genotoxins. Cells expressing either ERCC1N110A or ERCC1Y145A showed slightly increased sensitivity to UV when compared with wild-type cells and corrected UV20 cells. However, UV20 cells transduced with ERCC1N110A/Y145A were significantly more sensitive to UV than either AA8 or corrected cell lines, although not as sensitive as UV20 cells, demonstrating that mutations in the XPA-interacting domain of ERCC1 affect long term survival following exposure to UV irradiation. Interestingly, all of the ERCC1 mutations studied here supported normal resistance to mitomycin C (Fig. 5B), cisplatin (Fig. 5C), and ionizing radiation (Fig. 5D) at levels comparable with AA8 cells or UV20 cells expressing wild-type ERCC1. These observations provide direct experimental evidence that the ERCC1-XPA interaction is required only for NER and not for ICL and DSB repair activities. The data of the ERCC1N110A/Y145A mutant demonstrate that the functions of ERCC1-XPF in NER can be separated from its functions in ICL and DSB repair by selectively blocking its interaction with XPA.
FIGURE 5.
Mutations in the XPA-binding domain of ERCC1 inhibit NER but not ICL or DSB repair. A–D, clonogenic survival assays to measure the sensitivity of CHO cell lines AA8 (WT) (red diamonds), Ercc1−/− UV20 (blue diamonds), and UV20 expressing ERCC1WT (green diamonds), ERCC1N110A (black diamonds), ERCC1Y145A (purple diamonds), or ERCC1N110A/Y145A (yellow diamonds) to UV-C (A), mytomycin C (MMC) (B), cisplatin (C), or ionizing radiation (D). The data are plotted as the percentage of colonies that grew on the treated plates relative to untreated plates ± S.E. (error bars). cDDP, cis-diamminedichloroplatinum(II).
DISCUSSION
Known Functional Domains of ERCC1-XPF
ERCC1-XPF is a structure-specific endonuclease that cleaves at junctions between single-stranded and double-stranded DNA, incising 3′ single-stranded DNA overhangs. Genetic and biochemical data demonstrate the importance of ERCC1-XPF in multiple genome maintenance mechanisms including NER, ICL repair, double-strand break repair, and telomere biology (39–42). Several functional domains have been identified in the ERCC1-XPF heterodimer; the two proteins interact through two helix-hairpin-helix (HhH) domains at their C termini (43), and these domains are also implicated in DNA binding (28, 44–46). Adjacent to the (HhH)2 domain, XPF contains the active site nuclease domain (29). The N-terminal half of XPF is made up of an SF2 type helicase-like domain. This domain lacks conserved ATP-binding domains and is deficient in ATP binding and helicase activity with a possible role in single-stranded/double-stranded DNA junction binding (47, 48). ERCC1, although evolutionarily related to XPF, is considerably smaller, lacking the helicase domain (49). The N-terminal region of ERCC1 is of unknown structure and function that is expendable for NER (43). Its central domain is structurally homologous to the nuclease domain of XPF but lacks the essential acidic residues of the active site (46, 49). Instead, the groove contains basic and aromatic residues and appears to have single-stranded DNA binding activity (46, 50).
Our present knowledge of ERCC1-XPF therefore suggests a number of interaction sites with DNA, but less is known about how ERCC1-XPF is recruited to different DNA repair pathways through protein-protein interactions. The only protein whose interaction with ERCC1 is understood in some detail is XPA (24–26). Structural studies of the XPA peptide bound to ERCC1 show that it contacts ERCC1 through a conserved GGGF motif and that it undergoes a disorder-to-order transition upon binding ERCC1 (27, 28).
Conserved Residues in the XPA-binding Domain of ERCC1 Are Required for NER but Not Other DNA Repair Pathways
In the present study, we demonstrated the importance of the XPA-binding pocket in the central domain of ERCC1 for NER activity but not other DNA repair functions. Among the proteins with mutations in the XPA-binding pocket in ERCC1 investigated, ERCC1N110A/Y145A showed the most severe effects and was almost completely devoid of NER activity in vitro and in vivo (Figs. 2B and 4). This defect in NER is due to a weakened interaction with XPA because the protein is not recruited to sites of UV damage in cells (Fig. 3), whereas the nuclease activity of ERCC1N110A/Y145A-XPF protein is unaffected (Fig. 2A). UV20 cells that lack functional ERCC1 protein are sensitive to UV irradiation, the cross-linking agents cisplatin and mitomycin C, and to ionizing radiation. Expression of ERCC1N110A/Y145A, which is devoid of NER activity, corrects the defects in other repair processes (Fig. 5), demonstrating a clear separation of function in ERCC1-XPF. We speculate that these point mutations in a transgenic animal would result in a mild phenotype similar to NER-deficient Xpa−/− mice (51) rather than the severe phenotype of Ercc1−/− or Xpf−/− mice (19, 21–23).
How Is ERCC1-XPF Recruited to DNA Repair Pathways Other Than NER?
Although these data and our previous work (27) defined the region in ERCC1 required for its function in NER through interaction with XPA, we know less about how ERCC1-XPF is recruited to sites of ICL and DSB repair. Several additional protein partners were reported for ERCC1 and XPF. ERCC1 interacts with the mismatch repair protein MSH2, and it has been suggested that MSH2 and ERCC1 work together in a replication-independent ICL repair pathway (52, 53). However, the sensitivity of MSH2-deficient cells to cross-linking agents is much less pronounced than that of ERCC1-deficient cells (54), making it unlikely that the interaction with MSH2 is required for the major ICL repair pathways. XPF is also reported to interact with hRad52, an association that might direct ERCC1-XPF to sites of homologous recombination or single-strand annealing (55).
The Drosophila homolog of XPF, MEI-9, interacts with ERCC1 as well as MUS312. Mus312 mutants and a Mei-9 mutant are hypersensitive to cross-linking agents but not UV irradiation, strongly suggesting a role in ICL repair (56). Recent studies have identified SLX4 as a human homolog of MUS312 (57–60). SLX4 interacts with a number of endonucleases, including ERCC1-XPF, MUS81-EME1, and SLX1. It is therefore likely that SLX4 serves as a platform to coordinate the activity of various endonucleases and has a key role in targeting ERCC1-XPF to sites of ICL repair.
Which Function of ERCC1-XPF Causes Resistance to Cisplatin in Tumor Cells?
In addition to its role in protecting cells against the deleterious effects of DNA damage, ERCC1 is a potential target for cancer chemotherapy. Numerous studies have correlated the levels of ERCC1 in tumors as well as polymorphisms in the Ercc1 gene with response to platinum- based chemotherapy and survival (61, 62). A common perception is that this resistance to cisplatin is due to enhanced levels of NER (63). By contrast, other studies have indicated that the treatment of cells with small interfering RNA against ERCC1, but not against XPA, renders cells sensitive to cross-linking agents (64). Our studies show directly that a defect in the interaction between ERCC1 and XPA that disrupts NER has no major effect on the cellular sensitivity to the chemotherapeutic agents cisplatin and mytomycin C. It therefore appears that NER mediated by ERCC1 is not a key determinant of cellular sensitivity to cisplatin. Cisplatin, like other ICL-forming agents, forms a variety of adducts including 1,2 and 1,3-intrastrand cross-links as well as 1,2-interstrand cross-links (65). Among these, the 1,3-intrastrand cross-link is an excellent NER substrate, whereas the major, but less distorting, 1,2-intrastrand cisplatin adduct is less well repaired by NER and may be shielded from NER by additional proteins (66–71). The 1,2-interstrand cisplatin, on the other hand, does not appear to be recognized by the NER machinery at all (67). The sensitivity of ERCC1-deficient cells to cisplatin is therefore almost certainly due to the presence of the 1,2-interstrand cross-links, where ERCC1-XPF plays a role in the unhooking and/or homologous recombination step (40, 72). These observations again emphasize the importance of finding additional sites for protein-protein interactions in ERCC1-XPF and interacting proteins that target the heterodimer to sites of ICL and DSB repair.
Supplementary Material
Acknowledgment
We thank Arthur J. Campbell for help with Fig. 1.
This work was supported, in whole or in part, by National Institutes of Health Grants GM080454 (to O. D. S.), CA092584 (to O. D. S. and T. E.), and ES016114 (to L. J. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. 1 and 2.
- NER
- nucleotide excision repair
- ICL
- interstrand cross-link
- XP
- xeroderma pigmentosum
- ERCC
- excision repair cross-complementing
- (6-4)PP
- (6-4)photoproduct
- CHO
- Chinese hamster ovary
- PBS
- phosphate-buffered saline
- DSB
- double strand break
- WT
- wild type.
REFERENCES
- 1.Friedberg E. C., Walker G. C., Siede W., Wood R. D., Schultz R. A., Ellenberger T. (2005) DNA Repair and Mutagenesis, 2nd Ed., ASM Press, Washington, D. C. [Google Scholar]
- 2.Gillet L. C., Schärer O. D. (2006) Chem. Rev. 106, 253–276 [DOI] [PubMed] [Google Scholar]
- 3.Hanawalt P. C., Spivak G. (2008) Nat. Rev. Mol. Cell Biol. 9, 958–970 [DOI] [PubMed] [Google Scholar]
- 4.Sugasawa K., Ng J. M., Masutani C., Iwai S., van der Spek P. J., Eker A. P., Hanaoka F., Bootsma D., Hoeijmakers J. H. (1998) Mol. Cell 2, 223–232 [DOI] [PubMed] [Google Scholar]
- 5.Sugasawa K., Okuda Y., Saijo M., Nishi R., Matsuda N., Chu G., Mori T., Iwai S., Tanaka K., Tanaka K., Hanaoka F. (2005) Cell 121, 387–400 [DOI] [PubMed] [Google Scholar]
- 6.Volker M., Moné M. J., Karmakar P., van Hoffen A., Schul W., Vermeulen W., Hoeijmakers J. H., van Driel R., van Zeeland A. A., Mullenders L. H. (2001) Mol. Cell 8, 213–224 [DOI] [PubMed] [Google Scholar]
- 7.Riedl T., Hanaoka F., Egly J. M. (2003) EMBO J. 22, 5293–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fousteri M., Vermeulen W., van Zeeland A. A., Mullenders L. H. (2006) Mol. Cell 23, 471–482 [DOI] [PubMed] [Google Scholar]
- 9.Evans E., Moggs J. G., Hwang J. R., Egly J. M., Wood R. D. (1997) EMBO J. 16, 6559–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapias A., Auriol J., Forget D., Enzlin J. H., Schärer O. D., Coin F., Coulombe B., Egly J. M. (2004) J. Biol. Chem. 279, 19074–19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakasugi M., Sancar A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6669–6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Laat W. L., Appeldoorn E., Sugasawa K., Weterings E., Jaspers N. G., Hoeijmakers J. H. (1998) Genes Dev. 12, 2598–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camenisch U., Dip R., Schumacher S. B., Schuler B., Naegeli H. (2006) Nat. Struct. Mol. Biol. 13, 278–284 [DOI] [PubMed] [Google Scholar]
- 14.Staresincic L., Fagbemi A. F., Enzlin J. H., Gourdin A. M., Wijgers N., Dunand-Sauthier I., Giglia-Mari G., Clarkson S. G., Vermeulen W., Schärer O. D. (2009) EMBO J. 28, 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocquet V., Lainé J. P., Riedl T., Yajin Z., Lee M. Y., Egly J. M. (2008) EMBO J. 27, 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogi T., Lehmann A. R. (2006) Nat. Cell Biol. 8, 640–642 [DOI] [PubMed] [Google Scholar]
- 17.Moser J., Kool H., Giakzidis I., Caldecott K., Mullenders L. H., Fousteri M. I. (2007) Mol. Cell 27, 311–323 [DOI] [PubMed] [Google Scholar]
- 18.Lehmann A. R. (2003) Biochimie 85, 1101–1111 [DOI] [PubMed] [Google Scholar]
- 19.Niedernhofer L. J., Garinis G. A., Raams A., Lalai A. S., Robinson A. R., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W., Theil A. F., Vermeulen W., van der Horst G. T., Meinecke P., Kleijer W. J., Vijg J., Jaspers N. G., Hoeijmakers J. H. (2006) Nature 444, 1038–1043 [DOI] [PubMed] [Google Scholar]
- 20.Jaspers N. G., Raams A., Silengo M. C., Wijgers N., Niedernhofer L. J., Robinson A. R., Giglia-Mari G., Hoogstraten D., Kleijer W. J., Hoeijmakers J. H., Vermeulen W. (2007) Am. J. Hum. Genet. 80, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWhir J., Selfridge J., Harrison D. J., Squires S., Melton D. W. (1993) Nat. Genet. 5, 217–224 [DOI] [PubMed] [Google Scholar]
- 22.Weeda G., Donker I., de Wit J., Morreau H., Janssens R., Vissers C. J., Nigg A., van Steeg H., Bootsma D., Hoeijmakers J. H. (1997) Curr. Biol. 7, 427–439 [DOI] [PubMed] [Google Scholar]
- 23.Tian M., Shinkura R., Shinkura N., Alt F. W. (2004) Mol. Cell. Biol. 24, 1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park C. H., Sancar A. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5017–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Elledge S. J., Peterson C. A., Bales E. S., Legerski R. J. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5012–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L., Peterson C. A., Lu X., Legerski R. J. (1995) Mol. Cell. Biol. 15, 1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsodikov O. V., Ivanov D., Orelli B., Staresincic L., Shoshani I., Oberman R., Schärer O. D., Wagner G., Ellenberger T. (2007) EMBO J. 26, 4768–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripsianes K., Folkers G. E., Zheng C., Das D., Grinstead J. S., Kaptein R., Boelens R. (2007) Nucleic Acids Res. 35, 5789–5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enzlin J. H., Schärer O. D. (2002) EMBO J. 21, 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivji M. K., Moggs J. G., Kuraoka I., Wood R. D. (1999) Methods Mol. Biol. 113, 373–392 [DOI] [PubMed] [Google Scholar]
- 31.Dunand-Sauthier I., Hohl M., Thorel F., Jaquier-Gubler P., Clarkson S. G., Schärer O. D. (2005) J. Biol. Chem. 280, 7030–7037 [DOI] [PubMed] [Google Scholar]
- 32.Salmon P., Kindler V., Ducrey O., Chapuis B., Zubler R. H., Trono D. (2000) Blood 96, 3392–3398 [PubMed] [Google Scholar]
- 33.Salmon P., Trono D. (2006) Curr. Protoc. Neurosci. Chapter 4, Unit 4.21 [DOI] [PubMed] [Google Scholar]
- 34.de Laat W. L., Appeldoorn E., Jaspers N. G., Hoeijmakers J. H. (1998) J. Biol. Chem. 273, 7835–7842 [DOI] [PubMed] [Google Scholar]
- 35.Biggerstaff M., Szymkowski D. E., Wood R. D. (1993) EMBO J. 12, 3685–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vuuren A. J., Appeldoorn E., Odijk H., Yasui A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. (1993) EMBO J. 12, 3693–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagi T., Wood R. D., Takebe H. (1997) Mutagenesis 12, 41–44 [DOI] [PubMed] [Google Scholar]
- 38.Rolig R. L., Lowery M. P., Adair G. M., Nairn R. S. (1998) Mutagenesis 13, 357–365 [DOI] [PubMed] [Google Scholar]
- 39.Sijbers A. M., de Laat W. L., Ariza R. R., Biggerstaff M., Wei Y. F., Moggs J. G., Carter K. C., Shell B. K., Evans E., de Jong M. C., Rademakers S., de Rooij J., Jaspers N. G., Hoeijmakers J. H., Wood R. D. (1996) Cell 86, 811–822 [DOI] [PubMed] [Google Scholar]
- 40.Niedernhofer L. J., Odijk H., Budzowska M., van Drunen E., Maas A., Theil A. F., de Wit J., Jaspers N. G., Beverloo H. B., Hoeijmakers J. H., Kanaar R. (2004) Mol. Cell. Biol. 24, 5776–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad A., Robinson A. R., Duensing A., van Drunen E., Beverloo H. B., Weisberg D. B., Hasty P., Hoeijmakers J. H., Niedernhofer L. J. (2008) Mol. Cell. Biol. 28, 5082–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X. D., Niedernhofer L., Kuster B., Mann M., Hoeijmakers J. H., de Lange T. (2003) Mol. Cell 12, 1489–1498 [DOI] [PubMed] [Google Scholar]
- 43.Sijbers A. M., van der Spek P. J., Odijk H., van den Berg J., van Duin M., Westerveld A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. (1996) Nucleic Acids Res. 24, 3370–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishino T., Komori K., Ishino Y., Morikawa K. (2005) Structure 13, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 45.Newman M., Murray-Rust J., Lally J., Rudolf J., Fadden A., Knowles P. P., White M. F., McDonald N. Q. (2005) EMBO J. 24, 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsodikov O. V., Enzlin J. H., Schärer O. D., Ellenberger T. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11236–11241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sgouros J., Gaillard P. H., Wood R. D. (1999) Trends Biochem. Sci. 24, 95–97 [DOI] [PubMed] [Google Scholar]
- 48.Nishino T., Komori K., Tsuchiya D., Ishino Y., Morikawa K. (2005) Structure 13, 143–153 [DOI] [PubMed] [Google Scholar]
- 49.Gaillard P. H., Wood R. D. (2001) Nucleic Acids Res. 29, 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripsianes K., Folkers G., Ab E., Das D., Odijk H., Jaspers N. G., Hoeijmakers J. H., Kaptein R., Boelens R. (2005) Structure 13, 1849–1858 [DOI] [PubMed] [Google Scholar]
- 51.de Vries A., van Oostrom C. T., Hofhuis F. M., Dortant P. M., Berg R. J., de Gruijl F. R., Wester P. W., van Kreijl C. F., Capel P. J., van Steeg H., et al. (1995) Nature 377, 169–173 [DOI] [PubMed] [Google Scholar]
- 52.Lan L., Hayashi T., Rabeya R. M., Nakajima S., Kanno S., Takao M., Matsunaga T., Yoshino M., Ichikawa M., Riele H., Tsuchiya S., Tanaka K., Yasui A. (2004) DNA Repair 3, 135–143 [DOI] [PubMed] [Google Scholar]
- 53.Zhang N., Lu X., Zhang X., Peterson C. A., Legerski R. J. (2002) Mol. Cell. Biol. 22, 2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Q., Christensen L. A., Legerski R. J., Vasquez K. M. (2005) EMBO Rep. 6, 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motycka T. A., Bessho T., Post S. M., Sung P., Tomkinson A. E. (2004) J. Biol. Chem. 279, 13634–13639 [DOI] [PubMed] [Google Scholar]
- 56.Yildiz O., Majumder S., Kramer B., Sekelsky J. J. (2002) Mol. Cell 10, 1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fekairi S., Scaglione S., Chahwan C., Taylor E. R., Tissier A., Coulon S., Dong M. Q., Ruse C., Yates J. R., 3rd, Russell P., Fuchs R. P., McGowan C. H., Gaillard P. H. (2009) Cell 138, 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Svendsen J. M., Smogorzewska A., Sowa M. E., O'Connell B. C., Gygi S. P., Elledge S. J., Harper J. W. (2009) Cell 138, 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muñoz I. M., Hain K., Déclais A. C., Gardiner M., Toh G. W., Sanchez-Pulido L., Heuckmann J. M., Toth R., Macartney T., Eppink B., Kanaar R., Ponting C. P., Lilley D. M., Rouse J. (2009) Mol. Cell 35, 116–127 [DOI] [PubMed] [Google Scholar]
- 60.Andersen S. L., Bergstralh D. T., Kohl K. P., LaRocque J. R., Moore C. B., Sekelsky J. (2009) Mol. Cell 35, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gossage L., Madhusudan S. (2007) Cancer Treat. Rev. 33, 565–577 [DOI] [PubMed] [Google Scholar]
- 62.Martin L. P., Hamilton T. C., Schilder R. J. (2008) Clin. Cancer Res. 14, 1291–1295 [DOI] [PubMed] [Google Scholar]
- 63.Olaussen K. A., Fouret P., Kroemer G. (2007) N. Engl. J. Med. 357, 1559–1561 [DOI] [PubMed] [Google Scholar]
- 64.Cummings M., Higginbottom K., McGurk C. J., Wong O. G., Köberle B., Oliver R. T., Masters J. R. (2006) Biochem. Pharmacol. 72, 166–175 [DOI] [PubMed] [Google Scholar]
- 65.Jamieson E. R., Lippard S. J. (1999) Chem. Rev. 99, 2467–2498 [DOI] [PubMed] [Google Scholar]
- 66.Huang J. C., Zamble D. B., Reardon J. T., Lippard S. J., Sancar A. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10394–10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamble D. B., Mu D., Reardon J. T., Sancar A., Lippard S. J. (1996) Biochemistry 35, 10004–10013 [DOI] [PubMed] [Google Scholar]
- 68.Moggs J. G., Yarema K. J., Essigmann J. M., Wood R. D. (1996) J. Biol. Chem. 271, 7177–7186 [DOI] [PubMed] [Google Scholar]
- 69.Moggs J. G., Szymkowski D. E., Yamada M., Karran P., Wood R. D. (1997) Nucleic Acids Res. 25, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guggenheim E. R., Xu D., Zhang C. X., Chang P. V., Lippard S. J. (2009) Chembiochem 10, 141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu G., Lippard S. J. (2009) Biochemistry 48, 4916–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Silva I. U., McHugh P. J., Clingen P. H., Hartley J. A. (2002) Nucleic Acids Res. 30, 3848–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.